Abstract
Timely resolution of inflammation is crucial for normal wound healing. Resolution of inflammation is an active biological process regulated by specialized lipid mediators including the lipoxins and resolvins. Failure of resolution activity has a major negative impact on wound healing in chronic inflammatory diseases that is manifest as excess fibrosis and scarring. Lipoxins, including Lipoxin A4 (LXA4), have known anti-fibrotic and anti-scarring properties. The goal of this study was to elucidate the impact of LXA4 on fibroblast function. Mouse fibroblasts (3T3 Mus musculus Swiss) were cultured for 72 hours in the presence of TGF-β1, to induce fibroblast activation. The impact of exogenous TGF-β1 (1 ng/mL) on LXA4 receptor expression (ALX/FPR2) was determined by flow cytometry. Fibroblast proliferation was measured by bromodeoxyuridine (BrdU) labeling and migration in a “scratch” assay wound model. Expression of α-smooth muscle actin (α-SMA), and collagen types I and III were measured by Western blot. We observed that TGF-β1 up-regulates LXA4 receptor expression, enhances fibroblast proliferation, migration and scratch wound closure. α-SMA levels and Collagen type I and III deposition were also enhanced. LXA4 slowed fibroblast migration and scratch wound closure at early time points (24 hours), but wound closure was equal to TGF-β1 alone at 48 and 72 hours. LXA4 tended to slow fibroblast proliferation at both concentrations, but had no impact on α-SMA or collagen production by TGF-β1 stimulated fibroblasts. The generalizability of the actions of resolution molecules was examined in experiments repeated with resolvin D2 (RvD2) as the agonist. The activity of RvD2 mimicked the actions of LXA4 in all assays, through an as yet unidentified receptor. The results suggest that mediators of resolution of inflammation enhance wound healing and limit fibrosis in part by modulating fibroblast function.
Keywords: Docosahexaenoic Acids, Lipoxins, Resolvin D2, Inflammation, Wound Healing, Collagen
1. Introduction
Wound healing is a complex process involving three sequential, yet overlapping phases: inflammation, proliferation and remodeling [1]. Phagocytic cells release growth factors, and produce cytokines to regulate the subsequent proliferative phase [1, 2]. The proliferative phase involves the formation of granulation tissue and revascularization, regulated by fibroblasts and endothelial cells. During the final maturation stage, the extracellular matrix is remodeled, leading to the tissue repair. Aberrations in any step of the reparative process are likely to result in impairment, with the potential for development of chronic wounds and ulcers, especially inflammation [3]. Chronic inflammation delays epithelialization (wound closure) and interferes with the remodeling phase that results in poor wound outcome such as increased fibrosis and scarring. Myofibroblasts differentiated from fibroblasts regulate wound healing, secret extracellular matrix (ECM), and are responsible for the contractility of scar tissue [4]. Myofibroblasts express α-smooth muscle actin (α-SMA) in stress fibers for contractile activity [5]. Scar formation has been linked to the number of myofibroblasts and to the extracellular environment, including inflammation, which halts tissue remodeling causing fibrotic scars [6].
Differentiation of myofibroblasts involves both mechanical stimulus to the cells by tensile forces and chemical stimuli by growth factors, such as TGF-β [5]. TGF-β regulates fibrotic responses in wound healing; it is produced by inflammatory cells, such as macrophages, fibroblasts, myofibroblasts and epithelial cells [7]. TGF-β has pleiotropic actions that are temporal and concentration dependent, such as inflammation, angiogenesis, fibroblast proliferation, collagen synthesis and deposition, and remodeling of the new extracellular matrix [8, 9]. TGF-β has three isoforms. While TGF-β3 appears to reduce scarring, TGF-β1 and β2 are key factors promoting scar formation [7]. Connective tissue growth factor, a downstream mediator of TGF-β1, has been shown to be involved in fibrosis, scar contractility, and deposition of collagen types I and III in the ECM [10]. During the entire process of healing, orchestrated resolution of inflammation is crucial for restoration of homeostasis and tissue integrity. Uncontrolled inflammation results in chronic, non-healing wounds or excessive scarring [11].
