Abstract
Nicotine addiction is a chronic brain disorder that is characterized by dysphoria upon smoking cessation and relapse after brief periods of abstinence. It has been hypothesized that the negative mood state associated with nicotine withdrawal is partly mediated by a heightened activity of brain stress systems. Animal studies suggest that blockade of vasopressin 1b (V1b) receptors diminishes high levels of drug intake in dependent animals and attenuates the emotional response to stressors. The goal of the present studies was to investigate the effect of acute and chronic treatment with the V1b receptor antagonist SSR149415 on the negative mood state associated with nicotine withdrawal in rats. An intracranial self-stimulation (ICSS) procedure was used to assess mood states and nicotine dependence was induced using minipumps. The nicotinic receptor antagonist mecamylamine was used to precipitate withdrawal. Mecamylamine elevated the brain reward thresholds of the nicotine dependent rats, which reflects a negative mood state. Mecamylamine did not affect the brain reward thresholds of the saline-treated control rats. Chronic treatment with SSR149415 completely prevented the elevations in brain reward thresholds associated with nicotine withdrawal while acute treatment only partly prevented nicotine withdrawal. These data suggest that chronic treatment with V1b receptor antagonists may prevent the dysphoria associated with smoking cessation and thereby improve relapse rates.
Keywords: Nicotine, withdrawal, ICSS, dysphoria, vasopressin 1b receptor, SSR149415
1. Introduction
Nicotine addiction is a chronic brain disorder that is characterized by dysphoria upon smoking cessation and relapse after periods of abstinence [1]. The World Health Organization estimates that there are one billion smokers worldwide and that about 6 million people die each year from smoking or second hand smoke exposure [2]. Smoking has detrimental effects on human health and increases the risk for cancer, cardiovascular disorders, chronic obstructive pulmonary disease, and dementia and Alzheimer's disease [3-5].
The acute rewarding and cognitive enhancing effects of nicotine play an important role in the initiation of smoking [1, 6]. However, it has been suggested that negative mood states play an important role in the transition from experimenting with cigarettes to high levels of smoking and in the maintenance of smoking and relapse [7]. This is supported by the observation that people who are trying to quit smoking are most likely to relapse during the first week when the negative affective withdrawal symptoms are most severe [8, 9]. People with depression are more likely to relapse than people without a mood disorder [10, 11]. Furthermore, preclinical studies with cocaine indicate that animals that display the greatest deficit in reward function during withdrawal display the greatest increase in drug intake over time [12]. Therefore, it is critical to understand the neuronal mechanisms that mediate the negative mood state associated with smoking cessation.
Animal models have been developed to study the negative mood state associated with smoking cessation. In particular, the intracranial self-stimulation (ICSS) procedure has been widely used to study the effects of drugs of abuse on brain reward function [13-15]. Acute administration of drugs of abuse lowers ICSS / brain reward thresholds which is indicative of a potentiation of brain reward function. In contrast, cessation of chronic drug administration leads to elevations in brain reward thresholds, which is indicative of a negative mood state. Previous studies have shown that acute nicotine administration lowers brain reward thresholds and that the administration of nicotinic receptor (nAChR) antagonists to nicotine dependent rats or cessation of nicotine administration leads to elevations in brain reward thresholds [16, 17]. The US Food and Drug Administration (FDA)-approved smoking cessation drugs bupropion (Zyban®) and varenicline (Chantix®) prevent the elevations in brain reward thresholds associated with nicotine withdrawal in rats [17, 18]. Therefore, this animal model can be used to identify new treatments that diminish nicotine withdrawal and improve relapse rates in humans.
Preclinical studies point to a critical role for brain stress systems in nicotine addiction [19]. Nicotine withdrawal increases the release of CRF in the brain and drugs that block the CRF type 1 (CRF1) receptor prevent the negative mood state associated with nicotine withdrawal [20, 21]. Recent studies also point to a critical role for vasopressin in regulating mood states [22]. Vasopressin is expressed in brain sites that regulate mood states such as the paraventricular nucleus of the hypothalamus, bed nucleus of the stria terminalis, and the lateral septum [23, 24]. Plasma vasopressin levels and vasopressin mRNA levels in the hypothalamus are increased in people with depression and antidepressants decrease vasopressin mRNA levels in the hypothalamus of rats with high levels of anxiety [25-27]. Vasopressin mediates its effects via the activation of the vasopressin type 1a receptor (V1a), V1b receptor, and V2 receptor, but there is extensive evidence that the effects of stress on mood states are mediated via the V1b receptor [22, 28]. The role of vasopressin and the V1b receptor in nicotine addiction is rather unexplored, but studies with other drugs of abuse suggest that the V1b receptor plays a role in drug addiction. The V1b receptor antagonist SSR149415 decreases alcohol intake in Sardinian alcohol-preferring rats and in alcohol-dependent rats with high levels of alcohol intake but does not affect alcohol intake in animals that are not dependent and have low levels of alcohol intake [29, 30]. Furthermore, cessation of high levels of cocaine administration to rats leads to the release of adrenocorticotropic hormone (ACTH) and corticosterone and this is attenuated by V1b receptor blockade [31]. Several studies have shown that chronic but not acute administration of a V1b receptor antagonist has antidepressant-like effects [32, 33]. This would suggest that long-term blockade of V1b receptors induces adaptations that provide protection against the aversive effects of stress or drug withdrawal.
