Abstract
Background
Recent evidence suggests that the insular cortex may play an important role in cognitive and emotional processes that facilitate drug use but it is unclear whether changes to the insula would result in sustained abstinence. To better understand the role of the insula in maintaining abstinence, we examined quitting patterns in smokers with acute damage to their insula relative to other regions.
Design
Prospective cohort study with 3 months follow-up, beginning June 2013 and ending May 2014.
Setting
Three acute care hospitals in Rochester, NY.
Participants
One-hundred-fifty-six current smokers hospitalized for acute ischemic stroke; 38 with insular infarctions and 118 with non-insular infarctions, assessed by 3 neuroradiologists.
Measurements
Self-reported smoking status (seven-day point prevalence and continuous abstinence), complete abstinence from any nicotine product, and disruption of smoking addiction (defined by criteria on smoking status, difficulty of quitting, and urge) were assessed at three months post-stroke. Time to relapse (in days) after discharge was also assessed.
Results
Insular damage was associated with increased odds of three-month continuous abstinence (OR=3.71, 95% CI: 1.59, 8.65) and complete cessation from any nicotine product (OR=2.72, 95% CI: 1.19, 6.22). Average time to relapse was longer in the insular-damaged group (17.50 days, SD=19.82) relative to non-insular damage (10.42 days, SD=18.49). Among quitters, insular damage was also associated with higher relative odds of experiencing a disruption of addiction compared to non-insular damage (adjusted OR=5.60, 95% CI: 1.52, 20.56).
Conclusions
These findings support the potential role of the insular cortex in maintaining smoking and nicotine abstinence. Further research is needed to establish whether the insula may be a novel target for smoking cessation interventions.
Keywords: Insular cortex, nicotine, cessation, stroke, smoking
1. Introduction
Tobacco dependence is regarded as a chronic disease in which long-term management strategies are needed to address the strong neurophysiological reward response, physical withdrawal symptoms, and the psychological response to environmental, social, and cultural cues associated with its use [1]. Although many smokers are aware of the increased health risks and have a desire to quit, only one third make an attempt to quit annually [2]. Even with current pharmacotherapies for tobacco dependence, 66.8 to 81.0 percent of smokers motivated to quit relapse by six months [1, 2]. Given the high prevalence of relapse, it is important to further evaluate potential mechanisms responsible for this addictive behavior so that successful strategies for cessation intervention can be developed.
The craving for nicotine has been suggested to originate from long-term adaptations within specific neural systems that promote escalating drug use, difficulty quitting, and relapse [3]. Mesocorticolimbic regions of the brain, including the ventral tegmental area (VTA), amygdala, nucleus accumbens (NAc), prefrontal cortex (PFC), and hippocampus have observed molecular adaptations when exposed to addictive drugs in preclinical studies [4, 5] and have been the primary target for smoking cessation pharmacotherapies such as bupropion [6] and varenicline [7]. Recently, the insular cortex – the cerebral cortex beneath the sylvian fissure surrounded by the temporal, frontal, and parietal opercula – has been of particular interest due to the potential role it plays in conscious urges, notably for cravings associated with addictive drugs [3]. The central and peripheral effects of nicotine are implicated in conscious pleasure induced by cigarette use. Smoking-related cues are believed to result in interconnections from the amygdala and orbitofrontal cortex/ ventromedial PFC triggering a representation of the euphoria produced by nicotine in the insula and consequently insular projections to the NAc core, thus motivating nicotine dependent behaviors [3].
To date, only two experimental animal studies have examined the effects of insular cortex inactivation on drug seeking and have demonstrated the integrity of the insula to facilitate motivation to take methamphetamine [8] and nicotine [9]. Human studies of stroke-induced lesions have also shown insular damage to be associated with higher odds of cessation [4, 10, 11], although one study obtained a null finding [12]. These studies, however, had relatively small sample sizes, only examined cessation of cigarette smoking and did not address subsequent use of other nicotine products such as nicotine replacement therapies (NRT) and other forms of tobacco.
We previously demonstrated that acute insular damage by ischemic stroke among current cigarette smokers resulted in reduced and less severe withdrawal symptoms during hospitalization, a period of forced abstinence [13]. Although this may be the mechanism through which cessation occurs, it is important to characterize quitting patterns in these individuals to better understand the durability of the insula in maintaining abstinence. We hypothesized that, among current cigarette smokers admitted for acute ischemic stroke, those with damage to their insular cortex would be more likely to quit by three months post-stroke and experience complete cessation from all forms of nicotine, compared to smokers with non-insular damage. Three-month follow-up was chosen as a clinically-relevant timeframe in which the neuroadaptations that underlie addiction last after quitting [14]. To account for the possibility that insular function may be restored with time, particularly for smaller lesions, we also hypothesized that smokers with insular damage would relapse later – after discharge – than those with non-insular damage. In an effort to confirm the findings reported by Naqvi et al. [4], we also hypothesized that, among the quitters, those with insular damage would be more likely to experience a “disruption of addiction” relative to individuals with non-insular strokes.
