Abstract
To find the first restorative treatment for spinal cord injury (SCI), researchers have focused on stem cell therapies. However, one obstacle is the lack of an implantable cell scaffold that can support efficient motor neuron (MN) differentiation and proliferation. We aimed to overcome this through the use of an RGD functionalized novel biomimetic polyurea, optimized to encourage efficient differentiation of MNs. Images taken after 14-days showed increased differentiation (~40%) of hNSCs into MNs as well as increased cell count on the biomimetic polymer compared to PDL-Laminin coating, indicating that the RGD-polyurea provides a favorable microenvironment for hNSC survival, having promising implications for future SCI therapies.
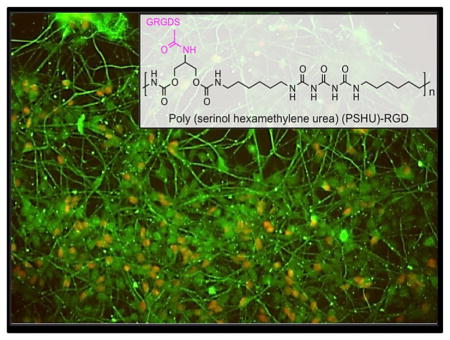
Keywords: biomaterials, biomimetic polymer, neural stem cells, motor neuron, spinal cord injury
1. Introduction
Substantial research has been conducted to investigate the signals responsible for promoting stable proliferation and differentiation of human neural stem cells (hNSCs). This task has proved to be extremely challenging due to the complex progression of signals required for efficient differentiation.[1] In the human body, hNSCs are present in stem cell niches that control the self-renewal or differentiation of the stem cells.[2,3] The signals that control differentiation in vivo are multifaceted including cell–cell interactions, cell–biomolecule interactions, and cell–extracellular matrix (ECM) interactions. Due to the complexity of these signals, most efficient motor neuron (MN) induction protocols rely on some forms of gene transfection to bypass the need for induction signals similar to those present within a stem cell niche.[1,4] Although effective, gene transfection methods may not be suitable for cell transplantation due to their complicated and variable characteristics. For this reason, researchers have begun manipulating bioactive polymers scaffolding in order to control cellular behavior.[5] We propose the use of a synthetic polymer that can be fine-tuned and manipulated to mimic the signals found in a stem cell niche. The goal of this biomimetic polymer would be to harness the correct signals in order to permit human motor neuron (hMNs) induction and in turn be a promising cell scaffold for stem cell spinal cord injury (SCI) treatment.
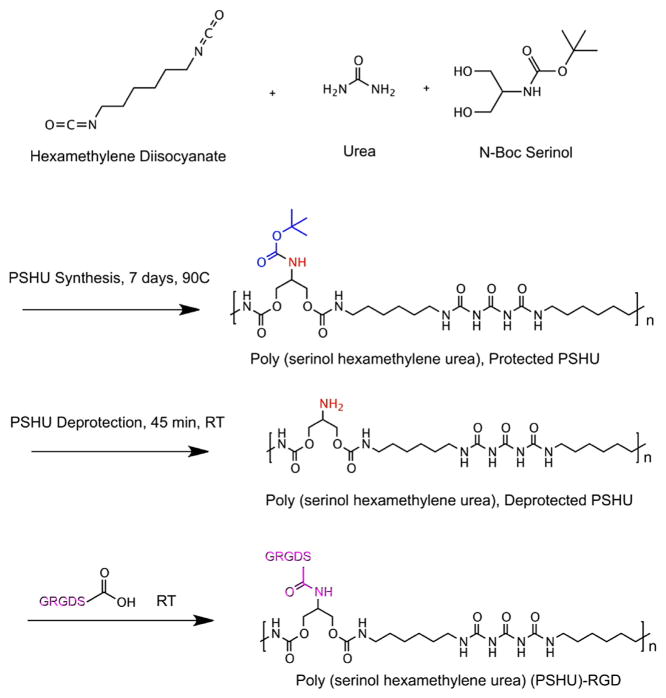
Poly (serinol hexamethylene urea) (PSHU) (Figure 1) was employed due to its protein-like backbone structure and its potential to attach a large quantity of biomolecules (18 potential linkages per molecule). The protein-like backbone structure of the polymer may provide a cellular environment more similar to the naturally occurring proteins within the extracellular matrix. We predicted that the hNSCs would respond positively to this biomimetic polymer structure. FT-IR was employed to show the similarities between this polymer and collagen, the most abundant protein in the ECM responsible for structural support of cells (Figure S1). In addition, functional aspect of this polymer is extremely beneficial for these purposes because achieving a high concentration of biomolecules for cell–biomolecule interactions plays a crucial role in stem cell survival and differentiation.