Abstract
Background
Clove oil of Eugenia caryophyllata (Myrtaceae) is a light yellowish fluid obtained from dried flower buds. Clove oil is used traditionally to relieve toothache.
Aim
The aim of the present work was to study the anti-inflammatory, antinociceptive and antipyretic potential of clove oil in mice.
Methods
Analgesic activity was examined using acetic-acid-induced abdominal constrictions and the hot plate test. Carrageenan-induced paw edema and brewer's-yeast-induced pyrexia were used to investigate the anti-inflammatory activity and the antipyretic effects, respectively. The oil was administered intraperitoneally (i.p.) at a dose of 33 mg/kg body weight and the effects were compared with reference drugs.
Results
In the antinociceptive test, mice treated with clove oil exhibited significantly decreased acetic-acid-induced writhing movements by a maximum of 87.7% (p<0.01) compared with a decrease of 77.7% (p<0.01) in response to aspirin injection (100 mg/kg, intraperitoneal, i.p.). Similarly, in the hot plate test, clove oil significantly increased the reaction latency to pain after 60 min by 82.3% (p<0.05) compared with morphine value of 91.7% (p<0.01). In addition, clove oil and indomethacin produced anti-inflammatory effects, as demonstrated by respectively 50.6% (p<0.05) and 70.4% (p<0.01) inhibition of mouse paw edema induced by carrageenan. Furthermore, clove oil significantly attenuated the hyperthermia induced by yeast at ΔT-max by 2.7°C (p<0.001), and time of peak effects was 30–180 min compared with a paracetamol value ΔT-max of 3.2°C (p<0.001). The estimated i.p. LD50 of clove oil was 161.9 mg/kg. Phytochemical screening of the oil showed the presence of eugenol.
Conclusion
The present findings demonstrate the potential pharmacological properties of clove oil and provide further a support for its reported use in folk medicine.
Keywords: Eugenia caryophyllata, clove oil, eugenol, antinociceptive, anti-inflammatory, antipyretic, mice
Over the centuries, plants have been used for their medicinal values. However, many plants have yet to be studied and relatively few scientific researches have been carried out. Therefore, assessment of medicinal plants is of special interest because of rich heritage and the continuous use among large portion of people.
Clove is an evergreen plant 10–20 cm tall found throughout the hot tropical climates. It was originally from Asian tropical countries. Clove, Syzygium aromaticum L. (Eugenia caryophyllata, syn.), belongs to the family Myrtaceae. It has a strong phenolic smell and sharp pungent taste. The dried flower bud is commonly used as a spice. The oil, steam distilled from clove buds, is a colorless to light yellowish fluid heavier than water and freely soluble in alcohol (1).
Humans have consumed clove over a long period of time. In addition to its flavoring properties, it is known for its medicinal advantages. In Ayurveda, Chinese medicine and Western herbalism, clove has been valued as a herbal remedy. For years, clove has been used as antispasmodic, carminative and to improve peristalsis (1). The essential oil of clove is used for dental emergencies, mainly for symptomatic relief of toothache and inflammation in the mouth and throat. In this regard, clove oil has been reported to be used in preparation of certain toothpastes and mouth washes (1). In the recent past, studies have demonstrated that clove is antimutagenic (2), antioxidant, antithrombotic, antiparasitic and anti-inflammatory (3). In addition, clove oil is stated to possess antibacterial, antiviral and antifungal properties (4–6).
Evidence from numerous studies has shown the several constituents of clove, mainly eugenol (70–90%), tanene (13%) and fixed oil (10%) (7). Different studies evaluated the effects of these compounds on physiological parameters and their potential health-related significance. Studies in the arthritic rat model have shown that eugenol exhibits anti-inflammatory and anti-arthritic activities (8). Feng and Lipton (9) have demonstrated that eugenol significantly reduced fever when given to rabbits made febrile by the injection of interleukin 1. These findings provide support for some of the traditionally accepted medicinal uses of clove oil. However, the ethno-pharmacological properties of the native oil as it is used traditionally have not been investigated. Therefore, to provide more information, the present study aimed to study the oil available in public markets and pharmacy shops for dentistry purposes for possible antinociceptive, antipyretic and anti-inflammatory potential in mice.
