Abstract
Objective
This study compared the effects on cartilage and meniscus matrix catabolism and biosynthesis of several adipokines implicated in osteoarthritis.
Design
Cartilage and meniscus explants were cultured for 1 or 9 days in serum-free medium alone or with 0.02, 0.2, or 2μg/ml of leptin, visfatin, adiponectin, or resistin. Media were supplemented with 3H-proline or 35S-sodium sulfate to evaluate protein and sulfated glycosaminoglycan (sGAG) accumulation on the last day of culture. Explants were assayed for radiolabel, sGAG, and DNA contents. Cultured media were assayed for sGAG, nitrite and lactate dehydrogenase.
Results
Cartilage tissue was minimally affected by adipokines, with only the highest resistin dose increasing sGAG release and nitrite production compared to controls. In sharp contrast, meniscus tissue was responsive to several adipokines, with elevated sGAG and nitrite release following treatment with resistin, leptin, or visfatin. Cartilage sGAG content was unaltered by adipokine treatment whereas meniscal sGAG content significantly decreased with resistin dosage. Protein (3H) incorporation was unaffected by adipokine treatment in both tissues. sGAG (35S) incorporation did not significantly vary with adipokine treatment in cartilage but was inhibited by treatment with leptin, visfatin, and resistin in meniscus.
Conclusion
Our results indicate that meniscal tissue is more susceptible to adipokine-stimulated catabolism than is cartilage. Resistin had the strongest effect of the adipokines tested, inducing sGAG release in both tissues and depleting sGAG content in meniscus. These results suggest that increased adipokine levels due to obesity or joint injury may alter the mechanical integrity of the knee joint through biological pathways.
Keywords: meniscus, cartilage, adipokines, degeneration, obesity, osteoarthritis
Introduction
Obesity in developed nations is at epidemic levels, with prevalence among US adults estimated at 36% in 2009–10 [1] and predicted to reach about 50% by 2030 [2]. Obesity is a major risk factor for hip and knee osteoarthritis (OA), with every 5 unit increase in body mass index (BMI) doubling the risk of OA [3]. The association between obesity and OA has been viewed as predominantly biomechanical [4], but overloading may not be the only link. Knee OA incidence and progression were more strongly associated with fat mass than with BMI or total mass [5], and obesity has been identified as a strong risk factor for hand OA [6, 7], suggesting that systemic, biologic links between obesity and OA may exist in addition to direct mechanical effects.
Adipose tissue is now recognized as a metabolically active organ [8] that secretes biologically active factors collectively described as adipokines. Altered adipokine levels have been implicated in diabetes, cardiovascular disease, metabolic syndrome, and inflammatory disorders including rheumatoid arthritis [9]. With observations of adipokines including leptin [10], visfatin [11], adiponectin and resistin [12] in the synovial fluid of osteoarthritic joints, roles for adipokines in the onset and progression of OA have been hypothesized [13]. In the knee, the synovium and infrapatellar fat pad have been identified as major adipokine producers [14, 15]. Elevated leptin [10, 16], resistin [17, 18], and visfatin [19, 20] levels in both serum and synovial fluid have been associated with increased OA progression or severity, but the association of adiponectin with OA remains unclear [21, 22].
In OA joints, synovial fluid concentrations range around 10–20 ng/mL for leptin [10, 14, 16], 10–40 ng/mL for visfatin [19, 20], 0.3–2.3 μg/ml for adiponectin [12, 14, 23], and 3–50 ng/mL for resistin [12, 14, 17]. Increased BMI correlates with elevated synovial fluid concentrations of leptin [10] but lower synovial fluid concentrations of adiponectin [21, 24]. While studies have reported no correlation between synovial fluid concentrations of visfatin [20] or resistin [24] and BMI, both correlated with OA severity.
Various adipokines have induced cell-mediated cartilage catabolism in vitro. Leptin induced production of proinflammatory factors and enhanced matrix metalloproteinase (MMP) expression in OA cartilage [25, 26]. Visfatin stimulated chondrocyte aggrecanase expression and MMP production [11], while adiponectin induced production and expression of several MMPs and proinflammatory factors [22, 27]. Resistin induced production of proinflammatory factors and inhibited matrix synthesis in mouse cartilage [17] and increased aggrecanase and MMP expression by human chondrocytes [28]. Particularly relevant to the potential involvement of adipokines in OA development, serum and synovial fluid level of resistin were elevated following traumatic joint injury (median resistin level approximately 3 ng/ml one week following injury) [17].
However, little is known about the effects of adipokines on joint tissues other than articular cartilage. A recent investigation demonstrated that 100ng/ml visfatin increased nitric oxide production and MMP activity or matrix degradation in adult porcine cartilage and meniscus [29]. Particularly given growing evidence that meniscal degeneration is an early event in knee OA that accompanies or precedes cartilage degeneration [30, 31], a more detailed understanding of the effects of catabolic mediators on meniscal metabolism is desirable. The goals of this study were to evaluate dose-dependent catabolic and anti-anabolic effects of leptin, visfatin, adiponectin and resistin on cartilage and meniscus tissue explants, and to examine the relative susceptibility of these tissues to each adipokine.
Method
Materials
Immature bovine stifles were from Research 87 (Marlborough, MA). High glucose Dulbecco’s modified Eagle’s medium (DMEM) was from HyClone (Logan, UT). Non-essential amino acids (NEAA) and proteinase K were from Life Technologies (Carlsbad, CA). N-(2-hydroxyethyl)-piperazine-N′-2-ethanesulfonic acid (HEPES), gentamicin and phosphate buffered saline (PBS) were from Mediatech (Manassas, VA). Insulin-transferrin-selenous acid premix (ITS+) was from BD Biosciences (Franklin Lakes, NJ). L-ascorbic acid 2-phosphate, proline, ammonium acetate, 1,9-dimethyl-methylene blue (DMMB), shark chondroitin sulfate, sodium nitrite, bisbenzimide (Hoechst 33258), and calf thymus DNA were from Sigma (St. Louis, MO). Sulfanilamide reagent and naphthylethylenediamine dihydrochloride solution were from Ricca Chemical (Arlington, TX). The CytoTox-ONE™ Homogeneous Membrane Integrity assay kit was from Promega (Madison, WI). 3H-proline and 35S-sodium sulfate were from Perkin Elmer (Waltham, MA). Recombinant human leptin was from Shenandoah Biotechnology (Warwick, PA), recombinant human visfatin was from Novus Biologicals (Littleton, CO), and recombinant human adiponectin and resistin were from Biovendor (Candler, NC).
