Summary
Genetic and clinical association studies have identified disrupted-in-schizophrenia 1 (DISC1) as a candidate risk gene for major mental illness. DISC1 is interrupted by a balanced chr(1;11) translocation in a Scottish family, in which the translocation predisposes to psychiatric disorders. We investigate the consequences of DISC1 interruption in human neural cells using TALENs or CRISPR-Cas9 to target the DISC1 locus. We show that disruption of DISC1 near the site of the translocation results in decreased DISC1 protein levels due to nonsense-mediated decay of long splice variants. This results in an increased level of canonical Wnt signaling in neural progenitor cells and altered expression of fate markers such as Foxg1 and Tbr2. These gene expression changes are rescued by antagonizing Wnt signaling in a critical developmental window, supporting the hypothesis that DISC1-dependent suppression of basal Wnt signaling influences the distribution of cell types generated during cortical development.
Introduction
Schizophrenia and other major mental illnesses (MMIs) are widely regarded to result from a combination of genetic susceptibility and environmental insults. Clinical and genetic studies indicate that schizophrenia and other MMIs are likely diseases of altered circuitry resulting from disruptions in neurodevelopment (Harrison, 1999; Weinberger, 1995; Williams et al., 2009). The recent expansion of GWAS studies has identified many interesting but generally weak genetic linkages to MMI (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013; Ripke et al., 2013; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). There also are rare strong genetic variants that have been associated with mental illness, including various copy number variants (CNVs) and mutation of the gene disrupted in schizophrenia 1 (DISC1) (Millar et al., 2000; Mitchell, 2011; Sullivan et al., 2012). DISC1 was initially associated with mental illness upon the discovery that its coding sequence is interrupted by a balanced chr(1;11) translocation in a Scottish family, in which the translocation cosegregates with schizophrenia, bipolar disorder and major depression (Blackwood et al., 2001; Millar et al., 2000; St Clair et al., 1990). The diversity of phenotypes in subjects harboring the translocation supports the hypothesis that the translocation leads to a subtle underlying disruption in neural development that predisposes to MMI by increasing vulnerability to other environmental and genetic risk factors. While such rare variants are not likely to contribute significantly to the incidence of sporadic disease, they offer valuable opportunities for investigation. Here, we engineered a disease-relevant disruption of the DISC1 locus in an isogenic background to investigate if and how DISC1 mutation might lead to a subtle underlying disruption in development that predisposes to MMI.
DISC1 has been implicated in several neurodevelopmental processes, including proliferation, synaptic maturation, neurite outgrowth, and neuronal migration. In addition, many known DISC1 interacting proteins have independently been associated with neuropsychiatric diseases, further implicating this network of proteins in the pathophysiology of mental illness (reviewed in (Brandon and Sawa, 2011)). The vast majority of studies showing functions of DISC1 in neural development were performed in rodents. Dozens of splice variants of DISC1 have been identified in the developing human brain (Nakata et al., 2009), and the architecture of splice variant expression is not identical between humans and rodents (Ma et al., 2002; Taylor et al., 2003). The evolutionary divergence of cerebral cortex development in humans and rodents, coupled to differences at the DISC1 locus between species, raises the importance of interrogating the effects of disease-relevant disruption of DISC1 isoforms in a model of human neurodevelopment.
Here, we study the consequences of DISC1 disruption in isogenic stem cell lines generated using transcription activator-like effector nucleases (TALENs) or clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 to interrupt DISC1 near the site of the balanced translocation or in an exon common to all isoforms. Multiple isogenic clonal lines are compared for each genotype, allowing for careful study of the effects of genomic DISC1 interruption on gene expression and neuronal development. We show that disease-relevant DISC1 targeting decreases DISC1 protein expression, which in turn results in increased Wnt signaling in neural progenitor cells and changes in expression of markers of cell fate. DISC1-dependent Wnt signaling and changes in expression of cell fate markers can be reversed by antagonizing the Wnt pathway during a critical window in neural progenitor development. These experiments suggest that disruption of long DISC1 isoforms results in elevated basal Wnt signaling, which alters the identity of neural progenitors, thereby modifying Wnt responsiveness and neuronal identity. The data herein identify effects of DISC1 disruption on human cerebral cortical development, thereby shedding light on the functions of DISC1 relevant to the pathogenesis of major mental illness.
Results
Genomic DISC1 exon 8 interruption results in loss of DISC1 expression due to nonsense-mediated decay
In order to investigate the effects of DISC1 interruption at the site of the Scottish translocation, we introduced DISC1 frameshift mutations into control iPSCs. Mutations were introduced into exon 8 (within 10 codons of the site of the translocation) or exon 2 (intended to disrupt all known coding isoforms) (Fig 1). Using TALENs or CRISPR-Cas9, we generated isogenic human iPSC lines that are wild-type (wt) or have frameshift mutations in exon 8 (monoallelic: exon 8 wt/mut or “ex8wm”, biallelic: “exon 8 mut/mut” or “ex8mm”) or exon 2 (biallelic: exon 2 mut/mut or “ex2mm”). In the chr(1;11) balanced translocation, splicing of DISC1 exon 8 to the next two available exons on chr11 results in the introduction of a premature stop codon (PTC) within 2 or 61 codons. Exon 8 targeting generated indels that result in a PTC within 3 codons (exon 8 ins 1 bp) or 27 codons (exon 8 del 1 bp). Similarly, exon 2 targeting yielded biallelic indels resulting in a PTC within 12 codons (exon 2 ins 1 bp) or 65 codons (exon 2 ins 2 bp). Targeted cells maintained iPSC colony morphology, expressed iPSC markers (Fig 1E; Fig S1), and maintained a euploid karyotype (Fig S2). No predicted off-target cleavage events were detected using RNA-sequencing (see Supplemental Experimental Procedures). Multiple iPSC clones of each genotype were used for the studies described herein to minimize the potential effects of clonal variability (Table S1).
Figure 1.
Genome editing of the human DISC1 locus. (A) Diagram of the coding sequence of DISC1, with the sites of induced frame shifts and the Scottish chr(1;11) translocation indicated. (B) Diagram of the DISC1 genomic locus, with exons shown on the top line and sample transcripts shown below. A selection of alternatively spliced isoforms are shown in red. (C,D) Wild-type and mutant sequences around the exon 8 (C) or exon 2 (D) targeting sites, obtained by Sanger sequencing of targeted iPS colonies. Mutation sites are indicated with black arrowheads. (E) Immunostaining for pluripotent cell markers Nanog, Oct4, SSEA4, Sox2, and TRA-1-60 following genome-editing. Scale bar = 50 um. See also Figure S1.
Genomic DISC1 interruption has been hypothesized to cause expression of a truncated or mutant DISC1 or to decrease DISC1 expression from the mutated allele (Brandon and Sawa, 2011). The introduction of a PTC into a coding RNA often results in nonsense-mediated decay (NMD) if the PTC occurs prior to the last exon-exon junction of the transcript (Silva and Romão, 2009). Thus, we hypothesized that genomic DISC1 interruption would cause decay of PTC-containing mRNAs that extended at least one exon past the interruption. Given the complexity of human DISC1 splicing (Nakata et al., 2009), we chose to analyze transcription at multiple DISC1 coding regions.
