Abstract
Background
In rural areas in China and India, cardiovascular disease burden is high but economic and healthcare resources are limited. This study aims to develop and evaluate a simplified cardiovascular management program (SimCard) delivered by community health workers (CHWs) with the aid of a smartphone-based electronic decision support system.
Methods and Results
The SimCard study was a yearlong cluster-randomized controlled trial conducted in 47 villages (27 in China and 20 in India). 2,086 ‘high cardiovascular risk’ individuals (aged 40 years or older with self-reported history of coronary heart disease, stroke, diabetes, and/or measured systolic blood pressure ≥160 mmHg) were recruited. Participants in the intervention villages were managed by CHWs through an Android-powered “app” on a monthly basis focusing on two medication use and two lifestyle modifications. Compared with the control group, the intervention group had a 25.5% (P<0.001) higher net increase in the primary outcome of the proportion of patient-reported anti-hypertensive medication use pre-and-post intervention. There were also significant differences in certain secondary outcomes: aspirin use (net difference 17.1%, P<0.001) and systolic blood pressure (−2.7 mmHg, P=0.04). However, no significant changes were observed in the lifestyle factors. The intervention was culturally tailored and country-specific results revealed important differences between the regions.
Conclusions
The results indicate that the simplified cardiovascular management program improved quality of primary care and clinical outcomes in resource-poor settings in China and India. Larger trials in more places are needed to ascertain potential impacts on mortality and morbidity outcomes.
Clinical Trial Registration Information
clinicaltrials.gov. Identifier: NCT01503814.
Keywords: cardiovascular disease prevention, high-risk populations, China, India Asian, community, decision aids
Introduction
Cardiovascular diseases (CVD) are one of the leading causes of morbidity, mortality and disability in both high-income and low-and middle-income countries.1 In 2011, it accounted for 41% and 29% of the total deaths, respectively, in China and India2, 3 – two of the largest growing economies in the world. They also face similar challenges and opportunities in CVD prevention and control such as the rising CVD burden, large urban-rural health disparities, limited resources and capacity, a large population base and increasing access to mobile phones. Although there also exist differences in the healthcare system, socioeconomic environment and culture customs between China and India, there is a need to jointly evaluate cost-effective and sustainable strategies for CVD prevention in rural areas of both countries.
Clinical guidelines, built on ample scientific evidence, recommend a combined strategy of lifestyle modifications and appropriate medication use for CVD prevention and control, based on a high-risk approach, i.e., to identify and manage individuals at high CVD risk.4-10 Targeting high risk individuals would be potentially a high cost-effective approach, especially for resource-limited settings. Reliance on the overburdened and relatively small number of specialists to implement this strategy is not feasible or sustainable. Previous studies have shown that there are significant improvements in accessing healthcare facilities, disease screening and monitoring, and patients’ adherence to treatment by engaging community healthcare workers (CHWs).11-14 In addition, in order to build capacity and ensure the quality of implementation, the intervention needs to be simplified and tailored to the local contexts and adequate training and technical support needs to be provided to CHWs. The high penetration of cell phones even in resource-poor settings offers an unprecedented opportunity to provide such support to CHWs in CVD prevention and control.
Therefore, this study aims to develop and evaluate a simplified yet guideline-based multifaceted intervention program for cardiovascular management among individuals with high cardiovascular risk delivered by CHWs with the aid of a mobile-technology based electronic decision support system (EDSS) in rural China and India (the “SimCard” study).
Methods
Study design
The SimCard study is a single-blinded cluster-randomized controlled trial conducted in rural villages in Tibet Autonomous Region (hereafter “Tibet”), China and Haryana, India. This trial was coordinated by the George Institute for Global Health at Peking University Health Science Center in collaboration with Tibet University in China and the Public Health Foundation of India (PHFI) in India. Ethical approval of the study protocol was granted by the institutional review boards at Peking University Health Science Center, China, Public Health Foundation of India, and Duke University, USA. Written informed consents were obtained from all participants. We describe the study design below briefly with more details available in the published protocol.15
Study sites and participants
This trial was carried out in 27 villages from 15 townships (the administrative unit managing the village) in two counties of Tibet, China and 20 villages from one tehsil (an administrative unit for a group of villages) in Haryana State, India. The two regions are geographically close to each other with Tibet to the east and Haryana to the west of the Himalayan Mountains. Tibet lies in southwest China and has a population of 3 million. The two counties, Gongbujiangda County and Linzhou County are located in the eastern and central parts of Tibet respectively. Gongbujiangda County has jurisdiction over 9 townships with a total of 80 villages, and Linzhou County has jurisdiction over 10 townships with a total of 45 villages. In India, this trial was carried out in Ballabgarh tehsil (sub district) of District Faridabad in the north Indian state of Haryana. There are 82 villages in Ballabgarh. Villages from both countries were chosen based on the eligibility criteria of having (1) within high CVD burden region, (2) limited healthcare resources, (3) existing CHWs or qualified candidates who can be trained to fulfil their role, and (4) local government support. The average population size of the villages in the trial was 900 in China and 3,500 in India, respectively.
Inclusion criteria for the participants include being residents in the participating villages and having high cardiovascular risk (“high-risk” hereafter). For ease of operation, high-risk in this study was defined on the basis of age, disease history, and measured blood pressure. Thus individuals who were 40 years old and above with a self-reported history of (1) coronary heart disease, (2) stroke, (3) diabetes mellitus, or (4) measured systolic blood pressure (SBP) ≥160 mmHg were included in the study. Exclusion criteria were as follows: (1) having CVD-related complications that cannot be managed in a primary care setting; (2) having a malignancy or life-threatening disease; (3) individuals who are bed-ridden; (4) currently participating in any other clinical trials; and (5) unable to stay in the village longer than 8 months out of a year. Blood pressure was measured twice by trained interviewer using a validated Omron HEM-7201 automatic blood pressure monitor (Omron Healthcare, Kyoto, Japan). At the baseline, all age-eligible participants were screened by a brief questionnaire and blood pressure measurement. Only participants who were identified as high-risk were enrolled into the trial.
