Abstract
Purpose
To investigate the direct effect and therapeutic consequences of epidermal growth factor receptor 2 (HER2)-targeting therapy on expression of estrogen receptor (ER) and Bcl2 in preclinical models and clinical tumor samples.
Experimental design
Archived xenograft tumors from two preclinical models (UACC812 and MCF7/HER2-18) treated with ER and HER2-targeting therapies, and also HER2+ clinical breast cancer specimens collected in a lapatinib neoadjuvant trial (baseline and week 2 post treatment), were used. Expression levels of ER and Bcl2 were evaluated by immunohistochemistry and western blot. The effects of Bcl2 and ER inhibition, by ABT-737 and fulvestrant respectively, were tested in parental versus lapatinib-resistant UACC812 cells in vitro.
Results
Expression of ER and Bcl2 was significantly increased in xenograft tumors with acquired resistance to anti-HER2 therapy, compared with untreated tumors, in both preclinical models (UACC812: ER p=0.0014; Bcl2 p<0.001. MCF7/HER2-18: ER p=0.0007; Bcl2 p=0.0306). In the neoadjuvant clinical study, lapatinib treatment for two weeks was associated with parallel upregulation of ER and Bcl2 (Spearman’s coefficient: 0.70; p=0.0002). Importantly, 18% of tumors originally ER-negative (ER−) converted to ER+ upon anti-HER2 therapy. In ER−/HER2+ MCF7/HER2-18 xenografts, ER re-expression was primarily observed in tumors responding to potent combination of anti-HER2 drugs. Estrogen deprivation added to this anti-HER2 regimen significantly delayed tumor progression (p=0.018). In the UACC812 cells, fulvestrant, but not ABT-737, was able to completely inhibit anti-HER2-resistant growth (p<0.0001).
Conclusion
HER2 inhibition can enhance or restore ER expression with parallel Bcl2 upregulation, representing an ER-dependent survival mechanism potentially leading to anti-HER2 resistance.
Introduction
Breast cancer is a highly heterogeneous disease and multiple signaling pathways can mediate tumor initiation and progression. These same pathways can contribute to treatment resistance when the main driver is inhibited. Approximately 20-25% of breast tumors have overexpression and/or gene amplification of the human epidermal growth factor receptor 2 (HER2), which represents the dominant driver of tumor cell growth and survival. In breast cancer, HER2 is the leading member of the HER receptor family, which includes four tyrosine kinase receptors (HER1-4) (1). HER2 positivity is typically associated with an aggressive tumor phenotype and shorter survival (2, 3). The introduction of effective HER2-targeting treatments in clinical practice dramatically improved patient outcome. The monoclonal antibody trastuzumab (T) represents the first agent successfully developed and approved for HER2 inhibition. More recently, additional effective anti-HER2 agents, including lapatinib (L), a dual HER1/2 tyrosine kinase inhibitor, pertuzumab (P), a monoclonal antibody that blocks HER2 heterodimerization, and trastuzumab emtansine (TDM1), an antibody-toxin conjugate, have also been approved for clinical use. Despite the remarkable efficacy of these anti-HER2 agents, treatment resistance remains a major clinical problem. Different molecular mechanisms have been suggested to cause anti-HER2 resistance (4-6). First, acquired resistance to T is associated in preclinical studies with reactivation of the redundant HER receptor layer (6). Indeed, a more complete blockade of the HER receptors, achieved with various combinations of HER inhibitors (i.e. L+T and T+P), results in more effective tumor growth inhibition both in vitro and in vivo, compared with T alone (6-8). Consistent results have been reported also in the clinical setting in multiple randomized trials (9-13).
Even in the presence of effective and sustained HER inhibition, HER2-positive (HER2+) tumor cells can acquire treatment resistance. Here, treatment resistance arises from activation of various other escape pathways that can become alternative dominant drivers of cell growth and survival. Several pre-clinical studies suggest that one of these potential escape pathways is the estrogen receptor (ER) signaling network (6, 14). Indeed, we and others found that ER-positive (ER+)/HER2+ tumor cells with acquired resistance to L (LR) or L + T (LTR) show increased expression of ER as well as its downstream products such as the antiapoptotic protein Bcl2, compared to parental cells (6, 14). Of note, these resistant cells demonstrate persistent inhibition of the HER2 pathway (6). This suggests that activation of ER and Bcl2 may represent an alternative mechanism of cell survival in the presence of HER2 pathway blockade. Consistent with this pre-clinical evidence, ER positivity, reported in about half of all HER2+ tumors, is associated with reduced response to HER2-targeting therapies in the clinical setting (9).
Here, we report that ER and Bcl2 expression are simultaneously increased in breast cancer xenografts treated with anti-HER2 therapies. We also show that neoadjuvant treatment with lapatinib leads to a rapid increase in ER and Bcl2 expression in patients with HER2+ breast cancer and demonstrate that co-targeting ER or Bcl2 along with HER2-targeted therapy circumvents this type of resistance. We finally report that endocrine therapy delays tumor progression in the presence of restored ER expression in xenograft tumors treated with anti-HER2 therapy.
Methods
Protein extracts and immunoblots
Total protein fractions were extracted from fresh cell cultures and archived frozen xenograft tumors, for immunoblotting as previously described (6, 8). Antibodies against phosphorylated (p)-Tyr1248 HER2, total (t)-HER2, and βactin were purchased from Cell Signaling Technology (Beverly, Ma, USA); anti-ERα was from Abcam (Fremont, CA. USA); anti-PR and anti-Bcl2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA. USA). Biomarker expression levels evaluated by immunoblotting were quantified by measuring band intensity with the use of ImageJ and normalized by βactin expression.
Xenograft studies
Animal care was in accordance with the Institutional Animal Care and Use Committee (IACUC). For evaluating ER, Bcl2, and PR protein levels by immunoblotting, UACC812 and MCF7 HER2-18 xenograft tumors, which were treated with vehicle or anti-HER2 therapy, collected and stored previously in two independent published studies (6, 8), were used. We performed two additional in vivo experiments using MCF7 HER2-18 xenograft models. In the first experiment, mice bearing MCF7 HER2-18 tumors were treated with estrogen deprivation (ED) by estrogen (E2) pellet removal, starting from a tumor volume of ≈ 200 mm3. At the time of ED resistance (≈ 70 days), treatment with the anti-HER2 regimen T + P + gefitinib (TPG) was started and tumors were harvested after 7 or 14 days for ER evaluation, assessed by immunohistochemistry (IHC), as described below. In the second experiment, mice bearing MCF7 HER2-18 tumor xenografts were treated with ED until the development of resistance. At that point, animals were randomized to receive TPG with or without continuing ED. In all the aforementioned experiments tumor volume was measured weekly as previously reported (7).