Resolution of inflammation is an active process leading to coordinated, temporal clearance of pro-inflammatory cells facilitating healing [12]. The coordination between pro-inflammatory and pro-resolving processes actively prevents damage to self [13]. Damage to self occurs in non-resolving inflammation that is associated with chronic diseases, such as arthritis, periodontal disease, diabetes and cardiovascular diseases [14]. A deficiency in resolution of inflammation molecules likely plays a role in disease pathogenesis [15–19]. Active resolution of inflammation is mediated by the local biosynthesis of endogenous specialized pro-resolving lipid mediators (SPMs), which include the lipoxins, resolvins, protectins and maresins [11]. SPMs are enzymatically synthesized and induce diverse actions on a variety of cells through specific receptors [11, 20]. Target cells for the SPMs are not confined to the immune system; they also include the cells of structural tissues, such as bone [21]. Resolvins (Rv) are derived from the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), while lipoxins (LX) are from the omega-6 arachidonic acid. They effectively resolve inflammation in periodontal diseases [21, 22], asthma [23, 24], and colitis [25]. Lipoxins reduce inflammatory pain, block IL-1β transcription induced by TNF-α in microglial cells, and limit polymorphonuclear leukocyte infiltration into inflamed brain, skin, and peritoneum [26]. In an experimental model of periodontitis, RvE1 prevented chronic inflammation, tissue breakdown and resulted in wound healing with restoration (regeneration) of all lost tissues [22, 27] suggesting a major role in regulating wound healing and regeneration. These observations have been further supported using lipoxins in a large animal model of regeneration [28]. In diabetic wound healing, Tang et al. demonstrated that RvD1 enhances wound closure in mice with decreased accumulation of apoptotic cells, macrophages and the susceptibility to infection [29]. While the potential of SPM for promoting wound healing without scarring is promising, there is a critical need to understand the mechanism of action on fibroblasts.
Considering that SPMs have significant actions on regulation of wound healing in inflammatory diseases, we hypothesized that amplification of resolution of inflammation pathways during wound healing will regulate fibroblast function, modulate fibroblast migration and proliferation, and potentially extend actions to myofibroblast differentiation and the deposition of collagen type-I and III. The aim of this study was to elucidate the impact of LXA4, on fibroblast function in an in vitro model of wound healing assessing migration, proliferation, differentiation and collagen deposition as surrogates for wound repair and scar reduction.
2. Materials and Methods
2.1. Cell Culture, Experimental Conditions, Scratch Wound Closure
Fibroblasts (3T3 Mus musculus Swiss; CCL92; ATCC, Manassas, VA, USA) were cultured in 1% D-MEM supplemented with sodium-glutamine (4.0 mM), 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (GIBCO, Invitrogen, Carlsbad, CA, USA) at 37°C and 5% CO2. Medium was changed every 3 days. Cells were passaged using 0.025% trypsin in phosphate buffered-saline (PBS) containing 0.02% EDTA (GIBCO, Invitrogen, Carlsbad, CA, USA). 3×104 cells were seeded in a 12-well plates using the same medium until they reached confluence, then differentiated into myofibroblasts within 72 hours with TGF-β1 (1 ng/mL, Millipore, Billerica, MA, USA). In order to test the impact of SPMs, fibroblasts were incubated with or without LXA4 (10 and 100 nM; Cayman Chemical, Ann Arbor, MI, USA). In order to study cell migration and wound closure in vitro, a linear scratch wound (3mm wide) was created using a rubber policeman as previously described [30]. The cell monolayers were stained with 0.9% crystal violet after 24, 48 and 72 hours of culture to visualize the fibroblast migration. To measure wound closure, standardized computer images were taken. The distance between the wound margins was quantified using computer software (Image J; NIH). The scratches were analyzed by two individuals blinded to the experimental conditions.
2.2. Receptor Expression on Fibroblasts
Fibroblasts from each culture were seeded in 75 cm2 flasks at an initial density of 106 cells per flask. When the cells became 80% confluent, they were starved overnight and differentiated into myofibroblasts as described above. The medium was aspirated and the monolayer was washed twice with PBS. Cells were detached using trypsin/EDTA; spun at 500×g for 10 minutes, washed with PBS, and fixed with 4% paraformaldehyde. Two flasks were used for each experimental condition. Each flask yielded approximately 2×106 cells. In order to determine the expression of ALX (a.k.a. FPR2), cells (4×105) were treated with antibody buffer (5% normal donkey serum in PBS containing 0.03% sodium azide) and stained with phycoerythrin (PE)-conjugated mouse anti-human ALX/FPR2 antibody (1:200, Santa Cruz Biotechnology, Dallas, TX, USA) or isotype control (1:200), which is known to cross-react with mouse ALX. The cells were analyzed by fluorescence with FACScan (CellQuest software, eBiosciences, San Diego, CA, USA).