2. Materials and Methods
2.1 Study Population
The Measuring the Impact on Nicotine Dependence after Stroke (MINDS) study population consisted of adults aged 18 years and older who were admitted to one of three acute care hospitals in Rochester, NY with a diagnosis of acute ischemic stroke (ICD-9 code 434.91) and were active cigarette smokers at the time of stroke onset. Patients were eligible to participate if they met all of the following inclusion criteria: (1) smoked at least one cigarette per day during the month prior to their stroke and at least 100 in their lifetime; (2) were able to understand and speak the English language; (3) were stable enough, as determined by a nurse practitioner, to give autonomous informed consent and respond to the survey questions verbally or on paper; and (4) were willing to respond to the follow-up survey three months after hospital admission. Participants were excluded from follow-up evaluation if they had a subsequent stroke of any kind or were maintained in an environment where access to smoking was restricted.
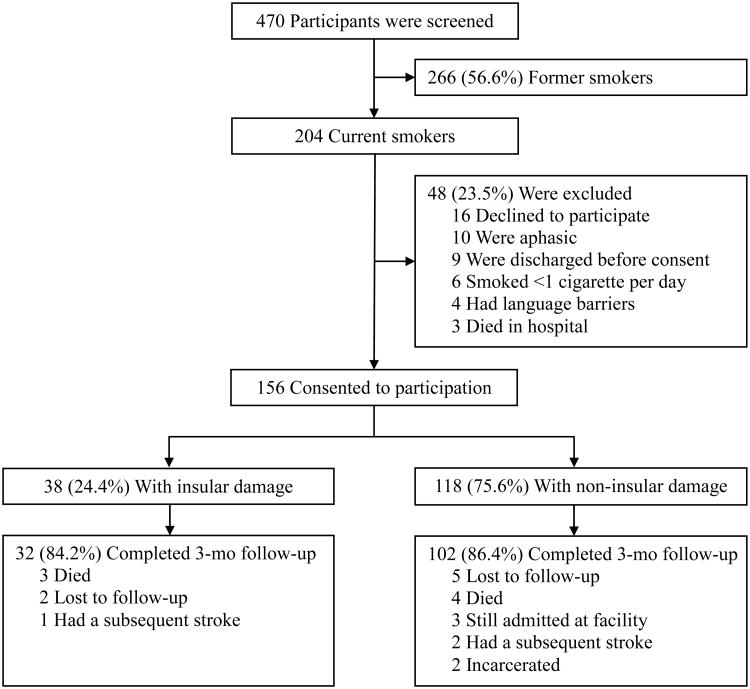
Figure 1 illustrates a flow diagram of recruitment and follow-up figures. A total of 470 ever smokers with acute ischemic stroke were identified between June 2013 and February 2014. Among the 204 (43.4%) who met the definition of a current smoker, 48 (23.5%) were excluded for not meeting the eligibility criteria; thus, 156 (76.5%) patients were enrolled into the study. Due to exclusions and losses to follow-up, 134 (85.9%) enrolled participants completed the three month follow-up assessment.
Figure 1.
Flow diagram of screening, exposure allocation, and follow-up
All participants signed informed consent forms. The institutional review boards at all participating institutions approved the study protocols and procedures.
2.2 Exposure Assessment
Acute infarctions to the insular cortex, the primary exposure region, and the aforementioned mesocorticolimbic structures were characterized by neuroradiologists (H.Z.W., E.M.S., B.E.S.) using standard of care computed tomography (CT) and magnetic resonance imaging (MRI) techniques in all participants. Participants with cerebral infarctions not in the insular cortex were considered unexposed. The neuroradiologists were blinded to information other than what is commonly clinically available. If evidence from CT and MRI were conflicting, findings from the MRI were more heavily taken into account than CT. In situations where MRI was contraindicated, only CT imaging was used. On MRI, diffusion weighted imaging was used to evaluate lesion location if completed within a few days from the stroke. If follow-up MRI scans were available, the fluid attenuated inversion recovery sequence was used to verify the initial MRI findings. The NAc and VTA were the two most difficult regions to evaluate as they are not routinely evaluated in practice and the standard MRI uses 5mm slices which may be too thick to detect lesions in these regions [15].
Exposure data were collected and managed using Research Electronic Data Capture (REDCap) [16] tools hosted at the University of Rochester. Lesions in 80 (51.3%) participants were identified using only MRI, 22 (14.1%) using only CT, and 54 (34.6%) using both MRI and CT. All participants were confirmed to have acute to subacute infarctions.