Randomization
Villages were randomized to intervention or control with stratification by country. In China, 27 villages were randomized to the intervention and control group with stratification by county and township. Villages in India were randomized without any stratification. A study-independent staff generated the randomization pattern through a central computerized process.
Intervention
The intervention was developed and simplified based on the international and national clinical guidelines for cardiovascular management.16-19 The key elements of the intervention were summarized as a ‘2+2’ model, which consisted of two therapeutic lifestyle modifications (smoking cessation and salt reduction) and the appropriate prescription of two medications (blood pressure lowering agents and aspirin). For blood pressure lowering agent, it was low-dose hydrochlorothiazide – 12.5 mg/day in China and calcium-channel blocker – 2.5 or 5 mg/day in India. The recommended dose for aspirin was 75-100 mg/day. All drugs were generally safe, low-cost and locally available. All hypertensive patients (SBP ≥140 mmHg) without contraindications would be prescribed the anti-hypertensive medication with a treatment target of 140 mmHg or lower while only patients with coronary heart disease, ischemic stroke, and diabetes but without contraindications such as bleeding or high blood pressure (SBP ≥160 mmHg) would be prescribed aspirin. The intervention model was designed to target the prevalent risk factors associated with CVD in these two areas. It was also designed to be easily implemented and incorporated into the existing local healthcare system.
In rural China, CHWs are village doctors who are not physicians but have received basic health professional training and are able to prescribe medications. In India, the CHWs were volunteer members of the community and not authorized to prescribe medicine. They were supported by a partnership with licensed physicians in healthcare centers responsible for providing clinical guidance and prescribing medications. The management program was delivered by trained CHWs in the intervention group on a monthly basis with the assistance of EDSS in the form of an Android-based “app” installed on smartphones. The development of the EDSS in this study was based on the ‘2+2’ intervention model and with easy acceptance and useability by the CHWs in mind to ensure successful implementation of the intervention. It consisted of prompts regarding the patient’s current lifestyle habits, blood pressure measurements, current medication use, previous medical history, new conditions, contraindications, and side effects. In India, the EDSS had a desktop component for the use of physicians to approve the drugs as suggested by the smartphone-based EDSS for CHWs.
Before the start of the intervention, all CHWs in the intervention group received initial one-day systematic training. After the training was provided, CHWs received the list of the participants who were identified as high-risk at baseline in their villages. CHWs were instructed to provide monthly follow-up visits for the high-risk participants under their management. These visits were conducted either in village clinics or another central place in the village or patient homes and included screening for new symptoms, diseases, and side effects since the last visit, measuring blood pressure, providing lifestyle counseling, and when appropriate, prescribing one or both medications. CHWs received refresher training every 3-4 months during the implementation of the intervention.
In both China and India, interventions were tailored to the local cultures and customs when possible. For example, special education was provided to alleviate concerns among Tibetan patients against western medicines. Health educational materials on lifestyle in China and India were all provided in local languages with cultural-specific images. Performance feedback and performance-based incentives to the CHWs were also part of the intervention package in China while Indian CHWs received fixed monthly honorariums. On average, the payment for CHWs in the intervention group for the entire duration of the intervention was US$400 in China and US$500 in India. The duration of the trial was designed to be one year long.
In the control group, usual cardiovascular management programs continued without additional intervention. However, in India, as stipulated by the ethics committee, the medications used in the intervention arm were also made available free of charge to patients in the primary care facilities serving the control villages, typically located 15-40 minutes by motorcycle from the villages. Main differences in implementation of the study in two countries are summarized in Table 1.
Table 1.
System and project-level differences in study implementation between China and India, SimCard Study
| Level | China | India |
|---|---|---|
| Healthcare system | Community healthcare workers (CHWs) are village doctors who are not physicians but received basic health professional training and are able to prescribe medicines. |
CHWs are volunteers from the community and not authorized to prescribe medicines. They are supported by a partnership with licensed physicians in healthcare centers responsible for providing clinical guidance and prescribing medications. |
|
| ||
| Participant recruitment | Trained medical students from Tibet University consented all age-eligible participants in the selected villages and screened them for high cardiovascular risk. Participants who were identified as high cardiovascular risk were enrolled into the study. |
CHWs visited all household in the selected villages and approached all age-eligible participants for eligibility check. Eligible participants were invited to attend a medical camp in the village. During the camp, a qualified physician examined the participant and enrolled them into the study after obtaining informed consent. |
|
| ||
| Intervention (country-specific adaptations made to suit local contexts) |
|
|
|
| ||
| Control | Usual care | Usual care, free medicines made available in primary healthcare centers (required by ethics committee) |
Outcomes
The primary outcome was the net difference between groups in the change in the proportion of patient-reported anti-hypertensive medication use from baseline to one-year follow-up.
A number of secondary outcomes were also evaluated in order to fully capture the effects of the intervention. The main secondary outcomes included between-group differences in the changes from baseline to one-year follow-up in: (1) the proportion of high-risk individuals taking aspirin; (2) mean systolic blood pressures of high-risk individuals; (3) the proportion of current smokers; (4) the proportion of high-risk individuals aware of the harms of a high-salt diet; (5) the proportion of high-risk individuals receiving monthly follow-ups from the CHWs; and (6) the proportion of high-risk individuals hospitalized.
The outcomes were assessed with data collected during baseline and post-intervention surveys from all high-risk individuals in both intervention and control villages in a standardized manner. The two survey questionnaires were identical and included information on demographics, lifestyle behaviors, medical care, medication use, and other relevant information. A few questions such as smoking were different between the Chinese and Indian questionnaires to reflect actual patterns in each country. Height, weight, waist circumference, and blood pressure were also measured according to standardized procedures in both surveys.
Sample size
This study was powered on the primary outcome, the proportion of high-risk individuals taking anti-hypertensive medication. In the design stage, we calculated the sample size based on the assumption of 2,000 high-risk individuals in 40 clusters (20 intervention villages and 20 control villages), an intra-cluster correlation coefficient (ICC) of 0.02 and two-sided alpha of 0.05.14, 20 The study had >90% power to detect a 10% net difference in the primary outcome. For the secondary outcome of SBP, assuming a standard deviation of the change in SBP of 15 mmHg among high-risk individuals, the study also had excellent power (>90%) to detect a 3 mmHg net difference with an ICC of 0.02.
