Abstract
Background
Prazosin (PRZ, an α1-adrenergic receptor antagonist) and naltrexone (NTX, a non-specific opioid receptor antagonist) each decrease alcohol drinking when administered to rats selectively-bred for high voluntary alcohol drinking (alcohol-preferring, or “P”), and the combination of PRZ+NTX decreases alcohol drinking more effectively than does either drug alone. Since drug responsiveness can depend on history of alcohol drinking and dependence, we investigated whether various schedules of PRZ and NTX administration, alone or in combination, are effective in decreasing alcohol drinking in male P rats with a history of protracted voluntary alcohol drinking, dependence and repeated withdrawals closely resembling human alcoholism.
Methods
Male P rats became alcohol-dependent during 1 year of ad libitum 24 h/day access to food, water and 20% alcohol with repetitive temporary alcohol withdrawals. Four sequential studies then addressed effects of oral PRZ (2 mg/kg) and NTX (10 mg/kg), alone or together, on alcohol drinking during: 1) daily alcohol access with daily drug treatment, 2) intermittent alcohol access with daily drug treatment, 3) intermittent alcohol access with occasional drug treatment, and 4) post-deprivation reinstatement of alcohol access.
Results
The combination of PRZ+NTX consistently suppressed alcohol drinking during daily or intermittent alcohol access conditions and when drug treatment was either daily or occasional. PRZ+NTX was consistently more effective than either drug alone. The reduction in alcohol drinking was not due to sedation, motor effects or malaise.
Conclusions
Both daily and “as-needed” treatment with PRZ+NTX are highly effective in suppressing daily, intermittent and post-deprivation alcohol drinking in male P rats with a protracted history of alcohol dependence and repeated withdrawals. This drug combination may be especially effective for treating individuals with long histories of heavy alcohol abuse, dependence and repeated relapse, as commonly encountered in clinical practice.
Keywords: prazosin, naltrexone, alcohol, alcoholism treatment, noradrenergic, opioid
INTRODUCTION
Alcoholism remains the most prevalent addictive disease, and development of effective treatments is a high priority. The US Food and Drug Administration (FDA)-approved pharmacotherapies for alcoholism – disulfiram, acamprosate and naltrexone (oral and injectable extended release) - all have limitations to their use and effectiveness (Garbutt, 2009), and clinical utilization rates remain low (Del Re et al., 2013). Additional pharmacotherapy targets, and additional strategies for utilizing available pharmacotherapies, are needed in order to maximize benefits of treatment while minimizing adverse side effects.
Brain opioidergic regulation has been studied extensively as a target for pharmacotherapy of alcohol use disorders, with a primary focus on naltrexone (NTX) - a non-subtype specific opioid receptor antagonist that reduces the reinforcing or euphoriant effects of alcohol (Froehlich et al., 2003; Froehlich and Li, 1993; Froehlich and Wand, 1996; Gianoulakis, 1989; Gianoulakis et al., 1996; Mitchell et al., 2012; O’Malley and Froehlich, 2003; Rasmussen et al., 1998; 2002). However, effectiveness of NTX in decreasing alcohol drinking is modest (Froehlich et al., 2003; O’Malley and Froehlich, 2003); it is not effective for all alcoholic individuals (Garbutt et al., 2005; Kranzler and Van Kirk, 2001; Krishnan-Sarin et al., 2007; O’Malley et al., 1992; Oncken et al., 2001) and, when it is effective, a significant number of alcoholic individuals fail to maintain treatment gains and subsequently relapse to heavy drinking (Anton et al., 2006; Garbutt et al., 2005; Krystal et al., 2001).
Noradrenergic regulation also plays an important role in regulation of alcohol drinking. Blocking norepinephrine synthesis by pharmacologic suppression of dopamine β-hydrolyase (DBH) activity (Amit et al., 1977; Davis et al., 1978; Colombo et al., 2014) or deleting the gene encoding DBH (Weinshenker et al., 2000) decreases alcohol self-administration in rodents. Administration of clonidine, an α2-adrenergic agonist that decreases presynaptic norepinephrine release, likewise decreases alcohol drinking by alcohol-preferring (P) rats (Rasmussen et al., 2014), an animal model of alcoholism (Bell et al., 2006). Compelling evidence for α1-adrenergic receptor-mediated regulation of alcohol drinking comes from studies with prazosin, an α1-adrenergic receptor antagonist that is non-subtype selective (similar affinities for α1A, α1B, α1D) and blocks brain α1-adrenergic signaling when administered systemically at clinically-relevant doses (Menkes et al., 1981; Rojawski and Aghajanian, 1982). Prazosin decreases alcohol withdrawal-induced operant alcohol intake in Wistar rats (Walker et al., 2008); decreases voluntary alcohol drinking by P rats (Rasmussen et al., 2009a; 2009b); increases latency to first operant response for alcohol by P rats (Verplaetse et al., 2012); reduces alcohol drinking throughout prolonged treatment of P rats (Froehlich et al., 2013a) or outbred rats (Skelly and Weiner, 2014); blocks initiation of alcohol drinking in P rats (Froehlich et al., 2013a); blocks development of alcohol conditioned place preference in mice when microinjected into the brain ventral tegmental area (Raskind et al., 2011); and decreases stress-induced reinstatement of alcohol seeking in rats (Lê et al., 2011). Prazosin also facilitates abstinence in treatment-seeking alcoholic individuals (Simpson et al., 2009) and decreases stress- and cue-induced alcohol craving in alcohol-dependent men and women (Fox et al., 2012). The similar α1-adrenergic receptor antagonist, doxazosin, likewise reduces alcohol drinking in P rats (O’Neil et al., 2013) and in alcohol-dependent patients with a high family history density of alcoholism (Kenna et al., 2015). This growing body of literature demonstrates that reducing CNS α1-adrenergic receptor-mediated activity reduces alcohol drinking and relapse in rodents and humans, providing a promising target for effective pharmacotherapy of alcohol use disorders.
Since either NTX or PRZ can decrease alcohol drinking in rodents and humans and both are safe, orally-active, well-characterized, inexpensive, FDA-approved for human use and well-tolerated in clinically-relevant doses, we hypothesized that combining these drugs may be useful for treating patients not responsive to either drug alone. This hypothesis was supported by our finding that combining PRZ with NTX in a single oral medication decreases alcohol drinking more consistently than does either drug alone when administered to P rats (Froehlich et al., 2013b).