Materials and methods
Animals
Inbred male Albino mice aged 2–3 months weighting 17–35 g and reared in our animal house were used in all experiments. Mice were maintained on standard husbandry condition of humidity and temperature (25±2°C) with a 12-h light/dark cycle, and had free access to animal pellets and water. Food was withheld overnight before the experiments while water was still provided ad libitum. All experiments were run during the day time. The experimental protocol, dated August 25, 2002, was reviewed and approved by the Ethics Committee for Research Programs (Faculty of Pharmacy, Tripoli University, Tripoli, Libya) under Ref. No. Pharm/23/09/2002/2003. Laboratory animal care and use was in strict accordance with the guidelines of animal welfare. The study was done on 110 mice (18–24 mice per experiment), in three groups of 6–8 mice for each experiment.
Drugs and chemicals
The following drugs were used: aspirin, indomethacin, Lambda-carrageenan Type IV (Sigma Chemical Co., St. Louis, USA), paracetamol (Bristol-Myers Squibb, Italy) and morphine (Lavoisier, France). The doses were selected according to previous studies (10, 11). All other chemicals were of analytical grade. Clove oil, (0.03% w/v, Al-Asi for antiseptic Co., Halib, Syria, batch no. 123) was bought from local market, Tripoli, Libya. The composition of the clove oil was 100% natural. The dose of clove oil (33 mg/kg body weight) was based on the literature (8). A bent blunted 25 g needle connected to a 1-ml syringe was used for i.p. administration.
Phytochemical screening
The oil was screened phytochemically for the presence of the phenolic substance eugenol. The method of analysis was a standard technique according to the color test procedure previously mentioned elsewhere (12, 13). Briefly, the presence of eugenol, the main phenolic constituent of clove oil, was tested by dissolving two drops of the oil in 1 ml 70% ethanol followed by addition of a few drops of 1% alcoholic ferric chloride.
Anti-inflammatory activity
The anti-inflammatory activity of clove oil was investigated by the use of carrageenan-induced paw edema test as described previously in mice (14). Briefly, in three groups of six mice each, 0.02 ml of a freshly prepared suspension of the phlogistic agent carrageenan in saline (10 mg/ml) was injected sub-plantar into the right hind paw of each mouse. Thirty minutes before carrageenan injection mice were treated i.p. by clove oil, negative and positive control mice received normal saline and indomethacin, respectively. Paw thickness was measured before and 3 h after carrageenan injection, using a micrometer (Mitutoyo, Japan). The increase in paw thickness was taken as an indicator of the degree of acute inflammation and edema. The percentage inhibition of the inflammatory response was determined for every mouse in comparison to saline-treated animals and computed using the following formula (14):
where EPT represents experimental paw thickness and CPT represents control paw thickness.
Analgesic activities
The writhing acetic acid test was used to evaluate the possible peripheral effects of the oil as an analgesic and was performed as previously described (14). Briefly, clove oil, aspirin, or an equivalent volume of saline (negative control) was given i.p. before the injection of 10 ml/kg of 0.6% v/v acetic acid. Each mouse was then placed in an observation box for 20 min. The number of constrictions of the abdominal muscles together with stretching of the hind limbs, an indication of writhing response, was counted. Antinociceptive activity was expressed as the number of abdominal constrictions in mice pre-treated with clove oil or aspirin relative to control animals. The formula used for the calculation of percentage of writhing inhibition was as follows (14):
Thermally induced pain in mice
The Eddy's hot plate test was used for assessment of possible centrally mediated analgesic effects of clove oil. This method was applied to assess response latencies as described (14). For two consecutive days before the experiments, mice were placed gently on a plate (Model-DS37) maintained at 25±1°C for 10 min each day. On the third day, each mouse was placed quietly on a hot plate at 55±1°C for a maximum of 25 sec. Licking of forepaws, shaking or jumping off the plate was recorded and used as a nociceptive response baseline reading. Clove oil was given i.p., whereas the central analgesic drug, morphine, used as a positive control, was injected subcutaneously (s.c.). Negative control mice received normal saline i.p. After 30 min, the latency time in each mouse was recorded to determine the analgesic response. Full analgesia was considered as a latency of 25 sec. The mean latency of nociceptive responses for each treatment group was calculated. The percentage of analgesia was calculated using the following formula (14):
where σ represents reaction time after treatment and ζ represents baseline recording.
Antipyretic test
The antipyretic test was done in the model of pyrexia induced by brewer's yeast (15). Temperature was measured with a lubricated digital thermometer (SK-1250 MC, Sato Keiryoki Mfg.) inserted approximately 2 cm into the rectum for 60 sec. Hyperthermia was induced in mice 18 h before treatment by s.c. injection below the neck of 1 ml/100 g body weight of a 20% w/v aqueous suspension of brewer's yeast. The first measurement was made immediately before the yeast injection. After 17 h, rectal temperature of each mouse was recorded. Each mouse exhibiting an increased rectal temperature of at least 0.7°C was selected for the investigation. This rectal temperature was indicated as baseline reading. Clove oil, paracetamol and saline were administered i.p. The response in each mouse was taken as the difference in rectal temperature after 0.5, 1, 2 and 3 h from the baseline reading. Percentage inhibition of fever (% if) was calculated according to the following formula:
where, PYT represents post-yeast temperature and PDT represents post-drug treatment.