To examine the effects of genomic DISC1 interruption on DISC1 expression, RNA was harvested from NPCs and differentiated neurons. Wild-type and DISC1-targeted iPSCs were differentiated to neural fates using an embryoid aggregate-based protocol (Muratore et al., 2014a)(Muratore et al., 2014b), which results in definitive neuroepithelium by day 17 and expression of neuronal makers past day 25. We characterized the expression of multiple isoforms of DISC1 by examining RNA expression of distinct exons. Day 40 neuronal qPCR analyses revealed that the presence of a single exon 8 mutant allele did not significantly decrease DISC1 exon 2 expression but did decrease expression of the longest DISC1 isoforms (Fig 2A,B). Biallelic exon 8 interruption significantly decreased DISC1 exon 2 and long DISC1 transcripts (Fig 2A,B). Surprisingly, biallelic exon 2 mutation did not reduce DISC1 expression (Fig 2A). We further investigated levels of various DISC1 isoforms in NPCs (day 17) and neurons (day 50) using a custom NanoString probeset, which included probes targeting multiple DISC1 exons. The NanoString analyses confirmed the qPCR data, where introduction of a PTC into exon 8 decreased expression of longer but not shorter DISC1 transcripts at days 17 and 50 (Fig 2C). Unexpectedly, exon 2 targeting appeared to decrease DISC1 expression at day 17 (Fig 2C), but either did not change or increased expression of various DISC1 isoforms at days 40–50 (Fig 2A–C).
Figure 2. DISC1 exon 8-disruption causes loss of full length DISC1 expression via NMD.
(A,B) Wild-type and DISC1-disrupted iPSCs were differentiated to day 40 neural fates and RNA was harvested for qRT-PCR of DISC1 exon 2 (A) or exons 12/13 (B). Expression is normalized to GAPDH and wt/wt levels. (C) iPSCs were differentiated to NPCs (day 17) and neurons (day 50) and RNA was harvested for NanoString. DISC1 probe expression is normalized to expression of 8 housekeeping genes and average wt/wt d17 levels. Statistics shown versus wt of corresponding differentiation day. (D) Representative Western blot of DISC1 in day 40 neuronal lysates. Full length DISC1 is indicated with blue arrows; novel truncated DISC1 is indicated by red arrows. The red asterisks denote a nonspecific band. (E,F) Quantification of full-length (E) or all (F) DISC1 from Western blots of day 40 neuronal lysates, normalized to GAPDH and wt/wt levels. (G,H) Day 40 neurons were treated with 100 ug/ml cycloheximide (CHX) or vehicle (DMSO) for 3 hrs, followed by RNA harvest and qRT-PCR for exon 2 (G) or the exon 12/13 junction (H) of DISC1. Expression normalized to GAPDH and average DMSO expression within genotype. All data derived from at least 5 independent differentiations, n is shown for each panel within bars or in the legend. Stats: (C) 2-way ANOVA, (A,B,E-H) 1-way ANOVA. Mean ± SEM shown. ns = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. See also Figure S3.
To investigate the effects of DISC1 interruption at the protein level, we performed Western blots of day 40 neuronal lysates and analyzed DISC1 expression using a monoclonal antibody generated against the C-terminus of DISC1. This antibody detected a single band of approximately 85 kDa, roughly corresponding to the predicted molecular weight of translated long (or “full-length”) DISC1 isoforms (L: 854 aa, Lv: 832 aa). Single allelic DISC1 exon 8 mutation resulted in a ~55% decrease in DISC1 protein expression, while biallelic exon 8 mutation led to total loss of detectable DISC1 protein (Fig 2D–F). Biallelic exon 2 targeting showed a total loss of full-length protein, but unexpectedly led to the expression of a shorter, novel DISC1 protein product in day 40 neural cells (Fig 2D, red arrows). This truncated protein was expressed at levels nearly identical to that of wild-type long isoform DISC1 (Fig 2F). Although unexpected, the truncated DISC1 protein resulting from exon 2 mutation provided an opportunity to analyze the effects of an independent DISC1 mutation in a system that was isogenic to our disease-relevant (exon 8-disruption) model. As several prior studies have demonstrated that misexpression of a mutant truncated DISC1 can phenocopy DISC1 knockdown (reviewed in Brandon and Sawa, 2011), we used our exon 2- and exon 8-targeted cells to explore whether these observations would hold true in a human neuronal context.
To determine whether the observed changes in DISC1 expression in the disease-relevant exon 8-targeted cells were due to NMD of PTC-containing mRNAs, we treated day 40 neurons with cycloheximide (CHX), which inhibits NMD by interfering with the “pioneer” round of translation required to detect the PTC (Ishigaki et al., 2001). Analysis of DISC1 expression after CHX treatment revealed significantly increased NMD in exon 8- but not exon 2-targeted cells (Fig 2G,H). These data suggest that introduction of a PTC into DISC1 exon 8, but not exon 2, results in NMD of PTC-containing transcripts.
We further explored the effects of DISC1 genomic disruption on DISC1 protein stability, using cycloheximide (CHX) to prevent translation in day 40 neurons. DISC1 protein was remarkably refractory to turnover under these conditions, showing very little or no degradation over 24 hours, and no significant alteration in protein stability with exon 8 or exon 2 mutations (Fig S3). Efficacy of the CHX treatment was confirmed by detecting APP levels, which showed a half life of ~4 hr for this protein, in agreement with previous reports (Lefort et al., 2012; Vieira et al., 2010). These results suggest that DISC1 exon 8 mutation results in NMD of longer DISC1 transcripts and exon 2 mutation causes expression of an N-terminally-truncated DISC1, but neither mutation destabilizes DISC1 protein.
Genomic DISC1 interruption alters expression of NPC fate markers
To examine the broader effects of DISC1 disruption, we characterized gene expression profiles and cell fate in wild-type and DISC1-targeted NPCs. Neural differentiation resulted in expression of transcription factors consistent with dorsal forebrain NPC fates across all genotypes at day 17 (Fig 3A). In order to quantitatively measure expression of a broad selection of neural markers, we used a custom NanoString codeset to assay RNA expression in rosette-selected day 17 NPCs. NanoString analyses showed that DISC1 exon 8 and exon 2 interruption significantly decreased FoxG1 and Tbr2 (EOMES) expression (Fig 3B). There also was decreased Sox1 and increased Pax6 expression in exon 2-targeted NPCs, suggesting that exon 2 mutation causes more dramatic changes than exon 8 interruption (Fig 3B,C). DISC1 interruption did not alter expression of other broad progenitor markers, such as Sox2, HES1, Nestin (NES), or Vimentin (VIM) (Fig 3B,C). The decrease in FoxG1 and Tbr2 RNA in the absence of decreased expression of other cortical progenitor markers argued against a deficit in telencephalic differentiation.
Figure 3. DISC1 disruption causes subtle alterations in NPC fate.
(A) iPSCs were differentiated to day 17 NPCs, rosette selected, plated for 48 hours, and immunostained for the NPC markers as shown. Scale bar = 50 um. (B,C) iPSCs were differentiated to day 17 NPCs, rosette selected, and RNA was harvested for NanoString. Gene expression is normalized to expression of 8 housekeeping genes and wt/wt levels. (D,E) NPCs were immunostained for Tbr2 (D) or FoxG1 (E), and co-stained with DAPI. Percentage of Tbr2- or FoxG1-expressing cells was calculated using the Cell Counter plugin in FIJI. Only Nestin-positive cells were counted. Number of cells counted: [Tbr2: wt/wt: 3916, ex8wm: 5873], [FoxG1: wt/wt: 5921, ex8wm: 5424]. Data derived from at least 5 independent differentiations. Statistics: (B,C) 2-way ANOVA, (D,E) 2-tailed t-test. Mean ± SEM shown. ns = not significant, * p < 0.05, ** p < 0.01, **** p < 0.0001.