2.3 Ascertainment of End Points
Participants were evaluated three months after the date of stroke – as a clinically relevant timeframe for neuroadaptations to occur after quitting [14] – to reassess their self-reported smoking status and levels of addiction severity. Smoking cessation was assessed using seven-day point prevalence of abstinence (refrainment from smoking for seven days prior to follow-up) and continuous abstinence (complete refrainment of smoking from discharge to follow-up) definitions. Complete abstinence from any nicotine product, including other forms of tobacco and NRT, was also assessed from discharge until follow-up. Date of smoking reinstatement was used to calculate time to relapse from hospital discharge, in days.
Disruption of smoking addiction among the quitters – a subjective tool to determine whether the urge to smoke was lost – was assessed using the following questions aimed at their quitting experience: (1) “How soon after your brain injury did you quit smoking?”; (2) “How difficult was it to quit smoking after your brain injury, on a scale of one to seven, with one being very easy and seven being very difficult?”; (3) “How many times have you started smoking again since your brain injury?”; and (4) “Have you experienced any urge to smoke since you (most recently) quit smoking?” Participants reporting having quit smoking less than one day after their stroke, rating the difficulty to quit as less than three, reporting never having smoked since their stroke, and having felt no urge to smoke since quitting were classified as having a “disruption of smoking addiction.” One rater (A.A.) conducted all the assessments and was naive to exposure status.
2.4 Statistical Analyses
Baseline characteristics of participants were described using relative frequencies for categorical measures and means and standard deviations (SD) for continuous measures. To test for a difference in seven-day point prevalence and continuous abstinence of smoking between insular and non-insular damaged participants, a chi-squared test for difference in proportions was used. A two-sided p-value of less than 0.05 was considered statistically significant. A logistic regression analysis was performed in which the binary dependent variable was continuous abstinence of smoking. In addition to crude models, fully-adjusted multivariable logistic regression models were used to control for covariates that were predictive of outcomes in this particular study as well as all a priori theoretical risk factors for the outcomes, including age [17], baseline Fagerström Test for Nicotine Dependence (FTND; asked retrospectively) [18], National Institutes of Health Stroke Scale (NIHSS) [19] score [20], post-admission NRT/ drug use (yes/no) [21], acute mesocorticolimbic infarction (yes/no) [22, 23], and intent to quit (prior to stroke) in the next month with an attempt in the past year (asked retrospectively; yes/no) [24, 25]. Identical analyses modeled the relative odds of complete abstinence from any nicotine or tobacco product at three months post-stroke, while excluding post-admission NRT/ drug use as a confounder since this is part of the outcome. Crude and adjusted odds ratios (OR) and 95% confidence intervals (CI) were calculated.
The number of relapse cases and mean (SD) days to relapse was enumerated by exposure group. To evaluate crude differences in the time to relapse between insular and non-insular damaged groups, survival analysis tests for equality were computed and Kaplan-Meier curves produced. Log-Rank (more sensitive to differences in survival at later points in time) and Wilcoxon (more sensitive to differences in survival at earlier points in time) test p-values of less than 0.05 were indicative of a significant difference between groups. Cox proportional hazard models were performed to test the association between insular damage and time to relapse. Crude and adjusted hazard ratios (HR) and 95% CI were calculated, controlling for age, baseline FTND, NIHSS score, post-admission NRT/ drug use, acute mesocorticolimbic infarction, and prior intent to quit.
To specifically evaluate whether those who quit experienced a complete “disruption of smoking addiction,” a logistic regression analysis was used in which the binary dependent variable was whether or not quitters met all four criteria. This analysis was limited to the subjects who quit smoking after discharge (n = 71, 45.5%) [4]. Crude and adjusted OR's and 95% CI were computed, controlling for the aforementioned covariates. All statistical procedures were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
3. Results
Of the 156 participants, acute to subacute infarctions in the insula were detected in 38 (24.4%) participants. The baseline characteristics of study participants, by exposure group, are described in Table 1. Demographic variables were well balanced between individuals with insular and non-insular infarctions. Participants with insular damage expressed a greater interest in quitting in the next month prior to hospitalization, had a greater stroke severity as indicated by the NIHSS score, and were more likely to also experience a concurrent mesocorticolimbic infarction as compared to the non-insular group.