PRZ and NTX effects on alcohol intake depend on history of alcohol drinking and dependence (Walker et al., 2008; Pettinati et al., 2006), so we now investigate whether PRZ and NTX, alone or in combination, decrease alcohol drinking in male P rats with a 1 year history of 24 h/day voluntary alcohol drinking that produces dependence and that spans the period from late adolescence to middle age. Introduction of imposed abstinences simulates the repeated withdrawals, temporary abstinences and relapses that are common to the clinical course of alcoholism, allowing assessment of whether combined PRZ+NTX treatment is likely to be effective in long-term alcoholic patients.
We were also interested in whether continuous drug treatments are more or less effective than treatments on an “as-needed” basis, i.e., only when drinking is likely to occur (Sinclair JD, 2001). Hence, effects of PRZ and/or NTX were assessed during either daily or intermittent alcohol access, together with either daily or occasional drug treatment. Since clinical treatment commonly begins during voluntarily- or externally-imposed abstinence, we also evaluated whether initiating PRZ+NTX treatment during imposed abstinence decreases alcohol intake during reinstatement of alcohol access.
We hypothesized that both daily and “as-needed” treatment with PRZ+NTX would be highly effective in suppressing daily, intermittent and post-deprivation alcohol drinking in male P rats with a protracted history of alcohol dependence and repeated withdrawals. If so, this drug combination potentially could provide an effective new option for treating heavy drinking subjects with long history of alcohol abuse, alcohol dependence and repeated episodes of abstinence and relapse consistent with characteristics of individuals commonly encountered in clinical practice.
MATERIALS AND METHODS
Subjects
Alcohol-naïve male rats (n = 64) from generation 71 of selective breeding for alcohol preference were provided by the Alcohol Research Resource Center of the Indiana Alcohol Research Center. The rats were received at 30 days of age and were group housed (3/cage) with controlled temperature (21 ± 1°C) and reversed 12-hour (h) light-dark cycle (lights-off at 10:00 h; procedures during dark periods were performed with dim red illumination). Chow (Laboratory Rodent Diet 5001; PMI Nutrition International, Brentwood, MO) and water were available ad libitum at all times throughout the study. Starting at 70 days of age, the rats were individually housed in clear plastic cages. Body weight (BW) was determined weekly (340 ± 4 g at start of study). All experiments were approved by the VA Puget Sound Health Care System IACUC and conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Alcohol and Water Intake
Alcohol solutions were prepared by diluting 95% alcohol (ethanol) with deionized water. During 24-h alcohol vs water access, positions of overhead ball-bearing sipper tube water and alcohol bottles were alternated daily. During tests of 2-h alcohol vs water intake, alcohol and water were presented in 2 glass drinking tubes (100 ml, BioServ, Frenchtown, NJ) with open-well side-arms extending through adjacent holes on the front of the cage, with positions alternated daily. Intakes were determined by weighing each tube to the nearest 0.1 g. Alcohol and water tubes on 2 empty cages provided estimated loss due to spillage and/or evaporation which was subtracted from intakes. Net alcohol intake was converted to g alcohol/kg BW.
Drugs
PRZ hydrochloride and/or NTX hydrochloride (Sigma-Aldrich, St. Louis, MO) were incorporated into pieces of sweetened gelatin as previously described (Froehlich et al., 2013a). PRZ (2 mg/kg), NTX (10 mg/kg), PRZ+NTX (2 and 10 mg/kg, respectively), or vehicle (water) alone in approximately 1.8 g of gelatin (determined by BW of each rat) were fed to the rats once/day, 45 minutes prior to onset of the 2-h alcohol access period, and were confirmed to be consumed within 15 min. We previously reported that these PRZ and NTX dosages in gelatin suppressed 2-h alcohol drinking in male P rats without a history of prolonged unlimited alcohol drinking (Froehlich et al., 2013b). The half-life of NTX in rats is 4 ± 0.9 h (Hussain et al., 1987); the half-life of prazosin in rats has not been determined, but is about 3 h in humans (Westfall and Westfall, 2006).
Prolonged Alcohol Drinking and Dependence
Starting at 70 days of age, 49 rats received 24 h/day, 7 day/week access to alcohol (starting at 10% v/v alcohol, increasing to 20% by week 4) for 17 weeks which then was restricted to 5 days/week for another 33 weeks. During weeks 27-28, alcohol access was restricted to 2 h/day (as training for limited access conditions to be used in later testing), after which 24-h alcohol access was restored. Fifteen rats did not receive alcohol. After 50 weeks of alcohol drinking, withdrawal was evaluated at 1500 h following 9-h alcohol deprivation, using the protocol of Macey et al. (1996) as previously described (O’Neil et al., 2013). In short, each rat was rated on abnormal body posture [broad-based stance or abnormal gait]; tail stiffness [rigid, awkwardly bent tail]; and ventromedial distal limb flexion when grasped by the scruff of the neck, using scales of 0 to 2 for each measure. The three scores were summed for a withdrawal rating of 0 - 6 (0 = undetectable, 6 = severe).
Experimental Design
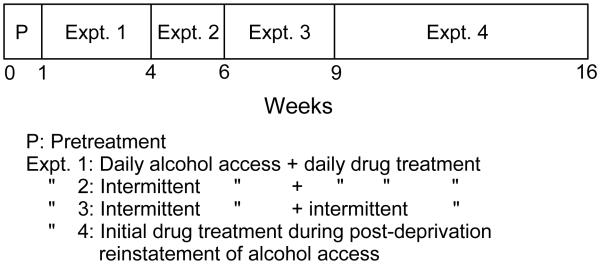
Following withdrawal testing, 15 rats that had not received alcohol (withdrawal controls) were removed from the study. The 49 middle-aged rats with a history of prolonged alcohol access were then given 2-h (11:00 to 13:00 h) free choice between 20% (v/v) alcohol and water for 5 days/week, and PRZ, NTX, PRZ+NTX or VEH effects on alcohol intake were investigated in 4 consecutive experiments (Fig. 1).
Figure 1.
Timeline of sequential studies. These studies were conducted after 1 year of ad libitum access to food, water and 20% alcohol, with repetitive temporary alcohol withdrawals, that produced alcohol dependence.
Pre-Treatment (week 0)
Each rat received a piece of sweetened gelatin (3 g/kg BW) at 45 min before each 2-h access to 20% alcohol vs water. Average 2-h alcohol intake during this week was used for counterbalanced assignment of rats to 1 of the 4 treatment groups (n = 12-13 rats/group) as previously described (Rasmussen et al., 2009a), assuring that the groups did not differ in baseline alcohol intake.