Immunohistochemistry
Archived formalin-fixed paraffin embedded (FFPE) tissue specimens collected in a neoadjuvant L trial were organized into 2-mm core tissue arrays and processed as previously described (6, 7, 15, 16). Tissue sections were incubated with primary antibody against ER (Vector Labs, Burlingame, CA, USA), PR, Bcl2, and Ki67 (Dako Cytomation, Carpinteria, CA, USA), t-HER2 (Thermo Scientific/ Neomarkers Waltham, MA, USA), or p-Tyr1221/1222 HER2 (Cell Signaling Technology, Beverly, MA, USA). Immunodetection was performed with the EnVision+ System (Dako). ER and PR expression was assessed according to the Allred score (17); Bcl2 and Ki67 were reported as percentage of positive cells. Levels of t- and p-HER2 were measured as signal intensity (0-3).
Cell line culture conditions and drugs for in vitro studies
The human breast cancer cell line UACC812, originally purchased from the American Type Culture Collection (Manassas, VA, USA), was cultured as previously reported (6). UACC812 cells resistant to lapatinib (LR) were derived from parental cells in vitro and maintained as previously described (6). Both parental and LR cell lines were authenticated by DNA sequencing and immediately frozen in our laboratory. Upon thawing, cell morphology, signaling, and sensitivity/resistance to L were maintained. L LC Laboratories, (Woburn, MA, USA) and ABT-737 (Selleckchem, Houston, Tx, USA) were dissolved in dimethyl sulfoxide (DMSO). Fulvestrant (Ful), AstraZeneca (London, UK), was prepared with ethanol.
Cell growth, apoptosis and signaling assays
In vitro UACC812 cell growth was assessed in the presence of different treatments using a methylene blue assay, as previously reported (6). Apoptosis was evaluated by using the Annexin V-FITC Apoptosis Detection Kit (Abcam, Cambridge, MA. USA). UACC812 cells (20,000/well) were plated 24 hours before starting each treatment. Annexin V was measured 24 hours after treatment initiation, according to the manufacturer’s instructions. Cells positive for Annexin V were counted by using the Celigo Cytometer (Cyntellect, San Diego, CA. USA). The results were expressed as fold change relative to control, and calculated by (number of Annexin V-positive cells/total number of cells) for each treatment, divided by (number of Annexin V-positive cells/total number of cells) for control. Both the cell growth and apoptosis assays were performed in quadruplicate. For signaling analysis cells were harvested after 72 hours of treatment.
Statistical Analysis
Normalized ER, Bcl2, and PR expression levels in UACC812 and MCF7 HER2 18 in vivo xenograft tumors were compared among different treatment arms by using one-way analysis of variance followed with Tukey–Kramer adjustment for multiple pairwise comparisons. In the neoadjuvant L study, clinical response rate was defined according to the response evaluation criteria in solid tumors (RECIST). Correlations among biomarkers at baseline and among biomarker changes were evaluated by Spearman’s rank correlation, whereas the association between biomarker changes and clinical response (complete or partial responders vs. no responders) was determined by the Wilcoxon rank sum test.
In vitro analysis, cell growth, and apoptosis in UACC812 parental and LR cells under various treatment conditions were assessed using one-way analysis of variance. Differences between groups were then determined by multiple pairwise comparisons with Tukey-Kramer adjustment. Error bars on plots represent standard error (SE).
Xenograft tumor growth curves were generated using the mean tumor volume at each time point. Error bars represented the standard error of the mean. Mice treated with anti-HER2 therapy (TPG) +/− endocrine therapy were divided into responders and non-responders. Response was defined as a decreased or stable tumor size after two consecutive measurements, compared with baseline. Tumor progression was defined as 1.5 fold increase of tumor volume compared with baseline. Time to progression was measured from the randomization day to the occurrence of tumor progression, and compared between treatment groups with Kaplan-Meier survival analysis and Generalized Wilcoxon test. Statistical analyses were done using SAS 9.2 (SAS institute Inc., Cary, NC).
Results
Acquired resistance to anti-HER2 therapy is associated with increased expression of ER, Bcl2, and PR, in two xenograft models in vivo
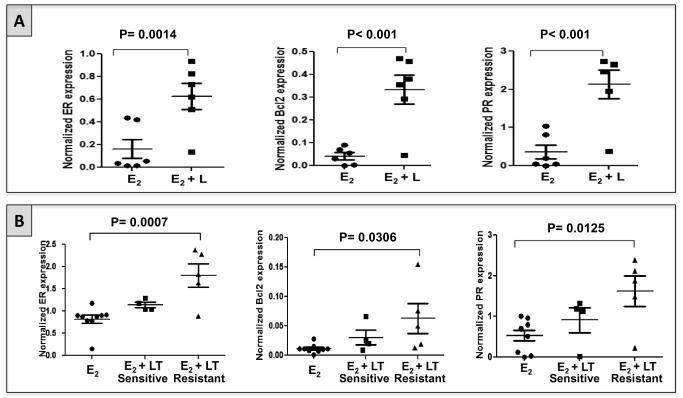
To test whether upregulation and/or reactivation of ER signaling occurs at the time of acquired anti-HER2 resistance in vivo, we assessed ER expression levels and ER’s downstream gene products Bcl2 and PR in archived xenograft tumors from two experiments previously performed in our laboratory. In the first experiment (6), mice bearing ER+/HER2+ UACC812 tumor xenografts were treated with L + E2 or with E2 alone (control). Tumor tissue was harvested at the time of treatment resistance. Expression of ER protein, measured by western blotting, was significantly higher in L + E2-treated tumors in comparison with E2-treated tumors (p=0.0014; figure 1A). Concordant changes were observed in Bcl2 and PR protein levels (figure 1A).