The goal of the present studies was to investigate the effects of acute and chronic treatment with the V1b receptor antagonist SSR149415 (V1a Ki 613 ± 144; V1b Ki 1 ± 0.3; V2 Ki 160 ± 26; Oxytocin receptor Ki 40 ± 4) on the negative mood state associated with nicotine withdrawal [34]. Somatic nicotine withdrawal signs are very mild in humans and relapse to smoking is more likely due to negative affective withdrawal signs than somatic signs [1, 7]. Therefore, the present studies will focus on negative affective signs associated with nicotine withdrawal. The rats were prepared with ICSS electrodes in the lateral hypothalamus to assess mood states. In addition, response latencies were assessed to determine whether the V1b antagonist has sedative effects or induces motor impairments. Nicotine dependence was induced using minipumps and withdrawal was precipitated with the nonselective and noncompetitive nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine [35]. The present studies suggest V1b receptor blockade might be a potential new treatment for the dysphoria associated with smoke cessation.
2. Material and Methods
2.1. Animals
Male Wistar rats (Charles River, Raleigh, NC, USA) weighing 250-300 g at the beginning of the experiments were used. The rats were housed with two per cage in a climate-controlled vivarium and maintained on a 12 h reversed light-dark cycle (lights off at 9 AM). The ICSS training sessions and testing were done during the first 4 h of the dark cycle. All subjects were treated in accordance with the National Institutes of Health guidelines regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Florida Institutional Animal Care and Use Committee.
2.2. Drugs
Nicotine, mecamylamine, and pentobarbital were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in sterile saline (0.9% sodium chloride). The V1b receptor antagonists SSR149415 ((2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3- dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide, isomer (−); Sanofi, Paris, France)[36] was dissolved in a mixture of dimethyl sulfoxide (DMSO) (5% v/v), Cremophor EL (5% v/v), and saline. Drug doses are expressed as salt with the exception of the nicotine dose which is expressed as free base.
2.3. Experimental design
In the first experiment, the effect of vehicle and 3 doses of SSR149415 (0.1, 0.5, 2 µg, intracerebroventricular [icv]) on mecamylamine-precipitated nicotine withdrawal was investigated. Rats (n=28) were prepared with ICSS electrodes in the brain, and when the brain reward thresholds were stable (less than 10% variation within a 5 day period) they were prepared with saline (n=12) or nicotine (n=16) pumps. At least 6 days later, the effect of SSR149415 on mecamylamine-precipitated nicotine withdrawal was investigated. There was a 6-day period between the implantation of the minipumps and the start of the mecamylamine injections to allow the development of dependence. The V1b receptor antagonist was administered 25 min before the administration of mecamylamine (3 mg/kg, subcutaneous [sc]), and 10 min after the administration of mecamylamine the rats were placed in the operant chambers to assess brain reward thresholds and response latencies. The pretreatment interval of SSR149415 was based on previous studies that reported behavioral and neuroendocrine effects 30 min after administration [36-38]. Furthermore, mecamylamine elevates the brain reward thresholds of nicotine dependent rats when administered 5-10 min before ICSS testing [21, 39]. The V1b antagonist SSR149415 was administered according to a Latin square design and there was a 4-day wash-out period between subsequent injections. This is sufficient time for the brain reward thresholds to return to baseline levels as the plasma half-life of mecamylamine is 1.2 h [40]. During the wash-out periods the rats were tested daily in the ICSS procedure. In the second experiment, the effect of chronic pretreatment with SSR149415 (0.5 µg per day for 6 days, icv) on nicotine withdrawal was investigated. Rats (n=38) were prepared with ICSS electrodes and when the brain reward thresholds were stable they received saline or nicotine pumps. About half the rats in each group received SSR149415 (saline-SSR149415 n=11, nicotine-SSR149415 n=10) and the other half received vehicle (saline-vehicle n=9, nicotine-vehicle n=8). The rats received one icv infusion per day and the first 5 infusions were given 1 h after the ICSS test sessions. The 6th and final infusion was given 25 min before the administration of mecamylamine (3 mg/kg, sc) and 10 min later the rats were placed in the operant chambers.