Experiment 1: Daily alcohol access, daily drug treatments (weeks 1-3)
Each rat received either PRZ (2mg/kg; n=12), NTX (10 mg/kg; n=12), PRZ+NTX (2 and 10 mg/kg, respectively; n=13) or vehicle (VEH; n=12) in gelatin 45 min before the daily 2-h alcohol access period, 5 days/week for 3 weeks. In week 2, it was observed whether each rat drank alcohol throughout the initial 10 seconds after placing alcohol and water tubes on the front of the cage.
Experiment 2: Intermittent alcohol access, daily drug treatments (weeks 4-5)
Each rat received the same daily (5 days/week) treatment that it had received in Experiment 1 (i.e., treatments were not re-randomized), but alcohol access was available only intermittently; i.e., for 2 h on days 1, 5 and 8 and for 1 h on day 11 (Fig. 3). On day 8, it again was observed whether each rat drank alcohol throughout the initial 10 sec after placing alcohol and water tubes on the cage. On days when alcohol was not provided, effects of drugs or VEH on consumption of [3% sucrose + 0.125% saccharin] vs water was determined during trials of 1 h (day 3), 5 min (day 6) or 2 min (day 9) (Fig. 3). On day 11, tail blood was collected after 1-h alcohol access and blood alcohol concentration (BAC) was determined by NAD-ADH enzymatic Ethanol Assay (Genzyme Diagnostics, Charlottetown, PE, Canada).
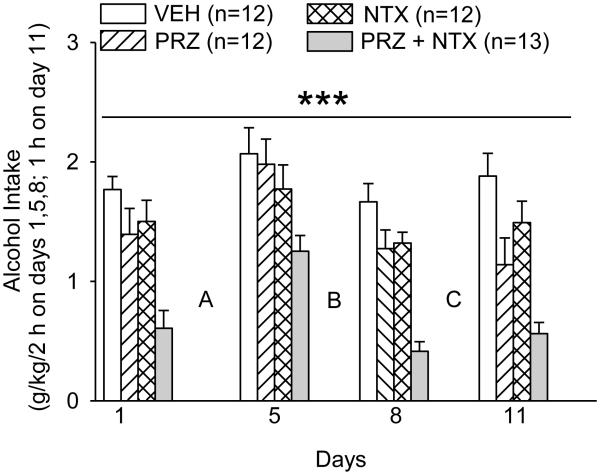
Figure 3.
Effects of oral prazosin (PRZ, 2 mg/kg) and naltrexone (NTX, 10 mg/kg), alone or in combination, on alcohol intake during intermittent 2-h alcohol access with daily drug treatments (Expt. 2; treatment weeks 4-5). A, B and C indicate tests of PRZ and NTX effects on 1 h, 5 min or 2 min intake of a solution of [3% sucrose + 0.125% saccharin] on days with no alcohol access (results presented in Fig. 4). Each bar represents data from 12-13 rats. *** p<0.001, PRZ+NTX vs vehicle (VEH), independent of day.
Experiment 3: Intermittent alcohol access, occasional drug treatments (weeks 6-8)
The rats continued to receive intermittent 2-h alcohol access for 3 additional weeks. On days 1, 11 and 16 (Fig. 4), alcohol access was preceded by the same treatment that the rat had received in Experiments 1 and 2. Rats that received PRZ, NTX or PRZ+NTX during Experiments 1-3 were then removed from further study.
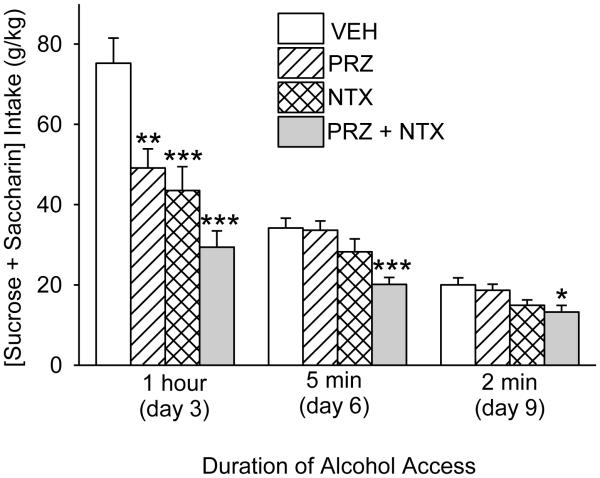
Figure 4.
Effects of oral prazosin (PRZ, 2 mg/kg) and naltrexone (NTX, 10 mg/kg), alone or in combination, on intake of a solution of [3% sucrose + 0.125% saccharin] during 1-h, 5-min or 2-min access in Expt. 2 (as noted in Fig. 3). Each bar represents data from 12-13 rats. *** p<0.001, ** p<0.01, * p<0.05 vs vehicle (VEH).
Experiment 4. Drug treatment during post-deprivation reinstatement of alcohol access (weeks 9-15)
The 12 rats that had received VEH treatment in Experiments 1-3 then received 24-h/day alcohol access for 4 weeks (weeks 9-12), followed by 2-week alcohol deprivation (weeks 13-14). In week 15, the rats received oral PRZ+NTX (2 and 10 mg/kg, respectively; n=6) or VEH (n=6) on each of 4 days. On treatment days 1 and 2, no alcohol was provided; on days 3 and 4, 2-h alcohol access was provided as in Experiments 1-3.
Data Analysis
Grubbs’ Test (Extreme Studentized Deviate Method) performed with GraphPad QuickCalcs (GraphPad Software, San Diego, CA) was used to eliminate outlying (p<0.05) alcohol or water intake values that were likely due to leakage or spillage. No more than 1 intake determination forany treatment X day was determined to be an outlier.
Alcohol or water intake was analyzed by 2-way (dose X day or dose X week) analysis of variance (ANOVA) with repeated measures on day (or week), followed – when significant overall F ratios were obtained – by Student-Newman-Keuls (SNK) pairwise comparisons. Main effect statistics are reported when there was not a significant interaction requiring dose X day (or week) comparisons. Intakes of [sucrose + saccharin] in 1 h, 5 min or 2 min trials, as well as BAC following 1-h alcohol intake, were each analyzed by 1-way ANOVA and pairwise SNK comparisons. Associations between BAC and alcohol intake were evaluated by Pearson’s product-moment correlation analyses. Analyses were performed with Sigmaplot 11 software (Systat Software, Inc., Chicago, IL) with significance at p<0.05. Data are presented as mean±SEM.