Figure 1. Expression of ER and its downstream gene products Bcl2 and PR increases at the time of acquired resistance to HER2-targeting therapy in two different HER2+/ER+ pre-clinical models in vivo.
A. Expression levels of ER, Bcl2, and PR in UACC812 tumor xenografts grown only in the presence of estrogen (E2; n=6) and treated with lapatinib in the presence of E2 (E2 + L; n=6). L-treated tumors showed a significant increase in the expression of ER, PR, and Bcl2, compared with tumors treated with vehicle.
B. Expression levels of ER, Bcl2, and PR in MCF7 HER2-18 tumor xenografts grown in presence of E2 with (n=9) or without (n=9) HER2-targeted therapy. Tumors treated with lapatinib plus trastuzumab (LT) were harvested either when still sensitive to the treatment (n=4) or at the time of acquired resistance (n=5)(8). LT-sensitive tumors showed a non-significant trend toward increase in ER expression, as well as in Bcl2 and PR levels, compared with control tumors (E2). LT-resistant tumors showed a significant increase in ER, Bcl2, and PR expression compared with control.
In the second experiment, ER+/HER2+ MCF7 HER2-18 xenografts were treated with a combination of L + T (LT) + E2, or with E2 alone (control). In this instance, tumor tissue was collected either after short-term treatment during the sensitive phase, or at the time of treatment resistance (8). ER expression levels were slightly increased in LT-sensitive tumors compared to E2-treated tumors, though this difference was not statistically significant (figure 1B). However, ER upregulation was marked and statistically significant in LT-resistant tumors, confirming the data in the UACC812 model (p: 0.0007; figure 1B). The changes in the levels of Bcl2 and PR were consistent with those of ER, showing a non-significant increase in LT-treated tumors harvested in the sensitive phase and a higher and statistically significant increase in the LT-resistant tumors, compared with control (figure 1B).
These results confirmed in vivo our previously conducted in vitro studies showing increased ER expression and transcriptional activity at the time of acquired resistance to potent anti-HER2 therapies (6). In addition, the marginal increase in ER expression and activity found in LT-treated MCF7 HER2-18 tumors while still sensitive to treatment suggests that ER reactivation may occur early after starting anti-HER2 therapy.
Neoadjuvant treatment with lapatinib results in rapid upregulation of ER and Bcl2 in breast cancer patients
To evaluate in the clinical setting the effect of anti-HER2 therapy on ER expression and activity, we analyzed tumor samples collected in a phase II neoadjuvant clinical trial enrolling patients with HER2+ primary breast cancer. Forty-nine patients were treated initially with L for 6 weeks, followed by trastuzumab plus docetaxel for 12 weeks, before surgery. For this study, tumor biopsies were performed at baseline and after 2 weeks of L treatment (supplementary figure 1). The expression levels of ER, PR, Bcl2, Ki67, and p- and t-HER2 were assessed by immunohistochemistry. The clinical results of this trial have been previously published (15). Thirty-five of the 49 (71%) tumors were available for baseline evaluation of both ER and Bcl2. Of those, 12 (34%) were ER+ and 23 (66%) ER-negative (ER−). As expected, a significant inverse correlation of ER expression with t- and p-HER2 and with Ki67 was found at baseline (table 1). On the other hand, ER expression strongly and positively correlated with both Bcl2 and PR levels, not surprising given that Bcl2 and PR are estrogen-regulated genes (table 1). Similarly, Bcl2 expression at baseline positively correlated with PR levels, and inversely correlated with t-HER2 expression (table 1).
Table 1.
Correlation among biomarkers at baseline.
| Marker | Correlation with ER Spearman’s coefficient |
P value |
|---|---|---|
| t-HER2 (N= 36) | −0.55 | 0.0005* |
| P-HER2 (N= 36) | −0.37 | 0.028* |
| Ki67 (N= 32) | −0.39 | 0.027* |
| PR (N= 34) | 0.57 | 0.0004* |
| Bcl2 (N= 35) | 0.75 | <0.0001* |
| Correlation with Bcl2 Spearman’s coefficient |
||
| t-HER2 (N= 35) | −0.43 | 0.0097* |
| P-HER2 (N= 35) | −0.23 | 0.1932 |
| Ki67 (N=32) | −0.22 | 0.2229 |
| PR (N= 35) | 0.53 | 0.0015* |
Statistically significant
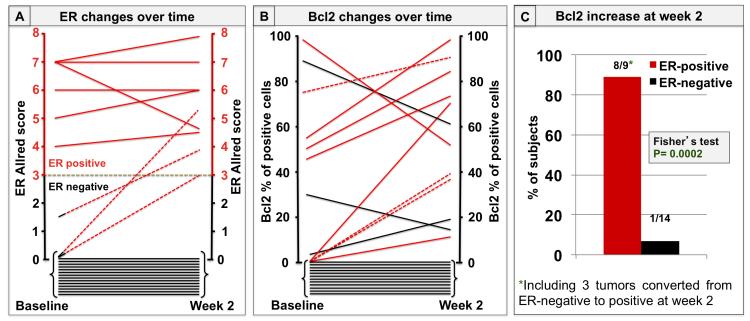
Subsequently, the changes in biomarker expression after 2 weeks of lapatinib treatment were analyzed. Twenty-three pairs of tumor biopsies (47%) were available for evaluation of ER and Bcl2 at both baseline and week 2. Of those, 6 (26%) were ER+ and 17 (74%) were ER− at baseline. At week 2, three of the 17 (18%) ER− tumors converted to ER+ (figure 2A). In addition, in the 6 tumors that were originally ER+, ER expression increased further in 3 cases, remained stable in two, and decreased in only one case. All five tumors with stable or increased ER expression had an increase in Bcl2 levels, whereas the one tumor with ER reduction had a parallel decrease of Bcl2 expression (figure 2B). In addition, all three tumors converting from ER− to ER+ had a parallel increase in Bcl2 (figure 2B). On the other hand, among the 14 tumors that were ER− both at baseline and at week 2, two had a decrease and only one had an increase in Bcl2 expression. The rest were Bcl2-negative at both baseline and week 2 (figure 2B).
Figure 2. ER and Bcl2 changes upon neoadjuvant treatment with lapatinib.