[6] Extensive efforts have been made to determine which biomolecules in the ECM are important in regulating NSC function in vivo and thus could be used to mimic the cell–biomolecule interactions to guide stem cell fate. The RGD sequence, an integrin-binding motif found in fibronectin and laminin (major components of the ECM), was found to be implicated in outside–inside cell signaling that can affect cell proliferation, migration, and cell survival in most tissues.[7] It has also been determined that integrin binding motifs, specifically RGD, are involved in supporting attachment, spreading, and differentiation of hNSC in a dose-dependent fashion.[8,9] Therefore, incorporating enough of this RGD sequence into synthetic polymers to produce a synthetic scaffold has the potential to increase hNSC differentiation and proliferation.[10] Not only do cell–biomolecule interactions play a role in directing stem cell fate, but also cell–ECM interactions can help to modulate neural stem cell behavior and differentiation.[11] To cater to these interactions, we engineered this polymer to possess multiple peptide-mimicking bonds to increase biocompatibility of the polymer.
Figure 1.
Schematic synthesis of PSHU-RGD. The first stage is PSHU synthesis followed by the removal of the N-BOC groups from the PSHU backbone and finally RGD conjugation.
2. Experimental Section
Materials
N-BOC-Serinol, urea, hexamethylene diisocyanate (HDI), anhydrous chloroform, and anhydrous N,N-dimethylformamide (DMF) were purchased from Sigma-Aldrich (St. Louis, MO, USA). N-(3-Dimethylamino- propyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), 2,2,2-trifluoroethanol (TFE), and trifluoroacetic acid (TFA) were purchased from Alfa Aesar (Ward Hill, MA, USA). Anhydrous diethyl ether was purchased from Fisher Scientific (Pittsburgh, PA, USA). Anhydrous dichloromethane (DCM) was purchased from JT Baker (Phillipsburg, NJ, USA). The pentapeptide Gly–Arg–Gly–Asp–Ser (GRGDS) was purchased from Biomatik (Wilmington, DE).
Equipment
1H NMR spectra were recorded on a Varian-500 NMR (500 MHz, Varian) with CDCl3 as the solvent at 25 °C. Chemical shifts are in ppm using the solvent peak as the internal reference. Fourier transformed infrared (FTIR) spectra were recorded on a Nicolet 4700 (Thermo Fisher Scientific, Waltham, MA) spectrometer. All in vitro cell morphologies were examined on a Nikon DIAPHOT 300 equipped with CCD camera (SPOT RT 2.3.0, Diagnostic Instruments) using SPOT Advanced software for post-hoc analysis.
Synthesis of poly (serinol hexamethylene urea) (PSHU)
PSHU was synthesized by combining HDI (1.928 ml, 12 mmol), N-BOC-Serinol (1.147 g, 6 mmol), and urea (0.360 g, 6 mmol) in 6 ml of anhydrous DMF. This reaction was maintained at 90 °C for 7 days. After the 7-day reaction period, the solution was cooled to room temperature and rotary-evaporated at 70 °C to remove the DMF. Next, the product mixture was re-dissolved in a small amount of DMF (2–3 ml) and precipitated into cooled anhydrous diethyl ether (100 ml). The purification process was repeated twice and the solution was washed overnight in excess ether (100 ml) to remove any unreacted reactants and remaining solvent. Finally, the product was rotary-evaporated at 45 °C until dry; the final product was stored at room temperature until conjugation with RGD.