Acute toxicity study (LD50)
The protocol used for acute toxicity study has been described (16). Briefly, in all groups, 5% dextrose, ad libitum, was used to replace the withheld food and to avoid stress and starvation of mice during the experiment. LD50 was defined as 50% deaths within 96 h after clove oil administration. Mice were divided into five groups (n=4 each). Four groups of four mice each were given the oil i.p. at doses 50, 90, 162 and 292 mg/kg body weight, respectively. Group 5, used as the control, received water at 10 ml/kg. After injection, mice were observed continuously for the first 3 h for any toxic manifestation or behavioral changes. Thereafter, deaths were monitored every 2–12 h for up to 4 days. The LD50 was calculated using the following formula (17):
where, log m represents the log of LD50, log D a represents the log of the smallest of the four doses used, d represents the logarithm of the constant ratio between dosage levels, and f represents a constant factor obtained from Weil's tables.
Statistical analysis
The data were found to be normally distributed based on the K-S statistical goodness of fit test. LD50 (Table 2) was obtained algebraically as described above. The results are expressed as mean±SEM. The variance between groups was tested by ANOVA followed by Tukey's test. For the anti-inflammatory experiment (Fig. 1), the difference was calculated on the individual changes recorded between the paw thickness obtained before and 3 h after carrageenan injection. In the study of antinociceptive activities, comparison was done on the individual pain reactions in the abdominal constrictions test observed in control and test animals (Fig. 2), and between the individual reaction times obtained before and after treatment in the Eddy's hotplate test (Fig. 3). For the antipyretic test (Table 1), comparisons were made on the individual differences calculated between the rectal temperature at each record and the one obtained 17 h after yeast injection. p<0.05 was considered as significant values against respective control. GraphPad Prism (GraphPad Software Inc., version 3.0, San Diego, USA) was used for statistical analysis.
Table 2.
LD50 determination in mice
| Group | n | Dose (mg/kg body weight) | No. of deaths | % Mortality |
|---|---|---|---|---|
| 1 | 4 | 50 | 0/4 | 0 |
| 2 | 4 | 90 | 1/4 | 25 |
| 3 | 4 | 162 | 1/4 | 25 |
| 4 | 4 | 292 | 4/4 | 100 |
| 5 | 4 | Control | – | 0 |
Log m=log D a+d. (f+1).
Log m=log 50+log 1.8 (1+1)=2.209245.
LD50 (i.p.)=161.9 mg/kg.
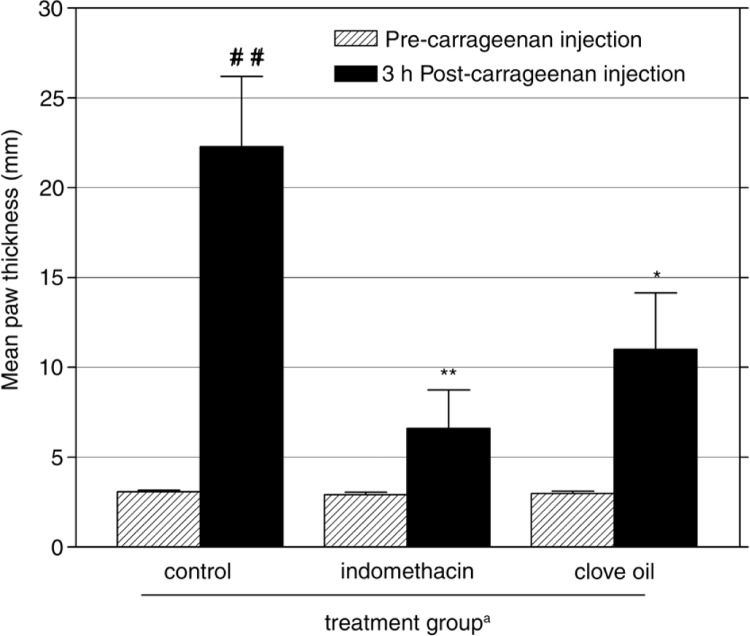
Fig. 1.