A. Each line represents changes in ER expression levels from baseline to week 2. ER levels are expressed as Allred scores. Solid red lines indicate tumors with ER-positive status at baseline (Allred score ≥ 3); dashed red lines indicate those three tumors that were ER-negative at baseline (Allred score < 3) and became positive at week 2; solid black lines indicate tumors with ER-negative status at baseline that remained negative at week 2 (14 tumors).
B. Each line represents Bcl2 expression changes from baseline to week 2. Bcl2 levels are expressed as percentage of positive cells. Solid red lines indicate Bcl2 changes in the 6 tumors with ER-positive status at baseline; dashed red lines indicate Bcl2 changes in the 3 tumors converting from ER-negative to positive; solid black lines indicate Bcl2 changes in the 14 tumors that were ER-negative at both baseline and week 2.
C. Percentage of tumors with a Bcl2 increase over time, according to ER status. Bcl2 increased at week 2 in 8 out of 9 of the ER-positive tumors, (including the 3 tumors converting to ER-positive), and in 1 out the 14 tumors that remained ER-negative.
Overall, Bcl2 expression increased in 8 of the 9 (89%) ER+ tumors (including the 3 tumors converting to ER+), and increased in only 1 of the 14 (7%) tumors that were persistently ER− (figure 2C). As expected, these changes in Bcl2 expression positively and significantly correlated with levels of PR expression (table 2). No correlation between changes in either ER or Bcl2 and clinical response rate, evaluated after 6 weeks of L treatment, was observed.
Table 2.
Correlation among biomarker changes after 2 weeks of lapatinib treatment.
| Changes in: | Correlation with Bcl2 changes Spearman’s coefficient |
P value |
|---|---|---|
| ER (N=23) | 0.70 | 0.0002* |
| PR (N=20) | 0.57 | 0.0076* |
Statistically significant
We assessed ER expression levels also in archived tissue samples from a previously described phase II neoadjuvant trial with trastuzumab (16). Importantly, in these HER2+ breast tumors treated with trastuzumab we found changes in ER expression that were consistent with those observed in the lapatinib trial (supplementary figure 2). In this instance, conversion from ER− to ER+ was observed in one out of 18 cases (6%; supplementary figure 2). Bcl2 expression was not assessed on tumor samples in that study due to insufficient tumor.
Altogether, these clinical results suggest that effective inhibition of the HER2 pathway can lead to rapid activation and restoration of ER expression and signaling and is associated with a parallel upregulation of Bcl2.
ER signaling inhibition is required to overcome lapatinib resistance in an ER+/HER2+ preclinical model in vitro
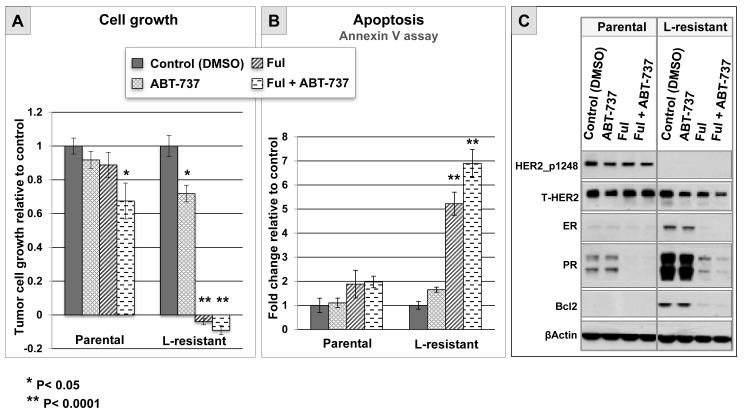
To establish whether the parallel changes of Bcl2 and ER observed in patient tumors treated with L could contribute to anti-HER2 resistance, we tested the effect of Bcl2 and ER inhibition in vitro using the UACC812 cell line model. We have previously shown that L-resistant UACC812 cells show a sustained inhibition of HER2 and its downstream signaling molecules (6). It is noteworthy that these resistant cells display increased expression of ER and Bcl2, similar to what we observed in tumor samples from patients treated with L. The effects of Bcl2 and ER inhibition by ABT-737 and fulvestrant, respectively, on tumor cell growth and apoptosis were tested in parental versus L-resistant UACC812 cells. The Bcl2 inhibitor alone significantly, although modestly, reduced cell growth in L-resistant cells compared with control (P=0.0029), whereas it had no effect in parental cells (figure 3A). In contrast, inhibition of ER signaling by fulvestrant led to complete growth arrest in L-resistant cells (p< 0.0001), but not in parental cells (figure 4A). The combination of ABT-737 with fulvestrant was not significantly superior to fulvestrant alone in L-resistant cells (p=0.7914; figure 3A). Next, we assessed apoptosis in L-resistant and parental cells after 24 hours of treatment with either ABT-737 or endocrine therapy. ABT-737 showed a limited and non-significant increase in L-resistant cells compared to control (p=0.6492), whereas no effect at all was observed in parental cells (figure 3B). In contrast, fulvestrant induced a significant increase in apoptosis in L-resistant cells compared to control (p< 0.0001), and no significant effect in parental cells (figure 3B). Finally, the combination of fulvestrant with ABT-737 resulted in a further increase in apoptosis in L-resistant cells compared to fulvestrant alone (p=0.0454; figure 3B). Western blot analysis of protein extracts collected after 72 hours of treatment confirmed sustained inhibition of HER2 activation, as well as upregulation of ER signaling in L-resistant UACC812 cells (figure 3C). As expected, treatment with fulvestrant completely eliminated ER and Bcl2 upregulation. These results suggest that Bcl2 inhibition alone may have only limited efficacy in reverting anti-HER2 resistance, whereas blocking ER signaling with fulvestrant may be more effective. These data also suggest that ER-mediated growth involves multiple genes, and is not solely due to Bcl2 increase.
Figure 3. Effects of pharmacologic inhibition of Bcl2 and ER in ER+/HER2+ cells with acquired resistance to lapatinib in vitro.
A. Tumor cell growth relative to control (DMSO) evaluated by methylene blue assay after 6 days of treatment with DMSO, ABT-737 (1 μM), fulvestrant (Ful; 0.1 μM), or the combination of ABT-737 with Ful, in parental and lapatinib (L)-resistant UACC812 cells.