Experimental Design
Independent experiments evaluated the effects of initial (1 day) and sustained (9 day) exposure to adipokines. Both experiments involved two substudies, each consisting of an untreated control group and three doses (0.02, 0.2 and 2 μg/ml) of two adipokines: leptin and visfatin in one substudy, adiponectin and resistin in the other. Tissue explants for each substudy were isolated from four immature bovine stifles (one each from four animals, total of sixteen donors), and samples from different donors and relative positions (anterior, central, posterior) were distributed across experimental groups (n=6–8 explants/tissue/condition).
Tissue Culture
Using a 4mm biopsy punch, full thickness explants were isolated from the lateral femoral condylar cartilage and the caudal surface of the radially middle region of lateral menisci from bovine stifles. Explants were cultured at 37°C, 5% CO2, and 95% relative humidity in basal, serum-free medium consisting of high glucose DMEM with 0.1 mM NEAA, 10 mM HEPES buffer, 50 μg/ml L-ascorbic acid-2-PO4, 50 μg/ml gentamicin, 0.4 mM proline, and 1% ITS+ premix. After overnight culture, explants were trimmed to 2 mm thickness retaining intact articular surfaces.
Radiolabeled cultured media were prepared with 20 μCi/ml of 3H-proline and 10 μCi/ml of 35S-sodium sulfate to label biosynthesis of proteins and sulfated glycosaminoglycans (sGAGs), respectively. In the first experiment, explants were cultured for 22 hours in radiolabeled basal medium alone (control) or supplemented with 0.02, 0.2, or 2 μg/ml of adipokines, as described above. In the second experiment, explants were cultured for 8 days in radiolabel-free media supplemented with adipokines, with media exchanges every 48 hours. Explants were then cultured for an additional 22 hours in media supplemented with radiolabels to assess chronic effects of adipokines on biosynthesis. Conditioned media were collected and stored at −20°C for later analysis.
Biochemistry
Explants were washed four times for 30 minutes each at 4°C in PBS supplemented with 0.8 mM sodium sulfate and 1.0 mM L-proline to remove unincorporated radiolabels. Explants were weighed, lyophilized overnight and re-weighed dry, then digested overnight at 60°C in 1 ml proteinase K (1mg/40mg cartilage wet mass, 1mg/20mg meniscus wet mass) buffered with 100 mM ammonium acetate. Digest radiolabel contents were measured using a liquid scintillation counter. Explant DNA contents were measured using the Hoechst 33258 assay [32] with calf thymus DNA standards. Sulfated glycosaminoglycan (sGAG) contents of digested explants and conditioned media were assayed using the dimethylmethylene blue (DMMB) assay [33] with chondroitin sulfate standards ranging from 0–100μg/ml. To estimate production of nitric oxide (NO), a cell signaling mediator in inflammation [34], nitrite contents of conditioned media were measured using the Griess assay [35] with sodium nitrite standards ranging from 0–100μM. Lactate dehydrogenase (LDH), a surrogate indicator of cell lysis, was measured in conditioned media from the 9-day culture studies using the CytoTox-ONE™ kit with purified LDH from fibrocytes as standards. As necessary, samples were diluted to remain in the linear region of each assay.
Data Analysis
Radiolabel incorporation of each explant was normalized to its DNA content. All data were log transformed to improve normality. Statistical analyses were performed using Minitab 17 (Minitab, Inc., State College, PA). For each experiment, responses for each adipokine were analyzed using general linear models (GLMs) and Bonferroni’s test for planned pairwise comparisons. Tissue and adipokine dose were the main factors in each analysis, with donor animal as a random factor and position (anterior, central, posterior) nested within tissue to account for baseline regional differences. The Anderson-Darling test was performed to verify normality of residuals; in rare instances individual outcomes had non-normal residuals due to outliers, but elimination of outliers did not alter main findings. Significance was set at p<0.05 and values are presented as means with 95% confidence intervals (lower limit, upper limit).
Results
sGAG Release
Immediate effects
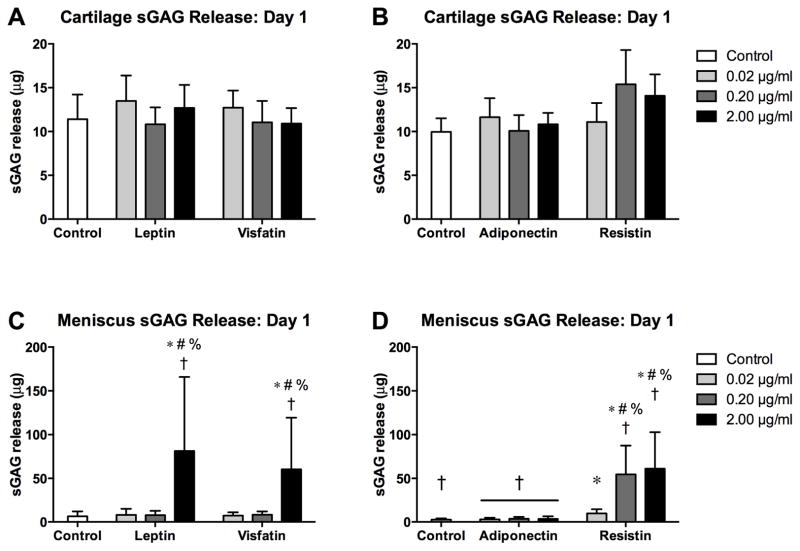
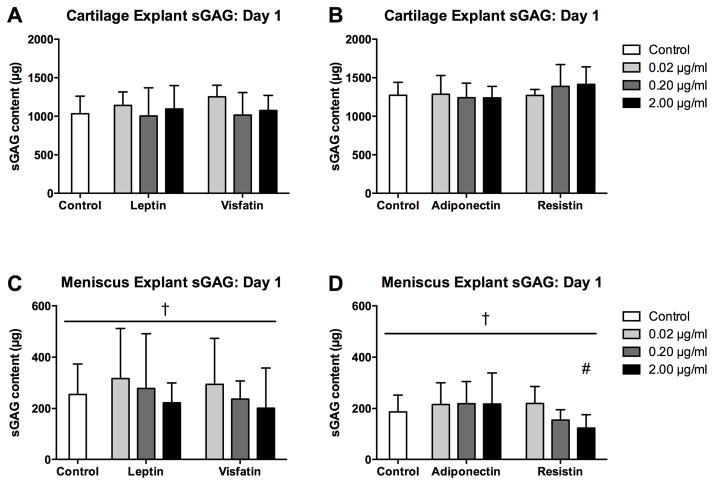
In the adiponectin-resistin substudy, cartilage controls and adiponectin at all doses exhibited higher sGAG release than corresponding meniscus groups (Figure 1; all p<0.001). For cartilage, none of the adipokines significantly elevated sGAG release on day 1 compared to tissue-matched controls. In contrast, meniscus sGAG release on day 1 was significantly greater than tissue-matched controls for leptin at 2 μg/ml, visfatin at 2 μg/ml and resistin at all doses (all p<0.001). Leptin, visfatin, adiponectin and resistin induced release of 24.1% (2.6%, 45.5%), 22.8% (5.0%, 40.5%), 1.4% (1.2%, 1.6%) and 32.0% (17.8%, 46.3%) of total sGAG, respectively, as compared to 2.3% (1.6%, 2.9%) and 1.4% (0.8%, 1.9%) for controls in the two substudies. Additionally, meniscus treated with leptin (2 μg/ml, p=0.032) visfatin (2 μg/ml, p<0.001) or resistin (0.2 μg/ml, p=0.001; 2 μg/ml, p<0.001) exhibited higher sGAG release than cartilage with corresponding treatments, despite the substantially lower sGAG contents of meniscus explants.