2.4. Cannula and electrode implantations
The rats were anesthetized with an isoflurane/oxygen vapor mixture and placed in a stereotaxic frame. The rats were then prepared with a cannula above the lateral ventricle and an electrode in the lateral hypothalamus / medial forebrain bundle as described previously [21, 41, 42]. The cannulas were implanted 2.5 mm above the lateral ventricle using the following flat skull coordinates: anterior posterior (AP) −0.9 mm, medial lateral (ML) ±1.4 mm, dorsal ventral (DV) −3.0 mm from dura. The electrodes were implanted in the lateral hypothalamus using the following coordinates: AP −0.5 mm, ML ±1.7 mm, DV −8.3 mm from dura (incisor bar 5 mm above interaural line). The cannulas and electrodes were secured with skull screws (Plastics One, Roanoke, VA, USA) and dental cement (Co-Oral-Ite Dental, Diamond Springs, CA, USA). When the studies were completed the rats were euthanized with an overdose of pentobarbital (150 mg/kg, ip), and cannula placements were verified by administering 5 μL of a 0.5% aqueous methyl blue solution at the injection site [21].
2.5. Osmotic minipump implantations
The rats were prepared with osmotic minipumps (model 2ML4, 28 day pumps, Durect Corporation, Cupertino, CA, USA) filled with either saline or nicotine. The pumps were implanted subcutaneously under isoflurane/oxygen anesthesia. The nicotine concentration was adjusted to compensate for differences in body weight and to deliver 3.16 mg/kg/day of nicotine base per day. Chronic administration of nicotine induces tolerance to the effects of nicotine and therefore continuous administration of nicotine does not affect baseline brain reward thresholds or response latencies in the ICSS procedure [21, 43].
2.6. Intracranial self-stimulation procedure
Rats were trained on a modified discrete-trial ICSS procedure [44], as described previously [21, 45]. The rats were tested in twelve operant conditioning chambers that are placed in sound-attenuating chambers (Med Associates, Georgia, VT, USA). After recovery from the intracranial surgeries, the rats were trained to turn a response wheel on a fixed-ratio (FR) 1 schedule of reinforcement. Each quarter turn of the wheel resulted in the delivery of a 0.5 s train of 0.1 ms cathodal square-wave pulses at a frequency of 100 Hz. After the acquisition of responding (100 reinforcements within 10 min), each trial began with the delivery of a non-contingent electrical stimulus, followed by a 7.5 s response window during which the animal could respond to receive a second contingent stimulus that was identical to the initial stimulus. A response during this 7.5 s window was labeled a positive response and the lack of a response was labeled a negative response. During the 2 s period immediately after a positive response, additional responses had no consequences. The inter-trial interval (ITI) that followed a positive response or the end of the response window (in the case of a negative response) had an average duration of 10 s. Responses that occurred during the ITI resulted in a further 12.5 s delay of the onset of the next trial. During the training period, the duration of the ITI and delay periods induced by time-out responses were gradually increased until animals performed consistently. Then brain reward thresholds were assessed by using a modification of the psychophysical method of limits. Test sessions consisted of four alternating series of descending and ascending current intensities starting with a descending series. Blocks of three trials were presented to the rats at a given stimulation intensity, and the intensity was altered systematically between blocks of trials by 5 µA steps. The initial stimulus intensity was set 40 µA above the baseline current-threshold for each animal. Each test session typically lasted 30 min and provided two variables: brain reward thresholds and response latencies. The brain reward threshold was defined as the midpoint between stimulation intensities that supported responding (i.e., positive responses on at least two of the three trials) and stimulation intensities that failed to support responding. Four threshold estimates were recorded and the mean of these values was taken as the final threshold. The time interval between the beginning of the non-contingent stimulus and a positive response was recorded as the response latency. The response latency for each test session was defined as the mean response latency on all trials during which a positive response occurred.
2.7. Statistical analyses
In the first and second experiment, baseline ICSS parameters (5-day averages, brain reward thresholds and response latencies) prior to minipump implantation were compared using one-way analysis of variance (ANOVA). The ICSS parameters during chronic nicotine or saline administration (days 1-6) were expressed as a percentage of the pre-pump implantation values (5-day average). These ICSS parameters were analyzed using two-way repeated measures ANOVAs with time as the within-subjects factor and pump content (nicotine vs. saline) as the between-subjects factor.
In the first experiment, ICSS parameters during the withdrawal tests were expressed as a percentage of the pre-test day baseline values and analyzed using two-way ANOVAs with pump content as the between-subjects factor and drug treatment (dose of SSR1494150) as the within-subjects factors. Absolute pre-test day brain reward thresholds and response latencies were compared using repeated measures one-way ANOVAs. In the second experiment, ICSS parameters during chronic SSR149415 or vehicle administration and during the withdrawal tests were expressed as a percentage of the pre-pump implantation values (5-day average). The effects of chronic administration of SSR149415 on ICSS parameters prior to the withdrawal phase were analyzed using three-way repeated measures ANOVAs with time (4 days, rats received SSR149415 for a total of 6-days but the drug was administered after the first ICSS session and the sixth session was the withdrawal test-day) as the within-subjects factor and drug (SSR149415 vs. vehicle) and pump content as the between-subjects factors. The effects of chronic SSR149415 on ICSS parameters during withdrawal were analyzed using two-way ANOVAs with pump content and drug treatment as the between-subjects factors.
Statistically significant results in the ANOVAs were followed by Bonferroni post hoc comparisons. The data were analyzed with GraphPad Prism version 6 and IBM SPSS Statistics version 22. Probability values less than 0.05 were considered significant.