B. Evaluation of apoptosis by annexin V assay after 24 hours of treatment with either DMSO (control), ABT-737 (1 μM), Ful (0.1 μM), or the combination of ABT-737 with Ful, in parental and L-resistant UACC812 cells.
C. Western blot evaluation of phosphorylated (p)-HER2 and total (t)-HER2, and ER and its downstream gene products PR and Bcl2, in parental and L-resistant UACC812 cells after 72 hours of treatment with either DMSO, ABT-737 (1 μM), Ful (0.1 μM), or the combination of ABT-737 with Ful.
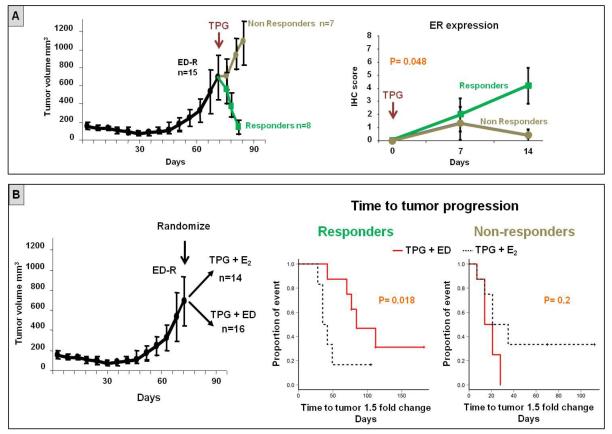
Figure 4. Endocrine therapy delays tumor progression in presence of restored ER expression in tumor xenografts treated with anti-HER therapy.
A. Mice bearing MCF7 HER2-18 tumor xenografts were subjected to estrogen deprivation (ED) until development of resistance, which was associated with complete loss of ER expression (18). At that time, the combination of trastuzumab, pertuzumab, and gefitinib (TPG) was added to ED. Anti-HER therapy induced tumor regression in 53% of mice (responders), whereas the remaining tumors continued to grow despite the inhibition of both ER and HER pathways. Restoration of ER expression was significantly associated with tumor response.
B. Mice bearing MCF7 HER2-18 tumor xenografts were randomized at the time of ED-resistance to receive TPG with or without continuing endocrine therapy (ED). The combination of TPG + ED was able to significantly delay tumor growth compared to TPG + E2 in the responders, but not in the non-responders.
Endocrine therapy delays tumor progression in presence of restored ER expression in xenograft tumors treated with anti-HER2 therapy
Anti-HER2 therapy with lapatinib or trastuzumab was associated in our neoadjuvant studies not only with upregulation of ER signaling in ER+ tumors, but also, in some cases, with conversion of ER expression from negative to positive. In turn, this restored ER activity, if left uninhibited, may provide alternative proliferation and survival signals to escape anti-HER2 therapy. We tested this hypothesis using an in vivo model mimicking the ER plasticity observed in our clinical specimens. Specifically, mice bearing ER+/HER2+ MCF7 HER2-18 tumor xenografts (N=15) received endocrine therapy with estrogen deprivation (ED; to mimic aromatase inhibitors) until development of endocrine resistance and complete loss of ER expression, as previously published (18). These ER−/HER2+ xenograft tumors were then treated with the potent anti-HER2 combination of T, P, and the HER1 inhibitor G (TPG), in association with continued ED (figure 4A). As expected, the addition of anti-HER2 to endocrine therapy determined tumor size reduction in a substantial fraction of mice (8 of the 15, 53%), whereas the remaining tumors were de novo resistant (figure 4A). Also, adding TPG therapy induced a progressive restoration of ER levels. This ER re-expression was limited to tumors that showed early response to TPG (within the first 2 weeks; figure 4A). Subsequently, we sought to test whether, despite an initial response to treatment, the observed re-expression of ER could mediate tumor survival and proliferation, and ultimately result in anti-HER2 resistance, in the absence of endocrine therapy. To test this, we randomized mice bearing ED-resistant ER− MCF7 HER2-18 tumors (N=30) to receive TPG in the presence (N=16) or absence (N=14) of continued endocrine therapy with ED (figure 4 B). Notably, in the presence of an initial response to treatment, where restored ER expression was observed, TPG combined with continued ED was associated with a significant delay in tumor progression, compared with TPG + E2 (p=0.018; figure 4B). On the other hand, within the group of mice that did not experience initial response to treatment (i.e. cases in which ER expression was not restored), no difference in time to tumor progression was observed between treatment arms (figure 4B).
Discussion
Compelling pre-clinical evidence (6, 8, 19) and results from multiple neoadjuvant trials (10-12, 20) demonstrate the superiority of anti-HER2 combinations over single-agent therapy in HER2+ breast cancer. However, the pathologic complete response (pCR) rates achieved by the combination anti-HER2 treatment strategies in these trials were remarkably lower in the ER+ compared to the ER− subset of patients (9-12, 20). We hypothesized that ER signaling, if left uninhibited, can become an alternative driver of cell growth and survival in ER+/HER2+ tumors in the presence of sustained HER2 inhibition, reducing the effect of anti-HER2 therapy. In this study, we confirm in two different in vivo xenograft models our previous in vitro analyses (6) showing increased ER expression and/or activity in the presence of acquired resistance to sustained HER2 inhibition in ER+/HER2+ cells. We also found a trend towards increased ER expression in LT-treated MCF7/HER2 xenograft tumors during the early sensitive phase of treatment. This may suggest that ER upregulation can occur soon after starting anti-HER2 therapy as a result of HER2 pathway inhibition, becoming more pronounced as treatment resistance evolves. Our data are in agreement with previous studies showing an inverse relationship between ER expression and HER pathway activity in breast tumors (21). As previously reported, hyperactivation of protein kinases downstream of HER receptors, such as p42/44 mitogen-activated protein kinase (MAPK) and PI3K/Akt, results in substantial reduction or complete loss of ER expression and activity (21-27). Although the molecular mechanisms causing this negative regulation are not completely clear, it has been hypothesized that HER pathway activity mediates inhibition of ER gene transcription and reduced ER protein stability (23-27). For example, Akt inactivates the Forkhead box protein FOXO3a that represents a key regulator of ER gene transcription (23). Likewise, hyperactive p44/42 MAPK signaling has been shown to downregulate ER at both mRNA and protein levels, via mechanisms involving epigenomic proteins and NF-kappaB (26, 28-30).
