Abstract
Prenatal alcohol exposure is associated with behavioral disinhibition, yet the brain structure correlates of this deficit have not been determined with sufficient detail. We examined the hypothesis that the structure of the anterior cingulate cortex (ACC) relates to inhibition performance in youth with histories of heavy prenatal alcohol exposure (AE, n = 32) and non-exposed controls (CON, n = 21). Adolescents (12–17 years) underwent structural magnetic resonance imaging yielding measures of gray matter volume, surface area, and thickness across four ACC subregions. A subset of subjects were administered the NEPSY-II Inhibition subtest. MANCOVA was utilized to test for group differences in ACC and inhibition performance and multiple linear regression was used to probe ACC-inhibition relationships. ACC surface area was significantly smaller in AE, though this effect was primarily driven by reduced right caudal ACC (rcACC). AE also performed significantly worse on inhibition speed but not on inhibition accuracy. Regression analyses with the rcACC revealed a significant group × ACC interaction. A smaller rcACC surface area was associated with slower inhibition completion time for AE but was not significantly associated with inhibition in CON. After accounting for processing speed, smaller rcACC surface area was associated with worse (i.e., slower) inhibition regardless of group. Examining processing speed independently, a decrease in rcACC surface area was associated with faster processing speed for CON but not significantly associated with processing speed in AE. Results support the theory that caudal ACC may monitor reaction time in addition to inhibition and highlight the possibility of delayed ACC neurodevelopment in prenatal alcohol exposure.
Keywords: Fetal alcohol spectrum disorders (FASD), Fetal alcohol syndrome (FAS), Prenatal alcohol exposure, Anterior cingulate cortex, Response inhibition, Structural neuroimaging
1. Introduction
Gestational alcohol exposure can result in adverse physical, neurological, cognitive, and behavioral consequences for the developing embryo and fetus. Fetal alcohol syndrome (FAS), a phenotype defined by a pattern of craniofacial dysmorphia, growth deficiencies, and central nervous system abnormalities is one of the more severe outcomes of prenatal alcohol exposure [1,2]. However, neurobehavioral deficits may be present even among individuals without all of the features required for FAS [3,4]. The non-diagnostic umbrella term fetal alcohol spectrum disorders (FASD) encompasses the broad range of clinical outcomes associated with prenatal alcohol exposure [5].
Impaired executive functioning, a construct including inhibitory control, cognitive flexibility, working memory, and sustained and selective attention [6] is thought to be a key feature of the neurobehavioral profile of FASD [7]. Here we focus on response inhibition, one aspect of executive functioning, conceptualized as the voluntary suppression of an automatic or impulse-driven action. On neuropsychological measures of response inhibition, prenatally alcohol-exposed individuals tend to demonstrate poor performance. For example, alcohol-exposed children were slower to complete inhibition tasks and made a greater number of inhibition errors relative to non-exposed controls [8] and also as compared to the normative mean score derived from the measure’s standardization sample [9]. Significant inhibition deficits have been documented over and above deficits in component skills such as word reading or color naming [8] and may not simply be attributable to lower IQ [10]. Analogous inhibitory deficits have also been reported in animal models of FASD [11–14].
Inhibitory control is critical for successful self-regulation and the completion of non-impulsive goal oriented behavior [15]. Disinhibition in childhood may lead to difficulties with social skills [16], academic functioning [17], and adolescent alcohol use [18]. Compromised impulse control may also contribute to the higher rates of disruptive behavior disorders [19] and attention-deficit/hyperactivity disorder (ADHD) [20,21] observed in alcohol-exposed children. While such problems are prominent in FASD, observations indicate that there may be treatment resistance or atypical treatment response to psychostimulant medications typically used to target impulsivity and related attention problems in children [22]. Understanding the neural underpinnings of inhibition in FASD may lead to the development of better treatment strategies for alcohol-exposed children with inhibitory deficits.
Neuroimaging studies of inhibition often focus on the anterior cingulate cortex (ACC), a medial region in the frontal cortex thought to be responsible for inhibitory processing and conflict/error monitoring. This is supported by a robust literature detailing increased ACC functional activation during inhibition tasks in healthy adults (e.g., [23–27]), typically developing children [28,29] and children with disorders that involve prominent impulse control difficulties such as ADHD [30], autism [31] and disruptive behavior disorders [32]. There is also mounting evidence that the structure of the ACC relates to inhibitory abilities. For example, Takeuchi and colleagues [33] demonstrated that greater ACC volumes were associated with better performance (speed and accuracy combined) on a Stroop interference task in healthy young adults. In healthy children, the sulcal pattern of the ACC predicted inhibitory speed later in development [34] but had no effect on inhibitory accuracy. ACC structure is also associated with children’s cognitive control, a broader executive functioning construct that encompasses response inhibition. For instance, youth with larger right ACCs were faster and more accurate on a task of controlled attention performance [35]. Moreover, ACC cortical surface area accounted for a significant proportion of variance in typically-developing children’s cognitive control performance and greater surface area was related to faster performance for younger children (<12 years old) [36].
Neuroimaging studies of response inhibition in FASD have almost exclusively focused on the functional correlates of inhibition. In this vein, two functional magnetic resonance imaging (fMRI) studies of inhibitory control using a go-no go task found increased blood oxygen level dependent (BOLD) response in the ACC and surrounding prefrontal and parietal regions, suggesting inefficient or immature processing in inhibitory fronto-parietal networks [37,38]. A more recent investigation using the stop signal task explored BOLD activation to inhibition conditions of varying difficulty in alcohol-exposed and non-exposed adolescents. Group differences in ACC and middle cingulate brain response were specific to the most difficult and demanding inhibition trials [39]. In addition, inhibition accuracy on the stop signal task was positively related to BOLD activation in the middle cingulate in both groups [39].
Although structural alterations in gray matter volume [40], white matter microstructure [41], and neuroanatomical maturation [42] have been reported in alcohol-exposed children, to our knowledge only one study to date has focused on the structural correlates of inhibitory control. Bjorkquist and colleagues examined the structure of the cingulate gyrus and found reduced gray and white matter volumes in alcohol-exposed children [43]. However, after adjusting for total brain volume to account for microcephaly associated with prenatal alcohol exposure, only cingulate white matter remained significantly reduced. Importantly, volumes analyzed in this study were parcellated into anterior and posterior anterior cingulate only, and thus may have been less sensitive to smaller subregional differences within the ACC. In addition, other structural variables such as cortical thickness and surface area were not examined. In cortical brain regions such as the ACC, volume is the product of cortical thickness and cortical surface area. Thickness and surface area are thought to be phenotypically and genetically independent [44–46], follow distinct developmental trajectories, and reach peak size at different stages of childhood and adolescence [47]. Thus, in measuring volume, signal from these independent metrics may be diluted and more subtle structural differences may go undetected.