Synthesis of RGD-conjugated PSHU
PSHU of 1 g was dissolved in 100 ml mixture of methylene chloride/trifluoroacetic acid (1:1, v/v). The BOC deprotection reaction was carried out at room temperature for 45 min. The mixture was rotary-evaporated at 45 °C to remove methylene chloride and TFA. The product was dissolved in anhydrous DMF (1ml). Once fully dissolved, this solution was purified by precipitation in excess cooled ether (100 ml). Finally, the product was rotary-evaporated at 45 °C, dried, and stored at room temperature.
To conjugate RGD, 100 mg of deprotected PSHU (0.1956 mmol amine groups) was mixed with 1.3 molar excess of GRGDS, N′-ethylcarbodiimide hydrochloride (EDC), and N-hydroxysuccinimide (NHS) in anhydrous DMF (1 ml). This conjugation reaction was performed overnight at room temperature and was finally precipitated in excess ether and dried using rotary evaporator.
Human embryonic stem cell culture conditions
Human embryonic stem cells (ESCs), UCSF-4 were cultured in mTesR media (STEMCELL) on Cellstart (Invitrogen) coating plate. For passaging, UCSF-4 cell colonies were cut into small squares using StemPro EZPassage Disposable Stem Cell Passaging Tool (Gibco) at a dilution of 1:10.
Neural stem cell Induction
hESCs (UCSF-4) were treated with 10ng/ml hLIF(Milipore), 3 μMCHIR99021 (Cellagentech), and 2 μM SB431542 (Cellagentech) in neural induction media, N2B27, containing DMEM/F12: Neurobasal (1:1), 0.5xN2, 1xB27, 1% Glutmax, for 10 d. The culture was then split 1:3 for the next passages using Accutase and expanded in neural induction media supplemented with 10 ng/ml hLIF, 3 μM CHIR99021, and 2 μM SB431542 on Cellstart coating plate.
PSHU-RGD and PDL/Laminin plate coating
To prepare the PSHU-RGD and PSHU-coated surfaces, the well plates were filled with the corresponding amount of polymers dissolved in 1 ml TFE. Following this, the plate was placed on a mechanical agitator for three days. While on the agitator, the solvent TFE will evaporate from the well leaving the polymer coated to the bottom of the well. The constant agitation provided while the solvent evaporates provided a more even coating of the polymer on the bottom of the well. Separately, poly-D-Lysine/Laminin was also coated according to vendor’s instruction.
hNSC culture
hNSCs (5×103 cells) were added in 24 plates coated with different materials: PSHU-RGD, PSHU, and Poly-D-Lysine/Laminin. For proliferation, N2B27 media with treatment of 1 uM retinoic acid (Sigma) and 100 nM SAG (Smoothened agonist, SHH Activator, EMD Chemicals) was used, and then the cells were terminally differentiated in the presence of 10 ng/ml BDNF and 10ng/ml GDNF(R&D systems) in N2B27 media.
Immunocytochemistry
The cells were fixed in 4% paraformaldehyde, followed by washing three times with PBS and incubated in blocking buffer, 0.1% Triton X-100 and 5% goat serum (Jackson ImmunoResearch) in PBS (Invitrogen/Gibco BRL), for 1 h at room temperature. The cells were then incubated with primary antibody overnight at 4 °C in incubation buffer, 0.1% Triton X-100 and 3% goat serum, in PBS. The following day, cells were washed with PBS 3 times and incubated with Alexa Fluorconjugated secondary antibodies (Invitrogen, 250×) in PBS containing 0.1% Triton X-100 and 3% goat serum for 1 h at RT. Nuclei were visualized by Hoechst 33342 staining (Sigma-Aldrich). Images were captured using a Nikon Diaphot 300 microscope. For neural cell marker, mouse or rabbit origin of βIII-tubulin (both 1:1000, Promega and Sigma) were used. For motor neuron markers, Islet-1 (Developmental Studies Hybridoma Bank, 1:50) and HB9 (Developmental Studies Hybridoma Bank, 1:50) were used.