Table 1. Baseline characteristics of study participant's.
| Type of acute infarction | P | ||
|---|---|---|---|
|
| |||
| Insula (n = 38) | Non-insula (n = 118) | ||
| Age, mean (SD) | 59.7 (12.1) | 59.7 (11.3) | 0.98 |
| Female, n (%) | 21 (55.3) | 50 (42.4) | 0.17 |
| White race, n (%) | 26 (68.4) | 78 (66.1) | 0.79 |
| Highest education completed, n (%) | 0.89 | ||
| < HS | 12 (31.6) | 33 (28.0) | |
| HS/GED | 21 (55.3) | 67 (56.8) | |
| College graduate or higher | 5 (13.1) | 18 (15.2) | |
| Household income, n (%) | 0.32* | ||
| < $40,000 | 23 (60.5) | 89 (75.4) | |
| $40,000 or higher | 5 (13.2) | 10 (8.5) | |
| Missing | 10 (26.3) | 19 (16.1) | |
| Marital status, n (%) | 0.41 | ||
| Married or living with partner | 10 (26.3) | 37 (31.3) | |
| Not married | 15 (39.5) | 33 (28.0) | |
| Divorced, separated, widowed | 13 (34.2) | 48 (40.7) | |
| Employment status, n (%) | 0.74 | ||
| Employed | 7 (18.4) | 27 (22.9) | |
| Unemployed | 14 (36.9) | 46 (39.0) | |
| Retired | 17 (44.7) | 45 (38.1) | |
| FTND, mean (SD) | 5.0 (2.1) | 5.2 (1.9) | 0.58 |
| Cigarettes per day, mean (SD) | 19.6 (20.7) | 18.8 (11.9) | 0.77 |
| Lifetime smoking years, mean (SD) | 37.7 (14.6) | 40.1 (13.6) | 0.35 |
| Intent to quit (prior to stroke) in the next month with attempt in past year, n (%) | 12 (31.6) | 19 (16.1) | 0.04 |
| Right handed, n (%) | 35 (92.1) | 105 (89.0) | 0.76* |
| Prior cerebral infarction, (%) | 18 (47.4) | 45 (38.1) | 0.33 |
| NIHSS score, mean (SD) | 8.8 (6.4) | 5.2 (5.2) | <0.001 |
| Length of stay (days), mean (SD) | 12.0 (13.1) | 10.6 (15.0) | 0.61 |
| Concurrent infarct in mesocorticolimbic structure, n (%) | 20 (52.6) | 35 (29.7) | 0.01 |
SD, Standard Deviation; FTND, Fagerstrӧ m Test for Nicotine Dependence; NIHSS, National Institutes of Health Stroke Scale.
Fisher's Exact test p-value used (not including missing subjects); >20% of cells have expected counts less than 5
Quit statistics in those who completed follow-up at 3 months post-stroke (n = 134) are shown in Table 2. Among the 102 non-insular damaged participants, 49 (48.0%) had quit at least 7 days prior to assessment and 38 (37.3%) had not initiated smoking at all since their stroke; hence, 11 (22.4%) of the 49 subjects smoked at least once before they quit at 3 months. Among the 32 insular damaged participants, 22 (68.8%) had quit by 3 months post-stroke, all of which remained abstinent since their discharge. The proportion quitting was significantly higher in the insular group using both seven-day point prevalence (p = 0.04) and continuous abstinence (p < 0.01) definitions.
Table 2. Quit statistics at 3 months post-stroke by exposure group.
| 7-day point prevalence | Continuous abstinence | ||||
|---|---|---|---|---|---|
|
|
|
||||
| n | n (%) | P* | n (%) | P* | |
| Non-insula | 102 | 49 (48.0) | - | 38 (37.3) | - |
| Any insula | 32 | 22 (68.8) | 0.04 | 22 (68.8) | <0.01 |
Chi-squared p-value for difference in proportions relative to non-insula exposure group
The odds of achieving continuous abstinence in the any versus no insular damage group appear in Table 3. Participants with insular damage were more likely to experience complete abstinence up to three months post-stroke compared to those with non-insular damage (adjusted OR = 2.45, 95% CI: 0.93, 6.49). Higher NIHSS scores, participants with a concurrent mesocorticolimbic infarct, and those with pre-stroke intent to quit had increased odds of quitting, while those with higher baseline dependence score and those who used post-admission NRT or drugs had decreased odds of quitting. Males (n = 74) with insular damage had 3.59 times the odds of continuous abstinence (95% CI: 0.72, 17.82) compared to non-insular damaged males. Among females (n = 60), those with insular damage only had a slight increase in continuous abstinence compared to non-insular damage (OR = 1.47, 95% CI: 0.40, 5.48). However, these stratum-specific estimates were statistically imprecise and the interaction was not statistically significant (p = 0.26).
Table 3. Crude and multivariable (adjusted) logistic regression modeling the odds of continuous abstinence (60 cases) at 3 months post-stroke associated with insular relative to non-insular damage (n = 134).
| Model | Primary exposure Covariates |
OR | 95% CI | P |
|---|---|---|---|---|
| Crude | Acute insular infarct | 3.71 | 1.59 - 8.65 | <0.01 |
| Adjusted | Acute insular infarct | 2.45 | 0.93 - 6.49 | 0.07 |
| Age | 1.01 | 0.98 - 1.05 | 0.52 | |
| Baseline FTND | 0.86 | 0.70 - 1.05 | 0.14 | |
| NIHSS score | 1.08 | 1.00 - 1.17 | 0.05 | |
| Post-admission NRT/ drug use | 0.57 | 0.26 - 1.27 | 0.17 | |
| Mesocorticolimbic infarct | 1.65 | 0.73 - 3.75 | 0.23 | |
| Intent to quit | 1.86 | 0.68 - 5.13 | 0.23 |
OR, Odds Ratio; CI, Confidence Interval; FTND, Fagerström Test for Nicotine Dependence; NIHSS, National Institutes of Health Stroke Scale; NRT, Nicotine Replacement Therapy.
