Abstract
Despite the effectiveness of agonist maintenance for opioid dependence, individuals can remain on waitlists for months, during which they are at significant risk for morbidity and mortality. Interim dosing, consisting of daily medication without counseling, can reduce these risks. In this pilot study, we examined the initial feasibility of a novel technology-assisted interim buprenorphine treatment for waitlisted opioid-dependent adults. Following buprenorphine induction during Week 1, participants (n=10) visited the clinic at Weeks 2, 4, 6, 8, 10 and 12 to ingest their medication under staff observation, provide a urine specimen and receive their remaining doses via a computerized Med-O-Wheel Secure device. They also received daily monitoring via an Interactive Voice Response (IVR) platform, as well as random call-backs for urinalysis and medication adherence checks. The primary outcome was percent of participants negative for illicit opioids at each 2-week visit, with secondary outcomes of past-month drug use, adherence and acceptability. Participants achieved high levels of illicit opioid abstinence, with 90% abstinent at the Week 2 and 4 visits and 60% at Week 12. Significant reductions were observed in self-reported past-month illicit opioid use (p<.001), opioid withdrawal (p<.001), opioid craving (p<.001) and ASI Drug composite score (p=.008). Finally, adherence with buprenorphine administration (99%), daily IVR calls (97%) and random call-backs (82%) was high. Interim buprenorphine treatment shows promise for reducing patient and societal risks during delays to conventional treatment. A larger-scale, randomized clinical trial is underway to more rigorously examine the efficacy of this treatment approach.
Keywords: Opioid abuse, Buprenorphine, Interim treatment, Technology
1. Introduction
Opioid misuse and dependence are reaching epidemic proportions in the United States (US), resulting in overdoses, premature death, infectious disease and economic costs of $56 billion annually (Becker et al., 2008; Birnbaum et al., 2011; Clausen et al., 2009; Hser et al. 2001; Jones et al., 2013; Paulozzi, 2012; SAMHSA, 2010a; Shah et al., 2008; Wisniewski et al., 2008). The problem is increasingly urgent in rural areas which often struggle with high rates of opioid misuse and a lack of available treatment options (Fortney & Booth, 2001; Lenardson & Gale, 2007; Rosenblum et al., 2011; Rounsaville & Kosten, 2000; Sigmon, 2014).
While opioid maintenance is the most efficacious treatment (Johnson et al., 2000; Mattick et al., 2014; Stotts et al., 2009), demand for treatment can exceed available capacity (Friedmann et al., 2003; Wenger & Rosenbaum, 1994). An alarming number of methadone clinics have waitlists, due in part to inadequate public funding, unfavorable zoning regulations and requirements for comprehensive care that increase their cost (Des Jarlais et al., 1995; Fountain et al., 2000; Gryczynski et al., 2009; Peles et al., 2012, 2013; Peterson et al., 2010). Many areas also lack office-based buprenorphine treatment capacity due to barriers to obtaining the special federal certification needed for prescribing buprenorphine, physicians’ concerns about induction logistics, reimbursement challenges, potential for medication nonadherence or diversion, and challenges in delivering psychosocial services to patients (Barry et al., 2008; Becker & Fiellin, 2006; Kissin et al., 2006; Netherland et al., 2009; Sigmon, 2015). Taken together, opioid-dependent individuals can remain on waitlists for months and are at significant risk for illicit drug use, criminal activity, infectious disease and mortality during this delay to treatment (Adamson & Sellman, 1998; Clausen et al., 2009; Cooper, 1989; Darke & Hall, 2003; Schwartz et al., 2009, 2011; Warner-Smith et al., 2001; Wenger & Rosenbaum, 1994).
One effort to reduce these risks has been to offer interim methadone treatment (IMT), in which methadone clinics can provide medication without accompanying psychosocial services on a temporary basis when only a waiting list would be otherwise available (Federal Register, 1993; IOM, 1995). IMT has been consistently demonstrated to reduce drug use, drug-related risk behaviors and criminal activity among patients awaiting entry into comprehensive treatment (Gruber et al., 2008; Schwartz et al., 2006, 2007, 2008, 2009a,b, 2011; Yancovitz et al., 1991). However, methadone treatment in the US is limited to licensed specialty clinics, it requires daily visits during the first few months of treatment and the medication poses considerable risk of overdose death if ingested by non-tolerant individuals (Luty et al., 2005). Further, federal IMT regulations mandate that patients ingest all doses under direct observation, requiring daily clinic visits (IOM, 1995). They also limit its duration to 120 days, with clinics required to discharge patients at that time if a slot has not become available. Despite successful efforts in Baltimore to bring IMT to scale (Schwartz et al., 2009), few other localities have used this approach. Taken together, these features have constrained the ability of IMT to treat opioid users while they await entry into standard opioid treatment.