RESULTS
During the 50th week of initial prolonged alcohol access, 24-h alcohol intake was 6.7 ± 0.2 g/kg, with alcohol preference (alcohol intake / [alcohol + water intake]) of 0.81 ± 0.03. After 9 h of imposed abstinence, alcohol withdrawal scores were increased relative to control rats without a history of alcohol drinking (3.9 ± 0.2 vs 1.1 ± 0.2, respectively; p<0.001, Student t-test). After limiting daily alcohol access to 2 h, alcohol intake during the pre-treatment week (week 0) was 2.4 ± 0.1 g/kg/2 h, with alcohol preference of 0.98 ± 0.02.
Experiment 1: Daily Alcohol Access, Daily Drug Treatment
Effects of daily alcohol access (5 days/week) and treatment before each alcohol access with PRZ (2 mg/kg), NTX (10 mg/kg), PRZ+NTX (2 and 10 mg/kg, respectively) or VEH are presented in Fig. 2.
Figure 2.
Effects of oral prazosin (PRZ, 2 mg/kg) and naltrexone (NTX, 10 mg/kg), alone or in combination, on alcohol intake during daily 2-h alcohol access with daily drug treatment (Expt. 1, treatment weeks 1-3). Each bar represents data from 12-13 rats. *** p<0.001, ** p<0.01, * p<0.05, independent of day. Veh: vehicle.
In week 1, there were significant effects of treatment, F(3, 45) = 7.94, p<0.001, and day, F(4, 179), p<0.001, on alcohol intake, but no treatment X day interaction. Only PRZ+NTX suppressed alcohol intake (p<0.01, relative to VEH). PRZ+NTX also suppressed alcohol intake relative to PRZ (p<0.001) or NTX (p<0.05). NTX suppressed alcohol intake, relative to PRZ (p<0.05).
In week 2, there was a significant effect of treatment, F(3, 45) = 8.71, p<0.001, on alcohol intake, but no effect of day and no treatment X day interaction. Only PRZ+NTX suppressed alcohol intake (p<0.001, relative to VEH). PRZ+NTX also suppressed alcohol intake relative to PRZ (p<0.001) or NTX (p<0.01).
In week 3, there were significant effects of treatment, F(3, 45) = 10.91, p<0.001, and day, F(4, 180) = 6.96, p<0.001, on alcohol intake, but no treatment X day interaction. Alcohol intake was suppressed by PRZ+NTX (p<0.001), PRZ (p<0.05) or NTX (p<0.05), relative to VEH. PRZ+NTX also suppressed alcohol intake relative to PRZ (p<0.05) or NTX (p<0.05).
In the first week of treatment, all rats in all treatment groups drank alcohol throughout the first 10 seconds following placement of alcohol and water drinking tubes on the cage. By the end of week 3, some rats did not, even though all rats were alert and commonly investigated the drinking tubes immediately following placement; the % that did drink alcohol in the first 10 sec was: VEH = 100; NTX = 83; PRZ = 50; PRZ+NTX = 15. The number of rats in each treatment group that did vs did not drink alcohol during the first 10 sec were compared for the first vs third week of 2-h alcohol access by 2×4 (week × treatment) Chi-square analysis; proportions of rats that decreased immediate alcohol drinking during the interval from week 1 to week 3 were significantly different among treatments (Chi-square = 22.14, 3 df, p<0.001).
Experiment 2: Intermittent Alcohol Access, Daily Drug Treatment
Effects of PRZ, NTX, PRZ+NTX or VEH treatment before each intermittent alcohol access are presented in Fig. 3. On 3 alcohol access days (days 1, 5 and 8 in Fig. 3), alcohol was available for 2 h, whereas on day 11 alcohol was available only 1 h before tail blood collection for determination of BAC. Nonetheless, analysis (1-way ANOVA with repeated measures on day) of intake in the VEH group on all 4 access days revealed no difference among days, suggesting that alcohol intake occurred primarily within the first h (consistent with observed behavior) and justifying inclusion of all 4 days in overall analysis of alcohol intake. Accordingly, 2-way repeated measures ANOVA on days 1, 5, 8 and 11 revealed significant effects of treatment, F(3, 45) = 13.77, p<0.001, and day, F(3, 135) = 20.287, p<0.001, on alcohol intake, but no treatment X day interaction. Alcohol intake was suppressed only by PRZ+NTX (p<0.001, relative to VEH); PRZ+NTX also suppressed alcohol intake relative to PRZ (p<0.001) or NTX (p<0.001).
On days when alcohol was not provided, treatment effects on 1-h intake of [3% sucrose + 0.125% saccharin] vs water were determined. There was a significant effect of treatment on 1-h [sucrose + saccharin] intake, F(3, 45) = 14.0, p<0.001; intake was suppressed by PRZ (p<0.01), NTX (p<0.001) or PRZ+NTX (p<0.001), relative to VEH (Fig. 4). Since all rats were observed to stop consuming the sweet solution within 45 min on day 3, access to [sucrose + saccharin] was limited to 5 min on day 6. During this 5 min access, there was a significant treatment effect on [sucrose + saccharin] intake, F(3, 45) = 7.2, p<0.001; PRZ+NTX suppressed [sucrose + saccharin] intake relative to treatment with VEH (p≤0.001), PRZ (p≤0.001) or NTX (p<0.05) (Fig. 4). When access to [sucrose + saccharin] was limited further to 2 min on (day 9, all rats were confirmed to drink the sweet solution throughout 2 min. Nonetheless, there was still a significant treatment effect on [sucrose + saccharin] intake, F(3, 45) = 4.1, p<0.05; PRZ+NTX suppressed intake relative to treatment with VEH (p<0.05) or PRZ (p<0.05) (Fig. 4).
BAC after 1-h alcohol access on day 11 was 0.064 ± 0.008, 0.009 ± 0.002, 0.007 ± 0.002 or 0.002 ± 0.000 % (w/v) in the VEH-, PRZ-, NTX- or PRZ+NTX-treated rats, respectively. There was a significant treatment effect on BAC, F(3, 43) = 45.0, p<0.001; PRZ, NTX or PRZ+NTX suppressed BAC (p<0.001 for each, relative to VEH). PRZ+NTX also suppressed BAC relative to PRZ or NTX (p<0.001 for each). BAC was positively correlated with preceding 1-h alcohol intake, r = 0.47, p<0.001.
