Abstract
Objectives
Prenatal diagnosis of critical congenital heart disease, that requiring surgical or catheter intervention in the first 30 days of life, allows for delivery at a specialized center which can reduce preoperative morbidity and mortality. We sought to identify risk factors for a missed prenatal diagnosis of critical congenital heart disease.
Methods
Patients presenting to the Children’s Hospital of Wisconsin with critical congenital heart disease from 2007-2013 were included. Those with a prenatal diagnosis were compared to those with a postnatal diagnosis.
Results
The cohort included 535 patients with prenatal diagnosis made in 326 (61%). The prenatal diagnostic rate improved from 44% in 2007 to 69% in 2013. Independent factors associated with a postnatal diagnosis were a lesion that required a view other than a 4 chamber view to make the diagnosis (p<0.0001), absence of another organ system anomaly (p<0.0001), and living in a higher poverty (p=0.02) or lower population density communities (p=0.002).
Conclusions
While the prenatal diagnostic rate for critical congenital heart disease is improving, those living in impoverished or rural communities are at highest risk of not having a diagnosis made prenatally. Interventions to improve prenatal detection of congenital heart disease should target these vulnerable areas.
Introduction
Malformations of the cardiovascular system remain the leading cause of infant mortality from congenital defects1. Those with critical congenital heart disease (CHD), defined as disease requiring surgical or catheter based intervention within the first 30 days of life, are at highest risk for early mortality. Prenatal detection allows for delivery at a surgical center capable of caring for these high risk infants and improves preoperative condition with conflicting data on the impact on mortality2-12.
While there are risk factors that increase the risk of CHD and therefore are indications for a fetal echocardiogram (i.e. maternal diabetes, family history of CHD), the vast majority of CHD occurs in low risk pregnancies13. As such, routine obstetric ultrasounds are the primary mechanism to identify CHD before birth. In spite of near universal performance of routine ultrasounds during pregnancy and estimates that over 90% of CHD should be detectable by prenatal ultrasound, actual detection rates remain low, ranging from 36-50% in the United States13-17. This study sought to determine the prenatal detection rate for critical CHD and determine risk factors for failure to make a prenatal diagnosis.
Methods
Population
After approval from the institutional review board, a retrospective chart review was performed. All patients presenting to the Children’s Hospital of Wisconsin requiring surgical or catheter based intervention at ≤ 30 days of age between January 1, 2007 and December 31, 2013 were included. Those with isolated patent ductus arteriosus or non-structural heart disease, such as congenital complete heart block, were excluded.
Definitions
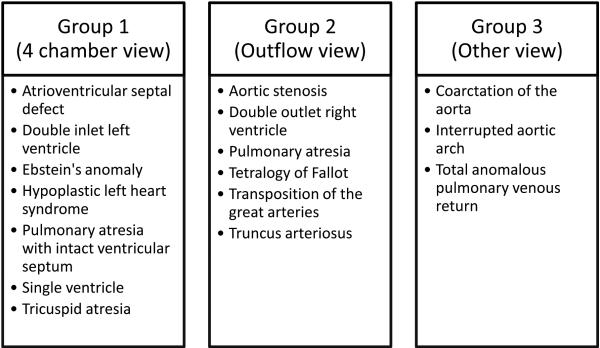
Socioeconomic variables including median household income, percentage below the poverty line and population density were based on the 2010 census data by zip code of the mother’s residence at the time of delivery. A population density of <500 people/sq mi was used to dichotomize rural and non-rural areas18 for graphical presentation; in analysis the continuous variable was used. The types of CHD were divided into three groups based on the echocardiographic views required to accurately identify the disease (Figure 1). Group 1 were those diseases identified by a four chamber view alone, Group 2 were those diseases identified by the addition of an outflow tract view, and Group 3 were those diseases identified by the need for unique views such as an aortic arch view or color and spectral Doppler evaluation of the pulmonary veins. Organ system anomalies were defined as those that could potentially be identified on routine obstetric ultrasound as these may impact referral for further evaluation prior to delivery.