Statistical analysis
All analyses on outcomes were at the individual level but accounted for clustering and were conducted under the principle of intention-to-treat.
For the primary outcome, the proportion of high-risk individuals treated with anti-hypertensive medication, a mixed logistic model with two sets of random intercepts-- for village, to account for the clustered study design, and for individual nested within village to account for the two outcome measurements on the same individual -- was used, and an unstructured covariance matrix structure was specified. All models included fixed effects for group (intervention vs. control), time (follow-up vs. baseline) and the group-by-time interaction. The model-based p-value associated with the interaction term was used to test a net difference in change (from baseline to follow-up) between groups. The descriptive data for each group and the net difference in change for each outcome were obtained from raw data. Analyses of other dichotomous outcomes adopted the same analytical model as the primary outcome. In case models failed to converge, other variance-covariance structures that produced convergence were used. For the continuous outcome of SBP, similar modelling strategies were adopted but mixed linear models instead of mixed logistic models were used.
Considering the potential cultural diversity across the two countries, pre-specified subgroup analyses by country were performed on all outcomes. The analyses were conducted using SAS software package (Version 9.3, SAS Institute Inc., Cary, NC, USA). All p values provided were from models without adjusting for any individual characteristics.
Results
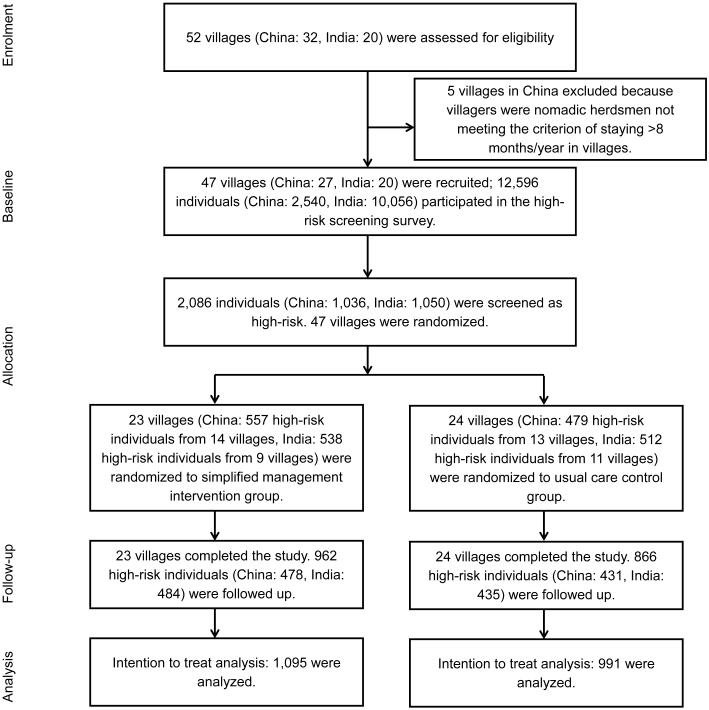
Patient recruitment started in January 2012, and the last patient completed the study in March 2014. Of the 52 potential villages assessed for eligibility in the study, 5 were excluded due to the fact that many residents in the villages were nomadic herdsmen who did not meet the criteria of staying in the villages at least 8 months in a year (Figure 1). In the remaining 47 villages surveyed, 2,086 individuals were identified as high-risk and recruited for the purposes of this study, with 23 villages (1,095 high-risk individuals) randomized to the intervention group and 24 villages (991 high-risk individuals) randomized to the usual care (control) group. Eighty-eight percent of individuals (1,828) at high-risk were successfully followed-up at the end of the study. Loss to follow-up was similar between the intervention (12.1%) and control (12.6%) groups.
Figure 1.
Trial Flow Chart.
Baseline characteristics
Baseline demographic and clinical characteristics of participants are provided in Table 2. The “typical” participant across both countries was a 60-year-old illiterate woman. In China, there were no statistical differences between subjects in the intervention group compared with those in the control group. In India, with the exceptions of the history of coronary heart disease (P<0.001) and history of diabetes (P=0.004), no significant differences were found.
Table 2.
Selected characteristics of high-risk individuals at baseline
| Characteristics (Mean, SD or %) |
Total
|
China
|
India
|
|||
|---|---|---|---|---|---|---|
|
Intervention
(N=1,095) |
Control
(N=991) |
Intervention
(N=557) |
Control
(N=479) |
Intervention
(N=538) |
Control
(N=512) |
|
| Age (years) | 59.7,11.7 | 60.4,11.8 | 59.5,11.6 | 59.3,11.3 | 59.9,11.8 | 61.5,12.1 |
| Female (%) | 65.4 | 66.8 | 72.0 | 70.8 | 58.6 | 63.1 |
| Education (%) | ||||||
| Illiterate | 59.3 | 61.9 | 62.0 | 63.7 | 56.4 | 60.3 |
| Primary school or under | 24.0 | 23.6 | 35.4 | 34.9 | 12.3 | 13.1 |
| Middle school | 6.2 | 4.6 | 2.4 | 1.1 | 10.2 | 7.8 |
| High school | 7.1 | 6.9 | 0 | 0.4 | 14.3 | 12.9 |
| College or above | 3.4 | 3.0 | 0.2 | 0 | 6.7 | 5.9 |
| Waist circumference (cm) | 89.2,11.6 | 89.9, 12.2 | 89.5, 10.0 | 88.8, 11.2 | 89.0, 13.0 | 90.9, 13.0 |
| Body mass index (kg/m2) | 23.6, 4.2 | 24.0, 4.4 | 23.0, 3.5 | 23.4, 3.8 | 24.1, 4.7 | 24.5, 4.8 |
| Overweight* (%) | 24.6 | 26.7 | 20.8 | 24.0 | 28.7 | 29.3 |
| Obesity* (%) | 7.2 | 9.6 | 4.6 | 5.1 | 10.0 | 13.9 |
| Physical activity+ (%) | ||||||
| Low | 6.1 | 7.1 | 4.3 | 4.5 | 7.7 | 9.6 |
| Moderate | 22.8 | 20.7 | 13.7 | 12.2 | 31.1 | 29.4 |
| High | 71.1 | 72.2 | 82.0 | 83.3 | 61.2 | 60.1 |
| Current smoker (%) | 36.7 | 37.5 | 37.5 | 36.5 | 35.9 | 38.7 |
| Disease history (%) | ||||||
| Coronary heart disease | 39.5 | 31.9 | 50.1 | 53.0 | 28.4 | 12.1 |
| Stroke | 10.4 | 9.9 | 6.8 | 9.4 | 14.1 | 10.4 |
| Diabetes | 13.4 | 9.8 | 2.9 | 1.9 | 24.3 | 17.2 |
| Hypertension^ (%) | 75.3 | 76.7 | 76.8 | 77.5 | 73.6 | 76.0 |
Based on the WHO definition, overweight was defined as BMI≥25 kg/m2 but <30 kg/m2 while obesity was defined as BMI≥30 kg/m2.