Effects of i.p. administration of clove oil and indomethacin (10 mg/kg) on mouse paw edema 3 h after injection of carrageenan. Mean±SEM of eight individual measurements of increase in paw thickness is presented (n=8 each mouse group). aAdministered 30 min before carrageenan injection. ## p<0.01 vs. pre-carrageenan injection (paired t-test), p<0.05, **p<0.01 vs. control mice (ANOVA followed by Tukey's test).
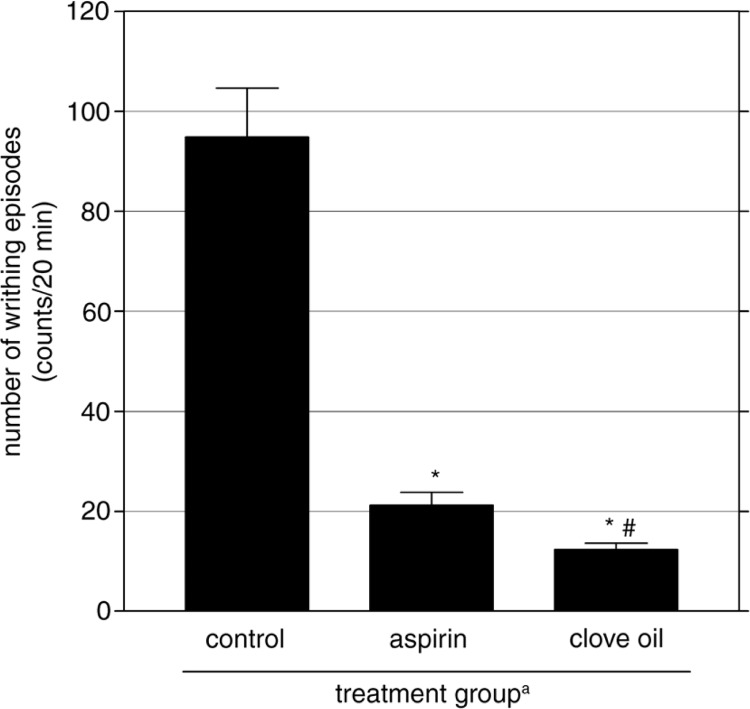
Fig. 2.
Effect of clove oil on the number of writhing induced by acetic acid injection in mice. Control mice received normal saline and positive control group was treated with aspirin (100 mg/kg). Mean±SEM of eight individual readings is presented (n=8 each group). aAdministered 0.5 h before acetic acid injection (0.6% v/v, 10 ml/kg, i.p.). *p<0.01 vs. control group. # p<0.05 vs. aspirin-treated mice.
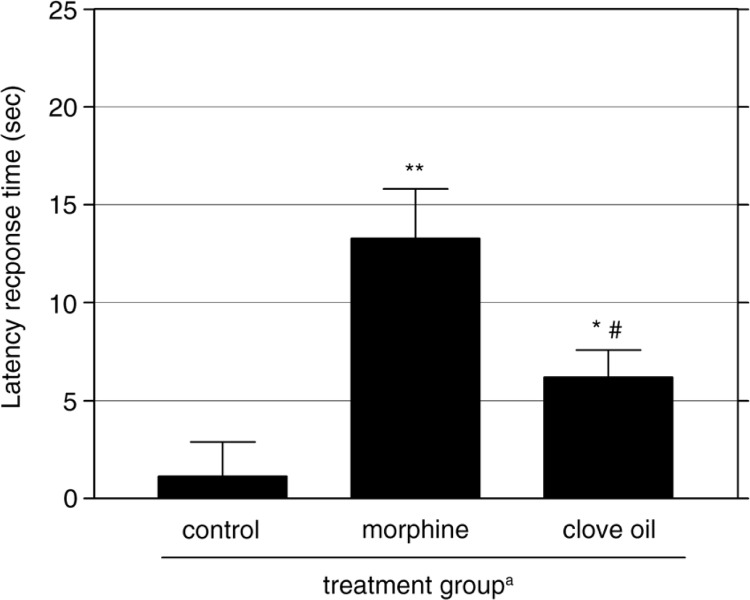
Fig. 3.
The effect of clove oil and morphine (5 mg/kg) against thermally induced pain by the hot-plate method in mice. Mean±SEM of eight individual readings is presented (n=8 each group). aAdministered 0.5 h before thermal stimulus at 55±1°C. *p<0.05, **p<0.01 vs. control mice. # p<0.05 vs. morphine-treated mice.
Table 1.