Experiment 3: Intermittent Alcohol Access, Occasional Drug Treatments
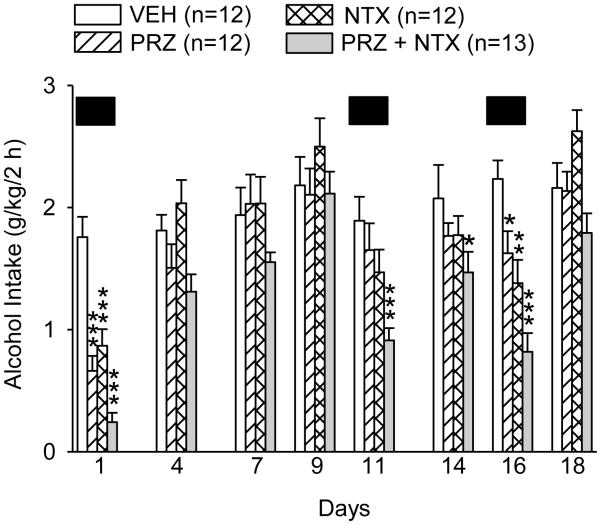
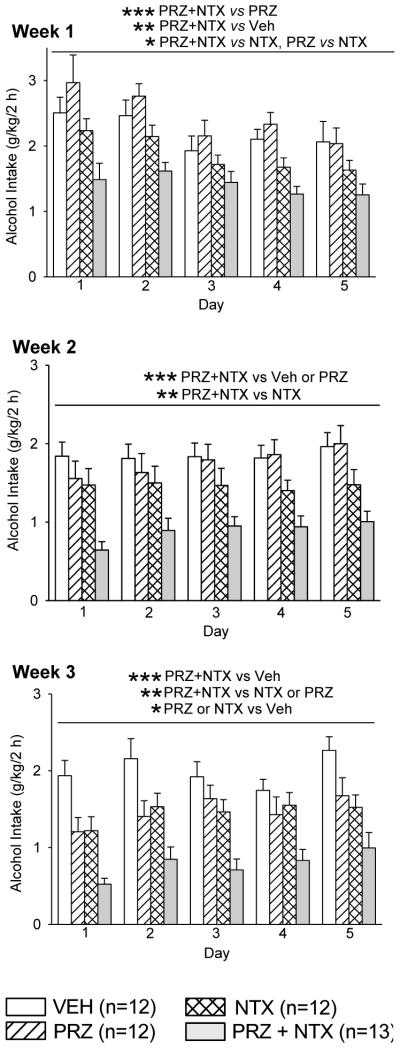
The effects of PRZ, NTX, PRZ+NTX or VEH treatment before only 3 of 8 intermittent alcohol access periods are presented in Fig. 5. Analysis of alcohol intake in the 8 alcohol access days revealed a treatment X day interaction, F(21, 315) = 3.72, p<0.001. On treatment day 1, alcohol intake was suppressed by PRZ, NTX or PRZ+NTX (p<0.001 for each, relative to VEH) and PRZ+NTX suppressed intake relative to NTX alone (p<0.05). On 3 subsequent intermittent alcohol access days with no treatments (days 4, 7, 9 in Fig. 5), there were no differences in alcohol intake among treatments. On the second drug treatment day (day 11), only PRZ+NTX suppressed alcohol intake (p<0.001, relative to VEH); PRZ+NTX also suppressed intake relative to PRZ (p<0.01) or NTX (p<0.05). On the next alcohol access day (day 14), with no treatment (day 14), alcohol intake nonetheless remained suppressed in rats treated with PRZ+NTX on the previous alcohol access day, 3 days prior (p<0.05). On the third treatment day (day 16), alcohol intake was decreased by PRZ (p<0.05), NTX (p<0.01) or PRZ+NTX (p<0.001); PRZ+NTX also suppressed intake relative to PRZ (p<0.01) or NTX (p<0.05).
Figure 5.
Effects of oral prazosin (PRZ, 2 mg/kg) and naltrexone (NTX, 10 mg/kg), alone or in combination, on alcohol intake during intermittent 2-h alcohol access with intermittent drug treatments (Expt. 3, treatment weeks 6-8). Solid black boxes (██) indicate days on which drug treatment was administered. Each bar represents data from 12-13 rats. *** p<0.001, ** p<0.01, * p<0.05 vs vehicle (VEH).
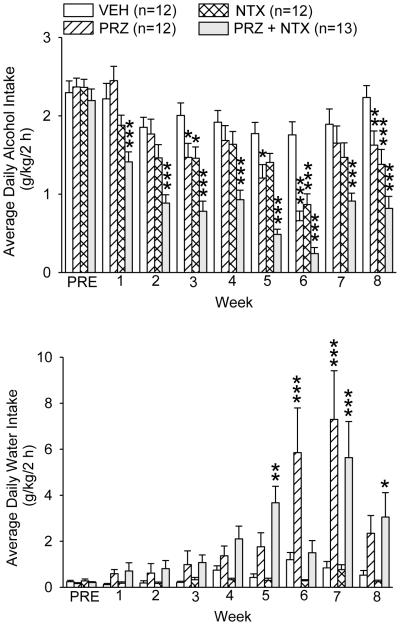
Maintenance of Treatment Effectiveness in 8 Consecutive Weeks
Although different alcohol access and treatment schedules were used, each rat received the same drug or VEH treatment throughout Experiments 1–3. Alcohol and water intakes on treatment days were further analyzed as weekly averages in all 8 weeks of the 3 experiments (Fig. 6),.revealing a treatment X week interaction, F(21, 315) = 2.58, p<0.001. PRZ suppressed alcohol intake in weeks 3 and 5 (p<0.05), 6 (p <0.001) and 8 (p<0.01), relative to VEH; NTX suppressed intake in weeks 3 (p<0.05), 6 and 8 (p<0.001). PRZ+NTX suppressed alcohol intake in all 8 weeks (p<0.001 in each). PRZ+NTX also suppressed alcohol intake relative to PRZ in 7 of the 8 weeks (p≤0.001 in weeks 1, 2, 4, 5 and 8; p<0.01 in 3 and 7); in week 6, suppression of intake by PRZ+NTX, relative to PRZ, was nearly significant (p=0.052). PRZ+NTX also suppressed alcohol intake relative to NTX in all 8 weeks (p≤0.001 in weeks 4-5; ≤0.01 in 2, 3, 6-8; <0.05 in 1). Analysis of average water intake on treatment days in all 8 weeks also revealed a treatment X week interaction, F(21, 315) = 4.04, p<0.001. PRZ increased water intake in weeks 6 and 7 (p<0.001 in each, relative to VEH). PRZ+NTX increased water intake in weeks 5, 7 and 8 (p<0.01, 0.001 and 0.05, respectively). Inspection of the results (Fig. 6) suggests a consistent trend for PRZ and PRZ+NTX to increase water intake in all weeks, although differences are not significant until week 5; no treatment suppressed water intake.
Figure 6.
