Abstract
A 58-year-old woman with a history of statin use presented with a 4-month history of progressive weakness of both shoulders and thighs. Laboratory and electromyography testing confirmed the presence of generalized proximal myopathy and ruled out connective tissue disease, malignancy, or active viral infection. Muscle biopsy was consistent with necrotizing autoimmune myopathy.
Keywords: statin, necrotizing myopathy, autoimmune, 3-hydroxy-3-methylglutaryl-coenzyme A reductase, antibody
Statins are among the most commonly prescribed medications worldwide and their use is expected to grow (1, 2). Statin use is associated with a wide spectrum of muscular disorders, the majority of which are due to direct myotoxicity and typically resolve with drug discontinuation (3). A novel immunogenic syndrome in which statin use triggers a 200/100 kDa 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibody response has recently been described (4, 5). The disease continues to progress despite drug withdrawal and requires therapy with immunosuppressive agents (3, 6, 7). Despite its rarity, with the exponential use of statins, many physicians are likely to encounter the syndrome.
We hereby describe a case of a 58-year-old woman with a history of statin use who presented with a 4-month history of progressive weakness of both shoulders and thighs. Symptoms progressed despite discontinuation of statin. Laboratory and electromyography testing confirmed the presence of generalized proximal myopathy and ruled out connective tissue disease, malignancy, or active viral infection. Muscle biopsy was consistent with necrotizing autoimmune myopathy. Her condition improved with immunosuppressive therapy. We discuss the pathogenesis and the therapeutic options for this condition.
Case report
A 58-year-old woman presented with a 4-month history of progressive weakness of the shoulders and thighs. At that point, she reported inability to walk up stairs or lift heavy objects. She had to pick up her legs to get out of the car. The patient could still open jars and use utensils. She denied any fever, chills, weight loss, anorexia, joint pain, dysphagia, Raynaud's phenomenon, skin rash, and heat or cold intolerance.
The patient's medical history was significant for hyperlipidemia which was initially treated with atorvastatin 40 mg daily for over a year. It was replaced with simvastatin 20 mg daily due to muscle pain. She was on simvastatin for 8 months when it was discontinued secondary to recurrence of muscle pain and weakness. Her past medical history also included diabetes mellitus type 2, hypertension, and hyperthyroidism. The latter was treated with radioactive ablation over 30 years ago. Her home medications at presentation were alprazolam, aspirin, glipizide, metformin, hydrochlorothiazide, lisinopril, and levothyroxine. She had a 43-pack-year smoking history but denied alcohol or drug use. The patient had no family history of autoimmune or neuromuscular diseases.
On physical examination, she had symmetric muscle weakness scoring 2 out of 5 on hips and 4 out of 5 on shoulders. She was unable to sit or stand without support. The muscle strength of neck, elbows, wrists, hands, knees, and ankles were normal. Deep tendon reflexes were intact with no sensory loss. There was no muscle atrophy or fasciculation. She had no skin rash or active arthritis.
Laboratory studies showed significantly elevated creatine phosphokinase (CPK) of 7,562 units/L (normal range 26–192 units/L) with normal thyroid-stimulating hormone of 1.9 µIU/mL (normal range 0.5–5.0 µIU/mL) and erythrocyte sedimentation rate of 19 (normal<20). Electromyography was performed on right upper and lower extremities and showed myopathic changes in the deltoid, hip flexors, and extensors.