To confirm our preclinical observation in the clinical setting, we used a unique series of tumor specimens collected in a neoadjuvant clinical trial using L monotherapy. The design of this study allowed us to confirm the presence of early changes in ER expression and signaling induced by HER2 pathway inhibition, in the absence of the confounding effects of co-administrated chemotherapy or endocrine therapy. Although these molecular changes have been previously described in patient tumors (14), our study represents the first prospective clinical trial reporting these findings in a serial collection of tissue specimens. Consistent with the upregulation of ER observed in patients treated with L, we found similar results in a different set of HER2+ tumors treated with neoadjuvant T, suggesting that ER upregulation can be induced also by other therapies targeting the HER2 pathway, such as T and its combination with P. This scenario might be the reason for a pCR rate of only 6% achieved in the Neo-Sphere trial by the combination of T+P (in the absence of chemotherapy chemo- and endocrine therapy) in ER+ patients, compared with 29% in those with ER− disease (10). In contrast, in two recent neoadjuvant trials, in patients with significantly larger ER+ tumors L+T combined with endocrine therapy, again in the absence of chemotherapy, overall achieved higher pCR rates (20, 31). Although cross-trial comparisons have limited value, these differences are consistent with preclinical data from our and other groups, and provide intriguing hypothesis for further clinical investigation.
In addition to increased ER expression upon HER2 inhibition in baseline ER+ tumors, we also found cases of baseline ER-negative tumors, which converted to ER+. Consistently, preclinical studies showed that inhibition of overactive HER pathway downstream kinases results in restoration of ER expression and endocrine sensitivity in breast cancer cells (21, 24, 25). To further confirm our intriguing clinical observations and the role of restored ER expression as a resistance mechanism for anti-HER2 therapy, we used an in vivo experimental ER−/HER2+ MCF7-HER2-18 xenograft model. Interestingly, an initial response to anti-HER2 combination regimen was significantly correlated with restoration of ER expression after two weeks of treatment. Although this finding could appear to contradict a role of ER in anti-HER2 resistance, we hypothesized that the observed ER re-expression may occur as an adaptive response to the effective blockade of HER2 signaling. On the other hand, in the tumors resistant ab initio to anti-HER2 therapy, the absence of ER increase may indicate that different signaling pathways mediate growth of these tumors. Importantly, addition of estrogen deprivation to anti-HER2 therapy in the presence of initial anti-HER2 treatment response and ER reactivation significantly delayed tumor progression compared with anti-HER2 therapy alone in the absence of estrogen deprivation. Collectively, these results may suggest that upregulation and/or restoration of ER represent an indicator of effective HER2 inhibition, but are also responsible for the activation of survival mechanisms that ultimately mediate anti-HER2 resistance.
The changes in ER expression levels observed in our preclinical models and in patient tumors treated with L were accompanied by increased expression of the ER target gene products PR and Bcl2. The latter has been implicated in the development of anti-HER2 resistance (32-34). In a recent study, reverse phase protein array (RPPA) analysis of in vivo HER2+ pre-clinical tumors revealed a L-induced upregulation of Bcl2 family members (35, 36). However, in that study the association of Bcl2 upregulation with ER was not assessed. Importantly, both our pre-clinical and clinical studies revealed that the upregulation of Bcl2 observed upon anti-HER2 treatment was entirely dependent on ER upregulation or restoration. Indeed, the changes in Bcl2 completely paralleled those in ER expression, and no discordance in the variation of these two biomarkers was observed in either xenograft or patient tumors. Moreover, in our in vitro UACC812 L-resistant cells with upregulation of both ER and Bcl2, inhibition of Bcl2 alone by ABT-737 only partially arrested resistant cell growth. In contrast, ER signaling blockade by fulvestrant completely reverted the resistant phenotype and abolished Bcl2 upregulation. Finally, the addition of ABT-737 to fulvestrant did not significantly enhance cell growth inhibition, compared with fulvestrant alone. These preclinical results imply that the pro-survival role of Bcl2 in anti-HER2-resistance is likely to be completely dependent on ER activity, and that additional ER-dependent survival and proliferation genes must be involved in anti-HER2 resistance. Our findings are clinically relevant as they suggest that inhibition of ER signaling may be needed in order to effectively overcome anti-HER2 resistance, whereas a Bcl2 inhibitor alone may not be effective in this setting.
The main limitation of our study is the relatively small number of available tumor biopsies for biomarker analyses. However, these tumor specimens, collected before and during the administration of only anti-HER2 therapy, are unique and represent the ideal setting to clinically confirm the direct effects of HER2 inhibition on ER and its transcriptional activity. We recognize that the upregulation of ER and Bcl2 observed in our study may at least partly be due to intra-tumor heterogeneity. If our findings were merely caused by tumor heterogeneity, we would expect to see upregulation as well as downregulation of these markers. However, we observed only upregulation in ER and Bcl2, suggesting that heterogeneity may not have a major contribution to our findings. Conversely, our observation may be the result of more rapid elimination of ER-negative subset of tumor cells by anti-HER2 therapy, resulting in selection of ER+ tumor cells that are more resistant to HER2-targeting treatments in absence of endocrine therapy.
Overall, our results emphasize the importance of combining endocrine with anti-HER2 therapy in ER+/HER2+ tumors. In ER−/HER2+ disease, the rapid ER re-expression and the resulting Bcl2 upregulation observed in clinical and preclinical tumors upon HER2 inhibition suggest the need to re-evaluate ER status, if feasible, during administration of anti-HER2 therapy, and to co-target ER if the tumor converts to ER+. Our data also suggest that the increase in cell apoptosis, obtained by adding anti-HER2 treatment to chemotherapy, may be jeopardized by an ER-dependent Bcl2 upregulation, if endocrine therapy is not given simultaneously. Therefore, the dogma that endocrine therapy cannot be given simultaneously with anti-HER2 therapy in the presence of chemotherapy, due to potential antagonism with the latter, should be re-challenged in HER2+/ER+ breast cancer, a concept currently being explored in the NRG B-52 trial (37).
Supplementary Material
Translational Relevance.