Figure 1.
Division of groups based on echocardiographic view required for diagnosis
Statistical analysis
Those with a prenatal diagnosis were compared to those with a postnatal diagnosis using Chi-squared for categorical variables or Wilcoxon rank sum test for continuous variables. Multivariable logistic regression with backwards elimination was performed to determine risk factors for a postnatal diagnosis. Data are presented as number with percent of total or mean ± standard deviation. Data analysis was performed using StataIC (StataCorp, College Station, Texas) with a p<0.05 considered significant.
Results
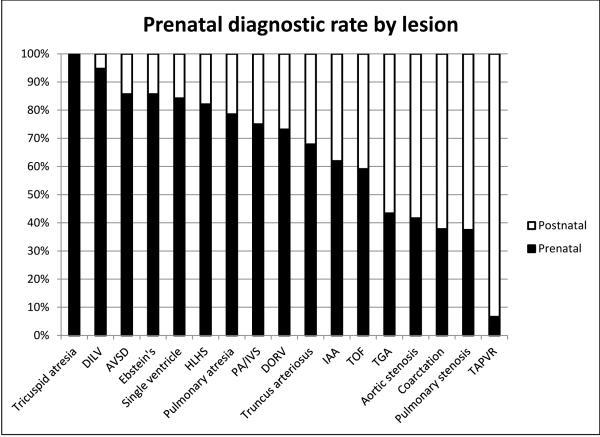
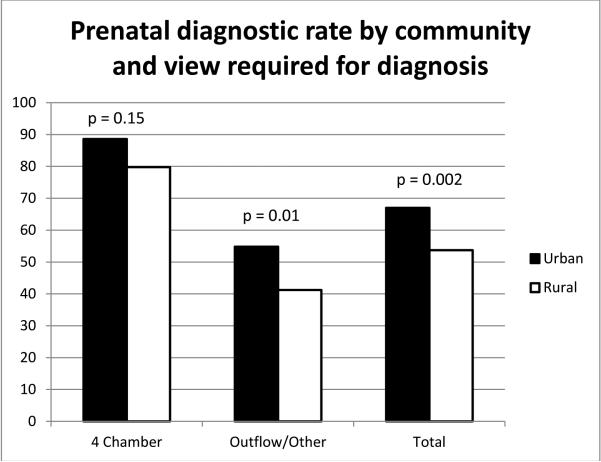
The cohort included 535 patients with 326 (61%) having a prenatal diagnosis. The percentage of prenatal diagnosis improved over time with 44% in 2007 and 69% in 2013. Prenatal diagnostic rates were best in tricuspid atresia (14/14, 100%) and worst in total anomalous pulmonary venous connection (1/14, 7%); see Figure 2. Comparison of characteristics between those diagnosed prenatally and those diagnosed postnatally can be seen in table 1. In multivariable analysis using the factors seen in Table 1, a lesion that required a view other than a 4 chamber view to make the diagnosis (p<0.0001), absence of another organ system anomaly (p<0.0001), and living in a higher poverty (p=0.02) or a lower population density zip code (p=0.002) were independently associated with a postnatal diagnosis of critical CHD. In analysis comparing rural and non-rural communities, if the CHD could be defined using the four chamber view alone, there was no significant difference in prenatal diagnostic rate between these communities. When outflow tract or other more complex views were needed to make the diagnosis, prenatal diagnostic rates were significantly lower in rural communities (Figure 3).
Figure 2.
Frequency of prenatal diagnosis by lesion. DILV – Double inlet left ventricle, AVSD – atrioventricular septal defect, HLHS – hypoplastic left heart syndrome, PA/IVS – pulmonary atresia with intact ventricular septum, DORV – double outlet right ventricle, IAA – interrupted aortic arch, TOF – tetralogy of Fallot, TGA – transposition of the great arteries, TAPVR – total anomalous pulmonary venous return
Table 1.