3. Results and Discussion
3.1. Polymer Characterization and Cell Survival Assay
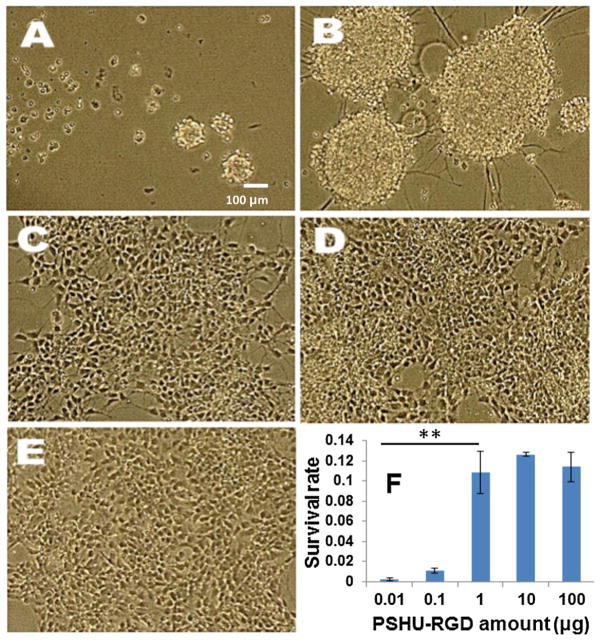
We began by synthesizing and characterizing a functionalizable biomimetic polymer, PSHU. 1H NMR confirmed the overall polymer structure (Figure S2 and S3). RGD was conjugated to all free amine groups on the PSHU backbone and FT-IR was used to confirm the conjugation of the GRGDS peptide to the free amine groups on the polymer backbone (Figure S4). Note that GRGDS was used instead of RGD in order to preserve the integrity of the entire RGD binding motif. Next, we moved to determine the effects of PSHU-RGD on hNSCs survival during motor neuron differentiation. To do this, we cultured hNSCs on a 24-well plate with varying amounts of PSHU-RGD for a 14 or 21-day period. Human embryonic stem cell (hESC)-derived hNSCs were cultured in N2B27 media supplemented with retinoic acid (ATRA) and purmorphamine (SAG) for the first 7 days to induce motor neuron differentiation and then N2B27 media supplemented with brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) for an additional 7 or 14 days to promote axonal extension. As shown in Figure 2, at the two lowest amounts of PSHU-RGD (0.01 and 0.1 μg), there is limited cell attachment after the 21-day culture period. The cells remained circular in shape and were aggregated to one another. This detachment and rounded morphology corresponds to poor cell health and the induction of apoptosis.[12] At the higher amounts of PSHU-RGD (1, 10, and 100 μg), cells showed good attachment and survival. These results indicate that at amounts above 1 μg, PSHU-RGD provided sufficient RGD ligands for cell survival.
Figure 2.
Phase contrast images of hNSC culture on PSHU-RGD at varied amounts: (A) 0.01 μg, (B) 0.1 μg, (C) 1 μg, (D) 10 μg, and (E) 100 μg after 21 days. The survival rate of each culture was also compared (F). ** corresponds p<0.0001.