In order to explore putative changes in the percentage of cells expressing FoxG1 and Tbr2, we quantified immunostaining in wild-type NPCs and NPCs with our disease model mutation, DISC1 exon 8 wt/mut. Tbr2 quantification revealed a significant decrease in the percentage of Tbr2-expressing cells (Fig 3D), demonstrating that the decreased Tbr2 RNA expression reflects fewer Tbr2+ cells. In contrast, although FoxG1 RNA expression was also decreased by DISC1 disruption, immunostaining quantification revealed that nearly 100% of NPCs expressed FoxG1 regardless of DISC1 mutation status (Fig 3E). This indicates that the FoxG1 gene expression data reflect a per-cell decrease in expression, rather than a decrease in the number of FoxG1+ cells.
DISC1 interruption subtly alters neuronal fate but not neuronal maturity
We further investigated effects of DISC1 disruption on expression of cell fate markers in iPSC-derived neurons. Differentiation for 40 days resulted in marker expression characteristic of lower- and upper-layer neurons (CTIP2, Tbr1, Cux1, Brn2, Satb2) as well as general neuronal markers (Tau, MAP2; Fig 4A). A population of NPCs persisted in these cultures (Nestin, Tbr2; Fig 4A). Neural cultures of all genotypes demonstrated spontaneous action potentials by day 42 when co-cultured with astrocytes (Fig 4B,C). Gene expression was assayed in day 50 neurons by NanoString, revealing a persistent decrease in Tbr2 and FoxG1 in DISC1 disrupted neurons (Fig 4D). There were no significant expression changes of general neuronal markers (Fig 4E). Exon 2 mut/mut neurons expressed lower levels of select mature neuronal genes VGLUT1 (SLC17A7), GRIN1, and MAP2 (Fig 4F). Exon 8 interruption did not significantly alter neuronal layer marker expression, while exon 2 interruption significantly decreased expression of cortical neuronal markers CTIP2 (BCL11B), FezF2, and Tbr1, with a trend for decreased expression of other cortical markers Satb1 and Cux1 (Fig 4F). These gene expression changes support the day 17 NPC data, where exon 8 interruption subtly affects expression of cell fate markers without impairing neuronal differentiation, and exon 2 interruption causes a broader dysregulation of neurogenesis.
Figure 4. DISC1 disruption does not alter neuronal differentiation capacity or maturity but causes subtle alterations in cell fate.
(A) iPSCs were differentiated to day 40 neuronal fates and immunostained for the markers labeled. Scale bar = 50 um. (B,C) Day 24 cells were dissociated and plated with human astrocytes on microelectrode arrays. (B) Examples of day 42 single unit waveforms are shown. (C) Example raster plots from day 47 neurons. Each row represents one electrode; 8 representative electrodes were selected from 1 recording of each of the genotypes as shown. (D–F) iPSCs were differentiated to day 50 neuronal fates and RNA was harvested for NanoString. Gene expression is normalized to expression of 8 housekeeping genes and wt/wt levels. (G,H) Quantification of Western blots for SYP (G) and SYN I (H) in day 40 neuronal lysates, normalized to Tau (similar results observed with normalization to MAP2 and GAPDH). (I) Representative Western blot of neuronal markers SYN I, SYP, MAP2, Tau, and loading control GAPDH in day 40 neuronal lysates. Same samples as shown in Fig 2D (GAPDH is shown in both panels for reference). Data derived from at least 8 independent differentiations. Statistics: (D–F) 2-way ANOVA, (G,H) 1-way ANOVA. Mean ± SEM shown. ns = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
We further investigated neuronal maturity by assessing expression of presynaptic proteins. Western blot analyses revealed no significant alterations in SYP or Syn I expression with DISC1 disruption (Fig 4G–I), in contrast to a different clinical DISC1 mutation (exon 12 Δ4bp (Sachs et al., 2005)), which was found to result in a dramatic increase in both (Wen et al., 2014).
RNA-sequencing supports a shift in gene expression profiles towards dorsal identities in DISC1-interrupted NPCs and neurons
In order to obtain a genome-wide view of gene expression changes, we performed RNA-sequencing (RNA-seq) on day 17 NPC samples and day 50 neuronal samples of wild-type and DISC1-disrupted cells. These data show that interruption at exon 8 did not alter global gene expression as dramatically as exon 2 (Fig 5, S4; Table S2). Examination of cell fate markers largely confirmed and extended the NanoString findings, suggesting a subtle shift in expression of cell fate markers with DISC1 interruption (Fig 5), including the decreased FoxG1 expression in DISC1-disrupted NPCs (Fig 5D). To investigate potential dorsal-ventral fate shifts, we evaluated expression of a subset of dorsal and ventral markers. As the “default” neuronal differentiation pathway of human stem cells generates dorsal, excitatory cortical neurons (Espuny-Camacho et al., 2013; Kim et al., 2011; Li et al., 2009), many markers characteristic of ventral or interneuron progenitors were not expressed in these cells (less than 10 reads in 10 samples, including: NKX2-1, NKX2-2, NKX6-2, FOXA2). Among telencephalic progenitor markers, expression of a selection of roof plate and dorsal progenitor markers was increased (Pax3, Pax7, Msx2, NeuroG2, Dbx2, Wnt8B) and ventral progenitor markers decreased (ASCL1, DLX1, DLX2, Gsx2) by DISC1 interruption (Fig 5A,B). Notably, Pax3, Pax7, NeuroG2, Msx2, Dbx2, Wnt8B, ASCL1, DLX1/2, GSX2, FoxG1, and Tbr2 are identified in transcriptome-wide comparisons (Table S2). While this RNA-seq analysis is limited by low sample numbers, it supports a subtle dorsal shift in cell fate with DISC1 interruption.
Figure 5. RNA-seq supports a dorsal fate shift in DISC1-targeted NPCs and neurons.
iPSCs were differentiated to NPCs (day 17) and neurons (day 50) and RNA was harvested for RNAseq. Counts were upper-quartile normalized. Expression of selected (A) Dorsal telencephalic markers, (B) ventral telencephalic markers, (C,D) NPC markers, (E,F) neuronal markers are shown. Statistics shown versus wt of corresponding differentiation day. Data derived from 2–6 independent differentiations. Statistics: 2-way ANOVA. Mean ± SEM shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. (G–J) Gene ontology analysis of exon 8 wt/mut vs. wt/wt day 17 (G,H) and day 50 (I,J) cells. See also Figure S4.
Gene ontology analysis identified genes involved in neural development, mental disorders, schizophrenia, Wnt signaling, cell adhesion, and other pathways as being significantly altered in our exon 8 wt/mut disease model at days 17 or 50 (Fig 5G–J). Comparison of differentially expressed genes across genotypes and to other existing data sets also highlights priority genes for future study based on their involvement in another model of DISC1-related mental illness (Wen et al., 2014) or in human neural progenitor development (Johnson et al., 2015) (Fig S4E–G).