2.3. Myofibroblast Differentiation
α-Smooth Muscle Actin (SMA, Sigma Chemical, St. Louis, MO, USA) expression was analyzed by Western blot. Fibroblasts were solubilized in RIPA buffer with proteinase inhibitors (Sigma Chemical, St. Louis, MO, USA) and centrifuged at 10,000×g for 10 min. Protein content was measured by the Bradford method [31]. The samples were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred onto polyvinylidene difluoride membranes (100V; 60 min). The membranes were blocked with 5% BSA for 60 min, and incubated overnight at 4°C with a polyclonal antibody against SMA and horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Dallas, TX, USA). Band density was measured using an imaging densitometer (ChemImager 5500 system, Alpha Innotech Corp., USA). The band densitometry in the TGF-β1 stimulated sample was used as the internal control.
2.4. Fibroblast Proliferation in Response to LXA4
104 cells were seeded per well in 96-well plates and treated with TGF-β1 in the presence or absence of LXA4 (10 and 100 nM). After 24 hours, 5-bromo-2′-deoxy-uridine (BrdU)-labeling (Calbiochem, EMD Millipore, Darmstadt, Germany) was quantified according to the manufacturer’s instructions. The absorbance was measured at 370 nm.
2.5. Collagen Deposition in Response to LXA4
Collagen deposition was measured as an indicator of extracellular matrix generation by fibroblasts by Western blotting, as described above. Membranes were incubated with polyclonal primary antibodies against collagen type-I (C18) or type-III (C15; Santa Cruz Biotechnology, Dallas, TX, USA).
2.6. Resolvin D2
Resolvin D2 is an SPM derived from DHA that exerts proresolving properties through an as yet unidentified receptor. In order to determine whether the properties of lipoxins mediated through ALX were generalizable to other SPM of different fatty acid origin; all experiments were repeated in an identical fashion using RvD2 as the SPM agonist at 10 and 100 nM.
2.7. Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) and corrected for multiple comparisons using Newman-Keuls correction. All values are reported as mean ± SEM. Statistical significance (p value) was set at 0.05.
3. Results
3.1. TGF-β1 Up-regulates Expression of ALX/FPR2 on Fibroblasts
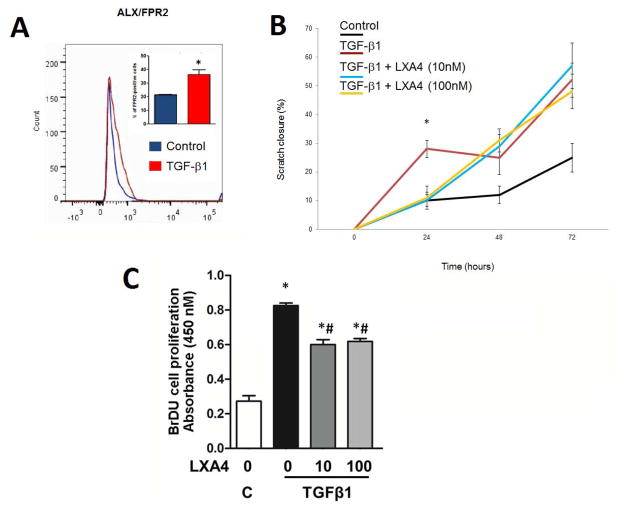
In order to determine the impact of LXA4 on normal fibroblasts, all assays were performed on resting fibroblasts in the absence of TGF-β1. Figure 1A shows that resting fibroblasts minimally expressed the ALX receptor. In functional assays (SMA up-regulation, Collagen I and III upregulation, migration), there is no response to SPM in the absence of TBF-β1 pretreatment (Supplemental Figure 1). TGF-β1 stimulates the upregulation of ALX 2-fold (p<0.05) and induces functional responses reported below.