We have been developing a novel interim opioid treatment to bridge delays in treatment access while surmounting the above barriers. Our intervention consists of four components, each selected to support delivery of an efficacious pharmacotherapy to waitlisted opioid-dependent adults while reducing the risk of medication nonadherence and diversion. First, we chose buprenorphine as the interim dosing medication because its pharmacological profile is associated with reduced abuse liability and overdose risk (Bickel & Amass, 1995; Johnson et al., 2003; Walsh et al., 1994, 1995). Buprenorphine is also available without the rigid regulatory regulations, daily observation of dosing and 120-day limit required for interim methadone. Second, we are using a computerized device to facilitate mobile buprenorphine dispensing while reducing risk of nonadherence or diversion. While pill bottles with Medication Event Monitoring System caps (MEMS; Aprex Corporation, Fremont, CA) have been used for years, they have substantive limitations. Patients can access all of their doses each time they open the bottle, and the cap only records a time-date stamp for each opening rather than the amount of medication removed. For the present study, we used the Med-O-Wheel Secure device (Addoz, Forssa, Finland), a portable, disc-shaped device which can hold several weeks’ worth of doses with each dose stored in a separate cell and only available during a predetermined 3-hour window. The Med-O-Wheel also includes locks and alarms to prevent tampering and access to tablets outside the preset time window. Third, we developed a mobile health (mHealth) platform for monitoring patients on a daily basis. mHealth applications hold significant potential for permitting delivery of monitoring, education and treatment beyond the confines of the medical office (Boyer et al., 2010). Particularly promising are those that provide customized content or monitoring via phone, as phone-based systems offer advantages of low cost, consistent delivery, expanded access, 24-hour availability, privacy and convenience (Crawford et al., 2005; Helzer et al., 2008; Kim et al., 2007; Moore et al., 2013; Rose et al., 2010; Stacy et al., 2009). Our mHealth system uses an Interactive Voice Response (IVR) platform to deliver automated calls to participants nightly to assess their drug use, withdrawal and craving. Finally, while biochemical verification via urine drug testing is the most objective method for evaluating recent drug use (Chermack et al., 2000; Fendrich et al., 2004; Kilpatrick et al., 2000; Preston et al., 1997; Wish et al., 1997), frequent visits are incompatible with resource-constrained settings and with rural areas where daily travel to treatment is challenging. We developed a random sampling approach whereby patients are contacted at random times via IVR and instructed to return to the clinic for urinalysis. Random sampling increases the effectiveness of urine monitoring, as patients remain unaware of when the next screen will be requested, reducing the possibility that they can tailor drug use to subvert monitoring (Harford & Kleber, 1978; Manno, 1986). At each random call-back, participants also must present their device for inspection by staff to ensure there is no indication of tampering, nonadherence or diversion.
In this 12-week pilot study, we sought to evaluate the initial feasibility of this novel interim buprenorphine treatment (IBT) in reducing illicit opioid use and drug-related risk behavior among opioid-dependent individuals awaiting entry into agonist maintenance. Our aim was to pilot the intervention in a small sample of waitlisted opioid abusers and identify any procedural adjustments indicated to be necessary prior to proceeding with a larger-scale randomized trial.
2. Methods
2.1 Participants
Opioid-dependent adults were recruited via flyers posted throughout the community and distributed to local opioid treatment providers. Eligible participants had to be ≥18 years old, in good health, meet DSM-V criteria for opioid use disorder, provide an opioid-positive urine specimen and be currently waitlisted with an opioid treatment program (OTP) or office-based buprenorphine provider. Those with a significant psychiatric or medical illness that could interfere with participation were excluded, as were those who were pregnant or nursing. Individuals dependent on sedative-hypnotics were also excluded, due to the medical risks and notably low success rates with sedative-dependent opioid abusers (Stitzer & Chutuape, 1999). The study was approved by the University of Vermont institutional review board and participants provided written informed consent prior to participating. All 10 individuals who were screened, deemed eligible and offered the study agreed to participate.
2.2 Study design
Participants completed buprenorphine induction during Week 1 (or longer if required), during which they attended the clinic daily. Thereafter, they visited the clinic at Weeks 2, 4, 6, 8, 10 and 12 to ingest their buprenorphine dose under staff observation, provide a urine specimen and receive their remaining doses in the Med-O-Wheel. Participants received calls daily from the IVR system, as well as IVR-generated random call-backs (approximately twice monthly) for an additional urinalysis and medication adherence check visit.
2.3 Assessments
Intake screening included a Demographic and Drug History Questionnaire, the psychoactive substance dependence section of the DSM-V (APA, 2013) and Addiction Severity Index (ASI; McLellan et al., 1985). Participants also completed a medical history, received a brief physical examination, provided a urine specimen and received $30. At each subsequent visit, their use of alcohol, prescribed and illicit drugs was assessed via Time-Line Followback (Sobell et al., 1988). At Weeks 4, 8 and 12, participants completed follow-up assessments consisting of modified versions of the intake battery, with an additional treatment satisfaction survey administered at Week 12.
2.4 Buprenorphine administration
Study medication consisted of buprenorphine/naloxone sublingual tablets (Amneal Pharmaceuticals, Bridgewater, New Jersey). During Week 1, participants attended the clinic daily and individualized dose adjustments were determined using the Clinical Institute Narcotic Assessment (Peachey & Lei, 1988) with the aim of stabilizing participants on a dose sufficient to achieve withdrawal suppression without intoxication or sedation (Sigmon et al., 2009, 2013). At subsequent visits during Weeks 2–12, participants ingested that day’s buprenorphine dose under nurse observation and received the remaining doses for the upcoming interval in the Med-O-Wheel. Each dose was secured in individually-locked compartments and available only during a predetermined 3-hour window each day. Participants were instructed to bring the device with them to both scheduled and random call-back visits (below). They could also return to the clinic between scheduled visits if concerns arose or a dose evaluation was needed.