The neurobehavioral effects of poor inhibition may be widespread in children with prenatal alcohol exposure, yet the neural basis underlying this deficit has not been adequately explored. We sought to examine whether the structure of the ACC related to behavioral performance on a standardized neuropsychological measure of inhibitory control in adolescents with prenatal alcohol exposure. While the majority of structural neuroimaging studies in FASD have focused on volumetric differences, we chose to examine volume, cortical surface area, and cortical thickness variables to gain a more comprehensive understanding of ACC neural structure.
On neuropsychological measures, we expected alcohol-exposed youth to demonstrate poorer inhibition completion time and inhibition error scores compared to non-exposed children, consistent with previous findings [8]. For the neuroimaging measures, we hypothesized that alcohol-exposed adolescents would have reduced ACC volume compared to non-exposed controls, as frontal lobe size reductions are a consistent finding in FASD [40]. This hypothesized difference in volume could be due to underlying differences in ACC surface area, ACC cortical thickness, or both. In light of recent findings suggesting that prenatal alcohol exposure impacts surface area to a greater degree than cortical thickness [48] as well as inconsistent reports of cortical thickness alteration in FASD (e.g., [49,50]), we predicted that alcohol-exposed adolescents would have reduced ACC area but not thickness. Finally, we expected that smaller ACCs would be associated with worse inhibition time and accuracy in both groups, as smaller ACC volume and area have been associated with poorer cognitive control in typically developing children.
2. Methods
2.1. Subjects
Fifty-five youth ages 12–17 years (M = 15.0, SD = 1.3) with (AE; n = 32) and without (CON; n = 21) histories of heavy prenatal alcohol exposure participated in this study as part of a larger ongoing project at the Center for Behavioral Teratology at San Diego State University. Subjects and their primary caregivers were recruited via professional referral, word of mouth, and community outreach. Written informed consent was obtained from each parent or legal guardian and assent was obtained from each subject in accordance with Institutional Review Boards at San Diego State University and University of California, San Diego. A financial incentive was provided for participation.
Prenatal exposure history was assessed retrospectively through a number of sources including medical history, birth records, social services records, and maternal report when available. While precise measures of maternal alcohol intake were often unavailable, adolescents were included in the AE group if mothers were reported to have had alcohol abuse or dependence during pregnancy. If maternal report was available, heavy prenatal alcohol exposure was defined as maternal consumption of ≥4 drinks per occasion at least once a week or ≥14 drinks per week on several occasions throughout pregnancy. A dysmorphologist with expertise in FAS (KLJ) evaluated all subjects and diagnosed 8 adolescents in the AE group with FAS. An FAS diagnosis was considered sufficient documentation of prenatal alcohol exposure for inclusion in the AE group. Subjects were excluded from the CON group if there was an indication of greater than minimal alcohol exposure, defined as no more than 1 drink per week on average in pregnancy and never more than 2 drinks per occasion in pregnancy. Subjects were also excluded from the CON group if they met criteria for ADHD, based on results from the National Institute of Mental Health Diagnostic Interview Schedule for Children (C-DISC-4.0; [51]). Using the same measure, 23 (72%) of AE subjects in the total neuroimaging sample and 16 (64%) of AE subjects in subsample with neuropsychological data met criteria for ADHD.
Additional exclusionary criteria for all participants were head injury with loss of consciousness >30 min, other known causes of cognitive deficiency, physical or psychiatric conditions that would prevent participation, primary language other than English, and MRI contraindications.
2.2. Measures
2.2.1. MRI data acquisition and analysis
MRI sessions were conducted using a Signa EXCITE (GE Healthcare, USA) 3.0 T scanner. One high-resolution T1-weighted anatomical scan (fast spoiled gradient sequence, TR = 8000 ms, TE = 3.1 ms, flip angle = 12°, matrix size = 256 mm × 192 mm, field of view = 24 cm, slice thickness = 1 mm, acquisition time = 7 min, 24 s) was acquired for each participant.
Surface-based registration and anatomical parcellation were performed using Freesurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu/). Automated processing procedures included motion correction, skull stripping, intensity normalization, Talairach transformation, determination of gray matter, white matter, and cerebrospinal fluid boundaries, and parcellation of the cerebral cortex according to gyral and sulcal anatomy [52–57]. Each dataset was visually inspected to confirm successful processing. Two individuals in the AE group were excluded due to motion artifact, bringing the total neuroimaging sample to 53 subjects. The boundaries of the ACC regions of interest (ROIs) were determined automatically using the Desikan atlas [52]. Four ACC ROIs were identified in each participant: right rostral ACC (rrACC), right caudal ACC (rcACC), left rostral ACC (lrACC), and left caudal ACC (lcACC). Volume, surface area, and cortical thickness values for each ROI were extracted.
2.2.2. Neuropsychological assessment
Subjects completed the NEPSY-II Inhibition subtest and the Wechsler Intelligence Scale for Children – fourth edition (WISC-IV; [58]) as a part of a neuropsychological battery administered in a larger, ongoing study. WISC-IV Full Scale IQ (FSIQ) data was unavailable for two subjects in the AE group. A subset of adolescents (n = 41) from the total neuroimaging sample who completed the NEPSY-II Inhibition subtest within 90 days (M = 18; SD = 20) of their MRI scan were analyzed to look at potential brain-behavior relationships between the ACC and inhibition.
The NEPSY-II has been used in a variety of developmental populations, including children with FASD [59]. The inhibition subtest (IN) is comprised of three conditions: (i) the Naming condition (IN-Naming) measures speeded naming of shapes (circles and squares) and directions of arrows (up and down) and is used as a measure of processing speed; (ii) the Inhibition condition (IN-Inhibition) measures inhibitory control, the ability to inhibit the prepotent response to name the shape or direction of an arrow; and (iii) the Switching condition (IN-Switching) measures set shifting and cognitive flexibility, in combination with inhibitory control abilities, as assessed by switching between naming and inhibiting responses.
IN-Inhibition completion time (inhibition speed measured in seconds) and IN-Inhibition errors (inhibition accuracy measured in number of errors made) were of primary interest in this study. While inhibition is a component of IN-Switching, IN-Switching is a complex task, tapping multiple components of executive functioning together. By contrast, IN-Inhibition is a more pure measure of inhibition. In addition, IN-Inhibition is more similar to Stroop-type interference tasks that form the foundation of the ACC-inhibition fMRI literature. Thus, IN-Inhibition was preferred over IN-Switching to examine potential relationships between ACC structure and inhibitory control.
2.3. Statistical analysis
All statistical analyses were conducted in Stata/SE 13 (StataCorp LP, College Station, TX).