Figure 1. Initial effects of adipokines on sGAG release.
sGAG contents of conditioned media from cartilage (A–B) and meniscus (C–D) explants treated with adipokines for 22 hours. Note the difference in scaling between cartilage and meniscus. *p<0.05 relative to tissue-matched controls, #p<0.05 relative to 0.02 μg/ml treatment, %p<0.05 relative to 0.20 μg/ml treatment, †p<0.05 relative to cartilage at the same treatment and dose.
Sustained treatment effects
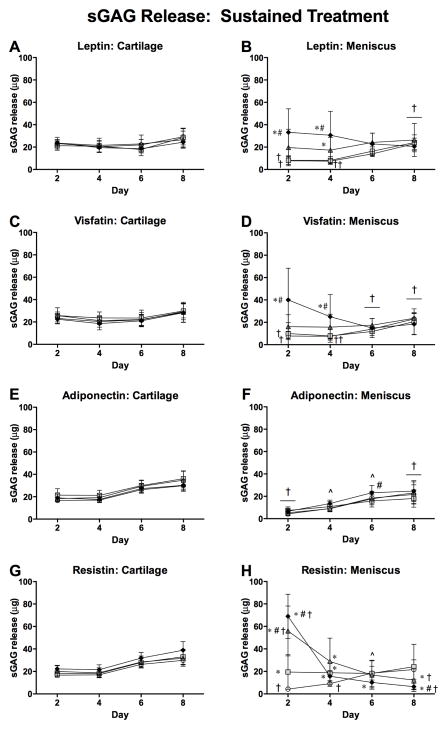
sGAG release from controls was greater for cartilage than for meniscus throughout the 9-day culture duration in both substudies (Figure 2). As a fraction of total measured sGAG, however, 8-day sGAG release from controls was significantly greater for meniscus than for cartilage (24.6% (19.8%, 29.5%) vs. 5.8% (5.3%, 6.2%)), consistent with previous observations [36]. Cartilage sGAG release was not significantly affected by any adipokine treatment throughout the culture duration. In contrast, meniscus sGAG release on day 2 was elevated by treatment with 2 μg/ml leptin, 2 μg/ml visfatin and all concentrations of resistin (all p<0.001 vs. controls). Elevated sGAG release continued through 4 days for meniscus treated with 2 μg/ml (p<0.001) or 0.2 μg/ml (p=0.023) leptin, 2 μg/ml visfatin (p=0.011), and all concentrations of resistin (all p<0.001). Later in the culture period, meniscus samples treated with resistin exhibited lower sGAG release than controls on day 6 at 2 μg/ml (p=0.003) and on day 8 at 0.2 μg/ml (p=0.003) and 2 μg/ml (p≤0.001), likely reflecting substantial sGAG depletion. Over 8 days, leptin, visfatin, adiponectin and resistin induced cumulative release of 29.6% (32.9%, 35.4%), 38.4% (23.1%, 53.8%), 35.8% (24.9%, 46.8%) and 62.7% (51.6%%, 73.8%) of measured sGAG, respectively, as compared to 20.0% (14.2%, 25.8%) and 29.3% (22.3%, 36.3%) for controls in the two substudies. Consistent with the results for immediate release, treatment with 0.2–2 μg/ml resistin elevated meniscus sGAG release relative to cartilage with the same treatment on day 2 of culture (both p<0.001), particularly notable given the substantial disparity in sGAG content between cartilage and meniscus tissues.
Figure 2. Effects of sustained adipokine exposure on sGAG release.
48-hour sGAG release to media from cartilage (left column) and meniscus (right column) explants treated with adipokines for 8 days. *p<0.05 relative to tissue-matched controls, #p<0.05 relative to 0.02 μg/ml treatment, †p<0.05 relative to cartilage at the same treatment and dose, ^p<0.05 relative to cartilage for some groups.
Nitrite Release
Immediate effects
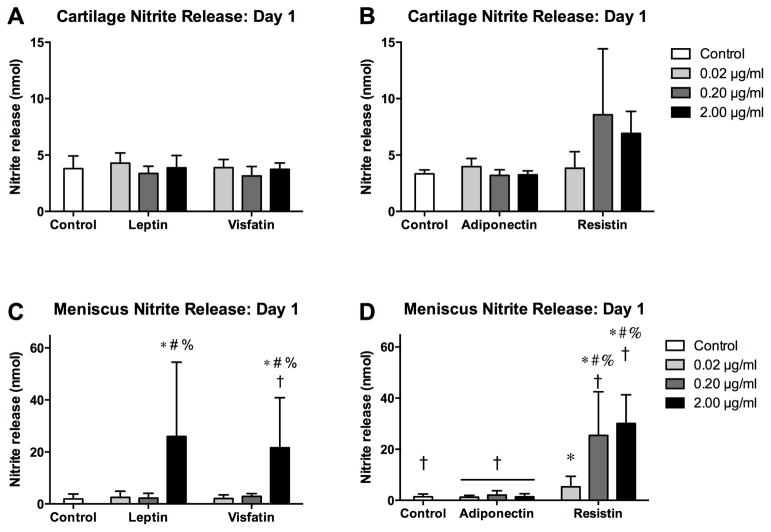
Nitrite release patterns on day 1 (Figure 3) generally paralleled sGAG release. In the adiponectin-resistin substudy, day 1 nitrite release was greater in cartilage for controls (p=0.035) and adiponectin groups (all p<0.001). In cartilage, nitrite release was not significantly elevated above controls for any adipokine. In contrast, leptin (2 μg/ml, p<0.001), visfatin (2 μg/ml, p<0.001) and all doses of resistin (0.02μg/ml, p=0.004; 0.2 and 2 μg/ml, p<0.001) significantly elevated day 1 nitrite release in meniscus above controls. Day 1 nitrite release was greater for meniscus than cartilage samples treated with visfatin (2 μg/ml, p<0.001) or resistin (0.2 μg/ml, p=0.042; 2 μg/ml, p=0.002), with a trend towards greater release with leptin (2 μg/ml, p=0.059).