2.5 Biochemical monitoring
Participants provided urine specimens under observation of a same-sex staff member at each visit. Specimens were immediately temperature tested and analyzed via enzyme multiplied immunoassay (Microgenics, Fremont, CA) for opioids (e.g., methadone, buprenorphine, oxycodone, hydrocodone, hydromorphone, heroin). If a participant failed to provide a scheduled urine sample, it was considered positive for illicit opioids. Samples were also tested for non-opioid drugs (e.g., cocaine, amphetamines, benzodiazepines, marijuana). Finally, participants provided a breath sample which was analyzed for recent alcohol use (ALCO-SENSOR III, Intoximeters, Inc., St. Louis, MO).
2.6 mHealth monitoring
The IVR system contacted participants each evening to assess any opioids or other drug or alcohol use, as well as opioid-related craving or withdrawal symptoms. Instances of use, craving or withdrawal prompted additional detailed questions (e.g., type and amount used, severity of craving and withdrawal), as well as provision of information about ongoing support meetings taking place in the community. Participants could also make inbound calls to the system at any time to complete their daily check-in if they anticipated missing the call.
2.7 Random call-backs
Participants were contacted via IVR approximately twice per month on a random basis and instructed to return to the clinic within 12 hours. For each call-back, they were instructed to refrain from taking that day’s dose and instead bring in their Med-O-Wheel device to ingest their medication under observation of the research nurse. The device was inspected for any evidence of tampering, nonadherence or diversion, and participants provided a staff-observed urine specimen.
2.8 Data Analyses
Analyses were performed on all subjects independent of early dropout or noncompliance, consistent with an intent-to-treat approach (Armitage, 1983). Missing urine samples were considered as opioidpositive for the primary analysis. Descriptive statistics were used to characterize baseline demographic and drug use variables and the percent of urine specimens testing negative for illicit opioids at each 2-week study visit. Overall percent abstinence and its corresponding 95% confidence interval were derived based on GEE modeling that accounts for correlated observations within each participant. Similar to prior studies (Fiellin et al., 2006), buprenorphine adherence was evaluated using a review of nurses’ notes, random call-back data and Med-O-Wheel monitoring. Participants were classified as adherent for a given day if adherence was documented by a nurse’s note or by the Med-O-Wheel having successfully dispensed a dose on that day. Repeated measures analyses of variance were used to examine changes in ASI composite scores and frequency of self-reported illicit opioid use across intake, 4-, 8- and 12-week follow-ups. Growth curves analyses using random intercept and slope models were used to describe temporal trends in withdrawal and craving. Analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Statistical significance was determined using α=.05.
3. Results
3.1 Participants
Demographic and drug use characteristics are presented in Table 1. Participants reported using opioids regularly for 8.4 ± 4.6 years, and 40% reported a lifetime history of intravenous drug use. While most endorsed a prescription opioid as their current primary drug of abuse, 60% reported a lifetime history of heroin use. Participants had been on a treatment waitlist for 5.5±6.3 months, with all currently waitlisted for a local OTP and 30% also waitlisted for office-based buprenorphine treatment. When asked to rate how troubled or bothered they were by their inability to access treatment from 0 (not at all) to 100 (extremely), participants’ mean rating was 89.6±16.
Table 1.
Demographic and Drug Use Characteristics (n=10)
| Age | 35.7(9.4) |
| Male, % | 50 |
| Caucasian, % | 100 |
| Employed full-time, % | 40 |
| Education, yrs | 12.1(0.2) |
| Duration of regular opioid use, yrs | 8.4(4.6) |
| Past-month opioid use, days | 29.3(0.8) |
| Ever used IV, % | 40 |
| Ever used heroin, % | 60 |
| Primary route of administration, % | |
| Oral/sublingual | 40 |
| Intranasal | 40 |
| Intravenous | 20 |
| Past year primary opioid of abuse | |
| Prescription opioid, % | 80 |
| Buprenorphine, %a | 63 |
| Mean daily dose, mg | 11.2 |
| Oxycodone, %a | 37 |
| Mean daily dose, mg | 126.7 |
| Heroin, % | 20 |
| Mean daily amount, bags | 13.8 |
| Addiction Severity Index (ASI)b | |
| Drug | .36 (.08) |
| Alcohol | .06 (.07) |
| Employment | .36 (.30) |
| Legal | .12 (.21) |
| Family/Social | .12 (.13) |
| Psychiatric | .19 (.22) |
| Medical | .41 (.37) |
Note: Values represent mean(SD) unless otherwise indidated.
The percentages of participants endorsing each prescription opioid subtype are based on the total of 8 primary prescription opioid users.
ASI composite scale scores range from 0–1.
3.2 Technology-assisted buprenorphine administration
Participants’ mean buprenorphine dose was 15.4 ± 4.0 mg. A total of 819 buprenorphine doses were administered during the study, with 161 ingested at the clinic under staff observation and 658 dispensed via Med-O-Wheel. All aspects of the technology-assisted buprenorphine administration went smoothly, and all devices were returned at the end of the study. Buprenorphine adherence was high, with participants taking their medication on 99% of study days.