2.3.1. Subject characteristics
Age, socioeconomic status (SES; Hollingshead Four Factor Index of Social Status [60]), days between neuropsychological assessment and MRI scan, and FSIQ were compared using independent 2-sample t-tests. Sex, race, ethnicity, and handedness were compared using Pearson’s chi square tests. All demographic variables were evaluated at an alpha of .05.
2.3.2. Evaluation of covariates
Given their theoretical relationship to inhibitory control and structural brain variables, age and sex were evaluated as potential covariates. For multivariate analysis of covariance (MANCOVA) and univariate analysis of covariance (ANCOVA) analyses, interactions between the covariates and independent variables were calculated to assess homogeneity of regression assumptions. Alpha was set at .05. Results indicated no significant group × age interactions for IN-Inhibition variables or ACC variables. In addition, there were no significant group × sex interactions, although the group × sex interaction for ACC surface area variables was marginally significant (p = .07). Homogeneity of regression assumptions were met. In addition, in our sample age was negatively correlated with rrACC (R2 = .22), lrACC (R2 = .18), and lcACC (R2 = .24) cortical thickness variables (ps < .01). Sex was significantly correlated with lrACC thickness (R2 = .09) and surface area (R2 = .11) variables (ps < .05), where being male was associated with thicker cortex and larger surface area. Thus, age and sex were retained as covariates.
As smaller head size is a common physical feature in FASD, structural neuroimaging comparisons of alcohol-exposed and non-exposed children often include ICV as a covariate to account for group differences in head size. ICV directly relates to cortical volume, cortical thickness, and cortical surface area. However, there are compelling reasons to avoid controlling for ICV in studies of cortical structure. Specifically, Im and colleagues demonstrated that cortical structure does not maintain geometric similarity with scaling [61]. As ICV increases, there is a lower than expected increase of cortical volume and thickness, but a higher than expected increase in cortical surface area. Thus, linear normalization for ICV can introduce confounding group differences by either over-scaling or under-scaling cortical measurements. We chose to conduct primary analyses without ICV. Secondary analyses including ICV were used to follow up significant findings. Homogeneity of regression assumptions were met as no group × ICV interactions were found.
Finally, while FSIQ may be related to both inhibition and structural brain variables, diminished IQ is intrinsic to and cofounded with prenatal alcohol exposure – the independent variable of interest. The extent literature illustrates that attempting to remove the variability associated with a representative group characteristic is methodologically tenuous and often statistically inappropriate in the context of neurodevelopmental disorders [62]. Because our objective is to characterize group differences in inhibitory control and brain structure, controlling for IQ may produce overcorrected or anomalous findings that are not fully representative of alcohol-related neurocognitive function. Therefore, FSIQ was not included as a covariate in subsequent analyses.
2.3.3. Neuropsychological data
For the NEPSY-II IN-Inhibition variables, completion time raw score (seconds) and errors raw score (number of errors) were compared between groups using MANCOVA, followed up by separate ANCOVAs for each dependent variable when appropriate. Although standard scores (correcting for age and sex) are available for these variables, raw scores were used so as not to overcorrect for these variables in the analysis, which included age and sex as covariates.
For all MANCOVA analyses, Box’s M test of homogeneity of covariance was evaluated using an alpha of .001. Wilk’s criterion (Λ) was used as the omnibus test statistic at an alpha of .05. For all ANCOVA analyses, Levene’s test was evaluated using an alpha of .001. To control for multiple comparisons, ANCOVA F statistics were evaluated at an alpha of .05/the number of family-wise comparisons.
2.3.4. ACC structure
To examine the main effect of group three between-subjects MANCOVAs were performed on each of the following sets of dependent variables: (a) Surface Area: rrACC area, rcACC area, lrACC area, lcACC area; (b) Volume: rrACC volume, rcACC volume, lrACC volume, lrACC volume; (c) Cortical Thickness: rrACC thickness, rcACC thickness, lrACC thickness, lrACC thickness. Age and sex were included as covariates. MANCOVAs were followed up by separate ANCOVAs for each dependent variable when appropriate. ICV was included as a covariate in secondary analyses.
Significant MANCOVAs were also followed up with linear discriminant analysis to measure the relative unique contribution of each ACC subregion to the significant group difference. Standardized discriminant function coefficients were extracted for each ACC subregion. These coefficients represent standardized weights that each ACC subregion was assigned in the MANCOVA to maximize the difference between groups. A cutoff of |.30| was used to determine the practical significance of the standardized discriminant function coefficients.
2.3.5. ACC-Inhibition relationships
Two sets of multiple linear regression analyses were used to examine the IN-Inhibition completion time–ACC relationship and the IN-Inhibition errors–ACC relationship. Given the strong correlations between ACC subregion structural variables, ACC variables were included in regression analyses only if they were found to have a practically significant unique contribution to the observed group difference (standardized discriminant function coefficient >|.30|) in the MANOVA follow up. IN-Inhibition completion time and IN-Inhibition errors were entered separately as the dependent variable with main effect of group, main effect of ACC, and the group × ACC interaction as the effects of primary interest. Age and sex were included as covariates. Six models were run in total. IN-Inhibition completion time was the dependent variable in Models 1–3 (Table 4) and IN-Inhibition errors was the dependent variable in Models 4–6 (Table 5). The nature of significant group × ACC interactions was explored via calculating the marginal effect of ACC for each group (taking the partial derivative with respect to ACC). As a follow up, regression analyses were also run separately for each group to further probe group × ACC interactions. In addition, predicted inhibition scores were calculated in order to graphically represent significant interactions.
Table 4.
Regression results for IN-Inhibition completion time.
| Variables | Model (1) Main effect of Group β (SE) |
Model (2) Main effect of ACC β (SE) |
Model (3) Group × ACC interaction β (SE) |
|---|---|---|---|
| Group | 13.78* (5.75) | 90.19** (27.94) | |
| R caudal ACC area | −0.03* (0.02) | 0.04 (0.03) | |
| Group × R caudal ACC area | −0.10** (0.03) | ||
| Age | −0.75 (2.38) | −1.77 (2.37) | −1.32 (2.17) |
| Sex | −8.48 (6.25) | −4.93 (6.29) | −13.41* (6.18) |
| Constant | 69.24 (35.65) | 115.93** (36.74) | 50.15 (39.16) |
| Observations | 41 | 41 | 41 |
| R2 | 0.18 | 0.16 | 0.36 |
ACC = anterior cingulate cortex.
p < 0.05.
p < 0.01.
Table 5.