Figure 3. Initial effects of adipokines on nitrite release.
Nitrite content of conditioned media from cartilage (A–B) and meniscus (C–D) explants treated with adipokines for 22 hours. Note the difference in scaling between cartilage and meniscus. *p<0.05 relative to tissue-matched controls, #p<0.05 relative to 0.02 μg/ml treatment, %p<0.05 relative to 0.20 μg/ml treatment, †p<0.05 relative to cartilage at the same treatment and dose.
Sustained treatment effects
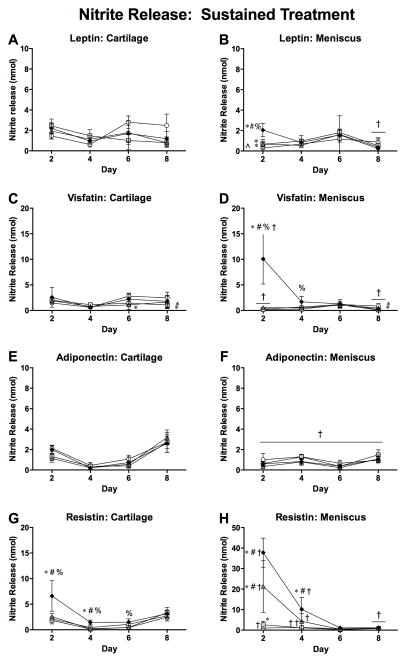
Dose-dependent nitrite release patterns (Figure 4) were similar to sGAG release patterns. On day 2 of both substudies, cartilage controls had greater nitrite release than meniscus controls (both p<0.001). Cartilage nitrite release was significantly increased relative to controls only by 2 μg/ml resistin on day 2 (p=0.001) and day 4 (p=0.004). Meniscus nitrite release on day 2 was elevated by treatment with all leptin doses (all p<0.001), 2 μg/ml visfatin (p<0.001) and all resistin doses (0.02 μg/ml, p=0.001; 0.2 and 2 μg/ml, p<0.001). Elevated nitrite release continued through day 4 of the culture duration for meniscus treated with 2 μg/ml resistin (p=0.004). Additionally, 2 μg/ml visfatin (p<0.001) and 0.2 and 2 μg/ml resistin (both p<0.001) elevated meniscus nitrite release on day 2 relative to cartilage with the same treatment, despite the lower baseline (control) nitrite production for meniscus.
Figure 4. Effects of sustained adipokine exposure on nitrite release.
48-hour nitrite levels in conditioned media from cartilage (left column) and meniscus (right column) explants treated with adipokines for 8 days. Note the differences in scaling. *p<0.05 relative to tissue-matched controls, #p<0.05 relative to 0.02 μg/ml treatment, %p<0.05 relative to 0.20 μg/ml treatment, †p<0.05 relative to cartilage at the same treatment and dose, ^p<0.05 relative to cartilage for some groups.
Lactate dehydrogenase release
LDH release, a surrogate measure of cell lysis, was not significantly above controls for any adipokine for either tissue, suggesting that the doses examined were not cytotoxic. All leptin doses decreased LDH release relative to controls on days 2, 4 and 8 (all p<0.01).
Explant Composition
Immediate effects
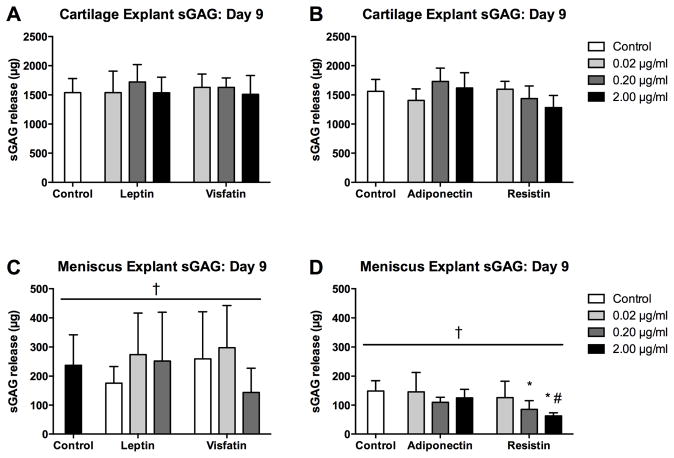
In both substudies, cartilage explants had greater water (p<0.001) and DNA (p<0.001) contents than meniscus explants, and water content was unaffected by any adipokine treatment. Cartilage DNA content was lower than controls for adiponectin at 0.02 μg/ml (p<0.001) but not for any other treatment, and meniscus DNA content did not vary with adipokine treatment. sGAG content (Figure 5) was greater in cartilage than in meniscus (p<0.001) in both substudies, indicative of differences in native tissue composition. Cartilage sGAG content did not vary with 1 day of adipokine treatment. Meniscus sGAG content after 1 day of culture was lower with 2 μg/ml resistin than with 0.02 μg/ml resistin (p=0.002), but otherwise showed no significant variations.
Figure 5. Initial effects of adipokines on explant sGAG content.
sGAG content of cartilage (A–B) and meniscus (C–D) explants treated with adipokines for 22 hours. Note difference in vertical axes between cartilage and meniscus. #p<0.05 relative to 0.02 μg/ml treatment, †p<0.05 relative to cartilage.
Sustained treatment effects
Cartilage explants after the 9-day culture had greater water (p<0.001), DNA (p<0.001) and sGAG (Figure 6, p<0.001) contents than meniscus explants. Adipokine treatments did not significantly alter water or DNA contents for either tissue or sGAG content for cartilage. Meniscus sGAG content was significantly decreased relative to tissue-matched controls by resistin at 0.2 (p=0.001) or 2 μg/ml (p<0.001), and was significantly lower at 2 μg/ml than at 0.02 μg/ml (p<0.001), but was not significantly altered by other adipokines.
Figure 6. Effect of sustained adipokine exposure on explant sGAG content.
sGAG content of cartilage (A–B) and meniscus (C–D) explants treated with adipokines for 22 hours. Note the difference in scaling between cartilage and meniscus. *p<0.05 relative to controls. #p<0.05 relative to 0.02 μg/ml treatment, †p<0.05 relative to cartilage.