The categorical score for physical activity was based on the International Physical Activity Questionnaire (IPAQ) scoring protocol.
Hypertension was defined as measured systolic blood pressure (SBP) ≥140 mmHg.
Primary outcome
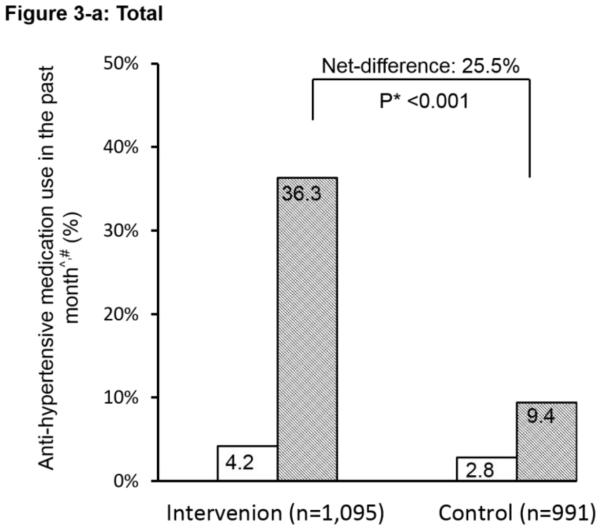
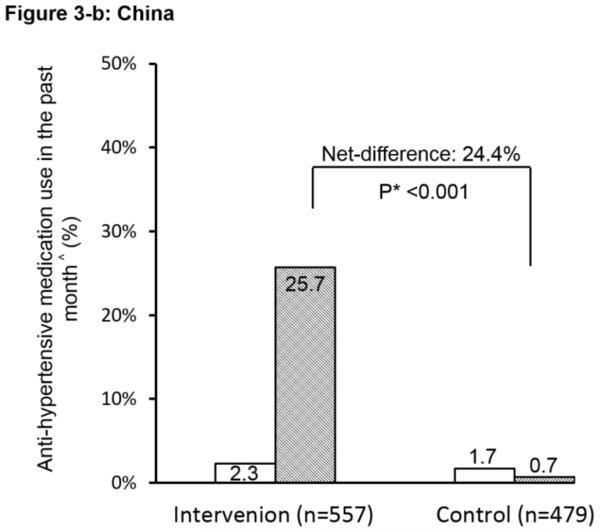
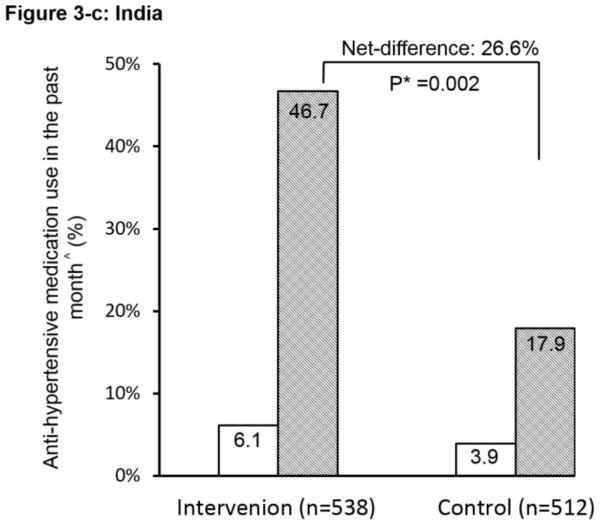
The ICC at baseline for the primary outcome of patient-reported anti-hypertensive medication use was 0.02. Pre-intervention use of anti-hypertensive medication was low (<7%) in both groups and in both countries (Figure 2). After the intervention, the proportion increased dramatically in the intervention group, reaching 36.3%, with a net pre-post difference between the two groups of 25.5% (P<0.001). The interaction between the countries on the primary outcome was significant (P<0.01). Country-specific difference was further explored. The net difference in China was 24.4% and in India was 26.6%; both were highly statistically significant between the intervention and control groups. These outcomes used all high-risk individuals as the denominator to show the overall impact. We conducted additional analyses on the primary outcome using only the subgroup of hypertensive patients identified at baseline (N=1,582). Results were similar (P<0.001) with net-difference of 27%, 24%, and 31% for the combined, China, and India data respectively.
Figure 2.

Effect of the simplified management program on the primary outcome among high risk individuals. ^: Anti-hypertensive medication use in the past month is defined as self-reported use of community healthcare workers-prescribed anti-hypertensive medication for ≥ 25 days in the past month. #: The interaction between China and India was significant (P<0.01). *: P-value for the net difference of pre-and-post intervention between the groups.
Secondary outcomes
In the analyses combining results from both countries, there was a significant net increase in the proportion of high-risk individuals taking aspirin (17.1%, P<0.001) and a significant reduction in mean SBP (−2.7 mmHg, P=0.04). Interaction between the countries on those variables was both significant (P<0.01 and P<0.05 respectively). In the country-specific analysis, the net increase in the proportion of high-risk individuals taking aspirin was higher in China (24.5%) than in India (9.8%), but both were highly significant (P<0.01) (Table 3). There was a significant net reduction in mean SBP in China (−4.1 mmHg, P=0.006) but a non-significant decline in India. Both countries had large net increases (>16%) in the proportion of high-risk patients receiving monthly follow-up but statistical significance was different for China (P<0.001) and India (P=0.20). We did not observe any change in tobacco use or knowledge on harmful effects of high salt intake in the combined results.