Regression results for IN-Inhibition errors.
| Variables | Model (4) Main effect of group β (SE) |
Model (5) Main effect of ACC β (SE) |
Model (6) Group × ACC interaction β (SE) |
|---|---|---|---|
| Group | 1.18 (1.23) | 0.02 (6.55) | |
| R caudal ACC area | −0.006 (0.003) | −0.006 (0.005) | |
| Group × R caudal ACC area | −0.00007 (0.008) | ||
| Age | −0.10 (0.51) | −0.19 (0.48) | −0.20 (0.51) |
| Sex | −1.95 (1.33) | −1.52 (1.28) | −1.52 (1.45) |
| Constant | 5.43 (7.61) | 11.70 (7.50) | 11.77 (9.18) |
| Observations | 41 | 41 | 41 |
| R2 | 0.08 | 0.14 | 0.14 |
ACC = anterior cingulate cortex.
2.3.6. ACC-processing speed post hoc analyses
Processing speed is an important component process underlying inhibition speed. Post hoc regression analyses were conducted to explore the role of processing speed in observed ACC-inhibition completion time relationships. IN-Naming completion time, which measures speeded naming independent of inhibition demands, was first entered as an independent variable in the original ACC-inhibition regression models. Next, a new set of regressions were conducted using IN-Naming as the dependent variable with the main effect of group, main effect of rcACC surface area, and the group × ACC interaction as the effects of primary interest (Models 6–9; Table 6). Age and sex were included as covariates. The nature of significant group × ACC interactions was explored via calculating the marginal effect of ACC for each group (taking the partial derivative with respect to ACC). Processing speed regression analyses were also run separately for each group to follow up group × ACC interactions. Predicted processing speed scores were calculated in order to graphically represent significant interactions.
Table 6.
Regression results for IN-Inhibition completion time.
| Variables | Model (7) Main effect of group β (SE) |
Model (8) Main effect of ACC β (SE) |
Model (9) Group × ACC interaction β (SE) |
|---|---|---|---|
| Group | 5.67 (2.83) | 40.97** (14.36) | |
| R caudal ACC area | −0.005 (0.008) | 0.03* (0.01) | |
| Group × R caudal ACC area | −0.04* (0.02) | ||
| Age | −1.07 (1.17) | −1.48 (1.21) | −1.10 (1.12) |
| Sex | 0.25 (3.08) | 1.35 (3.21) | −2.82 (3.17) |
| Constant | 55.82** (17.54) | 68.16** (18.73) | 33.39 (20.13) |
| Observations | 41 | 41 | 41 |
| R2 | 0.06 | 0.05 | 0.27 |
ACC = Anterior cingulate cortex.
p < 0.05.
p < 0.01.
3. Results
3.1. Subject characteristics
Groups did not differ significantly in age, sex, race, ethnicity, handedness, or socioeconomic status (see Table 1). As expected, the AE group scored lower on FSIQ (p < .001). For the subsample of participants with neuropsychological data obtained within 90 days of MRI scan, groups differed in mean time elapsed between test and scan. On average, subjects in the AE group experienced 12 days between test and scan (range 0–56 days) while those in the CON group experienced 26 days (range 1–61 days) between test and scan (p = .031). This 14-day mean difference between groups is unlikely to be practically significant.
Table 1.
Subject characteristics.
| AE M (SD) or % | CON M (SD) or % | Group difference | |
|---|---|---|---|
| Total neuroimaging sample | n = 32 | n = 21 | |
| Age [M (SD)] | 15.0 (1.3) | 15.1 (1.5) | ns |
| Sex [n (% female)] | 12 (38%) | 9 (43%) | ns |
| Handedness [n (% right handed)] | 28 (88%) | 20 (95%) | ns |
| Hollingshead [M (SD)] | 44.2 (12.8) | 48.7 (12.8) | ns |
| Race [n (% white)] | 19 (59%) | 13 (62%) | ns |
| Ethnicity [n (% hispanic)] | 11 (34%) | 6 (29%) | ns |
| FSIQ [M (SD)] | 87.1 (14.2) | 102.8 (12.1) | p < .001 |
| ADHD [n (% ADHD)] | 23 (72%) | – | |
| FAS [n (% FAS)] | 8 (25%) | – | |
| Subsample with neuropsychological data | n = 25 | n = 16 | |
| Days between test and scan [M (SD)] | 12 (17) | 26 (22) | p < .05 |
| Age [M (SD)] | 15.0 (1.1) | 15.3 (1.4) | ns |
| Sex [n (% female)] | 7 (28%) | 6 (38%) | ns |
| Handedness [n (% right handed)] | 22 (88%) | 15 (94%) | ns |
| Hollingshead [M (SD)] | 44.4 (12.7) | 48.8 (11.3) | ns |
| Race [n (% white)] | 15 (60%) | 9 (56%) | ns |
| Ethnicity [n (% hispanic)] | 8 (32%) | 3 (19%) | ns |
| FSIQ [M (SD)] | 87.6 (15.4) | 102.6 (13.9) | p < .01 |
| ADHD [n (% ADHD)] | 16 (64%) | – | |
| % FAS | 6 (24%) | – |
AE = children with prenatal alcohol exposure, CON = non-exposed control subjects, FAS = fetal alcohol syndrome, IQ = Wechsler Intelligence Scale for Children (WISC-IV) Full Scale IQ. ns = No significant difference, p > .05. FSIQ data was unavailable for two subjects in the AE group (n = 30) in the full neuroimaging sample.
3.2. Neuropsychological data
Box’s M test of homogeneity of covariance was non-significant for the IN-Inhibition dependent variables (ps > .001). Levene’s homogeneity test was also non-significant for each dependent variable (ps > .001). The main effect of group in the IN-Inhibition MANCOVA was significant (F(2, 36) = 3.27, p = .050). There were no main effects of age or sex. Follow up ANCOVAs showed that the AE group had significantly slower IN-Inhibition completion time relative to CON (F(1, 37) = 5.74, p = .022), but that groups did not differ significantly on IN-Inhibition errors (F(1, 37) = 0.93, p = .341), as presented in Table 2.
Table 2.
Descriptive data and univariate ANCOVA results for main effect of group for neuropsychological data.
| AE (n = 25) M (SD) | CON (n = 16) M (SD) | p | η2 | |
|---|---|---|---|---|
| NEPSY-II IN-Inhibition | ||||
| Inhibition completion time (seconds)* | 65.7 (20.6) | 52.5 (11.7) | .022 | .134 |
| Inhibition errors (number) | 3.7 (4.0) | 2.7 (3.5) | .341 | .025 |
AE = children with prenatal alcohol exposure, CON = non-exposed control subjects. Age and sex included as covariates in all analyses.
Remained statistically significant after correction for multiple comparisons (p < .025).