3.3 Opioid abstinence
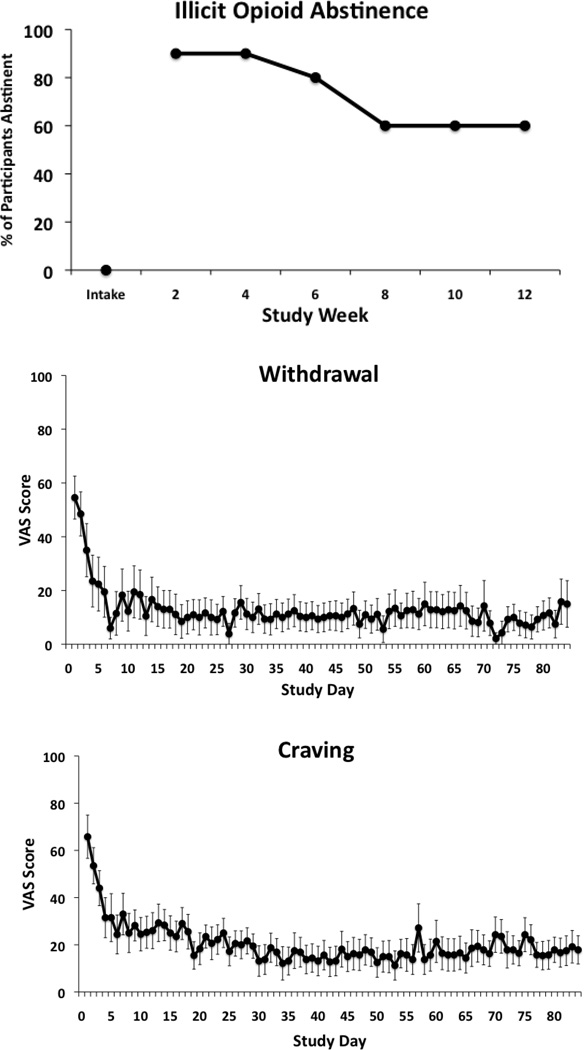
Participants achieved favorable rates of illicit opioid abstinence, submitting a total of 73.3% (95% CI: 47–89%) illicit opioid-negative specimens across the six scheduled study visits. Of the specimens categorized as opioid positive, 31% tested positive for illicit opioids and 69% were missing and thus presumed positive. Inspection of abstinence data over time showed an increase following intake in the percent of participants negative for illicit opioids, which peaked at 90% at the Week 2 and Week 4 assessments (Figure 1, upper panel). Abstinence rates declined somewhat in the final weeks, though 60% of participants were abstinent from illicit opioids at the final Week 12 visit.
Figure 1.
Participants reported a significant reduction in number of days of illicit opioid use in the past month, from 29.3±0.8 days at intake to 3.98±1.0, 0.14±0.1 and 0.35±0.2 days at the 4-, 8- and 12-week follow-ups, respectively (p<.001). Amount of money spent on illicit opioids during the past month showed a similar pattern, decreasing from a mean of $1,294 (range: $520 - $4000) at intake to only one subject reporting spending a total of $150 at Week 4 and no reported money spent at the 8- and 12-week assessments.
3.4 Clinical stability
Seventy percent (7/10) of participants were retained through the 12 weeks of treatment, with the three noncompleters dropping out at Weeks 3, 5 and 9. Participants reported significant reductions in IVR-assessed opioid withdrawal (p<.001) and craving (p<.001) during stabilization, after which levels remained low for the remainder of the study (Figure 1, middle and lower panels). With regard to adherence, 97% of the daily IVR calls were satisfactorily completed. Participants successfully completed 82% of random call-backs and, when the urine specimens collected at these random visits were examined, 96.3% tested negative for illicit opioids. With regard to other drugs of abuse, 92%, 100%, 100%, and 42% of scheduled urine specimens tested negative for cocaine, amphetamines, benzodiazepines and marijuana, respectively. Finally, there were significant changes in the Drug (p=.008) subscale score of the ASI from baseline to the 4- 8- and 12-week follow-up assessments, with all follow-up scores significantly lower than intake (p's<.05).
3.5 Treatment acceptability
At Week 12, participants’ satisfaction with the IBT intervention as a whole as well as its individual components was assessed, from 1 (not at all) to 5 (extremely). Mean ratings were 4.7±0.9 for IBT and 4.9±0.1, 4.9±0.1, 3.4±0.7 and 3.5±0.9 for the buprenorphine, Med-O-Wheel, IVR and random call-back components, respectively. When asked to identify the most valuable IBT component, all participants endorsed buprenorphine. The questionnaire also included an open-ended question inviting participants to offer suggestions for any changes that should be made to the IBT intervention. The most common suggestion, submitted by 40% of participants at the 12-week assessment, was to extend the length of IBT so that it remains in place until they gained entry into standard treatment. On a related note, while research staff made great efforts during the study to help participants find a treatment provider who could take them upon the conclusion of their participation, only one was able to transition immediately into an available treatment slot following the study.
4. Discussion
Despite the demonstrated effectiveness of agonist maintenance for opioid dependence, treatment capacity is inadequate to meet demand in many areas of the country. There is a critical need to develop innovative and effective approaches for bridging gaps in treatment access. In this pilot study, we sought to evaluate the feasibility of IBT for extending evidence-based pharmacotherapy to waitlisted opioid-dependent adults. While buprenorphine’s pharmacological and regulatory profile lends towards its use with reduced-intensity approaches, only a single study to date has evaluated its utility in an interim dosing paradigm for waitlisted individuals. That trial was conducted in Norway over a decade ago with heroin-dependent individuals awaiting methadone treatment (Krook et al., 2002). Participants were randomized to receive buprenorphine or placebo for 12 weeks, without psychosocial support. Buprenorphine treatment was associated with significantly greater retention and lower self-reported heroin use, though attrition was still high with two-thirds of patients having dropped out by Week 12. The investigators also used no objective measure of opioid abstinence, relying instead on patients to rate their recent drug use via visual analogue scales. Thus, the present study represents the first effort to our knowledge to evaluate buprenorphine’s potential utility in an interim dosing approach with abstinence confirmed biochemically.