This study confirms previous evidence of enhanced ER signaling as an adaptive cellular response to effective HER2 inhibition, using a unique series of preclinical and clinical tumor specimens collected before and during the administration of different anti-HER2 therapies, in the absence of the confounding effect of co-administered chemotherapy or endocrine therapy. We also describe a parallel upregulation of Bcl2 as a mechanism of survival entirely dependent on ER activity, and potentially leading to anti-HER2 resistance. All together our findings further emphasize the importance of combining endocrine with anti-HER2 therapy in ER+/HER2+ tumors, and suggest that therapeutic strategies aimed at targeting Bcl2 alone may not be beneficial to prevent/reverse anti-HER2 resistance. Moreover, the early changes in ER expression observed upon anti-HER2 therapy may support the need to re-evaluate hormone receptor status, if feasible, during administration of anti-HER2 therapy in ER−/HER2+ tumors.
Acknowledgments
Grant Support
This work was supported in part by: the Specialized Programs of Research Excellence (SPORE) Grants No. P50 CA58183 and CA 186784-01; the Dan L. Duncan Cancer Center and the Human Tissue Acquisition and Pathology (HTAP) Shared Resource Grant P30CA125123 from the National Cancer Institute; a Stand Up To Cancer Dream Team Translational Research Grant SU2C-AACRDT0409 (Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research), as well as research grants from the Breast Cancer Research Foundation, the Entertainment Industry Foundation/Lee Jeans, Cancer Prevention and Research Institute of Texas (CPRIT) program RP101499 – Baylor College of Medicine Comprehensive Cancer Training Program, and GlaxoSmithKline.
Footnotes
Authors’ Contributions
Conception and design: Mario Giuliano, Kent C. Osborne, Mothaffar F. Rimawi, and Rachel Schiff.
Development of methodology: Mario Giuliano, Huizhong Hu, Yen-Chao Wang, Xiaoyong Fu, Susan G. Hilsenbeck, and Rachel Schiff.
Acquisition of data (provided animals acquired and managed patients, provided facilities, etc.): Mario Giuliano, Huizhong Hu, Yen-Chao Wang, Xiaoyong Fu, Agostina Nardone, Sabrina Herrera, Sufeng Mao, Alejandro Contreras, Carolina Gutierrez, Carmine De Angelis, Nicholas J. Wang, Laura M. Heiser, Joe W. Gray, Sara Lopez-Tarruella, Anne C. Pavlick, Jenny C. Chang, Kent C. Osborne, Mothaffar F. Rimawi, and Rachel Schiff.
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Mario Giuliano, Tao Wang, Susan G. Hilsenbeck, Kent C. Osborne, and Rachel Schiff.
Writing, review, and/or revision of the manuscript: Mario Giuliano, Huizhong Hu, Yen-Chao Wang, Xiaoyong Fu, Agostina Nardone, Sabrina Herrera, Sufeng Mao, Alejandro Contreras, Carolina Gutierrez, Tao Wang, Susan G. Hilsenbeck, Carmine De Angelis, Nicholas J. Wang, Laura M. Heiser, Joe W. Gray, Sara Lopez-Tarruella, Anne C. Pavlick, Meghana V. Trivedi, Gary C. Chamness, Jenny C. Chang, C. Kent Osborne, Mothaffar F. Rimawi, and Rachel Schiff.
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Mario Giuliano, Yen-Chao Wang, Sufeng Mao, Alejandro Contreras, Carolina Gutierrez, Tao Wang, Susan G. Hilsenbeck, Meghana V. Trivedi, Gary C. Chamness, Anne C. Pavlick, Jenny C. Chang, Mothaffar F. Rimawi, and Rachel Schiff.
Study supervision: Mothaffar F. Rimawi and Rachel Schiff.
Disclosure of Potential Conflicts of Interest
C.K. Osborne served as consultant for Astra Zeneca, GlaxoSmithKline, Novartis, and Genentech. C.K. Osborne, J.C. Chang, M.F. Rimawi and R. Schiff report receiving a research grant from GlaxoSmithKline. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nature reviews Molecular cell biology. 2006;7(7):505–16. doi: 10.1038/nrm1962. Epub 2006/07/11. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. Epub 1987/01/09. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri R, Firgaira FA, Horsfall DJ, McCaul K, Setlur V, Kitchen P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11(10):1936–42. doi: 10.1200/JCO.1993.11.10.1936. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 4.Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Critical reviews in oncogenesis. 2012;17(1):1–16. doi: 10.1615/critrevoncog.v17.i1.20. Epub 2012/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tortora G. Mechanisms of resistance to HER2 target therapy. Journal of the National Cancer Institute Monographs. 2011;2011(43):95–8. doi: 10.1093/jncimonographs/lgr026. Epub 2011/11/02. [DOI] [PubMed] [Google Scholar]
- 6.Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers--role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011;13(6):R121. doi: 10.1186/bcr3067. Epub 2011/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99(9):694–705. doi: 10.1093/jnci/djk151. Epub 2007/05/02. [DOI] [PubMed] [Google Scholar]
- 8.Rimawi MF, Wiechmann LS, Wang YC, Huang C, Migliaccio I, Wu MF, et al. Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(6):1351–61. doi: 10.1158/1078-0432.CCR-10-1905. Epub 2010/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahta R, O’Regan RM. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast cancer research and treatment. 2012;135(1):39–48. doi: 10.1007/s10549-012-2067-8. Epub 2012/04/25. [DOI] [PubMed] [Google Scholar]
- 10.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 11.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–40. doi: 10.1016/S0140-6736(11)61847-3. Epub 2012/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(16):1989–95. doi: 10.1200/JCO.2011.39.0823. Epub 2012/04/12. [DOI] [PubMed] [Google Scholar]
- 13.Holmes VE FA, Liotta LA, Chang JC, Nagarwala YX, Danso M, McIntyre K, Osborne CR, Krekow LK, Gagnon RC, Mahoney JM, O’Shaughnessy JA. Correlation of Clinical and Molecular Findings with Pathologic Response to Preoperative Lapatinib and Trastuzumab, Separately or in Combination, Prior to Neoadjuvant Chemotherapy for HER2 Positive Breast Cancer. Cancer research. 2011;71(24, Supplement 3) Abstract P5-13-03. [Google Scholar]