The effect of clove oil and paracetamol on brewer's yeast-induced fever in mice
| Group | Control | Paracetamola | Clove oila | ||
|---|---|---|---|---|---|
| Dose (mg/kg) | – | 100 | 33 | ||
| Initial body temperature | 0 h | 37.4±0.21 | 37.1±0.22 | 37.2±0.12 | |
| Post-yeast temperature | 17 h | 39.2±0.25b* | 39.5±0.12b** | 39.0±0.17b** | |
| 0.5 h | 39.1±0.20b* | 37.4±0.30c# | 37.6±0.37c# | ||
| Rectal | % if | 4.34 | 3.96 | ||
| temperature (C°) | 1 h | 39.2±0.22b** | 36.7±0.24c## | 37.2±0.58c# | |
| Post-drug treatment | % if | 6.40 | 5.18 | ||
| 2 h | 39.0±0.23b** | 36.6±0.17c## | 36.3±0.32c## | ||
| % if | 6.03 | 6.86 | |||
| 3 h | 38.9±0.19b* | 36.3±0.27c## | 37.0±0.29c## | ||
| % if | 6.88 | 5.09 | |||
Values are expressed as mean±SEM rectal temperature (n=6 each group). ANOVA followed by Tukey's multiple comparison tests.
Administered 18 h after yeast injection
compared to 0 h (paired t-test)
compared to control group; % if=% inhibition of fever.
p<0.01
p<0.001
p<0.01
p<0.001 vs. control values.
Results
Anti-inflammatory activity
In control mice, carrageenan induced significant swelling of the mouse paw, which reached a maximum thickness of 22.3 (SEM±3.9) mm in 3 h (86.2% increase compared with before carrageenan injection, (p<0.01, Fig. 1), and gradually declined over the next hours (data not shown). The clove oil injected 30 min before carrageenan led to considerable inhibition of the induced edema. Clove oil significantly suppressed the increase in paw thickness by 50.6% (p<0.05) compared with control mice (Fig. 1). Indomethacin produced a greater edema inhibition (70.4% suppression, p<0.01) than that of clove oil compared with control group. The effect of indomethacin was not significantly different (p>0.05) from that of clove oil within the same period.
Antinociceptive activity
Acetic-acid-induced writhing in mice
In all treatment groups, mice showed no sign of abdominal constriction before acetic acid injection, among all controls and all clove-oil-treated animals. Twenty minutes after injection with acetic acid, mice that had received normal saline showed obvious abdominal constrictions (94.8±9.79) as judged by the number of writhing response related to that before injection. Compared with the control group, mice treated with clove oil exhibited fewer writhing responses (12.3±1.28 writhes). As Fig. 2 shows, clove oil significantly suppressed the development of acetic-acid-induced abdominal constrictions and stretching of hind limbs by 87.7% (p<0.01). The reference drug, aspirin, also effectively suppressed the number of writhes significantly by 77.7% (p<0.01) compared with control mice (Fig. 2). In this nociception model, the analgesic effect of clove oil was more prominent, and was significantly higher than that produced by aspirin (12.3 vs. 21.2 writhes, respectively, p<0.05).
Thermally induced pain in mice
The results of the hot plate test are presented in Fig. 3. Treatment with clove oil resulted in significant analgesic activity (p<0.05) compared with control group. The analgesic effect produced by clove oil was characterized by a considerable increase in the latency of the reaction to heat sensation from 1.1±1.77 sec to 6.2±1.39 sec (82.3% analgesia). The reference drug morphine also significantly delayed the reaction time by 91.7% (p<0.01) compared with control mice. The antinociceptive effect elicited by morphine was significantly stronger than that produced by clove oil (13.3±2.53 sec vs. 6.2±1.39 sec, respectively, p<0.05; Fig. 3).
Antipyretic activity
The effects of clove oil and paracetamol on yeast-induced pyrexia in mice are shown in Table 1. The s.c. injection of an aqueous suspension of brewer's yeast significantly increased the rectal temperature by 0.7°C after 17 h of administration. Control mice displayed an increase in rectal temperature from 37.4±0.21°C to 39.2±0.25°C (p<0.001). Mice treated with clove oil and paracetamol (positive control) exhibited significantly decreased rectal temperature compared with the control group. Clove oil showed significant suppression in rectal temperature by 2.7°C, from 39.0±0.17°C to 36.3±0.32°C (p<0.001), and the percentage inhibition of fever was 6.9%. The antipyretic effect of clove oil started 30 min after injection of the oil (p<0.01) and the reduction in rectal temperature was maintained for 3 h (p<0.001). Paracetamol also induced a significant reduction in rectal temperature of 3.2°C, from 39.5±0.12°C to 36.23±0.27°C, and the percentage inhibition of fever was 6.9% (p<0.001) for respective group. There was no significant difference between the antipyretic effect of clove oil and that produced by paracetamol. Normo-thermic mice did not show a decrease in rectal temperature after administration of clove oil (data not shown).