Comparison of prenatal and postnatal diagnosis groups
| Prenatal n = 326 |
Postnatal n = 209 |
p value | |
|---|---|---|---|
|
| |||
| Married, n (%) | 203 (63%) | 111 (54%) | 0.04 |
| Gravidity, n (%) | 0.12 | ||
| 1 | 101 (31%) | 57 (28%) | |
| 2 | 80 (25%) | 65 (32%) | |
| 3 | 62 (19%) | 30 (15%) | |
| >3 | 81 (25%) | 49 (24%) | |
| Male fetus, n (%) | 185 (57%) | 128 (61%) | 0.3 |
| Race/Ethnicity, n (%) | 0.16 | ||
| Black/African American | 30 (9%) | 17 (8%) | |
| Hispanic | 38 (12%) | 21 (10%) | |
| White/Caucasian | 227 (70%) | 138 (66%) | |
| Other | 31 (9%) | 33 (16%) | |
| View required for diagnosis, n (%) | <0.001 | ||
| 4 chamber view | 156 (48%) | 28 (13%) | |
| Outflow tract view | 117 (36%) | 91 (44%) | |
| Other | 53 (16%) | 90 (43%) | |
| Other anomaly present, n (%) | 70 (21%) | 16 (8%) | <0.001 |
| Private insurance, n (%) | 219 (68%) | 126 (63%) | 0.23 |
| Percent below poverty | 13.5 ± 9.8 | 14.1 ± 9.7 | 0.19 |
| 2518 ± | 1935 ± | ||
| Population density (people/sq mi) | 3338 | 3020 | <0.001 |
Figure 3.
Prenatal diagnostic rate by view required to make the lesion and by rural versus urban community
Discussion
This study demonstrates an overall improvement in the frequency of prenatal diagnosis of critical CHD over time, however there continues to be significant disparity in diagnosis between urban and rural communities. When adjusting for the view required for diagnosis and presence of other organ system anomalies, those living in impoverished or rural communities are at greatest risk for a missed prenatal diagnosis of critical CHD. In our study, the disparity between communities was most noticeable in defects that require advanced imaging views for accurate diagnosis. Lesions such as aortic stenosis, double outlet right ventricle, pulmonary atresia, tetralogy of Fallot, transposition of the great arteries, and truncus arteriosus which require an outflow tract view, and coarctation of the aorta, interrupted aortic arch, and total anomalous pulmonary venous return which require an aortic arch view or color and spectral Doppler imaging were less commonly identified in rural communities. It has previously been demonstrated that screening for CHD with only a 4 chamber view is insufficient and results in a sensitivity of only 40% for detection of CHD19. Recent changes to the guidelines for performance of obstetric ultrasound screening recognize this limitation, as routine evaluation of both the left and right ventricular outflow tract is recommended20.
We speculate that health care personnel working in impoverished, rural areas are less able to acquire the new skills required to adequately evaluate the outflow tracts or other complex views. This is likely a result of the rural location with greater isolation from peers and less ability to pursue further training in both image acquisition and interpretation. Training programs for those performing screening ultrasounds in the United Kingdom and Sweden have demonstrated a significant improvement in the detection of congenital heart disease21-24. This improvement has been demonstrated with minimal investment of time, with some programs lasting only 1-3 days23,24. Our data suggest that training programs in detection of CHD by screening obstetric ultrasound should be targeted to these impoverished, rural communities for the greatest impact. Supervision of imaging and specialized training on normal findings for more complex views has been demonstrated to be effective by Evans et al and could be a focus for continuing education25.
Recently, the United States Department of Health and Human Services recommended universal pulse oximetry screening for all neonates in an effort to improve early detection of CHD26. However, prenatal diagnosis offers the distinct advantage of allowing for delivery at a specialized cardiac care center. Morris et al demonstrated that patients with hypoplastic left heart syndrome born further from a surgical center were at higher risk for neonatal mortality, even independent of prenatal diagnosis11. This likely reflects the inability of those physicians and providers in the rural community to appropriately manage a critically ill neonate because of the equipment and expertise required. While pulse oximetry may improve detection, only through prenatal diagnosis can the location of delivery be altered and the risk of mortality and preoperative deterioration minimized.