Biosynthesis
Immediate effects
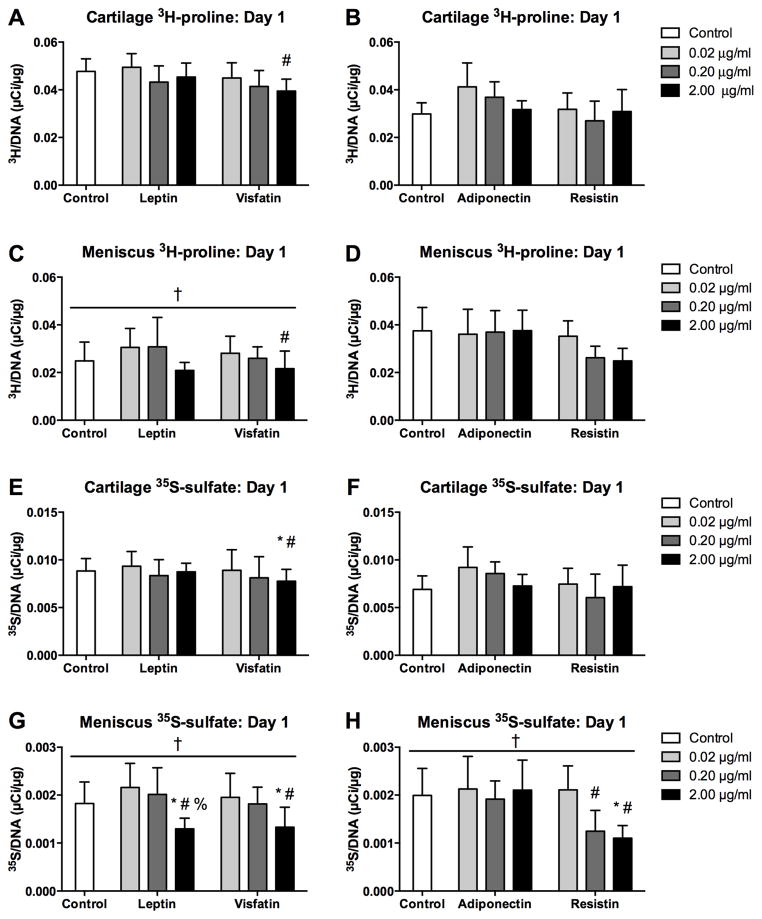
3H-proline incorporation on day 1 of culture (Figure 7A–D) was significantly greater for cartilage than for meniscus in the leptin-visfatin substudy (p<0.001) but not in the adiponectin-resistin substudy. 35S-sulfate incorporation on day 1 (Figure 7E–H) was significantly (and substantially) greater in cartilage than in meniscus explants in both substudies (p<0.001), consistent with the higher baseline sGAG synthesis rate of cartilage.
Figure 7. Initial effects of adipokines on incorporation rates.
Protein (3H-proline, A–D) and sGAG (35S-sulfate, E–H) incorporation rates of cartilage (A–B, E–F) and meniscus (C–D, G–H) explants over the first 22 hours of culture with adipokines. Note the difference in scaling between cartilage and meniscus. *p<0.05 relative to tissue-matched controls unless otherwise indicated, #p<0.05 relative to 0.02 μg/ml treatment, %p<0.05 relative to 0.20 μg/ml treatment, †p<0.05 relative to cartilage tissue.
Treatment with adipokines did not significantly alter 3H-proline incorporation on day 1 compared to tissue-matched controls. 3H-proline incorporation was lower with visfatin at 2 μg/ml than at 0.02 μg/ml (p=0.037) with no interaction with tissue, but there were no other significant effects of adipokine dose. In contrast, 35S-sulfate incorporation on day 1 exhibited dose-dependent responses to several adipokines. 35S-sulfate incorporation was lower for visfatin at 2 μg/ml than at 0.02 μg/ml (p=0.017) or controls (p=0.045), with no interaction with tissue. Leptin significantly affected meniscus 35S-sulfate incorporation, with lower values at 2 μg/ml than at 0.2 μg/ml (p=0.004), 0.02 μg/ml (p<0.001) or in controls (p=0.045). Similarly, resistin significantly affected meniscus 35S-sulfate incorporation, with lower values at 2 μg/ml than at 0.02 μg/ml (p=0.009) or in controls (p=0.028), and lower values at 0.2 μg/ml than at 0.02 μg/ml (p=0.043).
Sustained treatment effects
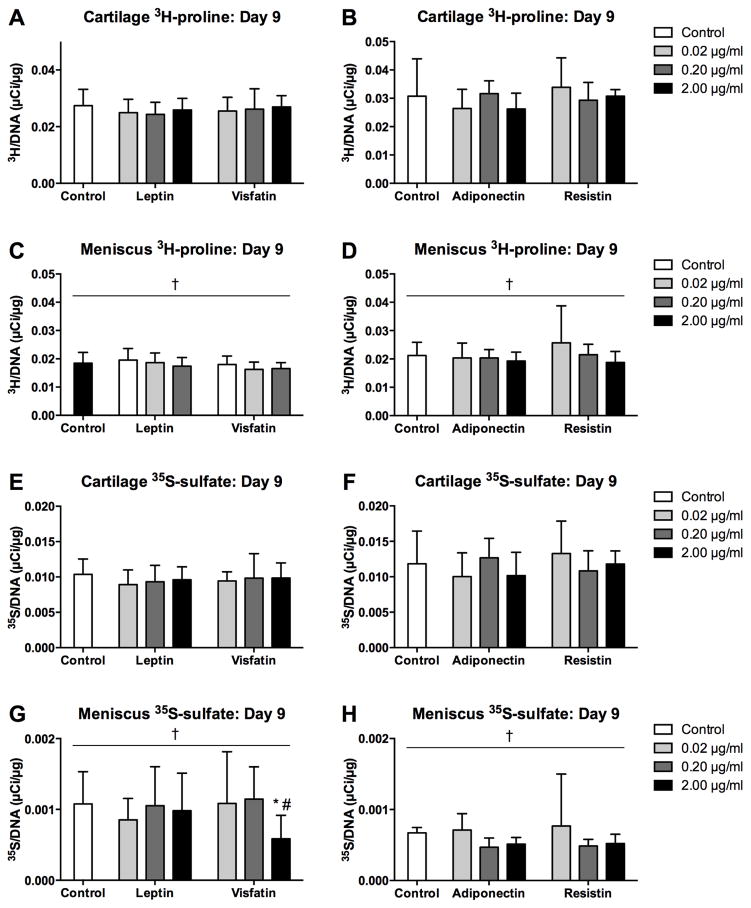
Effects of adipokines on radiolabel incorporation rates were fairly minor by day 9 of culture. Both 3H-proline incorporation and 35S-sulfate incorporation (Figure 8) were significantly greater in cartilage explants than in meniscus explants in both substudies (all p<0.001). 3H-proline incorporation was not significantly altered by any adipokine treatment in either tissue. For meniscus, 35S-sulfate incorporation was significantly lower with 2 μg/ml visfatin than with 0.02 μg/ml (p=0.023) or in controls (p=0.045), with no other significant effects of adipokines.