LD50 assessment
Table 2 shows that the median (i.p.) LD50 of clove oil was 161.9 mg/kg with a 95% Cl of 106.9–244.9 mg/kg. Mice treated with up to 50 mg/kg clove oil and observed for 96 h did not show abnormal symptoms, consistent with no decrease in food intake (data not shown). Furthermore, no deaths were observed. These data indicate that the toxicity of the clove oil is limited. Injection of doses ≥90 mg/kg body weight produced various observable effects, including in motor activity and respiration, within 45 min. Mice showed ataxia, sedation and decreased motor activity, piloerection, tremor, ptosis and 25% mortality. Also, a dose-dependent mortality was observed after increasing the doses: 292 mg/kg clove oil was 100% lethal (Table 2).
Phytochemical test
The color test used in this study showed the formation of a blue–green color, signifying the presence of the phenolic substance, eugenol, in the clove oil.
Discussion
It is well known that several mechanisms are involved in analgesic activity. The antinociceptive property of clove oil was examined using different mouse models. i.p. injection of acetic acid produces algesia by increasing endogenous substances, including prostaglandins in peritoneal fluids, such as PGF2α and PGE2, as well as histamine and serotonin. This model is generally used for assessment peripheral analgesics (18). The use of the thermal method is deemed to be selective for opioid-like substances, which are centrally acting analgesics in different animal models. It is commonly used particularly for evaluation of central pain at the supraspinal and spinal levels, since it encompasses neurogenic and central mechanisms of nociception (19). Our results showed that clove oil considerably inhibited acetic-acid-induced writhing in mice and increased pain latency time (the neurogenic pain) caused by thermal stimuli. These results indicated that clove oil possessed analgesic activity. In addition, the present study showed that clove oil has antipyretic and anti-inflammatory effects in experimental animals with a reasonable safety profile. These findings give a rational authentication for their traditional indications.
Carrageenan-generated paw edema is an established well-known experimental model for acute inflammation. This model has significant predictive value for evaluation of antiedematogenic compounds acting by interfering with the inflammatory mediators (14). Carrageenan as a phlogistic agent is non-antigenic and is devoid of apparent systemic activity (20). So far, several investigators have demonstrated that acute edema inflammation, due to carrageenan injection, has biphasic upshots. Histamine and serotonin trigger the first phase (21, 22), while, the later phase of inflammation in which the edema reaches its highest degree is elicited mostly by the release of prostaglandins (21, 22). It has been shown that prostaglandins encourage the formation of the inflammatory exudates during tenderness. This demagogic effect of prostaglandins is considerably attenuated by the use of non-steroidal anti-inflammatory drugs (NSAID) (23). Our observation that clove oil effectively suppressed this acute inflammation indicated that it has antiedematogenic activity. However, the mechanisms by which clove oil induced suppression of edema formation are not known at present. Although it cannot be excluded that abrogation of one of the two inflammatory mediators, histamine and serotonin, is responsible for the anti-inflammatory activity of clove oil, our observation that clove oil ameliorated edema formation during the late phase, 3 h after carrageenan injection, demonstrates that the effectiveness of clove oil is probably mediated through its interference with prostaglandins.
The usual classification of the antinociceptive drugs is based on their mechanism of actions, either on the central nervous system (CNS) or on the peripheral pathway. In this study, two different animal models were used with the goal of identifying potential peripheral or central effects of clove oil. Acetic-acid-induced abdominal constriction, as a model of visceral pain, is a highly sensitive method able to reveal the antinociceptive potential of drugs at doses that might appear inactive in other pain reliever patterns. So, this model is commonly used for the assessment of the peripheral pathway of analgesic drugs. Previous studies have demonstrated that acetic acid stimulates the pain nerve endings and induces contraction of abdominal muscle via the sensitization of the nociceptive receptor to the peripherally released prostaglandins, in particular PGE2α and PGF2α (18). It has been shown that inhibition of prostaglandins by aspirin and other related NSAIDs is involved in the protection against induction of pain (23). Therefore, although the mechanism of clove oil in this study is not clear, it seems likely that inhibition of prostaglandins contributes to its effectiveness in protecting mice from visceral pain. Thus, this and the observation that clove oil, when given before carrageenan, appeared to inhibit the late phase of inflammation, indicates that inhibition of prostaglandins peripherally is a mechanism by which clove oil induces its pharmacological effect. This conclusion is further supported by our findings that clove oil effectively inhibited yeast-induced fever in a mouse model of hyperpyrexia.