The observed changes in gene expression suggested that disease-relevant DISC1 exon 8 interruption did not drastically alter neuronal differentiation capacity or neuronal maturation but did result in significant changes in gene expression, including decreased FoxG1 and Tbr2 expression. FoxG1 is a marker of telencephalic progenitors (Molyneaux et al., 2007; Shimamura et al., 1995; Tao and Lai, 1992; Xuan et al., 1995), while Tbr2 is expressed in intermediate progenitor (IP) cells of the cortex (Englund et al., 2005; Hevner et al., 2006; Sessa et al., 2008). While FoxG1 is expressed broadly in the progenitor cells of the developing neocortex, it is particularly critical for differentiation of ventral forebrain neurons (Manuel et al., 2010; Martynoga et al., 2005; Xuan et al., 1995) and is expressed in a high-ventral to low-dorsal gradient in the developing forebrain (Danesin et al., 2009).
We hypothesized that DISC1-disruption might subtly alter neural cell fate via an established patterning pathway. The Wnt proteins are a major family of patterning molecules that are present in a dorsal to ventral gradient and act on neural progenitors to create a dorsal neuronal population (Ulloa and Martí, 2010). Artificially elevating Wnt signaling in the developing rodent cortex has been shown to decrease FoxG1 expression and cause a dorsal shift in neural identity (Backman et al., 2005). Wnts also have been shown to trigger IP differentiation and premature neuronal differentiation, thus reducing the abundance of Tbr2-positive cells in the cortex (Hirabayashi et al., 2004; Munji et al., 2011). Finally, DISC1 has been shown to participate in the Wnt signaling pathway via interactions with GSK3β (Mao et al., 2009) or D2R (Su et al., 2014). Since the observed changes in gene expression and cell fate could all be related to reported effects of increased Wnt signaling, and DISC1 has been shown to have multiple roles in the Wnt pathway, we next sought to investigate the Wnt signaling properties of DISC1-targeted neural progenitors.
DISC1-disrupted NPCs display elevated baseline Wnt signaling activity, independent of dorsal identity
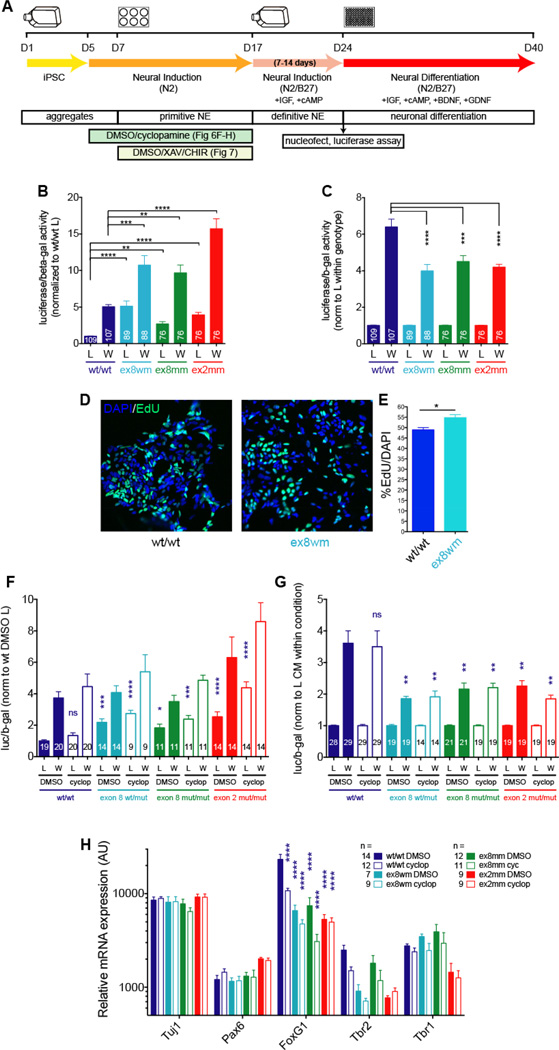
We investigated canonical Wnt signaling in NPCs derived from our isogenic wild-type and DISC1-disrupted iPSCs. We evaluated Wnt signaling by introducing a TCF/LEF-responsive luciferase (Super8xTOPFLASH) into cells by Amaxa nucleofection, followed by incubation with control (L) or Wnt3a (W) conditioned media (CM) for 24 hrs. Analysis of luciferase activity in cell lysates showed that DISC1-disrupted NPCs displayed decreased Wnt responsiveness, with a smaller fold change in luciferase activity after Wnt3a application relative to control NPCs (Fig 6C). However, this decreased Wnt response is driven by increased baseline Wnt signaling in DISC1-disrupted cells (wt vs. DISC1 mutant “L” CM luciferase activity, Fig 6B). Interestingly, this phenotype is not present in iPSCs (Fig S5), highlighting the cell-fate specificity of this DISC1-mediated alteration in Wnt signaling. These results suggest that DISC1 disruption decreases the Wnt response in human telencephalic NPCs by increasing basal Wnt signaling.
Figure 6. DISC1 interruption increases basal Wnt signaling and decreases Wnt responsiveness in NPCs, independent of Shh antagonism.
(A) Schematic of experimental design. iPSC-derived day 24 NPCs were nucleofected with plasmids encoding the Super8XTOPFlash luciferase reporter and pMIR-REPORT-beta-galactosidase (b-gal) as a marker of transfection efficiency. After 18 hr, media were changed to control (L) or Wnt3a (W) conditioned media (CM) for 24 hr, followed by cell lysis. Cell lysate luciferase activity (luc) was normalized to beta-galactosidase activity (b-gal) and wt/wt L levels (B), L levels within genotype (C), wt/wt DMSO L levels (D), or L levels within condition (E). (B,C) DISC1-disruption causes increased baseline Wnt signaling (B), which drives decreased Wnt response (C). (D,E) DISC1 interruption increases baseline NPC proliferation. NPCs were cultured in control CM for 24 hr, followed by addition of 10 uM EdU for 4 hr, and EdU withdrawal for 4 hr. (D) Example images of EdU and DAPI. (E) Quantification of %EdU/DAPI. Data derived from 4 independent differentiations. (F,G) Wild-type and DISC1-disrupted cells treated with vehicle (DMSO) or cyclopamine (cyclop) to dorsalize NPCs for days 5–17 do not alter baseline Wnt signaling (F) or Wnt response (G). Statistics in (F) versus wt DMSO L; statistics in (G) versus wt DMSO W. Sample numbers shown in bars. (H) RNA was harvested from day 40 neurons and used for Nanostring. Gene expression was normalized to all genes. Statistics shown versus wt DMSO. Mean ± SEM shown. Data derived from at least 3 independent differentiations. Stats: (B-G) 1 way ANOVA, (H) 2 way ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
We next explored the functional consequences of the observed increase in baseline Wnt signaling with DISC1 disruption by examining NPC proliferation in wild-type and DISC1 exon 8 wt/mut cells. EdU incorporation showed an increase in the basal proliferation rate of exon 8 wt/mut NPCs relative to wild-type cells (Fig 6D,E). These studies suggest that the increased baseline luciferase Wnt signaling activity in DISC1-disrupted NPCs results in increased baseline neural progenitor proliferation.