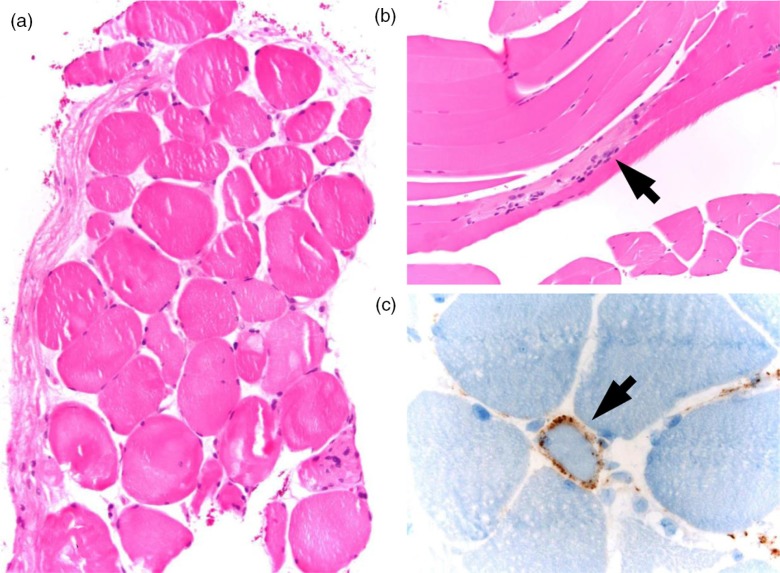
The patient subsequently underwent biopsy of the left quadriceps. Pathology showed abnormal variation in myofiber size (Fig. 1a) with pattern of degenerating and regenerating fibers (Fig. 1b). There were no rimmed vacuoles or inflammatory infiltrates. Immunohistochemical stain showed reactivity for major histocompatibility complex 1 (MHC-1) in 3–5% myofibers. The membrane attack complex (C5b-9) was positive in endomysial capillaries in a speckled pattern (Fig. 1c). The overall picture of marked necrosis of myofibers without inflammatory infiltrate strongly favored necrotizing autoimmune myopathy. Tests for autoantibodies were negative, including antinuclear antibody, rheumatoid factor, anti-Ro/SSA, anti-La/SSB, and anti-Jo-1. The myositis panel was negative for PL-7 Ab, PL-12Ab, EJ Ab, OJ Ab, SRP Ab, Mi-2 Ab, and Ku Ab. The patient was also seronegative for hepatitis A, B, and C infections. A computerized CT scan of the chest, abdomen, and pelvis was unremarkable for malignancy. The clinical diagnosis of statin-associated necrotizing myopathy was made given her history of statin exposure and the lack of evidence of connective tissue disease, active viral infection, or malignancy.
Fig. 1.
Muscle biopsy of left quadriceps. (a) Evidence of muscle fiber atrophy with abnormal variation in myofiber size. Note the absence of inflammatory infiltrates. (b) Regenerating muscle fibers (arrow). (c) Positive stain of membrane attack complex (arrow) in endomysial capillaries.
The patient was started on prednisone 60 mg daily which tapered down to 10 mg in 4 months. She was also given methotrexate up to 25 mg weekly. However, the patient's weakness was refractory to treatment with CPK level of 3,553 U/L at 4 months. Immunosuppressive therapy was escalated to azathioprine 100 mg daily then mycophenolate mofetil up to 3,000 mg, also without significant improvement at 8 months. Finally, the patient received rituximab 1,000 mg for two doses which resulted in dramatic improvement in her muscle strength, functional status, and CPK levels. The latter came down to a level of 751 units/L within 2 months after rituximab. After rituximab therapy, the patient's upper limb strength was back to baseline by 1 month and she was able to walk up stairs by 3 months’ time. She maintained her remission with prednisone 10 mg and mycophenolate mofetil 3,000 mg.
Discussion
Since the first commercial statin was introduced in 1982, this class of medications has gained widespread support in use for cardiovascular risk reduction (8). Rigorous evidence presented by the 2013 American College of Cardiology/American Heart Association Blood Cholesterol Treatment Task Force further solidified its role as the only cholesterol-lowering agent that showed mortality benefit so far (9). The new guideline is expected to expand the statin-eligible population in the United States from 43 to 56 million, a total increase of 13 million (2). This number further approaches 1 billion worldwide (1). Given the massive scale of statin use, adverse reactions are common and frequently encountered in the primary care setting. The spectrum of statin side effects include myalgia, myositis, abnormal elevation of liver enzymes, and overt rhabdomyolysis (3, 9). The majority of muscle damage by statins is secondary to its direct toxic effects, possibly via coenzyme Q10 depletion causing mitochondrial dysfunction. The injury is dose dependent and reversible after withdrawing the drug, with most recovery of symptoms occurring within 2–3 months (3).