As a secondary outcome, the association between insular damage and odds of complete abstinence from any nicotine product was also examined (Table 4). Only 40 (29.9%) participants completely refrained from any form of nicotine or smoking cessation product (i.e., cigarettes, electronic cigarettes, tobacco, hookah, NRT, bupropion, varenicline) from discharge to follow-up. Participants with insular damage had 1.79 times the odds (95% CI: 0.69, 4.67) of complete abstinence from any nicotine product compared to participants with non-insular damage in the fully adjusted model. While point estimates varied suggesting abstinence among males (OR = 3.12, 95% CI: 0.71, 13.80) but not females (OR = 0.99, 95% CI: 0.26, 3.84), the interaction was not statistically significant (p = 0.11).
Table 4. Crude and multivariable (adjusted) logistic regression modeling the odds of complete abstinence from any nicotine product (40 cases) at 3 months post-stroke associated with insular relative to non-insular damage (n = 134).
| Model | Primary exposure Covariates |
OR | 95% CI | P |
|---|---|---|---|---|
| Crude | Acute insular infarct | 2.72 | 1.19 – 6.22 | 0.02 |
| Adjusted | Acute insular infarct | 1.79 | 0.69 – 4.67 | 0.23 |
| Age | 1.02 | 0.98 – 1.06 | 0.31 | |
| Baseline FTND | 0.84 | 0.68 – 1.04 | 0.10 | |
| NIHSS score | 1.05 | 0.98 – 1.13 | 0.19 | |
| Mesocorticolimbic infarct | 2.42 | 1.05 – 5.57 | 0.04 | |
| Intent to quit | 1.26 | 0.44 – 3.60 | 0.67 |
OR, Odds Ratio; CI, Confidence Interval; FTND, Fagerström Test for Nicotine Dependence; NIHSS, National Institutes of Health Stroke Scale.
We next examined time to relapse by exposure group. Two participants in the non-insula group did not provide a relapse date and thus were excluded from these analyses. While 62 (62.0%) participants relapsed in the non-insular damaged group, only 10 (32.3%) relapsed in the insular damaged group. The average number of days to relapse from discharge was longer in the insular damaged group (mean = 17.50 days, SD = 19.82) relative to those with non-insular damage (mean = 10.42 days, SD = 18.49), and significant differences existed in the survival functions between groups using the log-rank test (p < 0.01) and the Wilcoxon test (p < 0.01) (Figure 2). Among participants with any insular damage, the instantaneous risk of relapse was 0.48 times that of participants with non-insular damage (95% CI: 0.24, 0.98) in the fully adjusted model (Table 5). Baseline dependence score and post-admission NRT/ drug use were suggested risk factors for time to relapse, whereas NIHSS score, mesocorticolimbic infarct, and pre-stroke intent to quit were protective factors. No differences were noted in gender-specific hazard ratio's for relapse time (p for interaction = 0.46).
Figure 2.

Kaplan-Meier curves for time to relapse between smokers with insular and non-insular damage. All censoring was done administratively due to end of study follow-up.
Table 5. The association between acute insular stroke and time to relapse (in days) using crude and multivariable (adjusted) Cox proportional hazards modeling (n = 132).
| Model | Primary exposure Covariates |
HR | 95% CI | P |
|---|---|---|---|---|
| Crude | Acute insular infarct | 0.37 | 0.19 - 0.73 | <0.01 |
| Adjusted | Acute insular infarct | 0.48 | 0.24 - 0.98 | 0.04 |
| Age | 1.00 | 0.97 - 1.02 | 0.73 | |
| Baseline FTND | 1.08 | 0.95 - 1.24 | 0.24 | |
| NIHSS score | 0.95 | 0.90 - 1.00 | 0.06 | |
| Post-admission NRT/ drug use | 1.35 | 0.82 - 2.21 | 0.24 | |
| Mesocorticolimbic infarct | 0.73 | 0.41 - 1.27 | 0.26 | |
| Intent to quit | 0.63 | 0.31 - 1.26 | 0.19 |
HR, Hazard Ratio; CI, Confidence Interval; FTND, Fagerström Test for Nicotine Dependence; NIHSS, National Institutes of Health Stroke Scale; NRT, Nicotine Replacement Therapy.