3.3. ACC structure
Box’s M test of homogeneity of covariance was non-significant for the Surface Area, Volume, and Thickness dependent variables (ps > .001). Levene’s homogeneity test was also non-significant for each dependent variable (ps > .001). The main effect of group in the MANCOVA for Surface Area was significant (F(4, 46) = 3.25, p = .020) indicating that groups differed on the combined surface area dependent variables, with AE having smaller surface area than CON. The Surface Area MANCOVA was non-significant after controlling for ICV (F(4, 45) = 2.20, p = .084). The main effect of group in the MANCOVA for Volume was marginally significant (F(4, 46) = 2.52, p = .054), but was clearly non-significant after controlling for ICV (F(4, 45) = 1.53, p = .210). The main effect of group in the MAN-COVA for Thickness (F(4, 46) = 0.28, p = .887) was also not significant. Notably, there was a main effect of age for the Thickness variables with greater age being associated with thinner cortex, after accounting for group and sex (F(4, 46) = 5.79, p < .001). No other significant effects of age or sex emerged for the ACC variables.
The significant MANCOVA for Surface Area was followed up with univariate ANCOVA analyses. As shown in Table 3, both rrACC (F(1, 49) = 8.81, p = .005) and rcACC (F(1, 49) = 13.07, p < .001) were significantly different between AE and CON groups after correction for multiple comparisons (alpha = .0125). When analyses were run controlling for ICV, the ANCOVA for rcACC remained significant (F(1, 48) = 8.65, p = .005) while rrACC did not (F(1, 48) = 4.71, p = .035).
Table 3.
Descriptive data and univariate ANCOVA results for main effect of group for neuroimaging data.
| AE (n = 32) M (SD) | CON (n = 21) M (SD) | p | η2 | |
|---|---|---|---|---|
| Anterior cingulate cortex surface area | ||||
| Right rostral ACC* | 619.9 (102.4) | 720.5 (158.8) | .005 | .152 |
| Right caudal ACC*,† | 689.1 (135.9) | 837.6 (163.7) | <.001 | .211 |
| Left rostral ACC | 778.6 (136.5) | 857.2 (165.7) | .039 | .084 |
| Left caudal ACC | 652.1 (140.7) | 715.5 (147.4) | .098 | .055 |
ACC = anterior cingulate cortex, AE = children with prenatal alcohol exposure, CON = non-exposed control subjects. Age and sex included as covariates in all analyses. Surface area measured in mm2.
After correction for multiple comparisons, only right rostral and right caudal ACC remained significantly different between groups (p < .0125).
After covarying for ICV, only the right caudal ACC remained significantly different between groups (p < .0125).
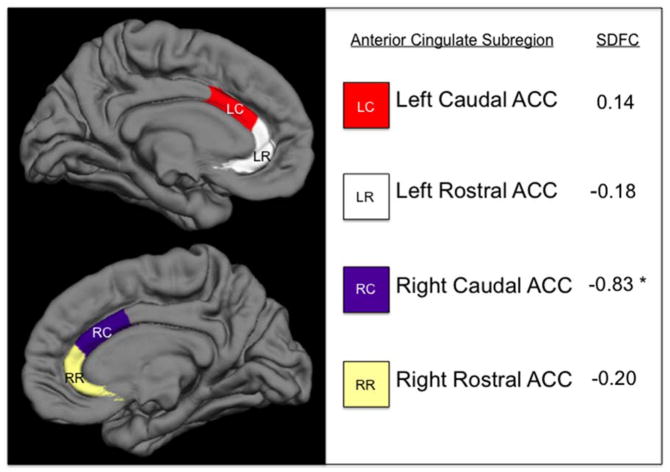
Standardized discriminant function coefficients from the significant Surface Area MANOVA are displayed in Fig. 1. Using a cutoff of |.30|, only the rcACC area (−0.83) was found to be practically significant, indicating that it makes a large unique contribution to the observed group difference. By contrast, the rrACC, lrACC, and lcACC areas each have low coefficient values (−0.20, −0.18, and 0.14, respectively), indicating that they contribute relatively little unique variance to the observed ACC group difference.
Fig. 1.
Anterior Cingulate Cortex Subregions with Standardized Discriminant Function Coefficients from Between Group MANCOVA.
ACC = Anterior Cingulate Cortex; SDFC = standardized discriminant function coefficients that maximize the observed difference between groups, controlling for age and sex. * = standardized discriminant function coefficients practically significant at > |.30|.
3.4. ACC-Inhibition relationships
Given the results from the univariate ANCOVAs and linear discriminant analysis, rcACC area was chosen for inclusion in regression analyses. Regression results for IN-Inhibition completion time are presented in Table 4. In Model 3 there was a significant group × ACC interaction (p = .006). The interaction effect demonstrates that for the AE group, a 1 SD reduction (182.7 mm2) in ACC area was associated with 0.65 SD (12.3 s; 95% CI: 7.4 s–17.3 s) slower inhibition time. On the other hand, for the CON group a 1 SD reduction (182.7 mm2) in ACC area was associated with 0.35 SD (6.5 s; 95% CI: 4.6 s–8.4 s) faster inhibition time. This interaction remained significant even when total surface area (p = .009) and ICV (p = .009) were separately included in the model.
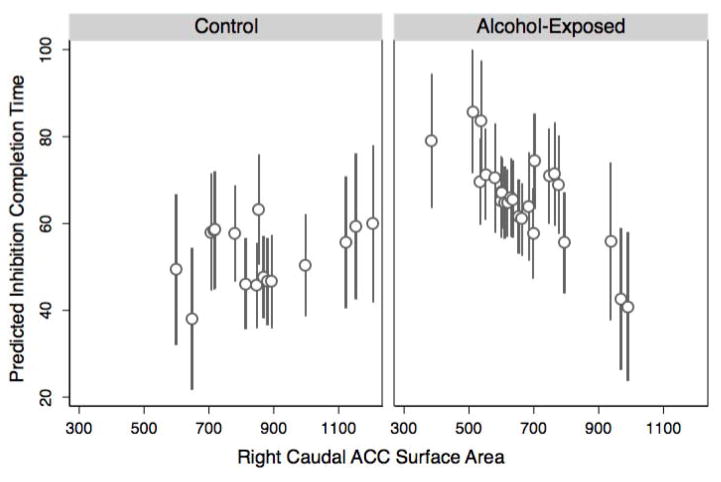
To follow up this interaction by examining simple main effects, regression analyses were run separately in each group. The main effect of ACC was significant for AE (β = −.068, p = .022), but non-significant for CON (β = .018, p = .409). Therefore, the significant group × ACC interaction reported above appears to be driven by a positive ACC-inhibition relationship in AE that is significantly different from a non-significant ACC-inhibition relationship in CON. In other words, accounting for age and sex, having a smaller rcACC area is associated with worse (i.e., slower) inhibition performance for alcohol-exposed youth, but is not associated with inhibition for controls (Fig. 2).
Fig. 2.