It is well known that NSAIDs usually do not raise the pain threshold in normal tissues, while narcotic drugs do (24). Studies have demonstrated that pain generated by a thermal stimulus is mediated centrally (19, 25). In this model, the effects of drugs on latency time of jumping responses or licking of the animal's front paws represents the analgesic effect on sensory receptor stimulation. Therefore, our observation that clove oil prolonged the reaction time, like morphine, demonstrates that the analgesic effect of clove oil was in part mediated centrally. These findings are in agreement with other reports illustrating that the analgesic effect of clove oil is CNS dependent (26). Our observation that higher doses of clove oil had a sedative effect might also explain the central effect observed in the hot plate test.
The present study shows that clove oil has antipyretic activity in mice made febrile by the use of dried yeast. A number of studies have demonstrated that yeast-induced pyrexia is a pathogenic fever (27, 28). In the hypothalamus, the production of prostaglandins, due to fever induction, sets the thermoregulatory center at a higher temperature. It is well known that NSAIDs decrease body temperature by inhibiting prostaglandin synthesis within the hypothalamus (29, 30). Therefore, we suggest that the antipyretic effect of clove oil in our model is mediated by inhibition of prostaglandin biosynthesis in hypothalamus.
The oil was devoid of signs of toxicity when given to mice. Though chronic toxicity was not studied here, absence of acute destructiveness symptoms explains its widespread use in folk medicine. Since the estimated i.p. LD50 of clove oil was 161.90 mg/kg, a five fold higher than the effective dose, it indicates that the dose required to produce the therapeutic effects is acceptably lower the toxic dose. Previous studies have demonstrated that eugenol, the major constituent of clove oil, has anti-inflammatory activity in rats, peripheral but not central analgesic effects in mice (31), and antipyretic activity in rabbits (9). In vitro experiments also showed that eugenol inhibits COX-2 enzyme in the macrophage cell line RAW264.7 (32). In addition, eugenol has been reported to inhibit prostaglandin synthesis in blood platelets (8). Hence, it seems likely that the peripheral potential effects of clove oil were attributed to their eugenol content, while the CNS effects is indorsed by other active ingredients found in the oil (7).
The present study has a few limitations. First, the use of the i.p. route of administration may limit extrapolation of our results since clove oil is not commonly used by this route. The second limitation is that because the experiments were performed a long time ago, new developments in this field are not taken into consideration. However, the present study gives clear evidence on some pharmacological properties of clove oil.
In summary, our data demonstrate that clove oil has valuable pharmacological properties in mice. We showed that pain, fever and acute inflammation, induced experimentally in mice, were considerably ameliorated by the use of clove oil. The effective dose of clove oil was sufficiently below the LD50 value. These findings need further experiments to elucidate the underlying mechanisms involved in the pharmacological effects of clove oil.
Acknowledgement
Part of the current data was extracted from a pharmacy student thesis (academic year 2002–2003). The authors thank the Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Tripoli University, Libya, for granting access to their facilities to conduct this research.
Conflict of interest and funding
All authors declare that no competing interests exist.
References
- 1.Milind P, Khanna D. Clove: a champion spice. IJRAP. 2011;2:47–54. [Google Scholar]
- 2.Kouidhi B, Zmantar T, Bakhrouf A. Anticariogenic and cytotoxic activity of clove essential oil (Eugenia caryophyllata) against a large number of oral pathogens. Ann Microbiol. 2010;60:599–604. [Google Scholar]
- 3.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharma. 2005;49:725–31. [PubMed] [Google Scholar]
- 4.Chaieb K, Zmantar T, Ksouri R, Hajlaoui H, Mahdouani K, Abdelly C, et al. Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical Candida species. Mycoses. 2007;50:403–6. doi: 10.1111/j.1439-0507.2007.01391.x. [DOI] [PubMed] [Google Scholar]
- 5.Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S, et al. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res. 2007;21:989–94. doi: 10.1002/ptr.2179. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Lee JS, Woo ER, Kim MK, Yang BS, Yu YG, et al. Isolation of virus-cell fusion inhibitory components from Eugenia caryophyllata . Planta Med. 2001;67:277–9. doi: 10.1055/s-2001-11993. [DOI] [PubMed] [Google Scholar]