Figure 1.
Fibroblasts express the LXA4 receptor, ALX/FPR2. LXA4 regulates fibroblast migration and inhibits TGF-β1 induced fibroblast proliferation. Panel A: TGF-β1 upregulates ALX receptor expression analyzed by flow cytometry (PE-conjugated anti-ALX/FPR2 receptor antibody; p<0.05, n=4). Panel B: TGF-β1 increases fibroblast migration and scratch closure significantly at 24 hours (p<0.05). LXA4 slows fibroblast migration at 24 hours at both doses, returning to TGF-β1 alone values at 48 and 72 hours (p<0.05). Panel C: TGF-β1 stimulated an increase in fibroblast proliferation, analyzed by BrdU incorporation, and it is reduced by LXA4 (n=8; * = p<0.05 vs. control; # = p<0.05 vs. TGFβ1 alone).
3.2. Scratch Wound Closure in Response to LXA4
Fibroblast migration in the “scratch” assay was used as a measure of fibroblast stimulation and function in response to TGF-β1. Fibroblast migration and scratch closure were significantly increased in response to TGF-β1 compared to the vehicle control at all time-points studied (p<0.05) (Figure 1B). In the presence of LXA4 (10 and 100 nM) a bi-phasic response was seen inhibiting fibroblast migration induced by TGF-β1 in the first 24 hours (p<0.05), with restoration of the response recovered after 48 hours.
3.3. TGF-β1 induced fibroblast proliferation is reduced by LXA4
TGF-β1 stimulated fibroblast proliferation was measured by BrdU incorporation (p<0.05). Stimulation was reduced significantly by LXA4 at both concentrations in the first 24 hours (Figure 1C). Negative controls were performed using only LXA4; no proliferation was observed in any group (data not shown).
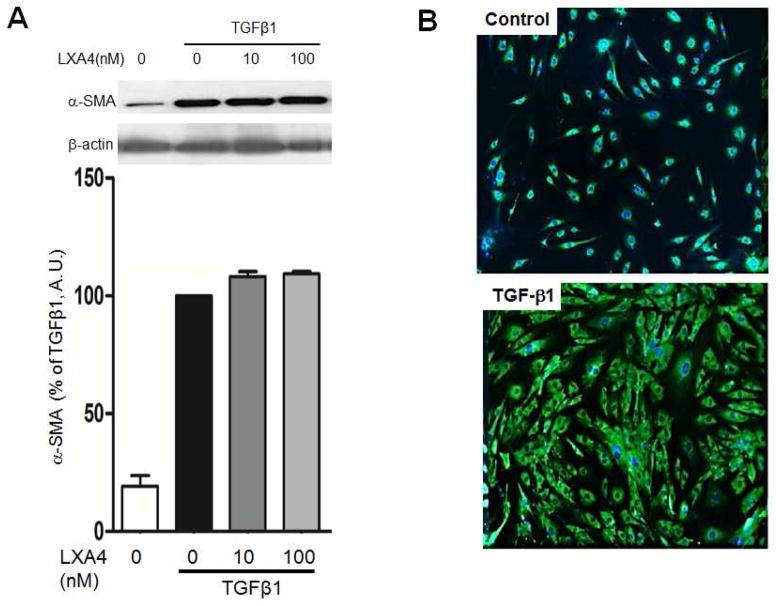
3.4. TGF-β1 stimulates myofibroblast differentiation
Alpha-smooth muscle actin (α-SMA) expression was used to assess myofibroblast differentiation. TGF-β1 stimulated α-SMA deposition after 12, 24 and 72 hours. LXA4 had no impact on myofibroblast differentiation at either concentration (Figure 2).
Figure 2.
Myofibroblast differentiation assessed by α-SMA protein expression. Panel A: TGF-β1 stimulates myofibroblast differentiation and α-SMA expression after 72 hours. LXA4 has no impact on myofibroblast differentiation (p<0.001, n=4). Panel B: α-SMA stained with FITC (green) and the nuclei with Hoechst (blue).