Participants achieved favorable rates of opioid abstinence, with 73% of specimens testing negative for illicit opioids and 60% of participants abstinent at the end of 3-month study. These initial pilot outcomes are promising and similar to (Schwartz et al., 2006, 2011) or better than (Yancovitz et al., 1991) previous studies evaluating IMT. They also compare favorably to the above interim buprenorphine study (Krook et al., 2002). Important to note is that these outcomes were achieved with substantially less monitoring than those earlier trials, all of which involved daily or near-daily clinic visits for observed dosing. Indeed, our IBT participants averaged four visits per month, which stands in striking contrast to the 24–28 visits per month in prior studies. The rates of illicit opioid abstinence achieved in this study with computerized buprenorphine dispensing and reduced in-person monitoring are also similar to or better than those seen in previous studies of more conventional buprenorphine and methadone maintenance (Fiellin et al., 2014; Ling & Wesson, 2003; Mattick et al., 2014).
The technology-assisted buprenorphine dispensing component appeared feasible in promoting medication adherence. Of the 819 doses administered during the study, 80% were dispensed via the Med-O-Wheel. With sufficient staff and participant training in how to use the devices, all aspects of the computerized dispensing went smoothly. Eighty-two percent of random call-backs were satisfied, which compares favorably to prior studies utilizing random call-backs without the technology-based component (e.g., 62% successful call-backs, Silverman et al., 2004). Our medication adherence outcomes also fare well against the only study to our knowledge using MEMS caps in buprenorphine-maintained patients (Fiellin et al., 2006). In that trial, buprenorphine adherence was moderate (71% of study days), varied widely across patients, and was significantly correlated with illicit opioid abstinence. The results of that study led the authors to conclude that the observed variability in adherence highlighted the need to measure buprenorphine adherence in future research and to monitor and encourage adherence in clinical practice to improve treatment outcomes.
Additional measures of clinical stability and acceptability were also promising. Participants reported significant reductions in the frequency of illicit opioid use, consistent with prior studies evaluating IMT (Gruber et al., 2008; Schwartz et al., 2009; Yancovitz et al., 1991). They maintained high levels of abstinence from illicit drugs other than marijuana and adherence to our daily monitoring system was high, with 97% of IVR calls satisfactorily completed. Participants also reported high levels of satisfaction with IBT and its individual elements, particularly the buprenorphine and Med-O-Wheel components. Taken together, IBT may be effective in reducing illicit opioid use and other risk behaviors among opioid-dependent individuals awaiting entry into more comprehensive opioid treatment.
Several strengths of this pilot study should be noted. First, it employed biochemical monitoring and random call-backs that included observed dose ingestion, medication adherence checks and staff-observed urinalysis. These methods permitted a rigorous evaluation of participants’ drug use despite a relatively lean schedule of clinic visits. Second, we leveraged technology to support buprenorphine dispensing and daily monitoring. While the Med-O-Wheel has begun to be used clinically in Finland where it is manufactured (Tacke et al., 2009; Uosukainen et al., 2013), this is the first study to our knowledge to rigorously evaluate its utility as a component of buprenorphine treatment for opioid dependence. With regard to the mHealth monitoring, IVR systems seem uniquely compatible with IBT and with opioid-dependent patients more generally (Moore et al., 2013). They are an excellent fit with resource-constrained settings, requiring no specialized equipment or extensive training. IVR hardware and software can support multiple clinic sites and have no on-site installation costs beyond telephone access. IVR systems also provide broad access for lower income and marginalized populations, as touch-tone phones are familiar, easy to use, and more widely available than computers. Finally, this pilot study extends our scientific knowledge about interim opioid treatment to new populations and settings. Whereas all prior studies on interim treatment were conducted with heroin-dependent patients (Gruber et al., 2008; Krook et al., 2002; Schwartz et al., 2006, 2007, 2009, 2011; Yancovitz et al., 1991), the majority of our participants were primary prescription opioid abusers. The prior studies were also conducted in urban areas (i.e., San Francisco, CA; Oslo, Norway; Baltimore, MD; New York, NY). This is the first to examine the utility of interim treatment in the rural and suburban areas which stand to significantly benefit from it.
Several limitations should also be noted. The primary limitation is that this was an unrandomized pilot trial with a limited sample size. A larger-scale randomized clinical trial is currently underway to more rigorously evaluate the efficacy of IBT in waitlisted opioid-dependent individuals randomly assigned to interim buprenorphine vs. continued waitlist control. Most participants endorsed a prescription opioid as their primary drug of abuse, which may limit the generality of our results to heroin-dependent individuals. Our randomized trial will include a greater proportion of primary heroin and intravenous users, which will allow us to examine the efficacy of IBT in a more representative sample. However, it is also worth noting that 60% and 40% of pilot study participants reported a lifetime history of heroin and intravenous drug use, respectively. Finally, while we used the term “interim buprenorphine treatment” to represent the technology-assisted dosing protocol being evaluated in this study, its duration was limited to 12 weeks and thus did not cover the full waiting period until a conventional treatment slot became available for many participants. However, if demonstrated to be efficacious, our IBT intervention could indeed be used to bridge any duration of delay until full treatment is available.