3. Results
3.1. Acute SSR149415 administration partly prevents the negative mood state associated with nicotine withdrawal
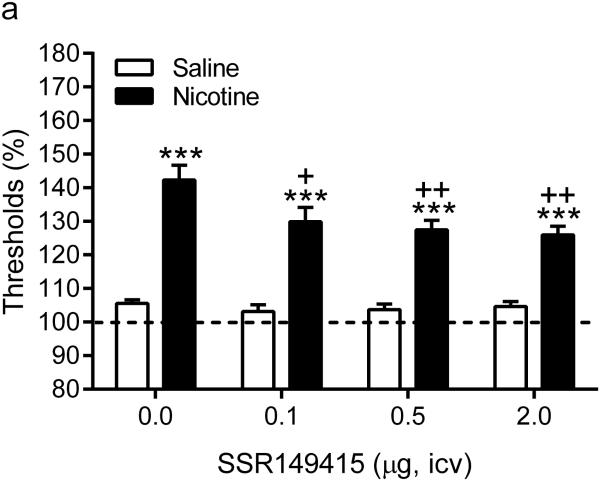
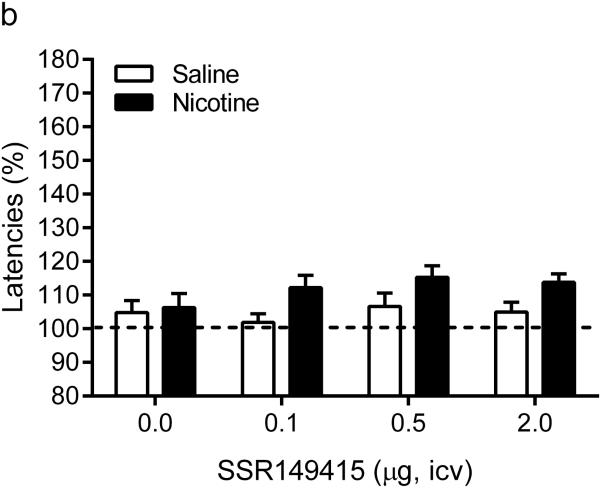
Before the implantation of the minipumps there were no differences in brain reward thresholds or response latencies between the saline and the nicotine group. Furthermore, there was no difference in response latencies between the nicotine and saline rats after the implantation of the minipumps (day 1-6). After the implantation of the minipumps there was a small (~10%), but significant, increase in the brain reward thresholds of the nicotine and saline rats (Time: F5,130=3.39, P<0.01). Repeated administration of SSR149415 did not affect the absolute pre-test day brain reward thresholds and response latencies (table 1). The administration mecamylamine elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated control rats (Pump: F1,26=182.0, P< 0.0001, Figure 1). Pretreatment with the V1b receptor antagonist SSR149415 attenuated the elevations in brain reward thresholds associated with nicotine withdrawal and did not affect the brain reward thresholds of the saline-treated control rats (Dose: F3,78=2.98, P<0.05). The post hoc analyses indicated that 0.1, 0.5, and 2 µg of SSR149415 diminished the elevations in brain reward thresholds in the nicotine withdrawing rats. However, the brain reward thresholds of the nicotine withdrawing rats treated with SSR149415 remained above baseline levels and thus the V1b antagonist did not completely prevent the negative mood state associated with nicotine withdrawal. Neither mecamylamine nor SSR149415 affected the response latencies of the saline-treated control rats.
Table 1.
Absolute baseline brain reward thresholds and response latencies on pre-test days.
|
Experimental groups
Pump / SSR149415 dose (μg, icv) |
Brain reward thresholds | |||
|---|---|---|---|---|
| 0 | 0.1 | 0.5 | 2 | |
| Saline | 124.1 ± 9.3 | 123.2 ± 6.5 | 124.9 ± 7.3 | 128.4 ± 9.1 |
| Nicotine | 121.5 ± 7.0 | 123.4 ± 6.9 | 123.1 ± 5.5 | 127.3 ± 6.4 |
| Latencies | ||||
| 0 | 0.1 | 0.5 | 2 | |
| Saline | 3.3 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 | 3.3 ± 0.1 |
| Nicotine | 3.4 ± 0.1 | 3.3 ± 0.1 | 3.2 ± 0.1 | 3.2 ± 0.1 |
Figure 1.
Acute treatment with SSR149415 partly prevents the negative mood state associated with nicotine withdrawal. Effect of SSR149415 on brain reward thresholds (A) and response latencies (B) during nicotine withdrawal. Asterisks (*** P<0.001) indicate elevated brain reward thresholds compared to the corresponding control group. Plus signs (+ P<0.05, ++P<0.01) indicate lower brain reward thresholds compared to nicotine withdrawing rats that received vehicle (dose 0, icv). Saline n = 12, Nicotine n = 16. Data are expressed as means ± SEM.