As a retrospective single center cohort study, these data are subject to some limitations. Most notably, no data on postnatal mortality prior to presentation at our institutional are available. Previous work has shown the number of deaths in Wisconsin from 2002-2006 from undiagnosed CHD to be less than 2 cases per year and this number is likely to be even lower given the improvement in prenatal detection noted over time in our study27. However, while postnatal death in undiagnosed patients may have little effect on our study, the rate of prenatal termination is unknown and likely more significant. With the inclusion criteria used, not all patients with each diagnosis were included, for example only severe forms of tetralogy of Fallot, those requiring early intervention, were included. An additional limitation is the use of zip codes to determine population density and poverty as there may be variation within zip codes.
Conclusions
While the rate of prenatal diagnosis of critical congenital heart disease has improved over time, those residing in impoverished, rural communities remain at greatest risk of being diagnosed postnatally. This difference in diagnostic rate is most notable for diagnoses requiring the outflow tract view and other more complex imaging. Additional training efforts targeted to these vulnerable communities may have the most significant impact.
What’s known:
Diagnostic rates for congenital heart disease prior to delivery are suboptimal and influenced by socioeconomic factors
What’s new:
Prenatal diagnostic rates for critical congenital heart disease are worse in impoverished rural communities
The effect is more notable when advanced views are required to make the diagnosis
Acknowledgments
Funding: This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: None declared
References
- 1.Lee K, Khoshnood B, Chen L, Wall SN, Cromie WJ, Mittendorf RL. Infant mortality from congenital malformations in the united states, 1970-1997. Obstet Gynecol. 2001;98(4):620–627. doi: 10.1016/s0029-7844(01)01507-1. [DOI] [PubMed] [Google Scholar]
- 2.Landis BJ, Levey A, Levasseur SM, et al. Prenatal diagnosis of congenital heart disease and birth outcomes. Pediatr Cardiol. 2013;34(3):597–605. doi: 10.1007/s00246-012-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar RK, Newburger JW, Gauvreau K, Kamenir SA, Hornberger LK. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol. 1999;83(12):1649–1653. doi: 10.1016/s0002-9149(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs IB, Muller H, Abdul-Khaliq H, Harder T, Dudenhausen JW, Henrich W. Immediate and long-term outcomes in children with prenatal diagnosis of selected isolated congenital heart defects. Ultrasound Obstet Gynecol. 2007;29(1):38–43. doi: 10.1002/uog.3900. [DOI] [PubMed] [Google Scholar]
- 5.Franklin O, Burch M, Manning N, Sleeman K, Gould S, Archer N. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart. 2002;87(1):67–69. doi: 10.1136/heart.87.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderon J, Angeard N, Moutier S, Plumet MH, Jambaque I, Bonnet D. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. J Pediatr. 2012;161(1):94–8. doi: 10.1016/j.jpeds.2011.12.036. e1. [DOI] [PubMed] [Google Scholar]
- 7.Oster ME, Kim CH, Kusano AS, et al. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol. 2014;113(6):1036–1040. doi: 10.1016/j.amjcard.2013.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics. 2001;107(6):1277–1282. doi: 10.1542/peds.107.6.1277. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet D, Coltri A, Butera G, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99(7):916–918. doi: 10.1161/01.cir.99.7.916. [DOI] [PubMed] [Google Scholar]
- 10.Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103(9):1269–1273. doi: 10.1161/01.cir.103.9.1269. [DOI] [PubMed] [Google Scholar]