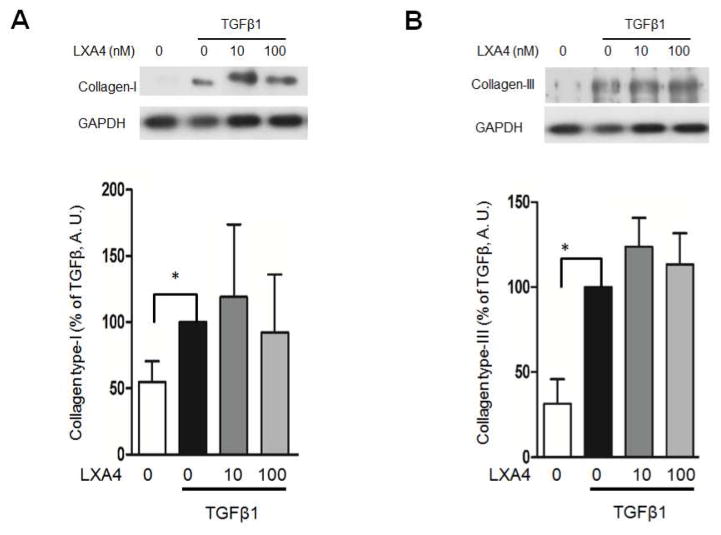
3.5. Collagen type-I and type-III expression is enhanced by TGFβ1
In order to determine the impact of LXA4 on TGF-β1 induced collagen deposition, collagen types I and III were assessed. As expected, TGF-β1 stimulated the expression of collagen types-I and III. LXA4 had no impact on TGF-β1 stimulation of collagen Type I or Type III deposition (p<0.05, Figure 3).
Figure 3.
TGF-β1 stimulates collagen type-I and III deposition by fibroblasts (p<0.05). LXA4 has no impact on collagen expression (A.U. = Arbitrary Units, n=4).
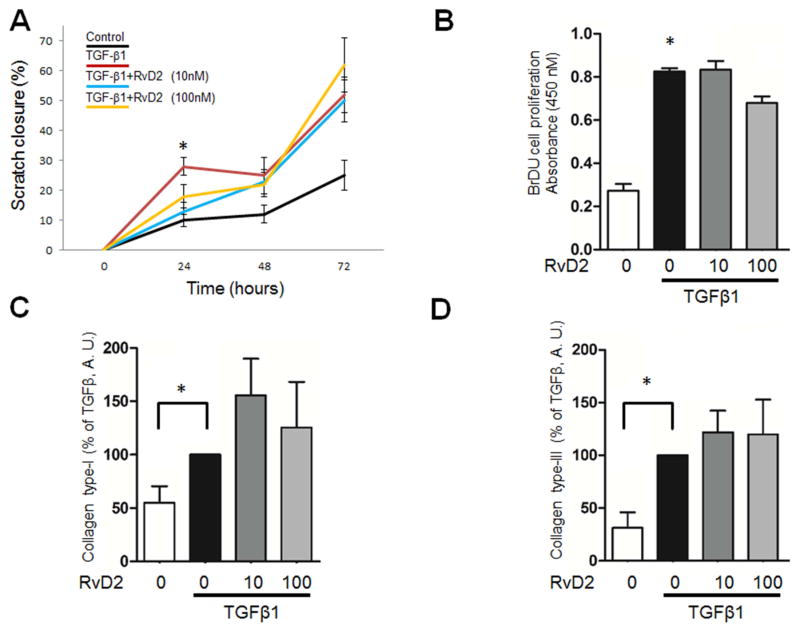
3.6. Actions of Resolvin D2 (RVD2)
Experiments were repeated as above using RvD2 in order to determine whether the actions of SPM on fibroblasts were limited to lipoxins. RvD2 exhibited the same properties qualitatively and quantitatively as LXA4 in the fibroblast proliferation, α-smooth muscle assays, the cell migration (scratch closure) and collagen production assays (Figure 4).
Figure 4.
RvD2 regulates fibroblast function in vitro. All experiments were repeated with RvD2; a resolution agonist derived from the n-3 fatty acid DHA. Panel A: RvD2 inhibits scratch wound closure induced by TGF-β1 at 24 hours (p<0.05); closure rate is restored at 48 and 72 hours. Panel B: RvD2 inhibits TGF-β1 induced cell proliferation (p<0.05; n=5). Panels C and D: RvD2 has no significant effect on Collagen expression (A.U. = Arbitrary Units, n=4).