3.2. Chronic SSR149415 administration completely prevents the negative mood state associated with nicotine withdrawal
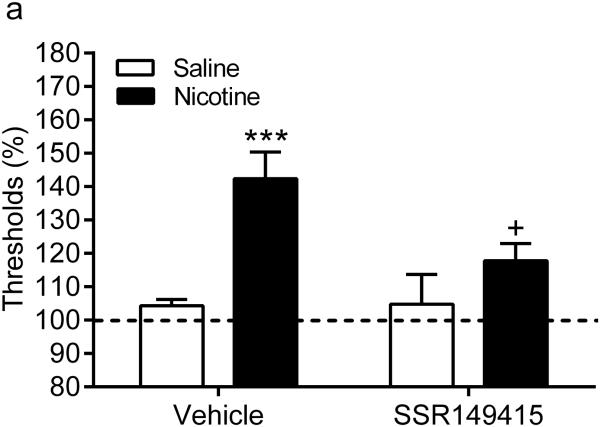
Before the implantation of the minipumps there were no differences in brain reward thresholds or response latencies between the experimental groups (saline-vehicle, saline-SSR149415, nicotine-vehicle, and nicotine-SSR149415). Chronic administration of nicotine or saline did not affect the brain reward thresholds or response latencies. Furthermore, chronic administration of SSR149415 did not affect the brain reward thresholds or response latencies of the nicotine and saline-treated rats (Table 2). The nAChR antagonist mecamylamine elevated the brain reward thresholds of the nicotine-treated rats and did not affect the brain reward thresholds of the saline-treated control rats (Pump: F1,34=19.73, P<0.0001, Figure 2). Chronic treatment with the V1b receptor antagonist SSR149415 prevented the elevations in brain reward thresholds associated with nicotine withdrawal and did not affect the brain reward thresholds of the saline-treated control rats (Drug: F1,34=4.39, P<0.05; Pump x Drug interaction: F1,34=4.79, P<0.05). The post hoc comparisons showed that the brain reward thresholds of the nicotine-treated rats that received SSR149415 were lower than those of the nicotine-treated rats that received vehicle. Furthermore, there were no difference in brain reward thresholds between the nicotine-SSR149415 rats and saline-vehicle rats, thus indicating that SSR149415 completely prevented the elevations in brain reward thresholds associated with nicotine withdrawal. The administration of mecamylamine, SSR149415, or both drug together did not affect the response latencies of the nicotine and saline-treated rats. Taken together, this study indicates that chronic treatment with SSR149415 prevents the elevations in brain reward thresholds associated with nicotine withdrawal and does not affect baseline brain reward thresholds or response latencies.
Table 2.
Effect of the V1b receptor antagonist SSR149415 on baseline brain reward thresholds and response latencies.
| Experimental groups | Brain reward thresholds | |||
| Day 2 | Day 3 | Day 4 | Day 5 | |
| Saline-Vehicle | 102.7 ± 1.5 | 100.9 ± 1.8 | 101.8 ± 2.1 | 99.6 ± 1.2 |
| Saline-SSR149415 | 102.3 ± 3.5 | 99.9 ± 4.3 | 103.6 ± 6.7 | 106.2 ± 5.9 |
| Nicotine-Vehicle | 103.3 ± 5.5 | 101.9 ± 5.2 | 97.9 ± 4.4 | 100.0 ± 4.1 |
| Nicotine-SSR149415 | 109.0 ± 5.8 | 105.9 ± 3.8 | 108.1 ± 3.0 | 106.7 ± 3.5 |
| Latencies | ||||
| Day 2 | Day 3 | Day 4 | Day 5 | |
| Saline-Vehicle | 99.0 ± 2.7 | 97.4 ± 2.6 | 97.8 ± 2.4 | 103.0 ± 1.6 |
| Saline-SSR149415 | 96.8 ± 4.4 | 96.3 ± 1.8 | 99.5 ± 3.3 | 100.7 ± 3.2 |
| Nicotine-Vehicle | 93.7 ± 2.8 | 99.5 ± 3.8 | 96.0 ± 3.1 | 97.7 ± 4.2 |
| Nicotine-SSR149415 | 100.0 ± 2.2 | 97.4 ± 2.6 | 96.4 ± 2.6 | 97.0 ± 2.3 |
The ICSS parameters are expressed as a percentage of pre-pump implantation values (5-day averages). Vehicle or SSR149415 was administered icv after ICSS testing on day 1 (data not shown) and after ICSS testing on days 2-5 (this table), and before the withdrawal session / ICSS testing on day 6 (see Fig. 2).
Figure 2.
Chronic treatment with SSR149415 completely prevents the negative mood state associated with nicotine withdrawal. Effect of chronic pretreatment with SSR149415 (0.5 µg) on brain reward thresholds (A) and response latencies (B) during nicotine withdrawal. Asterisks (*** P<0.001) indicate elevated brain reward thresholds compared to the corresponding control group. Plus sign (+ P<0.05) indicates lower brain reward thresholds compared to the nicotine withdrawing rats that received vehicle (icv). Saline-vehicle n = 9, saline-SSR149415 n=11, nicotine-vehicle n=8, nicotine-SSR149415 n=10. Data are expressed as means ± SEM.