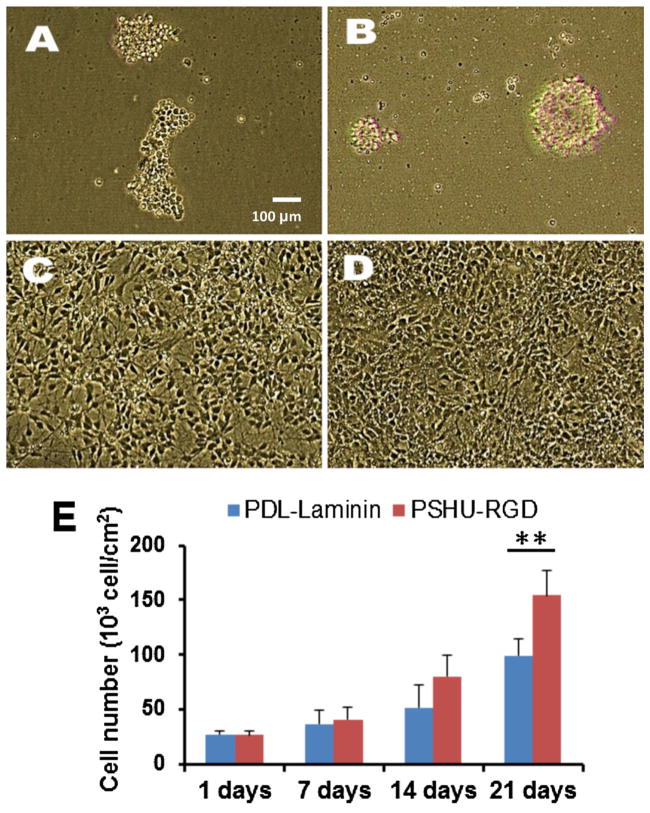
To observe the ability of PSHU-RGD at permitting cell survival during differentiation of hNSCs, we compared this synthetic biomimetic polymer coating to the widely used poly-d-lysine (PDL)-Laminin surface coating. PDL-laminin coating has shown to encourage hNSCs and MN proliferation and extensive neurite outgrowth.[13] We obtained phase contrast images of hNSCs cultured in the same fashion as described above on PSHU, PSHU-RGD, PDL-Laminin, and plain cell culture plate (Figure 3). We used the PSHU-RGD amount of 1 μg because the previous results indicated that there was no significant effect on hNSC survival during differentiation when the amount of PSHU-RGD was increased above 1 μg. Therefore, the following experiments were all conducted using 1 μg of polymer. The images of the cells cultured on the plain well plate (Figure 3A) and on PSHU (Figure 3B) showed little growth with cells mostly detached. This result signifies that cells were unable to attach to the polymer itself (PSHU) without the RGD ligands present. The cells cultured on PDL-Laminin (Figure 3C) and PSHU-RGD (Figure 3D) had significant cell survival and growth. Thus, it appears that the presence of RGD and more importantly the amount of RGD have a profound effect on hNSC survival.
Figure 3.
Phase contrast images of hNSC culture on various surfaces: (A) plain cell culture well plate, (B) PSHU, (C) PDL-Laminin, and (D) PSHU-RGD. Cell numbers cultured on PDL-Laminin and PSHU-RGD were also quantified at different time points (E). ** corresponds to p<0.0001.
To further compare the PSHU-RGD coating to the PDL-Laminin positive control, the number of cells was counted throughout the 21-day culture period (Figure 3E). Initially the cell count between the two groups remained fairly similar; however, after 7 days, the growth rate of the cells on the PSHU-RGD coating was larger than that of the PDL-Laminin coating. A significant difference in the cell count was observed between the PSHU-RGD and PDL-Laminin coating for both the 14-day and 21-day time points. Furthermore, after the 21-day culture period, the PSHU-RGD coating showed almost 50 k/cm2 more cells than the PDL-Laminin coating. This finding suggests that PSHU-RGD not only allows cell survival but may also encourage cell proliferation.
3.2. Motor Neuron Induction Analysis
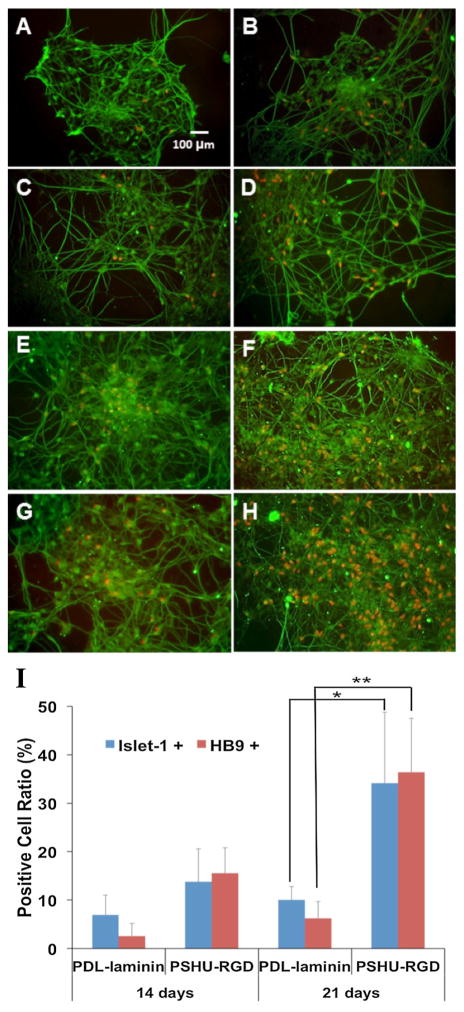
At this point, the MNs cultures were fixed and immunostained for HB9, a marker for maturing postmitotic motor neurons, and Islet 1, a marker for transcription factors involved in MN development and a mature motor neuron indicator.[14] Further analysis was conducted by immunostaining for β-III tubulin, a marker for mature motor neurons and axonal extension.