The fate-related gene expression changes and increased baseline Wnt signaling observed in DISC1-disrupted cells could be related or independent phenotypes. Altered cell fate could modify Wnt signaling by changing the pool of cells being assayed, increased baseline Wnt signaling could alter cell fate, or altered Wnt signaling and cell fate could be interrelated In a more complex manner. If dorsal NPC fates have different intrinsic Wnt signaling properties than more ventral NPCs, changes in Wnt signaling may be observed in dorsal-shifted NPCs. To assess whether the observed alteration in Wnt signaling could be induced in wild-type cells by forcing a dorsal fate, we pushed NPCs to more dorsal fates by antagonizing Shh signaling with cyclopamine during days 5–17 of differentiation (Chen et al., 2002; Wen et al., 2014). NPCs were rosette selected and cultured as neural aggregates without cyclopamine for ~13 days prior to Wnt signaling assays (Fig 6A). Cyclopamine pretreatment did not alter baseline levels of Wnt signaling or Wnt responsiveness in wild-type or DISC1-disrupted NPCs (Fig 6F,G). In parallel with Wnt assays, dissociated NPCs were plated and differentiated as neurons for 16 days, followed by RNA harvest. NanoString showed that a window of prior cyclopamine exposure did not alter expression of many cell fate markers, supporting previous studies demonstrating the “default” dorsal fate of NPCs differentiated from human pluripotent cells (Espuny-Camacho et al., 2013; Kim et al., 2011; Li et al., 2009). Cyclopamine treatment did decrease FoxG1 expression in wild-type cells (Fig 6H), suggesting that decreased FoxG1 expression in DISC1-disrupted cells can be mimicked in wild-type cells using cyclopamine to dorsalize NPCs. However, the increased baseline Wnt signaling and decreased Wnt-responsiveness of DISC1-disrupted NPCs is not induced in wild-type cells with cyclopamine treatment. Thus, shifting cells to a more dorsal identity is not sufficient to induce the altered Wnt signaling seen in DISC1- disrupted cells.
Wnt signaling and gene expression changes in DISC1-disrupted cells can be rescued with Wnt antagonism in a critical window of neurodevelopment
The elevation in baseline canonical Wnt signaling in DISC1-disrupted NPCs suggested an alternate mechanism, whereby DISC1 interruption alters cell fate via increased Wnt signaling. In order to test this hypothesis, we treated wild-type and DISC1 exon 8 wt/mut NPCs with either a Wnt agonist (CHIR99021) or antagonist (XAV939) during days 7–17 of differentiation. Immunostaining of NPCs showed dramatic morphological and gene expression changes with CHIR99021 treatment, including decreased FoxG1 and Tbr2 expression, and increased MAP2 expression, while XAV treatment had no qualitative effects on immunostaining of neural markers (Fig 7A). Neural rosettes were then cultured in suspension as neural aggregates in the absence of small molecules prior to assaying Wnt signaling. Prior Wnt agonism caused wild-type cells to mimic the decreased Wnt responsiveness of DISC1-targeted cells (Fig 7C). CHIR99021 treatment also caused a dramatic decrease in Wnt baseline signaling, likely due to the observed decrease in NPCs and increase in premature neuronal differentiation (Fig 7A,B). In contrast, prior Wnt antagonism with XAV939 decreased baseline Wnt signaling and increased Wnt responsiveness in DISC1 exon 8 wt/mut NPCs (Fig 7B,C). The altered Wnt response of DISC1-targeted NPCs can thus be rescued by prior Wnt antagonism during a 10-day window early in neural development.
Figure 7. Wnt antagonism in a critical early window rescues altered Wnt signaling and cell fate in DISC1-disrupted cells.
iPSCs were differentiated to NPCs and treated with vehicle (DMSO), Wnt antagonist XAV939 (XAV), or Wnt agonist CHIR99021 (CHIR) during days 7–17. After rosette selection at d17, small molecules were withdrawn. (A) Examples of immunostained NPCs (d19) are shown. Scale bar = 50 um. (B,C) Prior exposure to Wnt antagonist XAV939 decreases baseline Wnt signaling and increases Wnt responsiveness in NPCs, whereas Wnt agonist CHIR99021 decreases Wnt responsiveness. iPSC-derived NPCs were nucleofected with plasmids encoding the Super8XTOPFlash luciferase reporter and pMIR-REPORT-beta-galactosidase as a marker of transfection efficiency. After 18 hr, media were changed to control (L) or Wnt3a- (W) conditioned media (CM) for 24 hr followed by cell lysis. Cell lysate luciferase activity (luc) was normalized to beta-galactosidase activity (b-gal) and wt/wt DMSO L levels (B) or L levels within treatment condition (C). Sample numbers shown in bars. (D–F) RNA was harvested from day 40 neurons and used for Nanostring. (D,E) Expression of selected progenitor and neuronal, dorsal, ventral, and caudal markers are shown. (F) Expression of Wnt pathway genes are shown. Gene expression is normalized to all genes. Significance asterisks in dark blue are versus wt/wt DMSO; asterisks in light blue are versus ex8wm DMSO. All luciferase assay and Nanostring samples are independent of samples used in Fig 6. Mean ± SEM shown. Data derived from at 3 independent differentiations. Stats: (B,C) 1 way ANOVA, (D–F) 2 way ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Concurrent with Wnt signaling assays, NPCs were plated and differentiated as neurons for 16 days followed by RNA harvest and gene expression analyses. Antagonizing NPC Wnt signaling with XAV939 rescued decreased FoxG1, Brn2, and Tbr2 expression in exon 8 wt/mut neurons (Fig 7D,E). XAV939 also rescued increased DAB2 expression in exon 8 wt/mut cells, whereas CHIR99021 increased DAB2 levels in wt and exon 8 wt/mut neurons (Fig 7F).
Stimulating Wnt signaling with CHIR99021 pushed NPCs to spontaneously differentiate, decreasing expression of progenitor markers and cortical layer markers (Fig 7 D,E). CHIR99021 treatment also resulted in decreased expression of ventral marker GSX2 and increased expression of caudal genes HOXA1/2 and HOXB4, consistent with the effects of Wnt in dorsal and posterior patterning (Fig 7E). Furthermore, prior treatment of NPCs with CHIR99021 altered neuronal expression of Wnt pathway genes AXIN2, Irx3 and Six3 (Fig 7F). The reversal of gene expression changes in exon 8-targeted neurons with Wnt antagonism during NPC development suggests that altered cell identity in DISC1-disrupted cells is downstream of increased Wnt signaling. Wnt antagonism in DISC1 exon 8 wt/mut cells also rescued increased baseline Wnt signaling and decreased Wnt responsiveness in DISC1-targeted NPCs. These studies support a model in which altered Wnt signaling and cell identity are interdependent phenotypes in DISC1-disrupted NPCs and neurons (Fig S6).
Discussion
The study of genetic predispositions to mental illness, even if found only in a small subset of patients, will improve our understanding of the pathophysiology of these debilitating disorders. Both rare, highly-penetrant and common, weaker DISC1 variants have been associated with multiple MMIs (reviewed in (Brandon and Sawa, 2011)). The Scottish chr(1;11) translocation provides an opportunity to investigate the pathophysiology of a rare genetic alteration that has been strongly linked to MMI (Porteous et al., 2014). Animal models have identified critical neurodevelopmental roles for DISC1 (Brandon and Sawa, 2011). Due to differences in DISC1 splicing between human and rodent samples (Ma et al., 2002; Nakata et al., 2009), as well as the evolutionary divergence in development of the cerebral cortex, there is a need to complement animal models with studies of human neurons and glia. Furthermore, while DISC1 has been shown to function in a myriad of pathways, studies such as ours prioritize the developmental phenotypes relevant to increasing risk for MMIs.