4. Discussion
The goal of the present studies was to investigate the effect of acute and chronic treatment with the V1b receptor antagonist SSR149415 on the negative mood state associated with nicotine withdrawal. As reported previously by our group and others, the nAChR antagonist mecamylamine elevated the brain reward thresholds of the nicotine dependent rats and did not affect the brain reward thresholds of the saline-treated control rats [45, 46]. Acute treatment with SSR149415 partly prevented the negative mood state associated with nicotine withdrawal and chronic treatment with SSR149415 completely prevented the negative mood state associated with nicotine withdrawal. In the saline-treated control animals, acute or chronic treatment with SSR149415 did not affect brain reward function and did not induce sedative effects or motor impairments. The present studies suggest that chronic pretreatment (~1 week) with a V1b receptor antagonist may prevent the dysphoria associated with smoking cessation.
The present finding that chronic treatment with SSR149415 is more effective than acute treatment in preventing negative mood states is in line with previous studies that investigated the antidepressant-like effects of acute and chronic administration of SSR149415 [32, 33]. Several studies have investigated the effects of SSR149415 on depressive-like behavior using olfactory bulbectomized (OB) rats. Olfactory bulbectomy in rats induces a wide range of behavioral and neurochemical changes that are similar to those in depression and can be reversed by chronic treatment with antidepressants [47]. Iijima and Chaki investigated the effects of acute and chronic treatment with SSR149415 on hyperemotionality in the olfactory bulbectomy model of depression. They showed that acute treatment with SSR149415 does not affect hyperemotionality in OB rats but that chronic treatment with SSR149415 completely prevents hyperemotionality in OB rats [32]. Another study investigated the effects of acute and chronic treatment with SSR149415 on hyperactivity in OB rats. It was shown that acute treatment with SSR149415 does not affect hyperactivity but that chronic treatment with SSR149415 completely prevents hyperactivity [33]. Interestingly, the SSR149415-induced decrease in hyperactivity was even observed 1 week after the cessation of treatment with SSR149415. This pattern of results suggests that chronic treatment with SSR149415 induces neuroadaptations that mediate prolonged antidepressant-like effects.
The present findings support the hypothesis that brain stress systems play a critical role in nicotine withdrawal [1]. Previous studies have provided evidence for a role of the stress peptide CRF in nicotine addiction [19, 20]. Nicotine withdrawal has been associated with an increased release of CRF in the brain and CRF1 receptor antagonists decrease high levels of nicotine intake after a period of abstinence [20]. In addition, blockade of CRF1 receptors prevents the negative mood state associated with nicotine withdrawal [39]. Synergistic interactions between CRF and vasopressin have been detected at the level of the hypothalamus. CRF induces the release of ACTH from the anterior pituitary and this effect is potentiated by vasopressin [48, 49]. Chronic stress increases the production of vasopressin in CRF neurons and thereby potentiates the hypothalamic-pituitary-adrenal (HPA) axis response to stress [50, 51]. CRF and vasopressin are also expressed in extrahypothalamic brain sites that play a critical role in the regulation of mood states such as the central nucleus of the amygdala [52]. In addition, it has been shown that vasopressin levels are elevated in the amygdala during heroin withdrawal [53]. In previous studies, it was shown that CRF in the CeA plays a critical role in nicotine withdrawal [39, 45]. Therefore, it might be possible that withdrawal from nicotine leads to increased vasopressin release in the CeA and thereby potentiates the CRF-induced negative mood state.
It is also interesting to note that SSR149415 greatly decreases alcohol intake in alcohol dependent rats with high levels of alcohol intake but does not affect alcohol intake in non-dependent animals with low levels of alcohol intake [29]. Accumulating evidence suggests that low levels of drug intake are mediated by the positive reinforcing (i.e., reward) effects of drugs but that high levels of drug intake are mainly mediated by negative reinforcement processes (drug intake to prevent withdrawal or severe stress)[7]. For example, it has been shown that in rats with extended access to cocaine the brain reward thresholds are elevated (i.e., dysphoria) between self-administration sessions and drug intake increases of over time [12]. Furthermore, in the aforementioned study there was a positive correlation between the elevations in brain reward thresholds and the escalation of cocaine intake. Thus, animals with the most severe withdrawal symptoms displayed the greatest increase in drug intake over time. The present study showed that blockade of V1b receptors diminishes the negative mood state associated with nicotine withdrawal. Therefore, additional studies are warranted to investigate if blockade of V1b receptors diminishes the escalation of drug intake and the negative mood state that drives the escalation of drug intake.