[15] When cultured on the PDL-Laminin coating, we observed an increase between the 14 day culture and 21 day culture in the number of cells expressing HB9 and Isl1 as well as increased axonal extension after 21 days as seen through β-III tubulin staining. The cells after 21 days (Figure 4B and 4D) also developed a larger axonal network and increased length than at 14 days (Figure 4A and 4C). Compared to the PDL-Laminin coating, we observed that PSHU-RGD produced a larger number of cells expressing both HB9 and Isl1 after both 14 (Figure 4E and 4G) and 21 days (Figure 4F and 4H). Images displaying Hoechst 33342 staining with other immunostains (HB9 and Isl1) were included in the supplemental data (Figure S5) to preserve the clarity of Figure 4. It is also demonstrated that MNs cultured on PSHU-RGD versus PDL-Laminin produced a larger axonal network and increased axonal spreading possibly due to the increase in cell count also observed. Obtaining a balanced amount of not only integrin binding molecules but also surface charge with the PSHU-RGD coating likely caused this observation. We employed two surface characterization techniques to help elucidate the reasons behind these results. Beginning with zeta potential, we investigated the surface charge of both PSHU-RGD and PSHU (Figure S6, Left). The surface charge of PSHU-RGD was more positive than PSHU indicating the presence of RGD on the polymer backbone. This increase in positive surface charge caused by the increase in RGD could have resulted in improved cell attachment and cell spreading. Secondly, we measured the contact angle of PSHU-RGD at each polymer amount (Figure S6, Right). As the amount of PSHU-RGD was increased, the hydrophilicity of the surface was increased, likely caused by the large amount of peptide present. Finally, we calculated the theoretical amounts of RGD biomolecules in both the PSHU-RGD and PDL-Laminin coating. Calculations of the theoretical amount of RGD biomolecules are provided in the supplemental information (Equation S1). Although we were unable to fully divulge the mechanism behind PSHU-RGD’s effect on hNSCs, we hypothesize that the large amount of RGD biomolecules present in the PSHU-RGD coating (~1e+15) is responsible for the increased cell growth and attachment when compared to the PDL-Laminin coating (~6e+12).
Figure 4.
Fluorescent microscopy images of hNSCs cultured on PDL-Laminin (A–D) and PSHU-RGD (E–H). After culture for 14 days (A,C,E,G) and 21 days (B,D,F,H), cells were stained with HB9+ and βIII-tubulin (A,B,E,F), and Isl1+ and βIII-tubulin (C,D,G,H). Red dots indicate HB9+ and Isl1+ cells. Quantitative analysis comparing the ratio of cells expressing Isl1 and HB9 at 14- and 21-day cultures. * and ** corresopond to p<0.001 and p<0.0001 respectively (I).
The ratio of cells that were expressing Isl1 and HB9 for both the PSHU-RGD and PDL-Laminin coating was quantified (Figure 4I). At the 14-day time point, the ratio of cells producing Isl1 and HB9 was significantly larger on PSHU-RGD versus PDL-Laminin. After the 21-day time period, this difference was much more apparent. The PSHU-RGD coating achieved approximately 40% cells expressing HB9 and also approximately 40% of cells expressing Isl1, while the PDL-Laminin coating achieved less than 10% of cells expressing Isl1 and HB9. It should also be noted that these elevated ratios seen on the PSHU-RGD coating were accompanied by a higher total cell count producing a much more significant difference.