This initial pilot study represents a promising first step towards the development of an IBT that could reduce illicit drug use and drug-related risk behaviors among waitlisted opioid abusers. Controlled studies with larger sample sizes are needed to evaluate the efficacy of this intervention, as well as to identify the individual characteristics that may predict the patients who can succeed with such a low-intensity treatment vs. those who will require more intensive monitoring and support for good outcomes. Overall, however, providing interim opioid treatment as opposed to a waitlist when a formal treatment slot is unavailable stands to reduce drug-related risks and consequences to the patient and for society more generally.
Highlights.
Despite the effectiveness of opioid maintenance, treatment waitlists persist.
We developed an interim buprenorphine treatment (IBT) for waitlisted opioid abusers.
IBT significantly reduced illicit opioid use, withdrawal and craving.
IBT may reduce risks for patients and society during delays to treatment.
Acknowledgments
We thank Shoshana Aronowitz, RN, Betsy Bahrenburg, RN, and Theresa Krainz, RN for their assistance in conducting this study.
Funding Source: Preparation of this manuscript was supported in part by National Institutes of Health research (R34DA037385, Sigmon) and training (T32DA007242) grants, as well as a National Institute of General Medical Sciences center grant (P20GM103644). The funding agencies had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Dr. Schwartz provided a one-time consultation for Reckitt Benckiser with fees going to his employer, the Friends Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Author Sigmon conceptualized, developed the proposal and obtained funding for this study. Authors Meyer, Hruska, Ochalek, Brooklyn and Sigmon conducted the study. Authors Rose, Moore, Heil, Higgins and Schwartz provided ongoing and important scientific consultation regarding the treatment components related to their areas of expertise. Author Badger conducted all study analyses. All authors have reviewed and contributed to the study manuscript and approve of the final article.
Author Disclosures
Declarations: All authors declare that they have no conflicts of interest.
References
- Adamson SJ, Sellman D. The pattern of intravenous drug use and associated criminal activity in patients on a methadone treatment waiting list. Drug Alcohol Rev. 1998;17:159–166. doi: 10.1080/09595239800186961. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: Author; 2013. [Google Scholar]
- Armitage P. Exclusions, losses to follow-up, and withdrawals in clinical trials. In: Shapiro SH, Lewis TA, editors. Clinical Trials: Issues and Approaches. New York, NY: Marcel Dekker, Inc; 1983. pp. 99–113. [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, et al. Integrating buprenorphine treatment into office-based practice: A qualitative study. J Gen Intern Med. 2008;24:218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA. Provider satisfaction with office-based treatment of opioid dependence a systematic review. Subst Abuse. 2006;26:15–22. doi: 10.1300/j465v26n01_02. [DOI] [PubMed] [Google Scholar]
- Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: Psychiatric, medical and substance use correlates. Drug Alc Depend. 2008;94:38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L. Buprenorphine treatment of opioid dependence: A review. Exp Clin Psychopharmacol. 1995;3:477–489. [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Boyer EW, Smelson D, Fletcher R, Ziedonis D, Picard RW. Wireless technologies, ubiquitous computing and mobile health: application to drug abuse treatment and compliance with HIV therapies. J Med Toxicol. 2010;6:212–216. doi: 10.1007/s13181-010-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermack ST, Roll J, Reilly M, Davis L, Kilaru U, Grabowski J. Comparison of patient self-reports and urinalysis results obtained under naturalistic methadone treatment conditions. Drug Alcohol Depend. 2000;59:43–49. doi: 10.1016/s0376-8716(99)00106-4. [DOI] [PubMed] [Google Scholar]
- Clausen T, Waal H, Thoresen M, Gossop M. Mortality among opiate users: Opioid maintenance therapy, age and causes of death. Addiction. 2009;104:1356–1362. doi: 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- Cooper JR. Methadone treatment and acquired immunodeficiency syndrome. JAMA. 1989;262:1664–1668. [PubMed] [Google Scholar]
- Crawford AG, Sikirica V, Goldfarb N, Popiel RG, Patel M, Wang C, et al. Interactive voice response reminder effects on preventive service utilization. Am J Med Qual. 2005;20:329–336. doi: 10.1177/1062860605281176. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W. Heroin overdose: Research and evidence-based intervention. J Urban Health. 2003;80:189–200. doi: 10.1093/jurban/jtg022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Paone D, Friedman SR, Peyser N, Newman RG. Regulating controversial programs for unpopular programs for unpopular people: Methadone maintenance and syringe exchange programs. Am J Public Health. 1995;85:1577–1584. doi: 10.2105/ajph.85.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Register, Title 21. Codified at 58 CFR §495, part 291. 1993 [Google Scholar]
- Fendrich M, Johnson TP, Wislar JS, Hubbell A, Spiehler V. The utility of drug testing in epidemiological research: Results from a general population survey. Addiction. 2004;99:197–208. doi: 10.1111/j.1360-0443.2003.00632.x. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O’Connor PG. Primary care-based buprenorphine taper vs. maintenance therapy for prescription opioid dependence. JAMA Internal Medicine. 2014;174:1947–1954. doi: 10.1001/jamainternmed.2014.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortney J, Booth BM. Access to substance abuse services in rural areas. Recent Dev Alcohol. 2001;15:177–197. doi: 10.1007/978-0-306-47193-3_10. [DOI] [PubMed] [Google Scholar]