It is important to keep in mind that patients with MMI, with or without DISC1 disruption, do not show dramatic morphological alterations in the brain, and do not show global neuronal dysfunction. In order to identify relatively subtle developmental defects, and to attribute these to a particular mutation, it may be necessary to generate isogenic lines and interrogate multiple lines over several differentiations with high numbers of wells. To investigate the consequences of DISC1 interruption in human neurons with a controlled genetic background, we TALEN- and CRISPR-Cas9-targeted iPSCs. We found that genomic DISC1 interruption near the site of the Scottish chr(1;11) translocation causes loss of expression of longer DISC1 transcripts from the mutated allele by NMD, which increases baseline Wnt signaling and alters the transcriptional profile of NPCs and neurons. These data support a LOF model, wherein DISC1 interruption causes decreased full-length DISC1 expression and has downstream developmental consequences.
The limited evidence that exists from t(1;11) patients suggests that the translocation lowers DISC1 transcript and protein levels in patient-derived non-neuronal cells (Millar et al., 2005). Based on the genomic structure surrounding the t(1;11) breakpoint, if splicing occurs between DISC1 exon 8 and the next available exon on chr11, the resulting transcript would contain a PTC prior to the last exon of the chr11 gene. This fusion transcript should therefore also recruit the NMD pathway, as observed in our exon 8 mutant model. The common effect of exon 8 1 bp insertion or deletion on decreasing DISC1 expression suggests that the number of codons (3 or 27) preceding the PTC does not alter targeting of the transcript for NMD. The exon 2 mutant lines demonstrate that an independent DISC1 mutation can dysregulate many of the same processes as our disease-relevant exon 8 disruption, and has even stronger effects on neuronal cell identity. The phenotypes observed in exon 2 lines may result from a loss of long DISC1 isoforms and/or expression of the truncated protein. As exon 2 mutation does not dramatically alter DISC1 transcript structure by RNA-seq (Fig S4A), we hypothesize that the truncated DISC1 protein may result from forced usage of an alternate start codon downstream of the introduced frameshift mutation. It should be noted that because we have not generated the chr(1;11) translocation, we cannot assess for presence of a fusion transcript, evaluate the effects of disruption of the antisense lncRNA DISC2, or assess for alterations in expression of translocation-adjacent genes. In patients carrying t(1;11), there has been no evidence of expression of a truncated DISC1 or a DISC1 fusion protein with the nearest downstream chromosome 11 gene (DISC1FP1 or Boymaw) (Millar et al., 2005). However, there is evidence of expression of a fusion protein of DISC1 with the upstream gene, translin-associated factor X (TSNAX/TRAX) in the brain (Millar et al, 2000b). The balanced translocation may affect expression of this protein in a manner that is not recapitulated with our engineered mutations.
The results described herein reveal a role of DISC1 disruption in disordered neurodevelopment. Previous analyses of sporadic psychiatric disease with human iPSCs have also reported changes in neural development (Brennand et al., 2015; 2011; Yu et al., 2014). Another study used iPSCs with a distinct DISC1 mutation found in an American family, in which a 4 bp deletion in exon 12 co-segregates with schizophrenia (Sachs et al., 2005; Wen et al., 2014). Although the causality of this mutation remains unclear based on pedigree size and identification of the mutation in control subjects (Green et al., 2006), this mutation resulted in dysregulation of presynaptic biology, including increased presynaptic protein expression in cortical neurons (Wen et al., 2014). We do not observe an upregulation of presynaptic proteins with exon 8 or exon 2 DISC1 interruption. The differentiation methods used in that study included the use of dual SMAD inhibition and cyclopamine, which likely generates a different population of neural progenitors and neurons, which in turn may alter the resulting neuronal phenotypes (Hebert and Fishell, 2008). Interestingly, although the phenotypes described are divergent between our studies, a common subset of genes are dysregulated with the two models (Fig. S4F). Future studies with these models have the potential to provide a powerful system to identify convergent phenotypes of these disparate DISC1 mutations.
Here, we found that disease-relevant DISC1 disruption alters canonical Wnt signaling in human NPCs, as assessed by TCF/LEF-driven gene expression. We observed that DISC1 disruption decreases Wnt responsiveness in human NPCs, consistent with the decreased Wnt response upon DISC1 knockdown in murine NPCs (Mao et al., 2009). That study suggested a direct role for DISC1 in the Wnt signaling pathway, wherein DISC1 binds and inhibits GSK3β. Here we find that that the loss of long DISC1 isoforms drives decreased Wnt responsiveness via elevated baseline Wnt signaling. This difference may result from the use of human vs. rodent cells, the use of total DISC1 knockdown vs. disease-relevant loss of long DISC1 transcripts, or may suggest a different role for DISC1 in the Wnt signaling pathway.
The Wnt signaling pathway has been implicated in the pathophysiology of neuropsychiatric disorders, making the role of DISC1 in this pathway of particular interest (Freyberg et al., 2010). We found that DISC1 disruption causes altered NPC and neuronal gene expression profiles via increased Wnt signaling. Our data are consistent with rodent models in which increased Wnt signaling in the developing brain causes a dorsal NPC fate shift, decreases FoxG1 expression, and decreases Tbr2 expression by promoting IP differentiation (Backman et al., 2005; Munji et al., 2011). Wnt inhibition with XAV939 for a 10-day window in DISC1-targeted NPCs rescued changes in neural cell identity, suggesting that increased Wnt signaling with DISC1-disruption is upstream of observed gene expression differences. The increase in Tbr2 expression with XAV939 treatment upholds, and extends to human telencephalic cells, previous reports that Wnt antagonism causes expansion of Tbr2+ IPs (Fang et al., 2013; 2014; Munji et al., 2011). These studies suggest a model in which DISC1 interruption increases baseline Wnt signaling, which alters the pool of NPCs, resulting in decreased Wnt responsiveness.
Further work will be required to elucidate the exact mechanism by which DISC1-disruption alters Wnt signaling and fate-related gene expression profiles in human NPCs. We have identified numerous gene expression changes that result from DISC1 interruption by RNA-sequencing, which provide interesting candidates for future study. This study shows the utility of human iPSCs in modeling mental illness-associated defects in human neurodevelopment and strengthens the association between Wnt signaling, neurodevelopment, and major mental illness.
Experimental Procedures
Genome editing
Healthy control human iPSC line YZ1 was obtained from the UCONN Stem Cell Core (Zeng et al., 2010). TALENs were designed and constructed using a hierarchical ligation procedure (Sanjana et al., 2012). TALE monomer plasmids and TALEN backbone plasmids were a generous gift from Feng Zhang. Exon 8 TALENs were designed targeting unique genomic sequences in DISC1 exon 8. For CRISPR-Cas9 targeting, a unique genomic site in DISC1 exon 2 was cloned into a gRNA cloning vector. Targeting plasmids were electroporated into dissociated YZ1 iPSCs using the Amaxa 4D-Nucleofector X Unit (Lonza). 48 hours after transfection, iPSCs were dissociated and GFP+ cells were collected by FACS. Individual clones were screened for genomic mutation by PCR amplification around the target site, followed by Sanger sequencing.