4. Conclusion
Due to significant cell death during implantation, the initial number of cells implanted into the injury site far outweighs the surviving cells able to integrate into the surrounding tissue and restore function. Of course, encouraging cell attachment, differentiation, and proliferation through the use of a scaffold would significantly increase the number of surviving cells and thus the effectiveness of these cell therapies. Therefore, the development of cell scaffold that encourages efficient MN induction and survival without the alteration of the cell’s DNA may be sufficient to make stem cell therapies an institution in spinal cord injury treatment. To this end, we successfully designed a controllable polymer that supports the proliferation of hNSCs and the survival of MNs during differentiation. In summary, we anticipate that the controllable properties of this synthetic polymer that improve hNSCs differentiation and survival in vitro will have great implications on future stem cell therapies for SCI treatment.
Supplementary Material
Acknowledgments
This work was supported by the University of Colorado Denver start-up funding and NIH 1R21EY023711-01A1. Support for embryonic stem cell and motor neuron experiments came from the Walter and Lucienne Driskill Foundation and personal savings of Curt R. Freed, MD.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author
Contributor Information
Dr. Donghwa Yun, Department of Bioengineering, University of Colorado Denver, 12800 E. 19th Ave, Aurora, CO 80045-2560, USA
Dr. Young M. Lee, Division of Clinical Pharmacology and Toxicology, University of Colorado School of Medicine, 12700 E. 19th Ave, Aurora, CO 80045-2560, USA
Melissa R. Laughter, Department of Bioengineering, University of Colorado Denver, 12800 E. 19th Ave, Aurora, CO 80045-2560, USA
Dr. Curt R. Freed, Division of Clinical Pharmacology and Toxicology, University of Colorado School of Medicine, 12700 E. 19th Ave, Aurora, CO 80045-2560, USA
Dr. Daewon Park, Email: Daewon.Park@ucdenver.edu, Department of Bioengineering, University of Colorado Denver, 12800 E. 19th Ave, Aurora, CO 80045-2560, USA
References
- 1.Wada T, Honda M, Minami I, Tooi N, Amagai Y, Nakatsuji N, Aiba K. Plos One. 2009;4:e6722. doi: 10.1371/journal.pone.0006722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wichterle H, Lieberam I, Porter JA, Jessell TM. Cell. 2002;110:385. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 3.Conti L, Cattaneo E. Nat Rev Neurosci. 2010;11:782. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- 4.Hester ME, Murtha MJ, Song S, Rao M, Miranda CJ, Meyer K, Tian J, Boulting G, Schaffer DV, Zhu MX, Pfaff SL, Gage FH, Kaspar BK. Mol Ther. 2011;19:1905. doi: 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Townsend-nicholson A, Jayasinghe S. Biomacromolecules. 2006;7 doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]
- 6.Lutolf MP, Gilbert PM, Blau HM. Nature. 2009;462:433. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leone DP, Relvas JB, Campos LS, Hemmi S, Brakebusch C, Fassler R, ffrench-Constant C, Suter U. J Cell Sci. 2005;118:2589. doi: 10.1242/jcs.02396. [DOI] [PubMed] [Google Scholar]
- 8.Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli CH, ffrench-Constant C. Development. 1998;125:3167. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- 9.Saha K, Irwin EF, Kozhukh J, Schaffer DV, Healy KE. J Biomed Mater Res Part A. 2007;81A:240. doi: 10.1002/jbm.a.30986. [DOI] [PubMed] [Google Scholar]
- 10.Ananthanarayanan B, Little L, Schaffer DV, Healy KE, Tirrell M. Biomaterials. 2010;31:8706. doi: 10.1016/j.biomaterials.2010.07.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon WB, Park BH, Choi SK, Lee KM, Park JK. Bmc Biotechnol. 2012;12 doi: 10.1186/1472-6750-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawen A. Bioessays. 2003;25:888. doi: 10.1002/bies.10329. [DOI] [PubMed] [Google Scholar]
- 13.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Cell Stem Cell. 2008;3:637. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, Wiedau-Pazos M, Kornblum HI, Lowry WE. Stem Cells. 2009;27:806. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman PN, Lopata MA, Watson DF, Luduena RF. J Cell Biol. 1992;119:595. doi: 10.1083/jcb.119.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.