- 14.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7795–800. doi: 10.1073/pnas.0602468103. Epub 2006/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(2):166–73. doi: 10.1200/JCO.2009.27.7814. Epub 2010/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohsin SK, Weiss HL, Gutierrez MC, Chamness GC, Schiff R, Digiovanna MP, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(11):2460–8. doi: 10.1200/JCO.2005.00.661. Epub 2005/02/16. [DOI] [PubMed] [Google Scholar]
- 17.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(5):1474–81. doi: 10.1200/JCO.1999.17.5.1474. Epub 1999/05/20. [DOI] [PubMed] [Google Scholar]
- 18.Massarweh S, Osborne CK, Jiang S, Wakeling AE, Rimawi M, Mohsin SK, et al. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer research. 2006;66(16):8266–73. doi: 10.1158/0008-5472.CAN-05-4045. Epub 2006/08/17. [DOI] [PubMed] [Google Scholar]
- 19.Rexer BN, Arteaga CL. Optimal targeting of HER2-PI3K signaling in breast cancer: mechanistic insights and clinical implications. Cancer research. 2013;73(13):3817–20. doi: 10.1158/0008-5472.CAN-13-0687. Epub 2013/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimawi MF, Mayer IA, Forero A, Nanda R, Goetz MP, Rodriguez AA, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(14):1726–31. doi: 10.1200/JCO.2012.44.8027. Epub 2013/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Tarruella S, Schiff R. The dynamics of estrogen receptor status in breast cancer: re-shaping the paradigm. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(23):6921–5. doi: 10.1158/1078-0432.CCR-07-1399. Epub 2007/12/07. [DOI] [PubMed] [Google Scholar]
- 22.Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12(3):R40. doi: 10.1186/bcr2594. Epub 2010/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo S, Sonenshein GE. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Molecular and cellular biology. 2004;24(19):8681–90. doi: 10.1128/MCB.24.19.8681-8690.2004. Epub 2004/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayliss J, Hilger A, Vishnu P, Diehl K, El-Ashry D. Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(23):7029–36. doi: 10.1158/1078-0432.CCR-07-0587. Epub 2007/12/07. [DOI] [PubMed] [Google Scholar]
- 25.Brinkman JA, El-Ashry D. ER re-expression and re-sensitization to endocrine therapies in ER-negative breast cancers. Journal of mammary gland biology and neoplasia. 2009;14(1):67–78. doi: 10.1007/s10911-009-9113-0. Epub 2009/03/06. [DOI] [PubMed] [Google Scholar]
- 26.Creighton CJ, Hilger AM, Murthy S, Rae JM, Chinnaiyan AM, El-Ashry D. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer research. 2006;66(7):3903–11. doi: 10.1158/0008-5472.CAN-05-4363. Epub 2006/04/06. [DOI] [PubMed] [Google Scholar]
- 27.Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Mol Endocrinol. 2001;15(8):1344–59. doi: 10.1210/mend.15.8.0678. Epub 2001/07/21. [DOI] [PubMed] [Google Scholar]
- 28.Plotkin A, Volmar CH, Wahlestedt C, Ayad N, El-Ashry D. Transcriptional repression of ER through hMAPK dependent histone deacetylation by class I HDACs. Breast cancer research and treatment. 2014;147(2):249–63. doi: 10.1007/s10549-014-3093-5. Epub 2014/08/19. [DOI] [PubMed] [Google Scholar]
- 29.Holloway JN, Murthy S, El-Ashry D. A cytoplasmic substrate of mitogen-activated protein kinase is responsible for estrogen receptor-alpha down-regulation in breast cancer cells: the role of nuclear factor-kappaB. Mol Endocrinol. 2004;18(6):1396–410. doi: 10.1210/me.2004-0048. Epub 2004/04/02. [DOI] [PubMed] [Google Scholar]
- 30.Gionet N, Jansson D, Mader S, Pratt MA. NF-kappaB and estrogen receptor alpha interactions: Differential function in estrogen receptor-negative and -positive hormone-independent breast cancer cells. Journal of cellular biochemistry. 2009;107(3):448–59. doi: 10.1002/jcb.22141. Epub 2009/04/08. [DOI] [PubMed] [Google Scholar]
- 31.Rimawi MF, Wang T, Rexer B, Forero A, Wolff AC, Nanda R, et al. TBCRC023: A randomized multicenter phase II neoadjuvant trial of lapatinib plus trastuzumab, with endcorine therapy and without chemotherapy, for 12 vs. 24 weeks in patients with HER2 overexpressing breast cancer. Proceedings SABCS. 2014 doi: 10.1158/1078-0432.CCR-19-0851. Abstract S6-01. [DOI] [PubMed] [Google Scholar]
- 32.Lang JY, Hsu JL, Meric-Bernstam F, Chang CJ, Wang Q, Bao Y, et al. BikDD eliminates breast cancer initiating cells and synergizes with lapatinib for breast cancer treatment. Cancer Cell. 2011;20(3):341–56. doi: 10.1016/j.ccr.2011.07.017. Epub 2011/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witters LM, Witkoski A, Planas-Silva MD, Berger M, Viallet J, Lipton A. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep. 2007;17(2):465–9. Epub 2007/01/05. [PubMed] [Google Scholar]
- 34.Crawford A, Nahta R. Targeting Bcl-2 in Herceptin-Resistant Breast Cancer Cell Lines. Curr Pharmacogenomics Person Med. 2011;9(3):184–90. doi: 10.2174/187569211796957584. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoeller JJ, Gilmer TM, Selfors LM, Lu Y, Apple SK, Press MF, et al. Basement membrane localized tumor cells are protected from HER2-targeted therapy in vivo. Cancer research. 2012;72(24)(Supplement 3) doi: 10.1158/0008-5472.SABCS12-P4-08-05. [Google Scholar]
- 36.Zoeller JJ, Gilmer TM, Selfors LM, Lu Y, Mills GB, Brugge JS. Abstract 4834: Basement membrane localized tumor cells are protected from HER2-targeted therapy in vivo. Cancer research; Proceedings AACR Annual Meeting.2012. [Google Scholar]
- 37. https://clinicaltrials.gov/ct2/results?term=NRG+B-52+&Search=Search.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.