Table 6 displays the association between insular damage and experiencing a “disruption of addiction” among participants who self-reported having quit as of seven days prior to follow-up, of which 17 (77.3%) met the “disruption of smoking addiction” criteria in the insular group and 16 (32.7%) in the non-insular group. Therefore, quitters with any insular damage were more likely to experience a disruption of smoking addiction compared to those with non-insular lesions (adjusted OR = 5.60, 95% CI: 1.52, 20.56). Participants using NRT post-admission and those with higher baseline dependence scores were less likely to meet the criteria for a disruption of addiction. Participants with higher NIHSS score and those with a pre-stroke intent to quit were more likely to experience a disruption of addiction.
Table 6. Crude and multivariable (adjusted) logistic regression modeling the risk of experiencing a “disruption of addiction [4]” (33 cases) associated with insular relative to non-insular damage among the quitters* at 3 months post-stroke (n = 71).
| Model | Primary exposure Covariates |
OR | 95% CI | P |
|---|---|---|---|---|
| Crude | Acute insular infarct | 7.01 | 2.19 - 22.42 | <0.01 |
| Full | Acute insular infarct | 5.60 | 1.52 - 20.56 | 0.01 |
| Post-admission NRT/ drug use | 0.33 | 0.10 - 1.10 | 0.07 | |
| Age | 0.99 | 0.94 - 1.04 | 0.75 | |
| Baseline FTND | 0.90 | 0.66 - 1.22 | 0.50 | |
| NIHSS score | 1.08 | 0.97 - 1.21 | 0.15 | |
| Mesocorticolimbic infarct | 0.99 | 0.30 - 3.34 | 0.99 | |
| Intent to quit | 1.54 | 0.37 - 6.45 | 0.55 |
OR, Odds Ratio; CI, Confidence Interval; FTND, Fagerström Test for Nicotine Dependence; NIHSS, National Institutes of Health Stroke Scale; NRT, Nicotine Replacement Therapy.
Defined using 7-day point prevalence.
4. Discussion
We examined the relationship between insular damage and smoking cessation and found a higher proportion of quitters at three months post-stroke in the insular damaged group relative to those with non-insular damage. Remarkably, none of the smokers with insular damage who were abstinent at three months post-stroke had smoked at all since their stroke. This is an important finding that supports our overall hypothesis of the insula's role in maintaining abstinence and reducing the curiosity to smoke after insular adaptations. Changes to the insula could lead to an immediate and long-term termination of smoking behaviors due to the loss of motivation to smoke. Quit rates in our study were similar to those reported by Naqvi et al. [4] using a retrospective approach, Suñer-Soler et al. [10] at one year post-stroke, and Gaznick et al. [11] at three months post-stroke. However, they differed from Bienkowski et al. [12] who showed no difference in abstinence at three months between insular (37.5%) and non-insular (39.7%) lesioned groups.
Insular damage was associated with higher relative odds of complete abstinence relative to those with non-insular damage even after adjustment for various covariates. This is consistent with the study by Naqvi and colleagues showing higher relative odds of quitting but no significance (OR = 2.94, p = 0.10) [4]. Insular damaged participants may have quit smoking due to a disruption of smoking addiction, while non-insular damaged participants may have quit at a similar rate because of concerns about the negative health consequences of smoking. To distinguish urge from cessation, these two constructs must be measured separately [26]. Cessation may still occur even in the presence of urge, but without urge, relapse is less likely.
To confirm that cessation of cigarette smoking was not driven by the use of other pharmacologic interventions, we investigated the effects of insular lesions on complete abstinence from any nicotine product. As hypothesized, insular damage significantly increased the odds of complete abstinence relative to non-insular damage, suggesting the lack of alternative forms of nicotine needed after quitting from insular damage. This finding may be a consequence of our initial report showing a decrease in withdrawal symptoms among smokers with insular damage [13]. Not surprisingly, acute infarct in the mesocorticolimbic region was also associated with increased odds of complete abstinence from any nicotine, reinforcing our prior knowledge of its involvement in addiction [22, 23].
This study was also the first to explore differences in time to relapse between insular and non-insular exposure groups. We found that among participants who relapsed to smoking after discharge, the average days to relapse was significantly higher in those with insular damage compared to those without insular damage. The multivariable Cox proportional hazards models also showed participants with insular damage to relapse later on average compared to those with non-insular damage. This knowledge is important and supports our hypotheses given the possibility that acute infarctions shown on admission imaging may not have led to permanent cellular damage. Assuming the insula is responsible for maintaining addictive behaviors and cellular function can be regained after temporary damage from infarction, delayed relapse in those with insular lesions – moreso than non-insular lesions – may have been due to a temporary disruption in addiction until cellular regeneration occurred. This also assumes that cellular regeneration occurs at the same rate throughout the cerebral cortex. Further evaluation of lesions should be done to characterize the stability of damage.