Weekly average alcohol and water intakes during all 3 sequential experiments spanning drug treatment weeks 1-8. PRE: pretreatment week. Each bar represents data from 12-13 rats. *** p<0.001, ** p<0.01, * p<0.05 vs VEH. PRZ: prazosin, NTX: naltrexone, VEH: vehicle.
BW During 8 Consecutive Weeks of Treatment
Analysis of BW in the pre-treatment week and each of the 8 treatment weeks in Experiments 1 - 3 (data not shown) revealed no significant differences in BW among treatments or among weeks, and no treatment X week interaction. Mean ± SE BW before and after 8 weeks of treatment are shown in Table 1.
Table 1.
Body weights before and after 8 consecutive weeks of treatment
| Pre-treatment (g, mean ± SE) |
Week 8 (g, mean ± SE) |
|
|---|---|---|
| VEH | 616 ± 14 | 624 ± 14 |
| PRZ | 599 ± 15 | 612 ± 16 |
| NTX | 589 ± 18 | 595 ± 17 |
| PRZ + NTX | 587 ± 19 | 596 ± 21 |
Each value represents data from 12-13 rats VEH: vehicle; PRZ: prazosin; NTX: naltrexone.
Experiment 4: Drug Treatment During Post-Deprivation Reinstatement of Alcohol Access
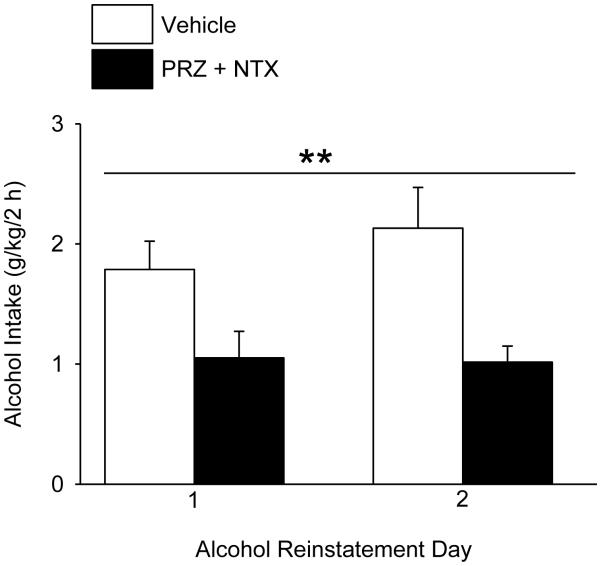
The effect of oral PRZ+NTX (2 and 10 mg/kg, respectively) vs VEH administration on each of the final 2 days of 2-week alcohol deprivation and at 45 min before 2-h alcohol access on each of 2 subsequent days is shown in Fig. 7. PRZ+NTX suppressed alcohol intake, relative to VEH (n=6/treatment), F(1, 11) = 13.89, p<0.01, independent of day (Fig. 7). PRZ+NTX increased water intake, F(1, 10) = 7.16, p<0.05, independent of day (average water intake: 0.21 ± 0.11 or 1.75 ± 0.74 g/kg/2 h for VEH- or PRZ+NTX-treated rats, respectively).
Figure 7.
Effects of the combined oral prazosin (PRZ, 2 mg/kg) + naltrexone (NTX, 10 mg/kg) treatment on alcohol intake during post-deprivation reinstatement of alcohol access (Expt. 4). Rats that had previously received only vehicle (VEH) control treatments in Experiments 1-3 were restored to unlimited 24-h alcohol access for 4 weeks, followed by 2 weeks of alcohol deprivation. The rats received either PRZ+NTX or VEH on the final 2 days of alcohol deprivation and on 2 days reinstatement of 2-h/day alcohol access. Each bar represents data from 6 rats. ** p<0.01, independent of day.
DISCUSSION
Unlimited (24 h/day) voluntary alcohol drinking by male P rats, starting at the transition from adolescence and continuing well into middle age, interrupted by repeated imposed abstinences, simulated the prolonged alcohol drinking history, repeated episodes of self- or externally-imposed alcohol abstinence, and alcohol dependence characteristic of many heavy-drinking patients seeking treatment for alcohol use disorders. We previously demonstrated that combining PRZ and NTX in a single oral medication decreases alcohol drinking more effectively than does either drug alone in male P rats without this prolonged history of extensive alcohol drinking and dependence (Froehlich et al., 2013b); here we evaluate whether these treatments are likewise effective in this animal model of heavy-drinking subjects with a prolonged history of alcohol dependence and withdrawals, and - if so - under what conditions.
Oral PRZ+NTX (2 and 10 mg/kg, respectively) suppressed 2-h/day alcohol intake on the first treatment day and consistently throughout 8 weeks of treatment. Oral PRZ alone or NTX alone were not effective until treatment week 3, and suppression of alcohol intake by PRZ or NTX was less consistent and smaller than that produced by PRZ+NTX. PRZ+NTX consistently decreased alcohol drinking under all conditions investigated (daily alcohol access with daily treatments; intermittent alcohol access with daily treatments; and intermittent alcohol access with occasional treatments). Under all conditions, PRZ+NTX decreased voluntary alcohol drinking more effectively than either drug alone, without loss of effectiveness during 8 weeks of treatment. When the subset of rats that had received only VEH during the 8 treatment weeks were provided with additional prolonged 24 h/day alcohol access followed by 2 weeks of imposed abstinence, PRZ+NTX treatment initiated 2 days before alcohol reinstatement and continuing during 2 days of 2-h/day alcohol access likewise suppressed alcohol intake, comparable to suppression in rats treated with PRZ+NTX for 8 weeks.
The oral PRZ and NTX doses used in this study (2 and 10 mg/kg, respectively) are the same as those previously demonstrated to produce additive suppression of alcohol drinking in male P rats without a history of prolonged unlimited alcohol drinking and dependence (Froehlich et al., 2013b). As previously discussed (Froehlich et al., 2013b), the oral NTX dose is higher than non-oral doses because NTX bioavailability is low when administered orally to rats (1% in rats vs 5-40% in humans). In contrast, bioavailability of oral PRZ is high, so doses that are effective intraperitoneally (Rasmussen et al., 2009a,b) are similarly effective orally (Froehlich et al., 2013a).