- Fountain J, Strang J, Griffiths P, Powis B, Gossop M. Measuring met and unmet need of drug misusers: Integration of quantitative and qualitative data. Eur Addict Res. 2000;6:97–103. doi: 10.1159/000019017. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Lemon SC, Stein MD, D’Aunno TA. Accessibility of addiction treatment: Results from a national survey of outpatient substance abuse treatment organizations. Health Serv Res. 2003;38:887–903. doi: 10.1111/1475-6773.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend. 2008;94:199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczynski J, Schwartz R, O’Grady K, Jaffe J. Treatment entry among individuals on a waiting list for methadone maintenance. Am J Drug Alcohol Abuse. 2009;35:290–294. doi: 10.1080/00952990902968577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford RJ, Kleber HD. Comparative validity of random-interval and fixed-interval urinalysis schedules. Arch Gen Psychiatry. 1978;35:356–359. doi: 10.1001/archpsyc.1978.01770270106010. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Rose GL, Badger GJ, Searles JS, Thomas CS, Lindberg SA, et al. Using interactive voice response to enhance brief alcohol intervention in primary care settings. J Stud Alcohol Drugs. 2008;69:251–258. doi: 10.15288/jsad.2008.69.251. [DOI] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotic addicts. Arch Gen Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Retting RA, Yarmolinsky A, editors. Institute of Medicine (IOM) Federal regulation of methadone treatment. Washington, DC: National Academy Press; 1995. [PubMed] [Google Scholar]
- Johnson RE, Chutaupe MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: How to use it right. Drug Alcohol Depend. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- Kilpatrick B, Howlett M, Sedgwick P, Ghodse AH. Drug use, self report and urinalysis. Drug Alcohol Depend. 2000;58:111–116. doi: 10.1016/s0376-8716(99)00066-6. [DOI] [PubMed] [Google Scholar]
- Kim H, Bracha Y, Tipnis A. Automated depression screening in disadvantaged pregnant women in an urban obstetric clinic. Arch Womens Ment Health. 2007;10:163–169. doi: 10.1007/s00737-007-0189-5. [DOI] [PubMed] [Google Scholar]
- Kissin W, McLeod C, Sonnefeld J, Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. J Addict Dis. 2006;25:91–103. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- Krook AL, Brørs O, Dahlberg J, Grouff K, Magnus P, Røysamb E, Waal H. A placebo-controlled study of high dose buprenorphine in opiate dependents waiting for medication-assisted rehabilitation in Oslo, Norway. Addiction. 2002;97:533–542. doi: 10.1046/j.1360-0443.2002.00090.x. [DOI] [PubMed] [Google Scholar]
- Lenardson J, Gale JA. Distribution of substance abuse treatment facilities across the rural-urban continuum. Portland, ME: US Department of Health and Human Services, Federal Office of Rural Health Policy; 2007. [accessed 4/9/15]. Available at: http://muskie.usm.maine.edu/Publications/rural/wp35b.pdf. [Google Scholar]
- Ling W, Wesson DR. Clinical efficacy of buprenorphine: Comparisons to methadone and placebo. Drug and Alcohol Dependence. 2003;70:S49–S57. doi: 10.1016/s0376-8716(03)00059-0. [DOI] [PubMed] [Google Scholar]
- Luty J, O’Gara C, Sessay M. Is methadone too dangerous for opiate addiction? BMJ. 2005;331:1352–1353. doi: 10.1136/bmj.331.7529.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno JE. Specimen collection and handling. NIDA Res Monogr. 1986;73:24–29. [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J. New data from the Addiction Severity Index: Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Moore BA, Fazzino T, Barry DT, Fiellin DA, Cutter CJ, Schottenfeld RS, et al. The Recovery Line: A pilot trial of automated, telephone-based treatment for continued drug use in methadone maintenance. J Subst Abuse Treat. 2013;45:63–69. doi: 10.1016/j.jsat.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherland J, Botsko M, Egan JE, Saxon AJ, Cunningham CO, Finkelstein R, et al. Factors affecting willingness to provide buprenorphine treatment. J Subst Abuse Treat. 2009;36:244–251. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ. Prescription drug overdoses: A review. J Safety Res. 2012;43:283–289. doi: 10.1016/j.jsr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br J Addict. 1988;83:193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M. Opiate-dependent patients on a waiting list for methadone maintenance treatment are at high risk for mortality until treatment entry. J Addict Med. 2013;7:177–182. doi: 10.1097/ADM.0b013e318287cfc9. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Sason A, Adelson M. Long waiting period to enter methadone maintenance treatment: Relation to patient characteristics and outcome. Eur Addict Res. 2012;18:149–152. doi: 10.1159/000336313. [DOI] [PubMed] [Google Scholar]
- Peterson JA, Schwartz RP, Mitchell SG, Reisinger HS, Kelly SM, O’Grady KE, et al. Why don’t out-of-treatment individuals enter methadone treatment programmes? Int J Drug Policy. 2010;21:36–42. doi: 10.1016/j.drugpo.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130–145. [PubMed] [Google Scholar]