Neuronal differentiation
Neuronal differentiation was performed using an embryoid aggregate-based protocol, as previously described (Muratore et al., 2014a) (Muratore et al., 2014b). For dorsal-ventral patterning, cells were treated with 2 uM cyclopamine (Santa Cruz) or vehicle (DMSO, Sigma) as indicated during days 5–17 of differentiation. For Wnt manipulation, cells were treated with 2 uM XAV939 (Stemgent), 3 uM CHIR99021 (Tocris), or vehicle (DMSO) as indicated during days 7–17 of differentiation. See Supplemental Experimental Procedures for details and media recipes.
qPCR
RNA was reverse transcribed and cDNA was used for qPCR with Fast SYBR Green Master Mix (Life Technologies) on a ViiA 7 System (Life Technologies). Data were normalized to GAPDH expression using the ΔΔCT method as previously described (Livak and Schmittgen, 2001). Primer sequences are listed in Supplemental Experimental Procedures.
NanoString gene expression analysis
Two 150-gene nCounter Custom CodeSets were designed by NanoString Technologies. One codeset was used to generate initial expression data (Fig 2–4; Table S3A). Data were analyzed by subtracting counts from a blank control and normalizing to 8 housekeeping genes (B2M, GAPDH, GUSB, HPRT1, LDHA, POLR2A, RPL13a and RPL27). After this codeset was depleted, another codeset was made, which greatly overlapped with the initial codeset – this codeset was used to generate patterning data (Fig 6–7; Table S3B). These data were analyzed with nSolver Analysis Software (NanoString Technologies) and normalized to the total gene set. Assays were performed according to the manufacturer’s instructions.
Immunocytochemistry and microscopy
Cells were fixed with 4% paraformaldehyde (Sigma), followed by membrane permeabilization and blocking with 0.2% Triton X-100 (Sigma) and 2% donkey serum (Jackson Immunoresearch). All quantifications were performed blinded to genotype using the Cell Counter plugin in FIJI. See Supplemental Experimental Procedures for a list of antibodies used.
Western blots
Lysates were prepared in a buffer containing 1% NP40, 10 mM EDTA, 150 mM NaCl, 50 mM Tris, cOmplete Protease Inhibitors and phosSTOP (Roche). Equal protein amounts were loaded onto 4–12% Bis-Tris NuPAGE gels (Life Technologies) and transferred to nitrocellulose membranes for blotting. Antibodies and dilutions are listed in Supplemental Experimental Procedures.
RNA-sequencing and accession numbers
RNA was extracted from day 17 or day 50 samples. Total RNA was converted into cDNA libraries using the TruSeq Stranded Total RNA-RiboZero Gold Sample Prep Kit (Illumina). RNA was sequenced on an Illumina HiSeq, using stranded paired-end reads, with ~50 millions reads per sample. Data deposited in the NCBI GEO database, GSE71289.
Differential gene expression analysis
Reads were mapped to the UCSC Human Reference Genome (hg19) using TopHat v2.0.10 (Kim et al., 2013). Reads mapping to genes were counted using htseq (v0.6.1) (Anders et al., 2015). Counts were upper quartile normalized using SVS (Golden Helix) for limited gene comparisons. Alternatively, differential gene expression analysis between lines was performed using the edgeR package in R with a false discovery rate of 0.05 (Robinson et al., 2010).
Luciferase assays
Control and Wnt3a conditioned media (CM) was produced using Wnt3a-expressing and control (“L”) cells (ATCC). CMs were generated according to the ATCC protocol. Neural aggregates (~day 27–31) were dissociated using Accutase. Cells were electroporated with Super8XTOPFlash (gift from Randall Moon) and pMIR-REPORT-beta-galactosidase (Life Technologies) using the Amaxa 4D-Nucleofector X Unit (Lonza). 16 hours later, media was changed to L or Wnt3a CM for 24 hours. Cells were then lysed and used for a firefly luciferase assay (Biotium) and beta-galactosidase assay (Promega). Luciferase activity was normalized to beta-galactosidase activity for each sample.
EdU incorporation
Neural aggregates (~day 27–31) were dissociated using Accutase and plated onto poly-ornithine/laminin coated 96-well plates at 25,000 cells/well. 16 hours later, media was changed to L CM for 24 hours. Cells were then incubated in L CM + 10 uM EdU for 4 hours, followed by incubation in L CM (without EdU) for 4 hours. Cells were then fixed as described above, and EdU staining was carried out using a Click-iT EdU Imaging Kit (Invitrogen).
To quantify the EdU signal, cells were imaged using an IN Cell Analyzer 2000 high content platform (GE Healthcare). Image sets were analyzed using the multi-target analysis algorithm in the IN Cell Workstation software (GE Healthcare).
Data collection and statistics
All data represent at least three independent experiments, with the minimum “n” and number of differentiations listed in each figure or figure legend, and utilizing the iPSC lines as shown in Table S1. Data were analyzed using GraphPad PRISM 6 software. Values are expressed as means ± SEM. Statistical significance was tested as indicated in figure legends.
Supplementary Material
Acknowledgements
We thank N. Sanjana and F. Zhang for help designing TALENs and providing TALE monomer and TALEN backbone plasmids; R. Hevner for sharing Tbr2 antibody; C. Zhou, F. Abawi, and P. Mouradian for technical assistance; and R. Nehme and K. Eggan for valuable feedback. This work is supported by funding from the Sackler Scholar Programme in Psychobiology, NIGMS grant T32GM007753, NIA grant T32AG000222, and NIMH grant 1F30MH103890-01A1 (PS), NIA grant 2R01AG006173-26 (DJS), a Young Investigator Award from the Brain & Behavior Research Foundation (TLY-P) and NIMH grants R00MH085004 and R01MH101148 (TLY-P). RNA-sequencing work was funded by a grant obtained through Expression Analysis (TLY-P).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
P.S. led and was involved in every aspect of the project. D.G.C. assisted with sample preparation, cell culture, and Western blots. K.H., J.B. and E.M. assisted with sample preparation and imaging. C.R.M. performed MEA experiments. M.L., H.Z., and K.K. assisted with RNA-seq analyses. T.L.Y-P., D.J.S., and P.S. designed the project and wrote the manuscript.