To assess the possibility that cessation in insular-damaged participants was driven by a loss of urge, we applied the methods used by Naqvi and colleagues [4]. They found no association between insular damage and quitting smoking on average after eight years of lesion onset, but reported that smokers with insular damage experienced a disruption of smoking addiction, characterized by quitting immediately following their stroke without difficulty, relapse, and urge. This provides support that subjective urges are necessary for maintaining nicotine dependence. Only one other study attempted to duplicate their results but found a null effect [12]. In our sample of quitters, insular damage was associated a significant disruption of smoking addiction, consistent with the original findings, but to a lesser magnitude than what they reported (OR = 22.05, p = 0.0005), although this was limited by their small sample size and retrospective design. We found pre-stroke intent to quit to be a driving factor in this analysis, although it is important to note that this variable was assessed after the quit date as a retrospective assessment, potentially biasing our findings towards the null.
Given the range of functions that the insula has been implicated in, it is important to understand whether insular adaptations influence other motivated or learned behaviors. Because the insular cortex has been suggested to play a role in mediating hunger and gustatory function [27, 28], we evaluated self-reported changes in eating habits from before the stroke to three months post-stroke and found no differences in eating behavior changes between the insular and non-insular damaged groups (p = 0.73). About 25% of participants in both groups reported consuming more food from baseline to follow-up. In the insular group, 34.4% decreased their food consumption, compared to 27.4% in the non-insular group. This finding appears to be consistent with the work of Gaznick and colleagues showing no changes in weight for any participants during the course of the study [11]. Changes in alcohol consumption also did not differ between groups. There is limited evidence to suggest that isolated insular ischemic strokes increase the risk for somatosensory deficits, aphasia, dysarthria, vestibular-like syndrome, and motor deficits [29, 27, 30], but the relevance to our study outcomes is unknown. Thus, our findings regarding smoking cessation appear to be rather specific and not likely confounded by concomitant changes in other behaviors or symptoms.
This study has strengths and limitations. Data were derived from a prospective cohort study, the largest human study to date to assess the relationship between insular cortex lesions and nicotine addiction. We present two definitions of cessation and disruption of smoking addiction to compare to prior studies, in addition to a new time to relapse outcome. These measures, however, were based on self-report with no objective corroboration. Because the location of the stroke was uncontrollable and “random”, exposure status should not have mediated one's intention to give false information. Unlike the previous studies which did not report information on pre-stroke smoking dependence, we retrospectively assessed these measures by asking participants about their cigarette dependency prior to their stroke and controlled for this in the statistical model, as dependent smokers may have more difficulty quitting compared to non-dependent smokers. Retrospective assessments, however, may have been subject to recall error, and recall error may be even more likely given their recent brain injury. Also, the possibility that patient responses to the questions regarding behaviors at baseline were more reflective of their feelings during admission should not be excluded. The risk for exposure misclassification also exists. If the quality of imaging was poor, there may have been some ambiguity as to the involvement of the insula for infarctions deep within the midbrain. Finally, results of this study are not generalizable to all cigarette smokers. The target population, smokers who experience a stroke, could be less “healthy” than other smokers. While there is evidence that smokers who undergo a serious medical event, such as stroke, are likely to make at least some effort to quit [20, 31], the amount of effort put forth is independent of the patient knowing exactly where in the brain the stroke occurred. In other words, the likelihood of a patient quitting smoking due to the stroke event alone should be equal between exposure groups. Therefore, the difference in three month abstinence between the insular and non-insular groups is likely to be a result of the insula's involvement.
5. Conclusions
Our findings of increased quit rates and longer time to relapse in smokers with insular cortex damage support the insula as a neurosubstrate potentially involved in the maintenance of smoking behaviors. Additional molecular-level and controlled experimental studies in animal models as well as observational human studies should examine whether damage in pathways leading to or away from the insula also results in similar patterns. Furthermore, this association should be tested with other addictions and drugs of abuse. If additional findings appear to be insula-specific, it may be a novel target for therapeutic interventions that prove effective in long-term smoking cessation.
Highlights.
Insular damage is associated with increased odds of 3-month continuous abstinence
Insular damage increases the odds of complete cessation for any nicotine product
Time to smoking relapse is higher on average in those with insular lesions
Those abstinent by 3 months with insular damage did not smoke at all post-discharge
The insula may be a target for long-term smoking cessation therapies
Acknowledgments
Special thanks to Adam Kelly, MD (Department of Neurology, University of Rochester Medical Center) for his input and contributions in the early phases of study design and implementation; the nurse practitioners and nursing staff at Strong Memorial Hospital, Highland Hospital, and Rochester General Hospital for assisting in the screening and identification of potentially eligible patients.
Sources of funding: This study was supported in part by the National Heart, Lung, and Blood Institute Preventive Cardiology Training Grant # T32 HL007937 and by the Clinical and Translational Science Institute Grant # UL1 RR024160 from the National Institutes of Health. The funders had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Conflicts of interest: None.