- 7.Öztürk A, Özbek H. The anti-inflammatory activity of Eugenia caryophyllata essential oil: an animal model of anti-inflammatory activity. Eur J Gen Med. 2005;2:159–63. [Google Scholar]
- 8.Sharma JN, Srivastava KC, Ee KG. Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology. 1994;49:314–8. doi: 10.1159/000139248. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Lipton JM. Eugenol: antipyretic activity in rabbits. Neuropharmacology. 1987;26:1775–8. doi: 10.1016/0028-3908(87)90131-6. [DOI] [PubMed] [Google Scholar]
- 10.Okechukwu PN, Ikujuni CM. The study of anti-inflammatory, anti-pyretic and anti-nociceptive activities of extract from leaves of Labisia pumila . Inter J Pharma Toxi Sci. 2012;2:12–27. [Google Scholar]
- 11.Narkhede MB, Ajmire PV, Wagh AE. Evolutions of antinociceptive and anti-inflammatory activity of ethanol extract of Murraya paniculata leaves in experimental rodents. Int J Pharm Pharm Sci. 2012;4:247–50. [Google Scholar]
- 12.Kotob FT, Yehia AM, Saleh MT. Colour reactions for some important volatile oil constituents. In: Kotob FT, Yehia AM, Saleh MT, editors. Pharmacognosy and natural product, practical manual. 1st ed. Tripoli (Libya): Faculty of Pharmacy, AL-Fateh University; 1982. pp. 69–70. [Google Scholar]
- 13.Kumar A, Lakshman K, Velmurugan C, Sridhar SM, Gopisetty S. Antidepressant activity of methanolic extract of Amaranthus Spinosus . Basic Clin Neurosci. 2014;5:11–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Taher YA. Antinociceptive activity of Mentha piperita leaf aqueous extract in mice. Libyan J Med. 2012;7 doi: 10.3402/ljm.v7i0.16205. 16205, doi: http://dx.doi.org/10.3402/ljm.v7i0.16205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valarmathi R, Rajendran A, Akilandeswari S, Senthamarai R. Study on antipyretic activity of a Mollugo pentaphylla Linn in albino mce. Inter J Pharm Tech Res. 2010;2:2388–90. [Google Scholar]
- 16.Weil CS. Tables for convenient calculation of median effective dose (LD50 or ED50) and instruction in their use. Biometrics. 1952;8:249–63. [Google Scholar]
- 17.AL-Sultan SI, Yehia AH. Acute toxicity of Euphorbia heliscopia in rats. Pak J Nutr. 2006;5:135–40. [Google Scholar]
- 18.Deraedt R, Jouquey S, Delevallee F, Flahaut M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;61:17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 19.Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. 1963;13:502–7. [PubMed] [Google Scholar]
- 20.Chakraborty A, Devi RK, Rita S, Sharatchandra K, Singh TI. Preliminary studies on anti-inflammatory and analgesic activities of Spilanthes acmella in experimental animal models. Indian J Pharma. 2006;36:148–50. [Google Scholar]
- 21.Crunkhorn P, Meacock SC. Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol. 1971;42:392–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 23.Inger L, Meek II, Mart AF, Harald EV. Non-steroidal anti-inflammatory drugs: an overview of cardiovascular risks. Pharmaceuticals. 2010;3:2146–62. doi: 10.3390/ph3072146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679–84. [PubMed] [Google Scholar]
- 25.Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol. 1977;69:167–71. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- 26.Hosseini M, Mina K, Hassan R. Analgesic effect of clove essential oil in mice. Avicenna J Phytomed. 2011;1:1–6. [Google Scholar]
- 27.Akio M, Tomoki N, Tatsuo W, Takuya O, Naotoshi M. Pattern differences in experimental fevers induced by endotoxin, endogenous pyrogen and prostaglandins. Am J Physiol. 1988;254:633–40. doi: 10.1152/ajpregu.1988.254.4.R633. [DOI] [PubMed] [Google Scholar]
- 28.Howard M. Fever: causes and consequences. Neurosci Biobehav Rev. 1993;17:237–69. doi: 10.1016/s0149-7634(05)80009-0. [DOI] [PubMed] [Google Scholar]
- 29.Clark WG, Cumby HR. The antipyretic effect of indomethacin. J Physiol. 1975;248:625–38. doi: 10.1113/jphysiol.1975.sp010992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tekoa LK, Elissa LM. Analgesia and anesthesia. In: Tekoa LK, Mary CB, editors. Text book of Pharmacology for women's health. 1st ed. London (UK): Jones and Bartlett; 2011. pp. 310–43. [Google Scholar]
- 31.Daniel AN, Simone MS, Gustavo S, Silvana MC, Ciomar AB, Roberto KN. Anti-inflammatory and antinociceptive activities of eugenol essential oil in experimental animal models. Braz J Pharmacogn. 2009;19:212–17. [Google Scholar]
- 32.Kim SS, Oh OJ, Min HY. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003;73:337–48. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]