Figure 8. Effects of sustained adipokine exposure on incorporation rates.
Protein (3H-proline, A–D) and sGAG (35S-sulfate, E–H) incorporation rates of cartilage (A–B, E–F) and meniscus (C–D, G–H) explants over the final 22 hours of 9-day cultures with adipokines.. Note the difference in scaling between cartilage and meniscus. *p<0.05 relative to tissue-matched controls, #p<0.05 relative to 0.02 μg/ml treatment, †p<0.05 relative to cartilage tissue.
Discussion
While systemic and local effects of obesity-related biochemical signals have received increasing attention as non-mechanical links between excess fat and joint degeneration, few studies have compared the effects of these factors on different tissues involved in early stage OA. The current studies evaluated catabolic and anti-anabolic effects on cartilage and meniscus explants of several adipokines present in synovial fluid and implicated in the development of OA. In meniscus explants, leptin, visfatin and resistin all stimulated sGAG release and nitrite production while inhibiting to varying degrees incorporation of newly synthesized sGAG. Effects on cartilage were relatively minor, with the only significant effects being initial reduction of matrix synthesis at the highest visfatin dose and elevated nitrite production with the highest resistin dose. Overall, our results indicate that meniscal tissue is more susceptible than cartilage tissue to metabolic alterations induced by these adipokines.
Interestingly, the greater relative effects of adipokines on meniscus are consistent with effects of interleukin-1 (IL-1) in similar culture conditions. Meniscus sGAG release was maximal immediately upon IL-1α treatment and steadily decreased until sGAG depletion [37] while cartilage exhibited more gradual sGAG release that peaked at about one week of treatment [38]. Although the patterns of catabolism induced by adipokines (primarily resistin) and IL-1 are similar, further studies are required to determine the extent to which common pathways are involved in the response, an issue with implications for potential pharmacologic interventions to address early obesity- or injury-related degeneration. It should be noted that we used media including high glucose and insulin to ensure high metabolic activity [39] and for consistency with previous studies examining IL-1 effects on these tissues [36, 38]. Like cytokines such as IL-1 and TNF-α [40], some adipokines may regulate glucose metabolism in cartilaginous tissues, and the effects of glucose levels (both in vitro and in vivo) on adipokine-stimulated tissue catabolism should be examined.
Several factors may contribute to the differential effects of adipokines on cartilage and meniscus tissues. Chondrocytes and meniscal fibrochondrocytes may respond differently to the adipokines, for example due to differences in receptor expression or differences in the cellular response to a given stimulus (e.g., different profiles of secreted proteases). The extracellular matrices (ECMs) may play a role via differential transport of adipokines into or through the tissues, differential transport of secreted proteases, or differential susceptibility of the ECMs to cell-mediated catabolism related to differences in ECM composition and structure. These intertwined factors could be separated to some extent by studying the responsiveness of isolated cells to exogenous factors, detailed analysis of protease expression patterns, or selective use of protease inhibitors. The microscale arrangement of the ECM and resident cells are quite different between cartilage and meniscus, with more mesoscale heterogeneity of meniscal matrix constituents and localization of fibrochondrocytes in the proteoglycan-enriched matrix compartment surrounding the primary circumferential collagen bundles [41, 42]. This different microstructural arrangement may play a role in the tissue-specific responsiveness to exogenous factors, as a greater fraction of the tissue sGAG is near the cells in meniscal tissue. Examination of the spatial distribution of protease expression and activity through histological methods may provide additional insights into the mechanisms behind the differential susceptibility.
Increased sGAG release was accompanied by increased nitrite release (indicative of NO production), suggesting the involvement of active inflammatory pathways. Visfatin (but not leptin) was previously reported to increase NO production in cartilage and meniscus explants and to increase sGAG release from cartilage explants [29]. Differences with our studies include culture media (serum-supplemented vs. serum-free), concentration ranges (up to 0.1 μg/ml vs. 2 μg/ml) and tissue source (adult porcine vs. juvenile bovine). Immature bovine tissue has often been used to study cartilage degradation in vitro as it provides a non-diseased blank slate to study pathological mechanisms. However, maturity can affect cell metabolism [43] and responses to catabolic stimuli [44]. While effects of recombinant human adipokines have been demonstrated on cartilaginous tissues from various species [17, 29, 45], caution should be exercised in directly extrapolating specific findings to human tissue without verification.
The dose ranges used in this study encompass physiologic and hyper-physiologic concentrations. For leptin [10, 14, 16], visfatin [19, 20], and resistin [12, 14, 17], reported concentrations in the synovial joints of OA patients approach the lowest dose in this experiment (20 ng/ml). It should be noted that in vivo adiponectin concentrations (up to 2 μg/ml [12, 14, 23]) can be orders of magnitude higher than those of the other adipokines tested, so the adiponectin doses in this study did not reach the clearly hyper-physiologic range.
In vivo animal studies with a single high dose injection of leptin into rat knees demonstrated a catabolic response in cartilage, with elevated MMP and aggrecanase expressions and proteoglycan depletion [46]. While this response could have been mediated through other joint tissues such as the synovium, in vitro culture with 10 μg/ml leptin upregulated MMP activity [25] and NO production [26] in human OA cartilage. In the current study, cartilage NO release was not modulated by leptin but NO production in meniscus was temporarily elevated, as observed previously for cartilage [26]. Leptin also stimulated elevated sGAG release in meniscus through the first four days of the culture period and inhibited sGAG incorporation in meniscus on day 1. Although leptin decreased LDH release in both cartilage and meniscus in this study, we found no significant effects on LDH release in a preliminary study. Similarly inconsistent effects of leptin on porcine myoblasts have been reported [47, 48].
Visfatin increased aggrecanase (ADAMTS-4 and -5) expression and MMP-3 and -13 synthesis by human OA chondrocytes in monolayer [11]. In the current study, cartilage was minimally affected by visfatin at the concentrations tested, which may be attributable to differences in species and disease state but may also reflect differences between monolayer and 3D tissue culture. Meniscus sGAG release and NO production were elevated by exposure to visfatin while initial protein and sGAG incorporations were inhibited at the highest dose. Inhibition of meniscal sGAG incorporation persisted through day 9, suggesting that sustained exposure to visfatin might affect tissue homeostasis.