It cannot be ruled out that non-vasopressinergic mechanisms contributed to the effects of SSR149415 on nicotine withdrawal. For example, it has been reported that SSR149415 modulates cholinergic transmission [54]. Acute and chronic administration of SSR149415 inhibits the release of acetylcholine in the hippocampus of rats [54]. Nicotine withdrawal has been associated with an increased release of acetylcholine in the brain and it has been suggested that the release of acetylcholine might contribute to the aversive aspects of nicotine withdrawal [55]. Therefore, it might be possible that SSR149415 at least partly prevents nicotine withdrawal by diminishing cholinergic transmission in the brain. It has also been reported that drugs that increase serotonergic or noradrenergic transmission attenuate the negative mood state associated with nicotine withdrawal [43, 56]. However, there is currently no evidence that SSR149415 affects serotonin or norepinephrine transmission in the brain [54, 57].
Taken together, the present study shows that acute administration of the V1b receptor antagonist SSR149415 partly prevents the negative mood state associated with nicotine withdrawal and that chronic administration of SSR149415 completely prevents the negative mood state associated with nicotine withdrawal. The present study supports the hypothesis that a dysregulation of brain stress systems contributes to the negative mood state associated with nicotine withdrawal. Therefore, pharmacological treatments that diminish the activity of brain stress systems during a smoking cessation attempt may prevent severe dysphoria and prevent relapse to smoking.
Highlights.
Nicotine withdrawal induces a negative mood state
Acute treatment with a V1b receptor antagonist partly prevents withdrawal
Chronic treatment with a V1b receptor antagonist completely prevents withdrawal
Blockade of V1b receptors does not affect reward function in control animals
Blockade of V1b receptors does not induce sedation or motor impairments
Acknowledgements
This research was funded by a National Institute on Drug Abuse grant (DA023575) to A. Bruijnzeel. We would like to thank Dr. Griebel (Sanofi, France) for providing the V1b receptor antagonist SSR149415.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. NeurosciBiobehavRev. 2012;36:1418–41. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].WHO WHO global report on trends in tobacco smoking 2000-2025. 2015.
- [3].Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385:899–909. doi: 10.1016/S0140-6736(14)60446-3. [DOI] [PubMed] [Google Scholar]
- [4].Pirie K, Peto R, Reeves GK, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133–41. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, et al. Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–3. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- [6].Rezvani AH, Levin ED. Cognitive effects of nicotine. BiolPsychiatry. 2001;49:258–67. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- [7].Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- [9].Jarvis MJ. Why people smoke. BMJ. 2004;328:277–9. doi: 10.1136/bmj.328.7434.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. JAMA. 1990;264:1541–5. [PubMed] [Google Scholar]
- [11].Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. JConsult ClinPsychol. 1996;64:791–8. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- [12].Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. NatNeurosci. 2002;5:625–6. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- [13].Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M. Severe deficit in brain reward function associated with fentanyl withdrawal in rats. BiolPsychiatry. 2006;59:477–80. doi: 10.1016/j.biopsych.2005.07.020. [DOI] [PubMed] [Google Scholar]
- [15].Wise RA, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmacology (Berl) 1995;117:130–6. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- [16].Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- [17].Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology. 2013;39:455–65. doi: 10.1038/npp.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–58. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- [19].Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain ResBrain ResRev. 2005;49:505–28. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [20].George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. ProcNatlAcadSciUSA. 2007;104:17198–203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bruijnzeel AW, Prado M, Isaac S. Corticotropin-Releasing Factor-1 Receptor Activation Mediates Nicotine Withdrawal-Induced Deficit in Brain Reward Function and Stress-Induced Relapse. BiolPsychiatry. 2009;66:110–7. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Griebel G, Stemmelin J, Gal CS, Soubrie P. Non-peptide vasopressin V1b receptor antagonists as potential drugs for the treatment of stress-related disorders. Curr Pharm Des. 2005;11:1549–59. doi: 10.2174/1381612053764797. [DOI] [PubMed] [Google Scholar]
- [23].De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–17. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- [24].Van Leeuwen FW, Caffe R. Vasopressin-immunoreactive cell bodies in the bed nucleus of the stria terminalis of the rat. Cell Tissue Res. 1983;228:525–34. doi: 10.1007/BF00211473. [DOI] [PubMed] [Google Scholar]
- [25].van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, et al. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–92. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- [26].Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: A preliminary report. BiolPsychiatry. 2006;60:892–5. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- [27].Keck ME, Welt T, Muller MB, Uhr M, Ohl F, Wigger A, et al. Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology. 2003;28:235–43. doi: 10.1038/sj.npp.1300040. [DOI] [PubMed] [Google Scholar]