Table 3.
Effect of the simplified cardiovascular management program on secondary outcomes
| Secondary outcomes (Mean, SD or %) | Intervention | Control | Net Difference | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre- Intervention |
Post- Intervention |
Pre- Intervention |
Post- Intervention |
|||
| Total | N=1,095 | N=991 | ||||
| Use of aspirin in the past month^,#, % | 6.0 | 20.6 | 4.7 | 2.2 | 17.1 | <0.001 |
| Mean systolic blood pressure#, mmHg | 161.3, 29.6 | 149.5, 26.1 | 161.4, 27.8 | 152.3, 27.2 | −2.7 | 0.04 |
| Current smoker, % | 36.7 | 37.5 | 37.5 | 36.3 | 2.0 | 0.42 |
| Awareness of harms of high salt diet#, % | 46.9 | 61.9 | 36.8 | 60.0 | −8.2 | 0.06 |
| Receiving monthly follow-up, % | 20.2 | 40.6 | 16.2 | 20.6 | 16.0 | <0.001 |
| Hospitalization during the past year, % | 14.5 | 11.7 | 11.4 | 12.5 | −3.9 | 0.09 |
| China | N=557 | N=479 | ||||
| Use of aspirin in the past month^, % | 7.0 | 26.8 | 5.6 | 0.9 | 24.5 | <0.001 |
| Mean systolic blood pressure, mmHg | 166.2, 30.8 | 153.6, 26.8 | 164.4, 28.8 | 155.9, 28.3 | −4.1 | 0.006 |
| Current smoker, % | 37.5 | 39.5 | 36.2 | 36.2 | 2.0 | 0.58 |
| Awareness of harms of high salt diet, % | 64.1 | 91.6 | 52.9 | 79.8 | 0.6 | 0.03 |
| Receiving monthly follow-up, % | 27.3 | 45.2 | 28.1 | 29.5 | 16.5 | <0.001 |
| Hospitalization during the past year, % | 15.3 | 10.9 | 12.5 | 13.7 | −5.6 | 0.07 |
| India | N=538 | N=512 | ||||
| Use of aspirin in the past month^, % | 5.0 | 14.5 | 3.7 | 3.4 | 9.8 | 0.003 |
| Mean systolic blood pressure, mmHg | 156.2, 27.4 | 145.6, 25.0 | 158.5, 26.5 | 148.7, 25.7 | −0.8 | 0.83 |
| Current smoker, % | 35.9 | 35.5 | 38.7 | 36.3 | 2.0 | 0.22 |
| Awareness of harms of high salt diet, % | 29.1 | 32.4 | 21.4 | 40.5 | −15.8 | <0.001 |
| Receiving monthly follow-up, % | 12.8 | 36.2 | 5.1 | 11.7 | 16.8 | 0.20 |
| Hospitalization during the past year, % | 13.6 | 12.6 | 10.4 | 11.3 | −1.9 | 0.53 |
“Use of aspirin” is defined as patient-reported use of community-health worker-prescribed aspirin for ≥ 25 days in the past month.
A significant interaction between China and India was found for this variable.
Additional analyses
We conducted additional analyses to adjust for baseline variables with significant differences between the intervention and control group (namely, history of coronary heart disease and diabetes in India). Such adjustments made no appreciable or negligible changes in the magnitude or p-values for the primary or secondary outcomes. To further investigate the influence of loss to follow-up on the results, we compared all 15 available baseline characteristics between the subjects who were followed-up and those lost to follow up. We found that there were significant differences in three variables - age, BMI and gender; however, the absolute differences for these variables were not large (Supplemental Table 1: 1.9 years for age, 0.9 kg/m2 for BMI) with little effect on the outcomes in the adjusted model (detailed results not shown). As a sensitivity analysis, we excluded one site (the only site) with a loss to follow-up rate >30% and rerun the analyses. The new results showed little effect on the main findings.
Discussion
Summary of findings
The combined results from China and India as well as country-specific results in the SimCard Trial provided clear evidence of the simplified cardiovascular management program’s effectiveness in increasing the proportion of high-risk individuals taking anti-hypertensive medication (the primary outcome) and aspirin. Results on SBP and regular monthly follow-up were significant in China but not in India. No significant changes from the intervention were observed in lifestyle factors in the overall results. These results indicate differential effects of the intervention on different outcomes in different settings.
The simplified and multi-faceted high-risk intervention strategy
Both population-based and high-risk strategies are necessary for CVD prevention and control. In resource-poor settings, it is a cost-effective choice to adopt a high-risk approach first and is in line with clinical guidelines on cardiovascular disease prevention and management. In addition, a comprehensive and integrated approach combining drug therapies and lifestyle modifications as opposed to strategies targeting single risk factors is required.18, 21-25 In this trial, the intervention focused on two lifestyle factors (smoking cessation and sodium reduction) and two low-cost and generally safe medications with demonstrated effects on risk reduction. As designed, the intervention was much simplified than all clinical guidelines including those for primary care to suit the contexts of our study sites and to ensure quality of implementation and sustainability in the long run.16, 18, 19, 26-28 Nonetheless, it was a multifaceted approach and if implemented successfully, relevant to not only CVD but also other chronic diseases affected by these lifestyle factors and hypertension. We found that the intervention was effective in changing physician behaviours including appropriate medication prescription, regular follow-up and lifestyle counseling, as well as patient behaviour in terms of medication use but not actual patient lifestyle changes. Country-specific analyses showed that reduction in blood pressure was evident in China only and even in China, the magnitude (4.1 mmHg) was modest, potentially due to the use of low dose single-drug regimen only.