Right Caudal ACC Surface Area Interaction Predicted Values Plotfor Inhibition Completion Time.
ACC = Anterior Cingulate Cortex; Plot displays predicted inhibition completion time performance based on regression Model 3. Controlling for age and sex, there is a significant group by ACC interaction (p = .006). Having a smaller right caudal ACC area is associated with worse (i.e., slower) inhibition performance for AE, but is not associated with inhibition for controls.
In Model 1 there was a significant main effect of group (p = .022) and in Model 2 there was a significant main effect of ACC (p = .036). The main effects must be interpreted in light of the significant group × ACC interaction detailed above. No main effects of age were evident in Models 1–3, though there was a significant main effect of sex (p = .037) in Model 3 only. Only after accounting for group, rcACC area, age, and the group × ACC interaction, being male was associated with faster inhibition speed.
Regression results for IN-Inhibition errors are presented in Table 5. No significant main effects or interactions emerged for Models 4–6.
3.5. ACC-processing speed post hoc analyses
When IN-Naming was added as a dependent variable to inhibition Models 1–3, there was no longer a significant group × ACC interaction. However, a main effect of ACC was evident in Model 2 (β = −.027, p = .009) where smaller rcACC was associated with slower inhibition performance regardless of group. No main effect of group emerged.
Results from regressions using IN-Naming completion time as the dependent variable are presented in Table 6. In Model 9 there was a significant group × ACC interaction (p = .014). The interaction effect demonstrates that for the AE group, a 1 SD reduction (182.7 mm2) in ACC area was associated with 0.30 SD (2.7 s; 95% CI: 2.3 s–3.2 s) slower processing speed. On the other hand, for the CON group a 1 SD reduction (182.7 mm2) in ACC area was associated with 0.60 SD (5.4 s; 95% CI: 4.2 s–6.3 s) faster processing speed. This interaction remained significant even when total surface area (p = .022) and ICV (p = .026) were separately included in the model.
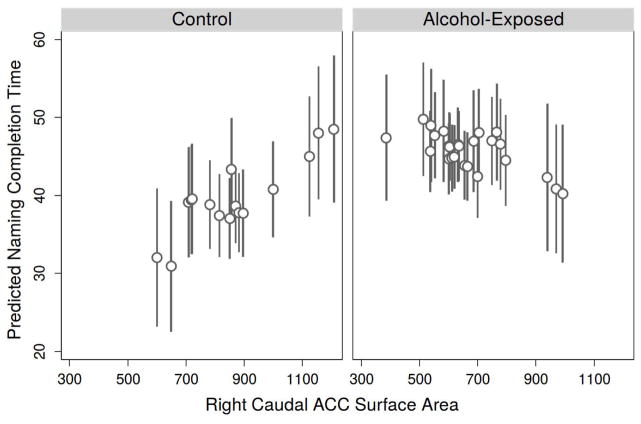
When processing speed regression analyses were run separately in each group to further probe this interaction, the main effect of ACC was significant for CON (β = .029, p = .008) but non-significant for AE (β = −.014, p = .365). In sum, accounting for age and sex, having a smaller rcACC area is associated with better (i.e., faster) processing speed for controls but is not associated with processing speed in alcohol-exposed youth (Fig. 3).
Fig. 3.
Right Caudal ACC Surface Area Interaction Predicted Values Plotfor Naming Completion Time(Processing Speed).
ACC = Anterior Cingulate Cortex; Plot displays predicted inhibition completion time performance based on regression Model 9. Controlling for age and sex, there is a significant group by ACC interaction (p = .014). Having a smaller right caudal ACC area is associated with better (i.e., faster) processing speed for controls but is not associated with processing speed in alcohol-exposed youth.
4. Discussion
This study is the first to examine the relationship between cortical surface area, inhibition, and processing speed in FASD. In partial support of our first prediction, alcohol-exposed adolescents exhibited worse performance on the inhibition test, albeit differences were limited to inhibition speed. Alcohol-exposed adolescents took significantly longer to complete the inhibition task, whereas the two groups did not differ in inhibition accuracy. This lack of significant group difference in inhibition errors is inconsistent with previous studies that have used the Delis–Kaplan Executive Functioning System (D-KEFS; [63] Color-Word Interference (CWI) task to probe inhibition in FASD [8,9]. However, the cognitive demands of the CWI task may not be directly comparable to the NEPSY-II IN task, as the CWI task requires lexical knowledge and reading skill while the IN task does not. On the other hand, another study reported no differences between alcohol-exposed versus control children on completion time or errors using the same NEPSY-II IN-Inhibition task used in the present study [59]. While alcohol-exposed adolescents in our sample made a higher number of errors on the inhibition task relative to controls, the magnitude of this accuracy difference (η2 = .024) was too small to reach statistical significance, perhaps due to the wide variance of total errors in both groups and our moderate sample size.
Regarding our second hypothesis, alcohol-exposed adolescents demonstrated structural alteration of the ACC. They had significantly smaller ACC surface area compared to non-exposed controls. Groups did not differ in ACC cortical thickness and the marginally significant group difference in ACC volume was thus likely driven by surface area reduction. This finding is consistent with the theory that surface area may be affected to a greater degree than cortical thickness in FASD [48]. In the context of the radial unit hypothesis of cortical development [45], the deleterious impact of prenatal alcohol exposure on the migration of radial cells [64] could be a causal mechanism for lasting alterations in brain surface area. Our study provides further evidence that a shift toward examining surface area in addition to volume in FASD neuroimaging investigations is warranted. If brain volume is studied in isolation the alcohol-related effects on ACC and other structures may go undetected.
Our analysis of ACC subregions indicates that the observed group difference in ACC surface area was driven primarily by reduction of the rcACC. The rcACC was the only subregion that was both statistically different between groups even after controlling for total surface area or ICV and had a significant unique contribution to the overall ACC surface area group difference. The caudal ACC region corresponds to the dorsal division of the ACC, which is thought to be specific to non-emotional cognitive control processes [65]. In line with this theory, we found that the surface area of the rcACC is associated with individual differences in non-emotional inhibitory control as measured by NEPSY-II IN-Inhibition completion time. While we expected that ACC size and inhibition performance would be positively related in both groups, our interaction model demonstrated that the relationship between ACC surface area and inhibition in fact differs by group. A decrease in rcACC surface area for alcohol-exposed children was associated with slower time to complete the inhibition task, but was not significantly associated with inhibition completion time for non-exposed controls. Controlling for either total cortical surface area or ICV did not diminish this relationship.