However, a different form of statin myopathy has been identified in recent years. Few case series identified groups of patients with prior exposure to statins who developed progressive muscle weakness and elevated CPK levels despite discontinuing the medications (7, 10). Muscle biopsy typically shows necrotizing myopathy characterized by muscle fiber degeneration, regeneration, and necrosis with minimal inflammation (11). MHC-1 staining and membrane attack complexes can be seen on non-necrotic muscle fibers (12). Most of these patients do not have underlying connective tissue disease. Malignancy and active viral infection such as hepatitis C should also be excluded. A screening myositis panel would be negative for antisynthetase antibodies including antisignal recognition particle (anti-SRP) (11). Christopher-Stine et al. discovered a novel antibody directed against proteins weighing 200 and 100 kDa in these patients (4). The antibody was eventually found to target the HMGCR (5). It was proposed that statins cause increased expression of HMGCR protein and an aberrant antigen recognizing process leading to the production of this autoantibody (5). Despite withdrawal of the statin, the overexpression of HMGCR in regenerating muscle cells sustains the immune response, further propagating the myopathy until immunosuppressive therapies are used to break the vicious cycle. Blood testing for HMGCR antibodies is not available commercially and is still limited to research purposes. Unfortunately, our patient was unable to get tested.
Treating patients with statin-associated necrotizing myopathy often require multiple immunosuppressive agents (6, 7, 10). Currently, there is no standard approach to therapy, but prednisone starting at 1 mg/kg/day with methotrexate is the most commonly used initial treatment (10, 11). For refractory patients, second-line choices include azathioprine, mycophenolate mofetil, cyclosporine, intravenous immunoglobulin, and rituximab (6, 7). In a 25 patient case series described by Grable-Esposito et al., 44% of patients had complete or near complete response to immunosuppression, and all patients had at least partial response (7). Long-term treatment is required due to frequent relapses upon tapering of immunosuppressants (6, 7). Our patient was difficult to treat as she failed prednisone, methotrexate, and azathioprine combination. However, she had a dramatic response to rituximab infusion and achieved complete remission in symptoms. The use of rituximab in refractory statin-associated necrotizing myopathy is promising but still awaits evaluation by well-designed clinical trials, a task limited by the rarity and poor awareness of the disease (11).
Conclusion
The benefits of statins are well established in reducing cardiovascular events of at risk populations (9). A novel myopathy mediated by HMGCR autoantibodies has been identified (5). It is characterized by muscle necrosis without inflammatory infiltrates. The condition should be suspected in patients who develop progressive muscle weakness and CPK elevation despite discontinuing statins for 2–3 months. Diagnosis is made by 1) proximal muscle weakness occurring during or after treatment with statins, 2) elevated serum CPK, 3) persistence of weakness and elevated CPK after discontinuation of statin, 4) muscle biopsy showing necrotizing myopathy without significant inflammation, and 5) absence of connective tissue, neoplastic, or viral disease (7, 11). Besides stopping statin use, immunosuppressive therapy is required for treatment. Rituximab can be considered in refractory cases (11). Further controlled studies are needed to define the optimal treatment approach.
Acknowledgements
We are indebted to Dr. Kevin Jenkins for review of the manuscript.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to reporting this case.
References
- 1.Ioannidis JPA. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2013;94305:E1. doi: 10.1001/jama.2013.284657. [DOI] [PubMed] [Google Scholar]
- 2.Pencina MJ, Navar-Boggan AM, D'Agostino RB, Williams K, Neely B, Sniderman AD, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422–31. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 3.Mohassel P, Mammen AL. The spectrum of statin myopathy. Curr Opin Rheumatol. 2013;25(6):747–52. doi: 10.1097/01.bor.0000434673.85515.89. [DOI] [PubMed] [Google Scholar]
- 4.Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–66. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme a reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padala S, Thompson PD. Statins as a possible cause of inflammatory and necrotizing myopathies. Atherosclerosis. 2012;222(1):15–21. doi: 10.1016/j.atherosclerosis.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41(2):185–90. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 8.Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(5):484–93. doi: 10.2183/pjab.86.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes GH, Zanoteli E, Shinjo SK. Statin-associated necrotizing autoimmune myopathy. Mod Rheumatol. 2014;24:862–4. doi: 10.1007/s10165-013-0837-8. [DOI] [PubMed] [Google Scholar]
- 11.Liang C, Needham M. Necrotizing autoimmune myopathy. Curr Opin Rheumatol. 2011;23:612–19. doi: 10.1097/BOR.0b013e32834b324b. [DOI] [PubMed] [Google Scholar]
- 12.Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord. 2007;17(2):194–200. doi: 10.1016/j.nmd.2006.10.007. [DOI] [PubMed] [Google Scholar]