The role of community healthcare workers
Our study has been designed on the basis of previous studies demonstrating the potential effectiveness of CHWs in shifting and sharing the tasks of healthcare professionals in maternal and child health and chronic disease prevention and control.11-14, 29 In particular, the SimCard trial learned the lessons from and adapted the intervention program in the Rural Andhra Pradesh Cardiovascular Prevention Study in India and the China Rural Health Initiative.29-31 Both studies targeted high cardiovascular risk individuals and implemented an intervention package delivered by CHWs. Main innovations in the present study were further simplifications of the intervention into the “2 (lifestyle factors) + 2 (medications)” model to suit the local contexts of more limited resources and capacity and the adoption of m-health technology (EDSS). Our results are generally consistent with previous studies in demonstrating the effectiveness of context-specific culturally appropriate intervention delivered by CHWs on certain indicators such as medication use but not on others such as lifestyle improvement.11-14
The use of mobile health technology
In our study, we used the mobile-phone-based EDSS to assist the CHWs in following up their high-risk individuals on a monthly basis. The use of mobile health was primarily in the form of an Android-based “app” installed on smartphones (EDSS) for assisting the CHWs in the intervention group for appropriate prescription of drugs and providing lifestyle recommendations. The use of EDSS was crucial in providing a centralized outlet of patient information database and guidance for a more efficient and appropriate administration of care by the CHWs. Although the trial was not designed to differentiate the impacts of the specific components of the intervention, it is conceivable that EDSS plays an important role in our finding of a positive effect on chronic disease management through reducing CHW training time, providing standardized evidence-based management plan, and enhanced user satisfaction.32-34 The EDSS can be further improved by integrating user-centered design, linking with local electronic health record system and engaging the patients to interact with the CHWs.
Interpretation of findings in China and India
China and India had similar results in certain outcomes such as increases in medication prescriptions as well as different patterns in other indicators including blood pressure. These findings may be explained by some important differences between the two countries in study implementation. First, although both regions had high disease burdens, the prevalence of hypertension among adults 18 years and older was higher in Tibet, China (51%) than in Haryana, India (25%) according to previous surveys.35, 36 In the present trial, baseline SBP was about 10 mmHg higher in Tibet than in Haryana, which may potentially partially explain the larger reduction in SBP observed in Tibet from increased medication use. Second, the CHWs in India were not authorized to prescribe medications and could therefore only serve as motivators for increasing compliance. They were volunteers provided with fixed honorariums instead of performance-based incentives. Third, because of ethical stipulations made by the review board in India, the two types of medications used in the intervention were also made readily available free of charge to the control groups. As such, the participants in the control group who were more inclined to proactively seek care from the health facilities after baseline screening were able to receive free medicines, potentially reducing the net between-group difference in blood pressure outcomes. This is evident from the control arm in India which had more than four-times the proportion of high-risk individuals on anti-hypertension treatment post-intervention (17.9%) as compared to pre-intervention (3.9%). These results from India were encouraging as they suggested that access to free medicine at the primary care setting had the potential to reap public health benefits.
We did not observe any significant changes for the lifestyle outcomes. This can be attributed to a number of reasons. First, the implementation of the lifestyle intervention was not intensive. The CHWs were only required to provide lifestyle counseling on smoking cessation and salt reduction on a monthly basis in the intervention groups. Second, the intervention period of 12 months might be too short to observe meaningful changes in lifestyle. Lastly, despite cultural tailoring of lifestyle interventions, people (particularly above 60 years of age) may be reluctant and it is difficult for adults to change their long-held lifestyle habits.
Strengths and limitations
To our knowledge, this was the first dual-country randomized controlled trial to evaluate the effectiveness of a simplified cardiovascular management program delivered by the CHWs with the aid of EDSS. To ensure the successful implementation of the trial, the study drew upon the rich experiences of several directly related programs in China and India and also received strong government supports from the local sites. The intervention model itself was designed with feasibility, acceptability, sustainability and scalability in mind. Additionally, the role of CHWs in this study was important as their active engagement correlated with improved clinical outcomes. Lastly, the use of EDSS in our study has shed much needed light on the benefits of utilizing m-health technology in helping CHWs in resource-limited settings.
Our study has several limitations. First, the results of this study may not be generalizable to healthcare settings without existing or available CHWs, who played key roles in managing high-risk individuals in the SimCard Trial. Second, this study was not able to evaluate the effectiveness of different components or specific measures of the simplified cardiovascular management program, e.g., any given lifestyle modification or prescription of appropriate medication. However, our results on the lack of impact on lifestyle factors and the significant results on clinical care indicators suggested that the pharmaceutical components in the “2+2” model may have played a bigger role for the observed improvements. Third, the imbalances of two baseline characteristics (history of coronary heart disease and history of diabetes) in India could have potentially affected the outcome assessment. We did a further analysis by adjusting those two variables. The effect on the outcomes was small with no change for interpretation of results.
Implications
Many regions in developing countries are now characterized as having triple burdens which include the longstanding problem of infectious diseases, the rapidly rising burden of non-communicable chronic diseases, and the serious lack of economic and healthcare resources coupled with a weak healthcare system.37, 38 Despite well-established national guidelines on chronic disease management, its uptake into routine clinical practices remains limited. A cost-effective, feasible, and culturally-tailored approach is urgently needed in these areas that have high burdens of diseases but limited resources. Our simplified cardiovascular management program provides a framework upon which the key components for effective CVD prevention and management can be based. These key components of the high-risk strategy include the following: effective, low-cost and safe medications, lifestyle recommendations addressing the main risk factors for CVD, active involvement of CHWs, and an EDSS administered through customized mobile technology.
Our trial was conducted in two countries with both similarities and differences in healthcare systems and certain aspects of the implementation to adapt to local contexts and ethic requirements. The major driver of our outcome appears to be the enhanced used of drugs while lifestyle changes did not influence the outcome in a significant way. The overall results demonstrated the effectiveness of the intervention model in improving quality of clinical care at the primary care level, the potential lack of impact of this low-intensity primary care-delivered model on lifestyle behavioural changes, and the need to tailor the intervention and implementation to local settings. The simplified cardiovascular management model tested in the SimCard study has the potential to be scaled up in more regions in China, India, and other countries to benefit a large number of disadvantaged populations. Larger context-specific trials are needed to enhance and refine the management program and to pinpoint cost-effective components of the model.