The role in cartilage catabolism of adiponectin, which is inversely correlated with BMI [21] but associated with OA severity, remains controversial. Adiponectin has been reported to upregulate TIMP-2 and downregulate interleukin-1β (IL-1β)-induced MMP-13 production by OA human chondrocytes [21], but has also been reported to increase NO and MMP production [22]. We observed no significant effects of adiponectin on cartilage or meniscus explants, again pointing to potential differences between monolayer and tissue culture.
Resistin downregulated collagen II and aggrecan expression and upregulated ADAMTS-4, TNF-α, and IL-1β expression in cultured human chondrocytes [28], and induced dose-dependent depletion of sGAG from mouse cartilage and inhibition of sGAG synthesis in human cartilage [17]. In our studies, resistin was the only adipokine to substantially stimulate cartilage sGAG release, although cartilage sGAG content and incorporation rates were not significantly altered. Our results show for the first time that resistin dramatically increased sGAG release from meniscal tissue and substantially reduced meniscus sGAG content, even after just 22 hours of exposure, and meniscal sGAG content was reduced to less than half that of controls after 9-day exposure to 2 μg/ml resistin.
The strong effects of resistin are particularly interesting, as resistin was elevated in the knee joint for several weeks following traumatic injury [17]. Both meniscus and cartilage tissues undergo immediate cell death following relatively moderate overload [49, 50], despite the greater resistance of meniscus to macroscopic failure. However, a greater susceptibility to inflammatory factors elevated as part of the injury response could contribute to earlier development of degeneration in meniscus than in articular cartilage. Meniscus has nearly an order of magnitude less proteoglycan content than cartilage, and small perturbations to the proteoglycan composition can induce larger relative loss of functional mechanical properties in meniscus than in cartilage [37, 38]. Following injury, the meniscus may be particularly susceptible to resistin-induced sGAG depletion even in the absence of macroscopic damage directly related to the trauma itself.
In conclusion, these studies demonstrated a greater susceptibility to adipokine-induced catabolism by meniscus tissue than by cartilage. Resistin was the most active of the four adipokines tested, inducing substantial sGAG depletion from meniscal tissue explants. This finding is particularly relevant as traumatic injury, independent of obesity, can elevate synovial concentrations of resistin in the knee joint. Based on these results, even a short-term exposure to resistin can have catabolic effects in meniscal tissue. Degradation of the meniscus then may stimulate cartilage degradation, either by altering the local mechanical environment or biologically stimulating catabolism. As obesity continues to be a national epidemic, understanding the systemic consequences of increased adiposity is important in evaluating its impact on the lifestyles of those at risk. Investigations into the complex interactions between biologic and mechanical factors in knee joint health are not only valuable to understand the etiology of osteoarthritis, but may also reveal novel targets for early diagnosis or pharmaceutical intervention.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (NIAMS R01AR052861) and a National Science Foundation Graduate Research Fellowship.
Role of the Funding Source
The sponsors (NIH through R01AR052861, NSF through a graduate research fellowship) had no involvement in the study design and execution, data interpretation, writing of the manuscript or the decision to submit the manuscript.
Footnotes
Contributions
Both authors participated in the conception and design of the studies and interpretation of data. JFN performed the studies and drafted the manuscript. Both authors participated in revising the manuscript and approved the final version. Both James Nishimuta (j.nishimuta@gmail.com) and Marc Levenston (levenston@stanford.edu) take responsibility for the integrity of the work presented.
Competing Interests Statement
Neither author has any competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James F. Nishimuta, Email: j.nishimuta@gmail.com.
Marc E. Levenston, Email: levenston@stanford.edu.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 3.Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. International Journal of Obesity. 2001;25:622–627. doi: 10.1038/sj.ijo.0801585. [DOI] [PubMed] [Google Scholar]
- 4.Stürmer T, Günther KP, Brenner H. Obesity, overweight and patterns of osteoarthritis: the Ulm Osteoarthritis Study. Journal of Clinical Epidemiology. 2000;53:307–313. doi: 10.1016/s0895-4356(99)00162-6. [DOI] [PubMed] [Google Scholar]
- 5.Teichtahl AJ, Wang Y, Wluka AE, Cicuttini FM. Obesity and knee osteoarthritis: new insights provided by body composition studies. Obesity. 2008;16:232–240. doi: 10.1038/oby.2007.30. [DOI] [PubMed] [Google Scholar]
- 6.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskeletal Disorders. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalichman L, Li L, Kobyliansky E. Prevalence, pattern and determinants of radiographic hand osteoarthritis in Turkmen community-based sample. Rheumatology International. 2009;29:1143–1149. doi: 10.1007/s00296-008-0815-1. [DOI] [PubMed] [Google Scholar]
- 8.Hutley L, Prins JB. Fat as an endocrine organ: relationship to the metabolic syndrome. The American Journal of the Medical Sciences. 2005;330:280–289. doi: 10.1097/00000441-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Neumann E, Frommer KW, Vasile M, Müller-Ladner U. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis and Rheumatism. 2011;63:1159–1169. doi: 10.1002/art.30291. [DOI] [PubMed] [Google Scholar]
- 10.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis and Rheumatism. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 11.Gosset M, Berenbaum F, Salvat C, Sautet A, Pigenet A, Tahiri K, et al. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis and Rheumatism. 2008;58:1399–1409. doi: 10.1002/art.23431. [DOI] [PubMed] [Google Scholar]
- 12.Schäffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Schölmerich J, et al. Adipocytokines in synovial fluid. Journal of the American Medical Association. 2003;290:1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 13.Hu P-F, Bao J-P, Wu L-D. Molecular Biology Reports. 2010. The emerging role of adipokines in osteoarthritis: a narrative review. [DOI] [PubMed] [Google Scholar]
- 14.Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis and Cartilage. 2006;14:690–695. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851–857. doi: 10.1136/ard.2010.140046. [DOI] [PubMed] [Google Scholar]