References
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with highthroughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman M, Machon O, Mygland L, van den Bout CJ, Zhong W, Taketo MM, Krauss S. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Developmental Biology. 2005;279:155–168. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, Beaumont KG, Kim HJ, Topol A, Ladran I, et al. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2015;20:361–368. doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes & Development. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesin C, Peres JN, Johansson M, Snowden V, Cording A, Papalopulu N, Houart C. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev. Cell. 2009;16:576–587. doi: 10.1016/j.devcel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RAM, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. Journal of Neuroscience. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Fang W-Q, Chen W-W, Fu AKY, Ip NY. Axin directs the amplification and differentiation of intermediate progenitors in the developing cerebral cortex. Neuron. 2013;79:665–679. doi: 10.1016/j.neuron.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Fang W-Q, Chen W-W, Jiang L, Liu K, Yung W-H, Fu AKY, Ip NY. Overproduction of upper-layer neurons in the neocortex leads to autism-like features in mice. CellReports. 2014;9:1635–1643. doi: 10.1016/j.celrep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Norton N, Peirce T, Grozeva D, Kirov G, Owen MJ, O'Donovan MC, Craddock N. Evidence that a DISC1 frame-shift deletion associated with psychosis in a single family may not be a pathogenic mutation. Mol Psychiatry. 2006;11:798–799. doi: 10.1038/sj.mp.4001853. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RAM, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neuroscience Research. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. 2001;106:607–617. doi: 10.1016/s0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Wang PP, Atabay KD, Murphy EA, Doan RN, Hecht JL, Walsh CA. Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat Neurosci. 2015 doi: 10.1038/nn.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-E, O'Sullivan ML, Sanchez CA, Hwang M, Israel MA, Brennand K, Deerinck TJ, Goldstein LSB, Gage FH, Ellisman MH, et al. Investigating synapse formation and function using human pluripotent stem cell-derived neurons. Proc Natl Acad Sci USA. 2011;108:3005–3010. doi: 10.1073/pnas.1007753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort R, Pozueta J, Shelanski M. Cross-linking of cell surface amyloid precursor protein leads to increased β-amyloid peptide production in hippocampal neurons: implications for Alzheimer's disease. Journal of Neuroscience. 2012;32:10674–10685. doi: 10.1523/JNEUROSCI.6473-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-J, Zhang X, Johnson MA, Wang Z-B, LaVaute T, Zhang S-C. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma L, Liu Y, Ky B, Shughrue PJ, Austin CP, Morris JA. Cloning and characterization of Disc1, the mouse ortholog of DISC1 (Disrupted-in-Schizophrenia 1) Genomics. 2002;80:662–672. doi: 10.1006/geno.2002.7012. [DOI] [PubMed] [Google Scholar]
- Manuel M, Martynoga B, Yu T, West JD, Mason JO, Price DJ. The transcription factor Foxg1 regulates the competence of telencephalic cells to adopt subpallial fates in mice. Development. 2010;137:487–497. doi: 10.1242/dev.039800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Developmental Biology. 2005;283:113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Human Molecular Genetics. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ. The genetics of neurodevelopmental disease. Curr Opin Neurobiol. 2011;21:197–203. doi: 10.1016/j.conb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. Journal of Neuroscience. 2011;31:1676–1687. doi: 10.1523/JNEUROSCI.5404-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LNP, Walsh DM, Selkoe DJ, Young-Pearse TL. The familial Alzheimer's disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Human Molecular Genetics. 2014a;23:3523–3536. doi: 10.1093/hmg/ddu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratore CR, Srikanth P, Callahan DG, Young-Pearse TL. Comparison and Optimization of Human iPSC Neuronal Differentiation Protocols. PLoS One. 2014b Aug 28;9(8):e105807. doi: 10.1371/journal.pone.0105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Lipska BK, Hyde TM, Ye T, Newburn EN, Morita Y, Vakkalanka R, Barenboim M, Sei Y, Weinberger DR, et al. DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proceedings of the National Academy of Sciences. 2009;106:15873–15878. doi: 10.1073/pnas.0903413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous DJ, Thomson PA, Millar JK, Evans KL, Hennah W, Soares DC, McCarthy S, McCombie WR, Clapcote SJ, Korth C, et al. DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol Psychiatry. 2014;19:141–143. doi: 10.1038/mp.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, O'Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, Bergen SE, Collins AL, Crowley JJ, Fromer M, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Biotechnol. 2013;45:1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs NA, Sawa A, Holmes SE, Ross CA, Delisi LE, Margolis RL. A frameshift mutation in Disrupted in Schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol Psychiatry. 2005;10:758–764. doi: 10.1038/sj.mp.4001667. [DOI] [PubMed] [Google Scholar]
- Sanjana NE, Cong L, Zhou Y, Cunniff MM, Feng G, Zhang F. A transcription activator-like effector toolbox for genome engineering. Nat Protoc. 2012;7:171–192. doi: 10.1038/nprot.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa A, Mao C-A, Hadjantonakis A-K, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Silva AL, Romão L. The mammalian nonsense-mediated mRNA decay pathway: to decay or not to decay! Which players make the decision? FEBS Lett. 2009;583:499–505. doi: 10.1016/j.febslet.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Singh KK, De Rienzo G, Drane L, Mao Y, Flood Z, Madison J, Ferreira M, Bergen S, King C, Sklar P, et al. Common DISC1 Polymorphisms Disrupt Wnt/GSK3β Signaling and Brain Development. Neuron. 2011;72:545–558. doi: 10.1016/j.neuron.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai L-H. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Gore A, Yan W, Abalde-Atristain L, Li Z, He C, Wang Y, Brodsky RA, Zhang K, Cheng L, et al. Whole-genome sequencing analysis reveals high specificity of CRISPR/Cas9 and TALEN-based genome editing in human iPSCs. Cell Stem Cell. 2014;15:12–13. doi: 10.1016/j.stem.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Su P, Li S, Chen S, Lipina TV, Wang M, Lai TKY, Lee FHF, Zhang H, Zhai D, Ferguson SSG. A Dopamine D2 Receptor-DISC1 Protein Complex may Contribute to Antipsychotic-Like Effects. Neuron. 2014;84:1302–1316. doi: 10.1016/j.neuron.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yu C, Qu J, Li M, Yao X, Yuan T, Goebl A, Tang S, Ren R, Aizawa E, et al. Targeted gene correction minimally impacts whole-genome mutational load in human-disease-specific induced pluripotent stem cell clones. Cell Stem Cell. 2014;15:31–36. doi: 10.1016/j.stem.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- Taylor MS, Devon RS, Millar JK, Porteous DJ. Evolutionary constraints on the Disrupted in Schizophrenia locus. Genomics. 2003;81:67–77. doi: 10.1016/s0888-7543(02)00026-5. [DOI] [PubMed] [Google Scholar]
- Ulloa F, Martí E. Wnt won the war: antagonistic role of Wnt over Shh controls dorso-ventral patterning of the vertebrate neural tube. Dev Dyn. 2010;239:69–76. doi: 10.1002/dvdy.22058. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Veres A, Gosis BS, Ding Q, Collins R, Ragavendran A, Brand H, Erdin S, Cowan CA, Talkowski ME, Musunuru K. Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell Stem Cell. 2014;15:27–30. doi: 10.1016/j.stem.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira SI, Rebelo S, Esselmann H, Wiltfang J, Lah J, Lane R, Small SA, Gandy S, da Cruz e Silva EF, da Cruz e Silva OA. Retrieval of the Alzheimer's amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol Neurodegener. 2010;5:40. doi: 10.1186/1750-1326-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346:552–557. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim N-S, Yoon K-J, Shin J, Zhang C, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014:1–15. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HJ, Owen MJ, O'Donovan MC. Schizophrenia genetics: new insights from new approaches. Br. Med. Bull. 2009;91:61–74. doi: 10.1093/bmb/ldp017. [DOI] [PubMed] [Google Scholar]
- Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- Yang L, Grishin D, Wang G, Aach J, Zhang C-Z, Chari R, Homsy J, Cai X, Zhao Y, Fan J-B, et al. Targeted and genome-wide sequencing reveal single nucleotide variations impacting specificity of Cas9 in human stem cells. Nature Communications. 2014;5:5507. doi: 10.1038/ncomms6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, Mei A, Mchenry L, Lisuk D, Grasmick JM, et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Reports. 2014;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu H-X, et al. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS ONE. 2010;5:e11853. doi: 10.1371/journal.pone.0011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.