Supplementary Material
Acknowledgements
We thank Drs. Elizabeth DeLong and Elizabeth Turner for providing biostatistical support. The China team would like to thank Mr. Luobu Zhandui, Mr. Baima Duoji, Ms. Zha Sang, and Ms. Ci Yang from Tibet University, Mr. Baima Ranjiu from Linzhou County Health Bureau, Mr. Pubu Ciren and Ms. Ci Deji from Gongbujiangda County Hospital, Ms. Christina Dong, Mr. Chris Lam, Ms. Wenjie Ma, Ms. Nancy Yang, and Mr. Alex Ewing for their great contributions during the design, implementation and dissemination of the study. We would also like to thank Omron (China) for providing the electronic blood pressure monitors in Tibet, China site. The India team would like to thank Ms Neeraj Sharma, Poonam Saini, Sonu Sharma, Sangeeta Rani, Savita Sharma, Sita Rani, Geeta Devi, Jitender, Kiran Devi, Lata Mann, Poonam Kumari, for their services as community health workers, Neelam Sinha for her supervisory role and Data Template for developing the EDSS.
Funding Sources: This study was funded in part with Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900027C. Additional support was received from the UnitedHealth Group Chronic Disease Initiative. LL Yan is also supported by the National Natural Sciences Foundation of China grants (71110107025, 71233001, and 71490732), US NIH R01 grant (R01AG023627), and UNFPA. D Prabhakaran was also supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services, and the UnitedHealth Group, Minneapolis, MN, USA under Contract No. HHSN268200900026C. VSA is supported by a Wellcome Trust Capacity Strengthening Strategic Award to the Public Health Foundation of India and a consortium of UK universities.
Footnotes
Disclosures: None.
References
- 1.Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, Baugh V, Bekedam H, Billo N, Casswell S, Cecchini M, Colagiuri R, Colagiuri S, Collins T, Ebrahim S, Engelgau M, Galea G, Gaziano T, Geneau R, Haines A, Hospedales J, Jha P, Keeling A, Leeder S, Lincoln P, McKee M, Mackay J, Magnusson R, Moodie R, Mwatsama M, Nishtar S, Norrving B, Patterson D, Piot P, Ralston J, Rani M, Reddy KS, Sassi F, Sheron N, Stuckler D, Suh I, Torode J, Varghese C, Watt J, Lancet NCDAG, Alliance NCD. Priority actions for the non-communicable disease crisis. Lancet. 2011;377:1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Cardiovascular Diseases C Report on cardiovascular diseases in china (2011) 2012 [Google Scholar]
- 3.Patel V, Chatterji S, Chisholm D, Ebrahim S, Gopalakrishna G, Mathers C, Mohan V, Prabhakaran D, Ravindran RD, Reddy KS. Chronic diseases and injuries in india. Lancet. 2011;377:413–428. doi: 10.1016/S0140-6736(10)61188-9. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KS. Cardiovascular diseases in the developing countries: Dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2002;5:231–237. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Lauer JA, Hutubessy RC, Niessen L, Tomijima N, Rodgers A, Lawes CM, Evans DB. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: A global and regional analysis on reduction of cardiovascular-disease risk. Lancet. 2003;361:717–725. doi: 10.1016/S0140-6736(03)12655-4. [DOI] [PubMed] [Google Scholar]
- 6.Gaziano TA, Steyn K, Cohen DJ, Weinstein MC, Opie LH. Cost-effectiveness analysis of hypertension guidelines in south africa: Absolute risk versus blood pressure level. Circulation. 2005;112:3569–3576. doi: 10.1161/CIRCULATIONAHA.105.535922. [DOI] [PubMed] [Google Scholar]
- 7.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: A cost-effectiveness analysis. Lancet. 2006;368:679–686. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 9.National vascular disease prevention alliance, guidelines for the assessment of absolute cardiovascualr disease risk National Heart Foundation of Australia: Melbourne. 2009 [Google Scholar]
- 10.Wu Y, Liu X, Li X, Li Y, Zhao L, Chen Z, Li Y, Rao X, Zhou B, Detrano R, Liu K, Cardiovascular U-PCSo. Cardiopulmonary Epidemiology Research G. China Multicenter Collaborative Study of Cardiovascular Epidemiology Research G Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in chinese adults. Circulation. 2006;114:2217–2225. doi: 10.1161/CIRCULATIONAHA.105.607499. [DOI] [PubMed] [Google Scholar]
- 11.Allen JK, Dennison-Himmelfarb CR, Szanton SL, Bone L, Hill MN, Levine DM, West M, Barlow A, Lewis-Boyer L, Donnelly-Strozzo M, Curtis C, Anderson K. Community outreach and cardiovascular health (coach) trial: A randomized, controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centers. Circ Cardiovasc Qual Outcomes. 2011;4:595–602. doi: 10.1161/CIRCOUTCOMES.111.961573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee S, Naik S, John S, Dabholkar H, Balaji M, Koschorke M, Varghese M, Thara R, Weiss HA, Williams P, McCrone P, Patel V, Thornicroft G. Effectiveness of a community-based intervention for people with schizophrenia and their caregivers in india (copsi): A randomised controlled trial. Lancet. 2014;383:1385–1394. doi: 10.1016/S0140-6736(13)62629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dye CJ, Williams JE, Evatt JH. Improving hypertension self-management with community health coaches. Health Promot Pract. 2014 doi: 10.1177/1524839914533797. doi: 10.1177/1524839914533797. [DOI] [PubMed] [Google Scholar]
- 14.Jafar TH, Hatcher J, Poulter N, Islam M, Hashmi S, Qadri Z, Bux R, Khan A, Jafary FH, Hameed A, Khan A, Badruddin SH, Chaturvedi N. Community-based interventions to promote blood pressure control in a developing country: A cluster randomized trial. Ann Intern Med. 2009;151:593–601. doi: 10.7326/0003-4819-151-9-200911030-00004. [DOI] [PubMed] [Google Scholar]
- 15.Ajay VS, Tian M, Chen H, Wu Y, Li X, Dunzhu D, Ali MK, Tandon N, Krishnan A, Prabhakaran D, Yan LL. A cluster-randomized controlled trial to evaluate the effects of a simplified cardiovascular management program in tibet, china and haryana, india: Study design and rationale. BMC Public Health. 2014;14:924. doi: 10.1186/1471-2458-14-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J, Jr., Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. 2012 update of the canadian cardiovascular society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Chinese Society of Cardiology of Chinese Medical Association EBoCJoC Chinese guidelines for prevention of cardiovascular diseases. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:3–22. [PubMed] [Google Scholar]