The ability to drink alcohol was not compromised by sedation, motor effects or malaise. After one week of treatment, all rats - regardless of treatment – drank alcohol throughout the first 10 seconds following presentation of alcohol and water tubes through the cage wall, confirming that all remained highly motivated, and capable, to drink alcohol. By week 3, all VEH-treated rats continued to drink alcohol in the first 10 seconds but many drug-treated rats did not, even though they were alert and investigated the tubes immediately. This delay in onset of alcohol drinking occurred most frequently in rats receiving daily PRZ+NTX and may reflect decreased reinforcing effects of alcohol. Development of aversive post-ingestional consequences of alcohol is unlikely since water and food intakes were not suppressed. Instead, PRZ or PRZ+NTX commonly increased water intake (Fig. 6). Stimulation of water drinking by PRZ, alone or combined with NTX, is consistent with previous studies (Rasmussen et al., 2009a,b, 2011; Froehlich et al., 2013a,b) and demonstrates that inhibition of alcohol drinking is not due to motor impairment. Increased water drinking due to blockade of α1-adrenergic neurotransmission may reflect hypotension-induced thirst or a centrally-mediated inhibitory effect of norepinephrine on water intake, as previously discussed (Froehlich et al., 2013b; O’Neil et al., 2013).
PRZ and/or NTX decreased intake of [sucrose + saccharin] during 1-h access, consistent with a report that these drugs can reduce sucrose-reinforced behaviors in P rats (Verplaetse and Czachowski, 2015). All treatment groups (including VEH) stopped drinking this sweet solution before 1-h access ended, suggesting that PRZ, NTX or PRZ+NTX each decreased the amount of sweet solution voluntarily consumed, reflecting decreased thresholds for satiety. Only PRZ+NTX decreased intake of the solution when available for 5 or 2 min. Since all rats were confirmed to drink the solution throughout the 2 min access, PRZ+NTX appears also to have decreased the rate of drinking sweet solution, potentially reflecting decreased avidity. Suppression of [sucrose + saccharin] drinking by PRZ and/or NTX suggests that effects of these drugs on alcohol drinking may be due in part to generalized suppression in intake of highly reinforcing substances, consistent with overlap of neural circuits in addiction and obesity (Volkow et al., 2008). However, daily PRZ and/or NTX for 8 weeks did not alter BW, suggesting that caloric intake and metabolic regulation were maintained.
PRZ and/or NTX also decreased BAC after 1-h alcohol access. BAC was positively correlated with alcohol intake, suggesting that lower BAC in drug-treated groups was secondary to reduction of alcohol intake.
Occasional PRZ+NTX treatment was highly effective in reducing intermittent alcohol drinking on the day of treatment, suggesting that this drug combination may be effective clinically when administered “as-needed”. This is consistent with evidence that NTX treatment only at times when drinking is anticipated to occur is more effective than chronic NTX (Sinclair JD, 2001), as well as with evidence that as-needed treated with the similar opioid antagonist, nalmefene, likewise reduced alcohol drinking in alcohol-dependent patients (Mann et al., 2013). Evidence that effects of PRZ+NTX treatment prior to one intermittent alcohol access can - without further treatment - carry over to a subsequent alcohol access 3 days later (Fig. 5) suggests that “as needed” treatment potentially even could provide benefit during a subsequent untreated episode of alcohol drinking, which requires additional investigation.
PRZ+NTX for treatment of alcohol use disorders offers potential advantages. PRZ may be most effective for individuals who drink to avoid aversive effects of excessive noradrenergic activation, such as anxiety and hyper-reactivity during abstinence, whereas NTX may be most effective for individuals who drink for reinforcing or euphoriant effects of alcohol. PRZ+NTX may thus be useful for treating multiple, probably overlapping, subject subpopulations as well as for decreasing vulnerability to alcohol drinking at multiple stages of alcohol abuse, addiction, withdrawal and abstinence. This and our preceding study (Froehlich et al., 2013b) also demonstrate that PRZ+NTX provides greater effectiveness than either drug alone. We presented evidence elsewhere that the combined effect in non-dependent male P rats is due to additivity of responses to the separate drugs (Froehlich et al., 2013b). However, under some conditions combining PRZ and NTX may also counteract an undesirable side effect of one of the drugs. For example, NTX blocks opioid suppression of forebrain noradrenergic neurons in the locus coeruleus, blocks opioid suppression of corticotropin-releasing factor (CRF) neurons innervating these noradrenergic neurons, and blocks opioid suppression of signaling in noradrenergic projection sites, including stimulatory noradrenergic innervation of CRF neurons (Koob, 2008; Dunn et al., 1992; Strahlendorf et al., 1980; Nakamura et al., 1982; Curtis et al., 1997), thus increasing activation of noradrenergic and CRF signaling that is suggested to increase (rather than decrease) stress responses and associated alcohol drinking under some conditions (Koob, 2008). Combining PRZ with the NTX likely decreases the undesired noradrenergic activation, potentially allowing full expression of NTX suppression of alcohol drinking - perhaps at lower doses - and decreasing potential noradrenergic-mediated adverse responses to NTX. Finally, PRZ+NTX may provide effective treatment for subjects who do not respond adequately to either drug alone.
In summary, the current results demonstrate that treatment with PRZ+NTX may provide especially effective suppression of alcohol drinking and relapse even in individuals with a long history of voluntary heavy alcohol drinking, dependence and relapses. This combination treatment is effective in a variety of alcohol drinking patterns (daily, intermittent, post-deprivation) and with treatment administered either chronically or “as needed”. The combination of these two safe, well-characterized and FDA-approved drugs thus offers great promise for effectively treating alcohol use disorders in a broad spectrum of patients.
Acknowledgments
SUPPORT: This material is based on work supported in part by resources from the VA Puget Sound Health Care System, the VA VISN 20 Mental Illness Research, Education and Clinical Center (MIRECC), and NIH Grants R01 AA018604, P20 AA017839 and P60 AA007611. P rats provided by the Indiana Alcohol Research Resource Center supported by NIH Grant R24 AA015512.