To our knowledge, only one study to date has reported a relationship between children’s ACC surface area and cognitive control, a construct that encompasses response inhibition. Fjell and colleagues found that ACC surface area was significantly positively related to cognitive control, but only for the younger children in their sample (ages 4–12) [36]. For individuals ages 12–21 the authors found no relationship between ACC surface area and cognitive control. The non-significant ACC-inhibition relationship we observed for control subjects ages 12–17 is in accord with Fjell and colleagues’ result in their older sample. Conversely, the positive ACC-inhibition relationship observed in our alcohol-exposed group directly mirrors that of more immature typically developing children in Fjell and colleagues younger sample. This suggests that alcohol-exposed children may experience delayed development or underdevelopment of the ACC surface. Of note, delayed cortical surface area maturation was recently identified in a large sample of children with ADHD [66]. Given the large number of subjects in the AE group who meet diagnostic criteria for ADHD (64% of subsample with complete neuroimaging and neuropsychological data), delayed maturation is a plausible explanation. Atypical cortical volume neurodevelopment, albeit within more posterior brain regions, has also been observed in FASD [42]. Longitudinal neuroimaging studies of surface area development are required to determine whether maturation of ACC surface area is delayed in FASD. Overall, children with histories of heavy prenatal alcohol exposure demonstrate an ACC-inhibition relationship that deviates significantly from that of non-exposed controls.
Because the ACC-inhibition association we observed was specific to inhibition speed and not inhibition accuracy, it is important to consider whether processing speed may play a causal role in our findings. In post hoc analyses we examined whether processing speed as measured by NEPSY-II IN-Naming accounted for the observed ACC-inhibition relationship. In this sample, the surface area of the rcACC was significantly associated with inhibition speed over and above the influence of processing speed. Specifically, when processing speed was included in the model, smaller rcACC surface area was associated with worse (i.e., slower) inhibition regardless of subject group. When processing speed was examined independent of inhibition speed, we found that processing speed was also related to ACC structure. A decrease in rcACC surface area was associated with faster processing speed for non-exposed controls but not significantly associated with processing speed in alcohol-exposed adolescents.
These results suggest that the relationship between ACC surface area and processing speed influences, but does not fully account for the relationship between ACC surface area and inhibition speed. Recent fMRI evidence suggests that the caudal ACC may monitor reaction time in addition to inhibition and conflict/error evaluation [67]. This expanded theory of caudal ACC function is consistent with the ACC-inhibition and ACC-processing speed structural relationships we report here. The potential mediating role of processing speed in ACC-inhibition relationships should be a future research target with a larger sample of children, particularly in children with FASD as slowed information processing and reaction time are thought to be key deficits in this population [68–70].
4.1. Limitations
There are a number of limitations to this investigation. First, the cross sectional nature of our study prevents examination of ACC and inhibition relationships across development. In addition, while there are benefits to the use of a hypothesis-driven a priori ROI analysis, executive functions are complex cognitive phenomena, and the ACC is thought to work in tandem with other regions such as the inferior frontal gyrus, dorsolateral prefrontal cortex, and striatal regions to give rise to successful inhibitory control. White matter microstructure connecting the ACC to other brain regions may play a key role in inhibition speed deficits, as white matter integrity has been associated with inhibition [71] and processing speed [41]. While we demonstrated that the ACC-inhibition relationship was not attributable to global reductions in cortical surface area or ICV, this study examined only one neural component of the broader response inhibition system. Future investigations using whole brain analysis and multimodal imaging techniques are likely to further increase our understanding of executive functioning deficits in FASD. Furthermore, impaired inhibition performance is also seen in a wide variety of developmental disorders. Future studies should include clinical contrast groups with inhibitory control deficits to better determine whether the observed relationships with the ACC are a distinguishing feature of prenatal alcohol exposure. For example, the known alterations of cortical surface area development in ADHD elevate the importance of neuroanatomical comparisons between alcohol-related and idiopathic attention, inhibition, and impulsivity impairments.
5. Conclusions
The primary objective of this study was to characterize brain-behavior relationships that may underlie clinically significant inhibitory dysfunction in FASD. Alcohol-exposed youth were slower to complete inhibition tasks and showed decreased ACC surface area, particularly in the rcACC subregion, which was associated with worse inhibition speed. That alcohol-exposed adolescents were accurate but slow to complete the inhibition task suggests that processing speed is an important component of efficient inhibitory control in FASD. The strong relationship between ACC surface area and inhibition speed (but not accuracy) and between ACC surface area and processing speed provides neuroanatomical support for the behavioral findings. Regarding clinical relevance, findings suggest that alcohol-exposed youth may benefit from ensuring adequate time is allotted for completion of tasks requiring cognitive control to account for processing speed deficits associated with alcohol’s effect on the developing ACC.
HIGHLIGHTS.
Prenatal alcohol exposed youth had reduced anterior cingulate (ACC) surface area.
Exposure associated with slow inhibition speed but no differences in accuracy.
Relations between ACC and inhibition speed differed based upon exposure history.
Smaller ACC area related to slower inhibition speed but only in exposed youth.
Smaller ACC area related to faster processing speed but only in control youth.
Acknowledgments
Research supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R01 AA019605, U24 AA014811, T32 AA013525, F31 AA022261, F31 AA022033, K99 022661; an American Fellowship from AAUW; and a National Science Foundation (NSF) Graduate Research Fellowship DGE-1247398.