Disclosures: All authors affirm having no conflicts of interest, including financial, personal or other relationships that could inappropriately influence, or be perceived to influence, our work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore M, Jaen C, Baker T, Bailey W, Benowitz N, Curry S, et al. Public Health Service Clinical Practice Guideline. Rockville, MD: U.S Department of Health and Human Services, Public Health Service; 2008. Treating tobacco use and dependence: 2008 update U.S. [Google Scholar]
- 2.Rigotti NA. Treatment of tobacco use and dependence. New England Journal of Medicine. 2002;346:506–512. doi: 10.1056/NEJMcp012279. [DOI] [PubMed] [Google Scholar]
- 3.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine-and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansvelder HD, Fagen ZM, Chang B, Mitchum R, McGehee DS. Bupropion inhibits the cellular effects of nicotine in the ventral tegmental area. Biochem Pharmacol. 2007;74:1283–1291. doi: 10.1016/j.bcp.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nocente R, Vitali M, Balducci G, Enea D, Kranzler HR, Ceccanti M. Varenicline and neuronal nicotinic acetylcholine receptors: a new approach to the treatment of co-occurring alcohol and nicotine addiction? Am J Addict. 2013;22:453–459. doi: 10.1111/j.1521-0391.2013.12037.x. [DOI] [PubMed] [Google Scholar]
- 8.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 9.Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265–671. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Suner-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Davalos A, et al. Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke. 2012;43:131–136. doi: 10.1161/STROKEAHA.111.630004. [DOI] [PubMed] [Google Scholar]
- 11.Gaznick N, Tranel D, McNutt A, Bechara A. Basal Ganglia plus insula damage yields stronger disruption of smoking addiction than Basal Ganglia damage alone. Nicotine Tob Res. 2014;16:445–453. doi: 10.1093/ntr/ntt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bienkowski P, Zatorski P, Baranowska A, Ryglewicz D, Sienkiewicz-Jarosz H. Insular lesions and smoking cessation after first-ever ischemic stroke: a 3-month follow-up. Neurosci Lett. 2010;478:161–164. doi: 10.1016/j.neulet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, et al. Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction. 2015 doi: 10.1111/add.13061. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawkins L, Powell JH, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104:850–858. doi: 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi V, Yousry TA, Muhlert N, Ron M, Golay X, Wheeler-Kingshott C, et al. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry. 2012;83:877–882. doi: 10.1136/jnnp-2012-303023. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kviz FJ, Clark MA, Crittenden KS, Warnecke RB, Freels S. Age and smoking cessation behaviors. Prev Med. 1995;24:297–307. doi: 10.1006/pmed.1995.1048. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. National Institute of Neurological Disorders and Stroke. Stroke Scale. http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf.
- 20.Bak S, Sindrup SH, Alslev T, Kristensen O, Christensen K, Gaist D. Cessation of smoking after first-ever stroke: a follow-up study. Stroke. 2002;33:2263–2269. doi: 10.1161/01.str.0000027210.50936.d0. [DOI] [PubMed] [Google Scholar]
- 21.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Corrigall WA, Franklin KBJ, Coen KM, Clarke PBS. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 23.Jiloha RC. Biological basis of tobacco addiction: Implications for smoking-cessation treatment. Indian J Psychiatry. 2010;52:301–307. doi: 10.4103/0019-5545.74303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rise J, Kovac V, Kraft P, Moan IS. Predicting the intention to quit smoking and quitting behaviour: extending the theory of planned behaviour. Br J Health Psychol. 2008;13:291–310. doi: 10.1348/135910707X187245. [DOI] [PubMed] [Google Scholar]
- 25.DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol. 1991;59:295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- 26.Shiffman S, West R, Gilbert D. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res. 2004;6:599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- 27.Cereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 2002;59:1950–1955. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]
- 28.Berman DE, Hazvi S, Neduva V, Dudai Y. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1-2 and formation of a memory trace. J Neurosci. 2000;20:7017–7023. doi: 10.1523/JNEUROSCI.20-18-07017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemieux F, Lanthier S, Chevrier MC, et al. Insular ischemic stroke: clinical presentation and outcome. Cerebrovascular diseases extra. 2012;2:80–87. doi: 10.1159/000343177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baier B, Conrad J, Zu Eulenburg P, et al. Insular strokes cause no vestibular deficits. Stroke. 2013;44:2604–2606. doi: 10.1161/STROKEAHA.113.001816. [DOI] [PubMed] [Google Scholar]
- 31.Ives SP, Heuschmann PU, Wolfe CD, Redfern J. Patterns of smoking cessation in the first 3 years after stroke: the South London Stroke Register. Eur J Cardiovasc Prev Rehabil. 2008;15:329–335. doi: 10.1097/HJR.0b013e3282f37a58. [DOI] [PubMed] [Google Scholar]