- 11.Morris SA, Ethen MK, Penny DJ, et al. Prenatal diagnosis, birth location, surgical center, and neonatal mortality in infants with hypoplastic left heart syndrome. Circulation. 2014;129(3):285–292. doi: 10.1161/CIRCULATIONAHA.113.003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright LK, Ehrlich A, Stauffer N, Samai C, Kogon B, Oster ME. Relation of prenatal diagnosis with one-year survival rate for infants with congenital heart disease. Am J Cardiol. 2014;113(6):1041–1044. doi: 10.1016/j.amjcard.2013.11.065. [DOI] [PubMed] [Google Scholar]
- 13.Stumpflen I, Stumpflen A, Wimmer M, Bernaschek G. Effect of detailed fetal echocardiography as part of routine prenatal ultrasonographic screening on detection of congenital heart disease. Lancet. 1996;348(9031):854–857. doi: 10.1016/S0140-6736(96)04069-X. [DOI] [PubMed] [Google Scholar]
- 14.Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease. J Pediatr. 2009;155(1):26–31. doi: 10.1016/j.jpeds.2009.01.050. 31.e1. [DOI] [PubMed] [Google Scholar]
- 15.Peiris V, Singh TP, Tworetzky W, Chong EC, Gauvreau K, Brown DW. Association of socioeconomic position and medical insurance with fetal diagnosis of critical congenital heart disease. Circ Cardiovasc Qual Outcomes. 2009;2(4):354–360. doi: 10.1161/CIRCOUTCOMES.108.802868. [DOI] [PubMed] [Google Scholar]
- 16.Pinto NM, Keenan HT, Minich LL, Puchalski MD, Heywood M, Botto LD. Barriers to prenatal detection of congenital heart disease: A population-based study. Ultrasound Obstet Gynecol. 2012;40(4):418–425. doi: 10.1002/uog.10116. [DOI] [PubMed] [Google Scholar]
- 17.Israel SW, Roofe LR, Saville BR, Walsh WF. Improvement in antenatal diagnosis of critical congenital heart disease implications for postnatal care and screening. Fetal Diagn Ther. 2011;30(3):180–183. doi: 10.1159/000327148. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services Rural Health Toolkit. U.S. Department of Health and Human Services Web site http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/defined.html. Updated 2014. Accessed December 12, 2014.
- 19.GonÇalves LF, Bronsteen R, Lee W. Fetal heart: A 4-chamber view is not enough. Clin Obstet Gynecol. 2012;55(1):266–280. doi: 10.1097/GRF.0b013e3182446df0. [DOI] [PubMed] [Google Scholar]
- 20.AIUM Practice Guideline for the Performance of Obsteric Ultrasound Examinations http://aium.org/resources/guidelines/obstetric.pdf. Updated 2013. Accessed May 2, 2013.
- 21.Asplin N, Dellgren A, Conner P. Education in obstetrical ultrasound--an important factor for increasing the prenatal detection of congenital heart disease. Acta Obstet Gynecol Scand. 2013;92(7):804–808. doi: 10.1111/aogs.12140. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho JS, Mavrides E, Shinebourne EA, Campbell S, Thilaganathan B. Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart. 2002;88(4):387–391. doi: 10.1136/heart.88.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter S, Heads A, Wyllie J, Robson S. Prenatal diagnosis of congenital heart disease in the northern region of england: Benefits of a training programme for obstetric ultrasonographers. Heart. 2000;84(3):294–298. doi: 10.1136/heart.84.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBrien A, Sands A, Craig B, Dornan J, Casey F. Impact of a regional training program in fetal echocardiography for sonographers on the antenatal detection of major congenital heart disease. Ultrasound Obstet Gynecol. 2010;36(3):279–284. doi: 10.1002/uog.7616. [DOI] [PubMed] [Google Scholar]
- 25.Evans W, Castillo W, Rollins R, et al. Moving towards universal prenatal detection of critical congenital heart disease in southern nevada: A community-wide program. Pediatr Cardiol. 2015;36(2):281–288. doi: 10.1007/s00246-014-0996-1. [DOI] [PubMed] [Google Scholar]
- 26.Mahle WT, Martin GR, Beekman RH, 3rd, Morrow WR, Section on Cardiology and Cardiac Surgery Executive Committee Endorsement of health and human services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129(1):190–192. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 27.Ng B, Hokanson J. Missed congenital heart disease in neonates. Congenit Heart Dis. 2010;5(3):292–296. doi: 10.1111/j.1747-0803.2010.00418.x. [DOI] [PubMed] [Google Scholar]