REFERENCES
- Amit Z, Brown ZW, Levitan DE, Ogren SO. Noradrenergic mediation of the positive reinforcing properties of ethanol. I. Suppression of ethanol consumption in laboratory rats following dopamine-beta-hydroxylase inhibition. Arch Int Pharmacodyn Ther. 1977;230:65–75. [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Maccioni P, Vargiolu D, Loi B, Lobina C, Zaru A, Carai MAM, Gessa GL. The dopamine β-hydroxylase inhibitory, nepicastat, reduces different alcohol-related behaviors in rats. Alcohol Clin Exp Res. 2014;38:2345–2353. doi: 10.1111/acer.12520. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- Davis WM, Smith SG, Werner TE. Noradrenergic role in the self-administration of ethanol. Pharmacol Biochem Behav. 1978;9:369–374. doi: 10.1016/0091-3057(78)90298-8. [DOI] [PubMed] [Google Scholar]
- Del Re AC, Gordon AJ, Lembke A, Harris AHS. Prescription of topiramate to treat alcohol use disorders in the Veterans Health Administration. Addict Sci Clin Pract. 2013;8:12. doi: 10.1186/1940-0640-8-12. 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor – norepinephrine interactions during stress. Ann N Y Acad Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fisher SM, Rasmussen DD. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for alcohol intake. Alcohol Clin Exp Res. 2013a;37:1552–1560. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer B, Rasmussen DD. Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcohol Clin Exp Res. 2013b;37:1763–1770. doi: 10.1111/acer.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Li TK. Opioid peptides. In: Galanter M, editor. Recent Developments in Alcoholism, Vol 11: Ten Years of Progress. Plenum Publishing Co.; New York: 1993. pp. 187–205. [PubMed] [Google Scholar]
- Froehlich J, O'Malley S, Hyytia P, Davidson D, Farren C. Preclinical and clinical studies on naltrexone: what have they taught each other? Alcohol Clin Exp Res. 2003;27:533–539. doi: 10.1097/01.ALC.0000057943.57330.AB. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Wand G. The neurobiology of ethanol/opioid interactions in ethanol reinforcement. Alcohol Clin Exp Res. 1996;20(8 S):181A–186A. doi: 10.1111/j.1530-0277.1996.tb01772.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat. 2009;36:S15–S23. [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gasfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW, Vivitrex Study Group Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. The effect of ethanol on the biosynthesis and regulation of opioid peptides. Experientia. 1989;45:428–435. doi: 10.1007/BF01952024. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, De Waele JP, Thavundayil J. Implication of the endogenous opioid system in excessive ethanol consumption. Alcohol. 1996;13:19–23. doi: 10.1016/0741-8329(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Aungst BJ, Kearney A, Shefter E. Buccal and oral bio-availability of naloxone and naltrexone in rats. Int J Pharm. 1987;36:127–130. [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, Leggio L. Role of the α1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2015 Jun 2; doi: 10.1111/adb.12275. doi: 10.1111/adb 12275 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O'Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry. 2007;62:694–697. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA, Veterans Affairs Naltrexone Cooperative Study 425 Group Naltrexone in the treatment of alcohol dependence. New England J of Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Lê A, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food self-administration in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Mann K, Bladström A, Torup L, Gual A, van den Brink W. Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol Psychiatry. 2013;73:706–713. doi: 10.1016/j.biopsych.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by α1-adrenoceptors in brain. Naunyn-Schmiedeberg's Arc Pharmacol. 1981;317:273–275. doi: 10.1007/BF00503830. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4:1–8. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Tepper JM, Young SJ, Ling N, Groves PM. Noradrenergic terminal excitability: effects of opioids. Neurosci Lett. 1982;30:57–62. doi: 10.1016/0304-3940(82)90012-x. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Froehlich JC. Advances in the use of naltrexone: an integration of preclinical and clinical findings. In: Galanter M, editor. Recent Developments in Alcoholism XVI: Research on Alcoholism Treatment. Kluwer Academic/Plenum Publishers; New York: 2003. pp. 217–245. [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Oncken C, Van Kirk J, Kranzler HR. Adverse effects of oral naltrexone: Analysis of data from two clinical trials. Psychopharmacology. 2001;154:397–402. doi: 10.1007/s002130000666. [DOI] [PubMed] [Google Scholar]
- O'Neil ML, Beckwith LE, Kincaid CL, Rasmussen DD. The alpha-1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2013;37:202–212. doi: 10.1111/j.1530-0277.2012.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Redia VA, Olson VG. Prazosin blocks development of ethanol, morphine, and cocaine conditioned place preference in mice. Alcohol Clin Exp Res. 2011;35(Suppl):66A. [Google Scholar]
- Rasmussen DD, Alexander LL, Malone J, Federoff D, Froehlich JC. The α-adrenergic receptor agonist, clonidine, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol. 2014;48:543–549. doi: 10.1016/j.alcohol.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The α1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009a;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Beckwith LE, Kincaid CL, Froehlich JC. Combining the α1-adrenergic receptor antagonist, prazosin, with the β-adrenergic receptor antagonist, propranolol, reduces alcohol drinking more effectively than either drug alone. Alcohol Clin Exp Res. 2011;38:1532–1539. doi: 10.1111/acer.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR. Chronic daily ethanol and withdrawal: 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcohol Clin Exp Res. 2002;26:535–546. [PubMed] [Google Scholar]
- Rasmussen DD, Bryant CA, Boldt BM, Colasurdo EA, Levin N, Wilkinson CW. Acute alcohol effects on opiomelanocortinergic regulation. Alcohol Clin Exp Res. 1998;22:789–801. [PubMed] [Google Scholar]
- Rasmussen DD, Federoff D, Froehlich JC. Prazosin reduces voluntary alcohol drinking in both non-deprived and alcohol-deprived rats selectively bred for alcohol dependence. Alcohol Clin Exp Res. 2009b;33:312. [Google Scholar]
- Rojawski MA, Aghajanian GK. Activation of lateral geniculate neurons by locus coeruleus or dorsal noradrenergic bundle stimulation: selective blockade by the α1-adrenoceptor antagonist prazosin. Brain Res. 1982;250:31–39. doi: 10.1016/0006-8993(82)90950-7. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte C, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD. Evidence about the use of naltrexone and for different ways of using it in the treatment of alcoholism. Alcohol Alcohol. 2001;36:2–10. doi: 10.1093/alcalc/36.1.2. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Weiner JL. Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain Behav. 2014;4:468–483. doi: 10.1002/brb3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahlendorf HK, Strahlendorf JC, Barnes CD. Endorphin-mediated inhibition of locus coeruleus neurons. Brain Res. 1980;191:284–288. doi: 10.1016/0006-8993(80)90334-0. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effects of prazosin, an alpha-1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36:881–886. doi: 10.1111/j.1530-0277.2011.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Czachowski CL. Low dose-prazosin alone and in combination with propranolol or naltrexone: effects on ethanol and sucrose seeking and self-administration in the P rat. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3896-z. Epub ahead of print, 2015 Mar 7, PMID: 25743758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. The effects of α1-noradrenergic receptor antagonism on dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Rust NC, Miller NS, Palmiter RD. Ethanol-associated behaviors of mice lacking norepinephrine. J Neurosci. 2000;20:3157–3164. doi: 10.1523/JNEUROSCI.20-09-03157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York, NY: 2006. pp. 237–295. [Google Scholar]