- 18.Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr., Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr., Tomaselli GF. 2013 acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 19.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F, European Association for Cardiovascular P, Rehabilitation European guidelines on cardiovascular disease prevention in clinical practice (version 2012) : The fifth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Int J Behav Med. 2012;19:403–488. doi: 10.1007/s12529-012-9242-5. [DOI] [PubMed] [Google Scholar]
- 20.Parker DR, Evangelou E, Eaton CB. Intraclass correlation coefficients for cluster randomized trials in primary care: The cholesterol education and research trial (ceart) Contemp Clin Trials. 2005;26:260–267. doi: 10.1016/j.cct.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Carey KM, Comee MR, Donovan JL, Kanaan AO. A polypill for all? Critical review of the polypill literature for primary prevention of cardiovascular disease and stroke. Ann Pharmacother. 2012;46:688–695. doi: 10.1345/aph.1Q621. [DOI] [PubMed] [Google Scholar]
- 22.Force USPST Aspirin for the prevention of cardiovascular disease: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 23.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: Analysis of 354 randomised trials. Bmj. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, Fonarow GC, Fortmann SP, Franklin BA, Galloway JM, Goff DC, Jr., Heath GW, Frank AT, Kris-Etherton PM, Labarthe DR, Murabito JM, Sacco RL, Sasson C, Turner MB, American Heart Association Council on E, Prevention American heart association guide for improving cardiovascular health at the community level, 2013 update: A scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation. 2013;127:1730–1753. doi: 10.1161/CIR.0b013e31828f8a94. [DOI] [PubMed] [Google Scholar]
- 25.Fortmann SP, Winkleby MA, Flora JA, Haskell WL, Taylor CB. Effect of long-term community health education on blood pressure and hypertension control. The stanford five-city project. Am J Epidemiol. 1990;132:629–646. doi: 10.1093/oxfordjournals.aje.a115705. [DOI] [PubMed] [Google Scholar]
- 26.Gupta R, Deedwania P. Interventions for cardiovascular disease prevention. Cardiol Clin. 2011;29:15–34. doi: 10.1016/j.ccl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Shroufi A, Chowdhury R, Anchala R, Stevens S, Blanco P, Han T, Niessen L, Franco OH. Cost effective interventions for the prevention of cardiovascular disease in low and middle income countries: A systematic review. BMC Public Health. 2013;13:285. doi: 10.1186/1471-2458-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang L, Patao C, Chuang J, Wong ND. Cardiovascular risk factor control and adherence to recommended lifestyle and medical therapies in persons with coronary heart disease (from the national health and nutrition examination survey 2007-2010) Am J Cardiol. 2013;112:1126–1132. doi: 10.1016/j.amjcard.2013.05.064. [DOI] [PubMed] [Google Scholar]
- 29.Yan LL, Fang W, Delong E, Neal B, Peterson ED, Huang Y, Sun N, Yao C, Li X, MacMahon S, Wu Y. Population impact of a high cardiovascular risk management program delivered by village doctors in rural china: Design and rationale of a large, cluster-randomized controlled trial. BMC Public Health. 2014;14:345. doi: 10.1186/1471-2458-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi R, Chow CK, Raju PK, Raju KR, Gottumukkala AK, Reddy KS, Macmahon S, Heritier S, Li Q, Dandona R, Neal B. The rural andhra pradesh cardiovascular prevention study (rapcaps): A cluster randomized trial. J Am Coll Cardiol. 2012;59:1188–1196. doi: 10.1016/j.jacc.2011.10.901. [DOI] [PubMed] [Google Scholar]
- 31.Chow CK, Joshi R, Gottumukkala AK, Raju K, Raju R, Reddy S, Macmahon S, Neal B. Rationale and design of the rural andhra pradesh cardiovascular prevention study (rapcaps): A factorial, cluster-randomized trial of 2 practical cardiovascular disease prevention strategies developed for rural andhra pradesh, india. Am Heart J. 2009;158:349–355. doi: 10.1016/j.ahj.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 32.Surka S, Edirippulige S, Steyn K, Gaziano T, Puoane T, Levitt N. Evaluating the use of mobile phone technology to enhance cardiovascular disease screening by community health workers. Int J Med Inform. 2014;83:648–654. doi: 10.1016/j.ijmedinf.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peiris D, Praveen D, Johnson C, Mogulluru K. Use of health systems and tools for non-communicable diseases in low- and middle-income countries: A systematic review. J Cardiovasc Transl Res. 2014;7:677–91. doi: 10.1007/s12265-014-9581-5. doi: 10.1007/s12265-014-9581-5. Epub 2014 Sep 11. [DOI] [PubMed] [Google Scholar]
- 34.Beratarrechea A, Lee AG, Willner JM, Jahangir E, Ciapponi A, Rubinstein A. The impact of mobile health interventions on chronic disease outcomes in developing countries: A systematic review. Telemed J E Health. 2014;20:75–82. doi: 10.1089/tmj.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan A, Shah B, Lal V, Shukla DK, Paul E, Kapoor SK. Prevalence of risk factors for non-communicable disease in a rural area of faridabad district of haryana. Indian J Public Health. 2008;52:117–124. [PubMed] [Google Scholar]
- 36.Zhao X, Li S, Ba S, He F, Li N, Ke L, Li X, Lam C, Yan LL, Zhou Y, Wu Y. Prevalence, awareness, treatment, and control of hypertension among herdsmen living at 4,300 m in tibet. Am J Hypertens. 2012;25:583–589. doi: 10.1038/ajh.2012.9. [DOI] [PubMed] [Google Scholar]
- 37.Mackay J, Mensah GA, World Health Organization. Center for Disease Control. Myriad Editions Limited . The atlas of heart disease and stroke. World Health Organization; Geneva: 2004. [Google Scholar]
- 38.World Health Organization . Global status report on noncommunicable diseases 2010. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.