- 16.Ku JH, Lee CK, Joo BS, An BM, Choi SH, Wang TH, et al. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clinical Rheumatology. 2009;28:1431–1435. doi: 10.1007/s10067-009-1242-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Ort T, Ma K, Picha K, Carton J, Marsters PA, et al. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthritis and Cartilage. 2009;17:613–620. doi: 10.1016/j.joca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Choe JY, Bae J, Jung HY, Park SH, Lee HJ, Kim SK. Serum resistin level is associated with radiographic changes in hand osteoarthritis: cross-sectional study. Joint Bone Spine. 2012;79:160–165. doi: 10.1016/j.jbspin.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Duan Y, Hao D, Li M, Wu Z, Li D, Yang X, et al. Increased synovial fluid visfatin is positively linked to cartilage degradation biomarkers in osteoarthritis. Rheumatology International. 2011 doi: 10.1007/s00296-010-1731-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen W-P, Bao J-P, Feng J, Hu P-F, Shi Z-L, Wu L-D. Increased serum concentrations of visfatin and its production by different joint tissues in patients with osteoarthritis. Clinical Chemistry and Laboratory Medicine. 2010;48:1141–1145. doi: 10.1515/CCLM.2010.230. [DOI] [PubMed] [Google Scholar]
- 21.Chen T-H, Chen L, Hsieh M-S, Chang C-P, Chou D-T, Tsai S-H. Evidence for a protective role for adiponectin in osteoarthritis. Biochimica et Biophysica Acta. 2006;1762:711–718. doi: 10.1016/j.bbadis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Lago R, Gomez R, Otero M, Lago F, Gallego R, Dieguez C, et al. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis and Cartilage. 2008;16:1101–1109. doi: 10.1016/j.joca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Honsawek S, Chayanupatkul M. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res. 2010;41:593–598. doi: 10.1016/j.arcmed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 24.de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JWJ, Lafeber FPJG, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis and Cartilage. 2012 doi: 10.1016/j.joca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Koskinen A, Vuolteenaho K, Nieminen R, Moilanen T, Moilanen E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clinical & Experimental Immunology. 2011;29:57–64. [PubMed] [Google Scholar]
- 26.Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage--mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators of Inflammation. 2009;2009:345838. doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Research & Therapy. 2011;13:R184. doi: 10.1186/ar3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Xing X, Hensley G, Chang L-W, Liao W, Abu-Amer Y, et al. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis and Rheumatism. 2010;62:1993–2003. doi: 10.1002/art.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNulty AL, Miller MR, O’Connor SK, Guilak F. Connective Tissue Research. 2011. The Effects of Adipokines on Cartilage and Meniscus Catabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuta S, Masaki K, Korai F. Prevalence of abnormal findings in magnetic resonance images of asymptomatic knees. J Orthop Sci. 2002;7:287–291. doi: 10.1007/s007760200049. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton JH, Hanley EN, et al. Analysis of meniscal degeneration and meniscal gene expression. BMC musculoskeletal disorders. 2010;11:19. doi: 10.1186/1471-2474-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 33.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 34.Amin AR, Abramson SB. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Current Opinion in Rheumatology. 1998;10:263–268. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Hensley K, Mou S, Pye QN. Nitrite Determination by Colorimetric and Fluorometric Griess Diazotization Assays. In: Hensley K, Floyd R, editors. Methods in Pharmacology and Toxicology: Methods in Biological Oxidative Stress. Totowa, NJ: Humana Press; 2003. pp. 185–193. [Google Scholar]
- 36.Wilson CG, Nishimuta JF, Levenston ME. Chondrocytes and meniscal fibrochondrocytes differentially process aggrecan during de novo extracellular matrix assembly. Tissue Eng Part A. 2009;15:1513–1522. doi: 10.1089/ten.tea.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson CG, Vanderploeg EJ, Zuo F, Sandy JD, Levenston ME. Aggrecanolysis and in vitro matrix degradation in the immature bovine meniscus: mechanisms and functional implications. Arthritis Research & Therapy. 2009;11:R173. doi: 10.1186/ar2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer AW, Wilson CG, Baum EJ, Levenston ME. Composition-function relationships during IL-1-induced cartilage degradation and recovery. Osteoarthritis and Cartilage. 2009;17:1029–1039. doi: 10.1016/j.joca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cigan AD, Nims RJ, Albro MB, Esau JD, Dreyer MP, Vunjak-Novakovic G, et al. Insulin, ascorbate, and glucose have a much greater influence than transferrin and selenous acid on the in vitro growth of engineered cartilage in chondrogenic media. Tissue Eng Part A. 2013;19:1941–1948. doi: 10.1089/ten.tea.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shikhman AR, Brinson DC, Valbracht J, Lotz MK. Cytokine regulation of facilitated glucose transport in human articular chondrocytes. J Immunol. 2001;167:7001–7008. doi: 10.4049/jimmunol.167.12.7001. [DOI] [PubMed] [Google Scholar]
- 41.Vanderploeg EJ, Wilson CG, Imler SM, Ling CH, Levenston ME. Regional variations in the distribution and colocalization of extracellular matrix proteins in the juvenile bovine meniscus. J Anat. 2012;221:174–186. doi: 10.1111/j.1469-7580.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kambic HE, McDevitt CA. Spatial organization of types I and II collagen in the canine meniscus. J Orthop Res. 2005;23:142–149. doi: 10.1016/j.orthres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis and Cartilage. 2009;17:971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arner EC. Effect of animal age and chronicity of interleukin-1 exposure on cartilage proteoglycan depletion in vivo. J Orthop Res. 1994;12:321–330. doi: 10.1002/jor.1100120304. [DOI] [PubMed] [Google Scholar]
- 45.Hui W, Litherland GJ, Elias MS, Kitson GI, Cawston TE, Rowan AD, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Annals of the Rheumatic Diseases. 2012 doi: 10.1136/annrheumdis-2011-200372. [DOI] [PubMed] [Google Scholar]
- 46.Bao J-P, Chen W-P, Feng J, Hu P-F, Shi Z-L, Wu L-D. Molecular biology reports. 2009. Leptin plays a catabolic role on articular cartilage. [DOI] [PubMed] [Google Scholar]
- 47.Will K, Kuzinski J, Kalbe C, Palin MF, Rehfeldt C. Effects of leptin and adiponectin on the growth of porcine myoblasts are associated with changes in p44/42 MAPK signaling. Domest Anim Endocrinol. 2013;45:196–205. doi: 10.1016/j.domaniend.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Will K, Kuzinski J, Palin MF, Hildebrands JP, Rehfeldt C. A second look at leptin and adiponectin actions on the growth of primary porcine myoblasts under serum-free conditions. Archiv Tierzucht. 2014;57:1–10. [Google Scholar]
- 49.Nishimuta JF, Levenston ME. Response of cartilage and meniscus tissue explants to in vitro compressive overload. Osteoarthritis and Cartilage. 2012;20:422–429. doi: 10.1016/j.joca.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kisiday JD, Vanderploeg EJ, McIlwraith CW, Grodzinsky AJ, Frisbie DD. Mechanical injury of explants from the articulating surface of the inner meniscus. Archives of Biochemistry and Biophysics. 2010;494:138–144. doi: 10.1016/j.abb.2009.11.022. [DOI] [PubMed] [Google Scholar]