- Rose GL, MacLean CD, Skelly J, Badger GJ, Ferraro TA, Helzer JE. Interactive voice response technology can deliver alcohol screening and brief intervention in primary care. J Gen Intern Med. 2010a;25:340–344. doi: 10.1007/s11606-009-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GL, Skelly JM, Badger GJ, MacLean CD, Malgeri MP, Helzer JE. Automated screening for at-risk drinking in a primary care office using interactive voice response. J Stud Alcohol Drugs. 2010b;71:734–738. doi: 10.15288/jsad.2010.71.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Cleland CM, Fong C, Kayman DJ, Tempalski B, Parrino M. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR. Treatment for opioid dependence: Quality and access. JAMA. 2000;283:1337–1339. doi: 10.1001/jama.283.10.1337. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Highfield DA, Jaffe JH, Brady JV, Butler CB, Rouse CO, et al. A randomized controlled trial of interim methadone maintenance. Arch Gen Psychiatry. 2006;63:102–109. doi: 10.1001/archpsyc.63.1.102. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Jaffe JH, O'Grady KE, Kinlock TW, Gordon MS, Kelly SM, et al. Interim methadone treatment: Impact on arrests. Drug Alcohol Depend. 2009b;103:148–154. doi: 10.1016/j.drugalcdep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Jaffe JH, Highfield DA, Callaman JM, O’Grady KE. A randomized controlled trial of interim methadone maintenance: 10-Month follow-up. Drug Alcohol Depend. 2007;86:30–36. doi: 10.1016/j.drugalcdep.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Jaffe JH, O’Grady KE, Das B, Highfield DA, Wilson ME. Scaling-up interim methadone maintenance: Treatment for 1,000 heroin-addicted individuals. J Subst Abuse Treat. 2009a;37:362–367. doi: 10.1016/j.jsat.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH. Interim methadone treatment compared to standard methadone treatment: 4-month findings. J Subst Abuse Treat. 2011;41:21–29. doi: 10.1016/j.jsat.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Mitchell SG, Peterson JA, Reisinger HS, et al. Attitudes toward buprenorphine and methadone among opioid-dependent individuals. Am J Addict. 2008;17:396–401. doi: 10.1080/10550490802268835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NG, Lathrop SL, Reichard RR, Landen MG. Unintentional drug overdose death trends in New Mexico, USA, 1990–2005: Combinations of heroin, cocaine, prescription opioids and alcohol. Addiction. 2008;103:126–136. doi: 10.1111/j.1360-0443.2007.02054.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC. Access to treatment for opioid dependence in rural America: Challenges and future directions. JAMA Psychiatry. 2014;71:359–360. doi: 10.1001/jamapsychiatry.2013.4450. [DOI] [PubMed] [Google Scholar]
- Sigmon SC. The untapped potential of office-based buprenorphine treatment. JAMA Psychiatry. 2015;72:395–396. doi: 10.1001/jamapsychiatry.2014.2421. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Badger GJ, Heil SH, Higgins ST. Brief buprenorphine detoxification for the treatment of prescription opioid dependence: a pilot study. Addict Behav. 2009;34:304–311. doi: 10.1016/j.addbeh.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Dunn K, Saulsgiver K, Patrick M, Badger GJ, Heil SH, et al. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry. 2013;70:1347–1354. doi: 10.1001/jamapsychiatry.2013.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Robles E, Mudric T, Bigelow GE, Stitzer ML. A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. J Consult Clin Psychol. 2004;72:839–854. doi: 10.1037/0022-006X.72.5.839. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Stacy JN, Schwartz SM, Ershoff D, Shreve MS. Incorporating tailored interactive patient solutions using interactive voice response technology to improve statin adherence: Results of a randomized clinical trial in a managed care setting. Popul Health Manag. 2009;12:241–254. doi: 10.1089/pop.2008.0046. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Chutuape MA. Other substance use disorders in methadone treatment: prevalence, consequences, detection, and management. In: Strain EC, Stitzer ML, editors. Methadone Treatment for Opiate Dependence. Baltimore, MD: Johns Hopkins University Press; 1999. pp. 86–117. [Google Scholar]
- Stotts AL, Dodrill DC, Kosten TR. Opioid dependence treatment: Options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–1740. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, MD: 2010. (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4856 Findings) [Google Scholar]
- Tacke U, Uosukainen H, Kananen M, Kontra K, Pentikäinen H. A pilot study about the feasibility and cost-effectiveness of electronic compliance monitoring in substitution treatment with buprenorphine–naloxone combination. J Opioid Manag. 2009;5:321–329. doi: 10.5055/jom.2009.0032. [DOI] [PubMed] [Google Scholar]
- Uosukainen H, Pentikainen H, Tacke U. The effect of an electronic medicine dispenser on diversion of buprenorphine-naloxone: Experience from a medium-sized Finnish city. J Subst Abuse Treat. 2013;45:143–147. doi: 10.1016/j.jsat.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: Partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Warner-Smith M, Darke S, Lynskey M, Hall W. Heroin overdose: Causes and consequences. Addiction. 2001;96:1113–1125. doi: 10.1046/j.1360-0443.2001.96811135.x. [DOI] [PubMed] [Google Scholar]
- Wenger LD, Rosenbaum M. Drug treatment on demand – not. J Psychoactive Drugs. 1994;26:1–11. doi: 10.1080/02791072.1994.10472597. [DOI] [PubMed] [Google Scholar]
- Wish ED, Hoffman JA, Nemes S. The validity of self-reports of drug use at treatment admission and at followup: Comparisons with urinalysis and hair assays. NIDA Res Monogr. 1997;167:200–226. [PubMed] [Google Scholar]
- Wisniewski AM, Purdy CH, Blondell RD. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J Addict Dis. 2008;27:1–11. doi: 10.1300/J069v27n01_01. [DOI] [PubMed] [Google Scholar]
- Yancovitz SR, Des Jarlais DC, Peyser NP, Drew E, Friedmann P, Trigg HL, Robinson JW. A randomized trial of an interim methadone maintenance clinic. Am J Pub Health. 1991;81:1185–1191. doi: 10.2105/ajph.81.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]