- [28].Birnbaumer M. Vasopressin Receptors. Trends in Endocrinology & Metabolism. 2000;11:406–10. doi: 10.1016/s1043-2760(00)00304-0. [DOI] [PubMed] [Google Scholar]
- [29].Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V(1b) receptors mediate the transition to excessive drinking in ethanol-dependent rats. AddictBiol. 2011 doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res. 2011;35:1876–83. doi: 10.1111/j.1530-0277.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou Y, Litvin Y, Piras AP, Pfaff DW, Kreek MJ. Persistent increase in hypothalamic arginine vasopressin gene expression during protracted withdrawal from chronic escalating-dose cocaine in rodents. Neuropsychopharmacology. 2011;36:2062–75. doi: 10.1038/npp.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Iijima M, Chaki S. An arginine vasopressin V1b antagonist, SSR149415 elicits antidepressant-like effects in an olfactory bulbectomy model. ProgNeuropsychopharmacolBiolPsychiatry. 2007;31:622–7. doi: 10.1016/j.pnpbp.2006.12.008. [DOI] [PubMed] [Google Scholar]
- [33].Breuer ME, van Gaalen MM, Wernet W, Claessens SE, Oosting RS, Behl B, et al. SSR149415, a non-peptide vasopressin V1b receptor antagonist, has long-lasting antidepressant effects in the olfactory bulbectomy-induced hyperactivity depression model. Naunyn Schmiedebergs ArchPharmacol. 2009;379:101–6. doi: 10.1007/s00210-008-0336-1. [DOI] [PubMed] [Google Scholar]
- [34].Hodgson RA, Higgins GA, Guthrie DH, Lu SX, Pond AJ, Mullins DE, et al. Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacology, biochemistry, and behavior. 2007;86:431–40. doi: 10.1016/j.pbb.2006.12.021. [DOI] [PubMed] [Google Scholar]
- [35].Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends PharmacolSci. 2010;31:580–6. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Serradeil-Le Gal C, Wagnon J, Simiand J, Griebel G, Lacour C, Guillon G, et al. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo- 2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. The Journal of pharmacology and experimental therapeutics. 2002;300:1122–30. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- [37].Bayerl DS, Klampfl SM, Bosch OJ. Central V1b receptor antagonism in lactating rats: impairment of maternal care but not of maternal aggression. J Neuroendocrinol. 2014;26:918–26. doi: 10.1111/jne.12226. [DOI] [PubMed] [Google Scholar]
- [38].Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. ProcNatlAcadSciUSA. 2002;99:6370–5. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, et al. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. PharmacolBiochemBehav. 2012;101:62–8. doi: 10.1016/j.pbb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barre L. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. JPharmSci. 2003;92:1051–7. doi: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- [41].Rylkova D, Boissoneault J, Isaac S, Prado M, Shah HP, Bruijnzeel AW. Effects of NPY and the specific Y1 receptor agonist [D-His(26)]-NPY on the deficit in brain reward function and somatic signs associated with nicotine withdrawal in rats. Neuropeptides. 2008;42:215–27. doi: 10.1016/j.npep.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–63. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- [43].Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- [44].Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. FedProc. 1979;38:2473–6. [PubMed] [Google Scholar]
- [45].Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–52. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].O'Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. NeurotoxicolTeratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- [48].Baker BI, Bird DJ, Buckingham JC. In the trout, CRH and AVT synergize to stimulate ACTH release. Regul Pept. 1996;67:207–10. doi: 10.1016/s0167-0115(96)00130-9. [DOI] [PubMed] [Google Scholar]
- [49].Giguere V, Labrie F. Vasopressin potentiates cyclic AMP accumulation and ACTH release induced by corticotropin-releasing factor (CRF) in rat anterior pituitary cells in culture. Endocrinology. 1982;111:1752–4. doi: 10.1210/endo-111-5-1752. [DOI] [PubMed] [Google Scholar]
- [50].Schmidt ED, Binnekade R, Janszen AW, Tilders FJ. Short stressor induced long-lasting increases of vasopressin stores in hypothalamic corticotropin-releasing hormone (CRH) neurons in adult rats. JNeuroendocrinol. 1996;8:703–12. [PubMed] [Google Scholar]
- [51].De Goeij DC, Jezova D, Tilders FJ. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res. 1992;577:165–8. doi: 10.1016/0006-8993(92)90552-k. [DOI] [PubMed] [Google Scholar]
- [52].Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- [53].Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–36. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- [54].Claustre Y, Rouquier L, Desvignes C, Leonetti M, Montegut J, Aubin N, et al. Effects of the vasopressin (V1b) receptor antagonist, SSR149415, and the corticotropin-releasing factor 1 receptor antagonist, SSR125543, on FG 7142-induced increase in acetylcholine and norepinephrine release in the rat. Neuroscience. 2006;141:1481–8. doi: 10.1016/j.neuroscience.2006.05.006. [DOI] [PubMed] [Google Scholar]
- [55].Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 2001;157:105–10. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- [56].Semenova S, Markou A. The alpha2 adrenergic receptor antagonist idazoxan, but not the serotonin-2A receptor antagonist M100907, partially attenuated reward deficits associated with nicotine, but not amphetamine, withdrawal in rats. EurNeuropsychopharmacol. 2010;20:731–46. doi: 10.1016/j.euroneuro.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Louis C, Cohen C, Depoortere R, Griebel G. Antidepressant-like effects of the corticotropin-releasing factor 1 receptor antagonist, SSR125543, and the vasopressin 1b receptor antagonist, SSR149415, in a DRL-72 s schedule in the rat. Neuropsychopharmacology. 2006;31:2180–7. doi: 10.1038/sj.npp.1301036. [DOI] [PubMed] [Google Scholar]