References
- 1.Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 3.Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–21. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- 4.Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–53. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand J, Floyd R, Weber M, O’Connor M, Riley E, Johnson K, et al. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 6.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 7.Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, et al. Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2013;37:517–28. doi: 10.1111/j.1530-0277.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–15. [PubMed] [Google Scholar]
- 9.Rasmussen C, Bisanz J. Executive functioning in children with fetal alcohol spectrum disorders: profiles and age-related differences. Child Neuropsychol. 2009;15:201–15. doi: 10.1080/09297040802385400. [DOI] [PubMed] [Google Scholar]
- 10.Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18:331–54. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll CD, Chen JS, Riley EP. Passive avoidance performance in rats prenatally exposed to alcohol during various periods of gestation. Neurobehav Toxicol Teratol. 1982;4:99–103. [PubMed] [Google Scholar]
- 12.Gallo PV, Weinberg J. Neuromotor development and response inhibition following prenatal ethanol exposure. Neurobehav Toxicol Teratol. 1982;4:505–13. [PubMed] [Google Scholar]
- 13.Olmstead MC, Martin A, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure increases disinhibition and perseverative responding in the adult guinea pig. Behav Pharmacol. 2009;20:554–7. doi: 10.1097/FBP.0b013e3283305e27. [DOI] [PubMed] [Google Scholar]
- 14.Riley EP, Lochry EA, Shapiro NR. Lack of response inhibition in rats prenatally exposed to alcohol. Psychopharmacology (Berl) 1979;62:47–52. doi: 10.1007/BF00426034. [DOI] [PubMed] [Google Scholar]
- 15.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychol. 2006;12:439–52. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Nordling JK, Yoon JE, Boldt LJ, Kochanska G. Effortful control in “hot” and “cool” tasks differentially predicts children’s behavior problems and academic performance. J Abnorm Child Psychol. 2013;41:43–56. doi: 10.1007/s10802-012-9661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, et al. Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction. 2013;108:1916–23. doi: 10.1111/add.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Disney ER, Iacono W, McGue M, Tully E, Legrand L. Strengthening the case: prenatal alcohol exposure is associated with increased risk for conduct disorder. Pediatrics. 2008;122:e1225–30. doi: 10.1542/peds.2008-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatara V, Loudenberg R, Ellis R. Association of attention deficit hyperactivity disorder and gestational alcohol exposure: an exploratory study. J Atten Disord. 2006;9:515–22. doi: 10.1177/1087054705283880. [DOI] [PubMed] [Google Scholar]
- 21.Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:e733–41. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- 22.Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15:258–67. doi: 10.1002/ddrr.67. [DOI] [PubMed] [Google Scholar]
- 23.Steele VR, Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, Stevens MC, et al. A large scale (N = 102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav Brain Res. 2013;256:529–36. doi: 10.1016/j.bbr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–36. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 25.Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 26.Gruber SA, Rogowska J, Holcomb P, Soraci S, Yurgelun-Todd D. Stroop performance in normal control subjects: an fMRI study. Neuroimage. 2002;16:349–60. doi: 10.1006/nimg.2002.1089. [DOI] [PubMed] [Google Scholar]
- 27.Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–42. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald KD, Zbrozek CD, Welsh RC, Britton JC, Liberzon I, Taylor SF. Pilot study of response inhibition and error processing in the posterior medial prefrontal cortex in healthy youth. J Child Psychol Psychiatry. 2008;49:986–94. doi: 10.1111/j.1469-7610.2008.01906.x. [DOI] [PubMed] [Google Scholar]
- 29.Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–5. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- 30.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–98. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 31.Chan AS, Han YM, Leung WW-M, Leung C, Wong VC, Cheung M-C. Abnormalities in the anterior cingulate cortex associated with attentional and inhibitory control deficits: a neurophysiological study on children with autism spectrum disorders. Res Autism Spectr Disord. 2011;5:254–66. [Google Scholar]
- 32.Gavita OA, Capris D, Bolno J, David D. Anterior cingulate cortex findings in child disruptive behavior disorders: a meta-analysis. Aggress Violent Behav. 2012;17:507–13. [Google Scholar]
- 33.Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, et al. Regional gray and white matter volume associated with Stroop interference: evidence from voxel-based morphometry. Neuroimage. 2012;59:2899–907. doi: 10.1016/j.neuroimage.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 34.Borst G, Cachia A, Vidal J, Simon G, Fischer C, Pineau A, et al. Folding of the anterior cingulate cortex partially explains inhibitory control during childhood: a longitudinal study. Dev Cogn Neurosci. 2014;9:126–35. doi: 10.1016/j.dcn.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, et al. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–9. [PubMed] [Google Scholar]
- 36.Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Jr, et al. Multimodal imaging of the self-regulating developing brain. Proc Natl Acad Sci U S A. 2012;109:19620–5. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal–striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31:1415–24. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien JW, Norman AL, Fryer SL, Tapert SF, Paulus MP, Jones KL, et al. Effect of predictive cuing on response inhibition in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2013;37:644–54. doi: 10.1111/acer.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ware AL, Infante MA, O’Brien JW, Tapert SF, Jones KL, Riley EP, et al. An fMRI study of behavioral response inhibition in adolescents with and without histories of heavy prenatal alcohol exposure. Behav Brain Res. 2014 doi: 10.1016/j.bbr.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21:102–18. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wozniak JR, Muetzel RL. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol Rev. 2011;21:133–47. doi: 10.1007/s11065-011-9162-1. [DOI] [PubMed] [Google Scholar]
- 42.Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 2012;32:15243–51. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjorkquist OA, Fryer SL, Reiss AL, Mattson SN, Riley EP. Cingulate gyrus morphology in children and adolescents with fetal alcohol spectrum disorders. Psychiatry Res. 2010;181:101–7. doi: 10.1016/j.pscychresns.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–6. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 46.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–35. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–6. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Rajaprakash M, Chakravarty MM, Lerch JP, Rovet J. Cortical morphology in children with alcohol-related neurodevelopmental disorder. Brain Behav. 2014;4:41–50. doi: 10.1002/brb3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–44. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou D, Lebel C, Lepage C, Rasmussen C, Evans A, Wyper K, et al. Developmental cortical thinning in fetal alcohol spectrum disorders. Neuroimage. 2011;58:16–25. doi: 10.1016/j.neuroimage.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 51.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 53.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 54.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–96. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 56.Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–29. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 57.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 58.Wechsler D. Manual for the Wechsler Intelligence Scale for Children-Fourth Edition Integrated. 4. San Antonio: Pearson; 2004. [Google Scholar]
- 59.Rasmussen C, Tamana S, Baugh L, Andrew G, Tough S, Zwaigenbaum L. Neuropsychological impairments on the NEPSY-II among children with FASD. Child Neuropsychol. 2013;19:337–49. doi: 10.1080/09297049.2012.658768. [DOI] [PubMed] [Google Scholar]
- 60.Hollingshead AB. Four factor index of social status. Yale J Sociol. 2011;8:21–51. [Google Scholar]
- 61.Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–91. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- 62.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15:331–43. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delis DC, Kaplan E, Kramer JH. Delis–Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 64.Bosco C, Diaz E. Placental hypoxia and foetal development versus alcohol exposure in pregnancy. Alcohol Alcohol. 2012;47:109–17. doi: 10.1093/alcalc/agr166. [DOI] [PubMed] [Google Scholar]
- 65.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 66.Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72:191–7. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neta M, Schlaggar BL, Petersen SE. Separable responses to error, ambiguity, and reaction time in cingulo-opercular task control regions. Neuroimage. 2014;99:59–68. doi: 10.1016/j.neuroimage.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005;29:1473–83. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- 69.Jacobson SW. Specificity of neurobehavioral outcomes associated with prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:313–20. doi: 10.1111/j.1530-0277.1998.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 70.Simmons RW, Wass T, Thomas JD, Riley EP. Fractionated simple and choice reaction time in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 2002;26:1412–9. doi: 10.1097/01.ALC.0000030563.14827.29. [DOI] [PubMed] [Google Scholar]
- 71.Treit S, Chen Z, Rasmussen C, Beaulieu C. White matter correlates of cognitive inhibition during development: a diffusion tensor imaging study. Neuroscience. 2014;276:87–97. doi: 10.1016/j.neuroscience.2013.12.019. [DOI] [PubMed] [Google Scholar]