4. Discussion
Active resolution of inflammation is a process driven by novel endogenous lipid mediators such as lipoxins and resolvins that rescue excessive or prolonged inflammatory responses to promote wound healing. The actions of resolution agonists are receptor mediated; a feed forward system rather than inhibition of enzyme pathways or use of receptor antagonism [17]. In this study, we show that two SPMs, LXA4 and RvD2, counter-regulate fibroblast migration and proliferation in fibroblast cultures at early time points suggesting that SPMs play an active role in limiting fibrosis in part through the limitation of fibroblast activity. The activity of fibroblasts at later time points is not inhibited suggesting SPM will not have a negative impact on wound healing overall. RvD2 and LXA4 do not impact myofibroblast differentiation or collagen deposition in the extracellular matrix induced by TGFβ1.
LXA4 binds to the formyl-peptide receptor type 2 (ALX), a unique G-protein coupled receptor that conveys proresolving signals induced by proteins, peptides, and lipid ligands [32]. We observed that fibroblasts express the receptor ALX and that TGF-β1 increases its expression. Resolvins derived from docosahexaenoic acid, such as RvD2, are synthesized by neutrophils during the resolution phase of inflammation and exhibit properties similar to lipoxins blocking the secretion of interleukin-1 beta (IL-1β) and tumor necrosis factor a (TNF-α), reducing neutrophil adhesion to the endothelium, and inhibiting neutrophil infiltration into the tissue [25]. The receptor for RvD2 has yet to be determined.
The presence of ALX on fibroblasts suggests that the proresolving mediators play a regulatory role in fibroblast function. Our data clearly indicate that both RvD2 and LXA4 modulate fibroblast activity. Proliferation and collagen deposition by fibroblasts are integral to wound healing. Fibroblast proliferation contributes to the formation of granulation tissue in the wound bed to begin the repair process and collagen deposition is essential for increasing the strength of the wound and allows the cells involved in angiogenesis and connective tissue construction to attach, grow, and differentiate. Earlier studies demonstrated that inhibition of collagen synthesis was observed in fibroblasts in the presence of the lipoxygenase-derived inflammatory lipid mediators 12(S)-12S-hydroxy-(5Z, 8Z, 10E, 14Z)-eicosatetraenoic acid [12(S)-HETE] [33] suggesting that the eicosanoids influence the metabolism of fibroblasts, which could impact the skin repair process.
Collagen types I and III are some of the most abundant collagens found in healing wounds. They are important in maintaining arterial stiffness, for example. These collagens are formed via a complex biosynthetic pathway involving intracellular and extracellular posttranslational modifications by various enzymes [34]. Some inflammatory cytokines, such as IL-1β and TNF-α down-regulate procollagen biosynthesis at the transcriptional level in various types of cells [35]. For example, Aoki et al. reported that IL-1β and NF-κB regulate collagen biosynthesis (types-I and III) in cerebral aneurysm walls. Pathologically, cerebral aneurysm is characterized by decreased collagen content resulting from a chronic inflammatory response in aneurysmal walls. The upregulation of IL-1β and NF-κB activation contributed to cerebral aneurysm progression [35] suggesting that unresolved inflammation has a negative impact on wound healing on two temporally distinct levels. First, early in healing, inflammation leads to excessive granulation tissue and fibroblast proliferation; late in healing, inflammation reduces collagen deposition and wound strength. The results reported here are in agreement with other studies; TGF-β1 induces both collagen type-I and III expression, but treatment with RvD2 and LXA4 does not interfere with deposition allowing the continued expression of collagens to take place by fibroblasts at later time points. At early time points, fibroblast migration and proliferation are slowed, which is consistent with limiting excess granulation tissue and subsequent fibrosis.
Previous studies have examined SPMs for their impact on wounds. Mustafa et al. showed that the treatment of human periodontal ligament cells (PDL; a fibroblast-like cell) with RvD1, reduces cytokine induced production of the pro-inflammatory mediator prostaglandin E2, and up-regulates LXA4 production. In addition, RvD1 significantly enhanced PDL proliferation, wound closure and fibroblast growth factor (FGF) release [36]. In another study, using a rat model of dorsal skin wound, Spite et al. showed that type-2 diabetes alters the resolution of inflammation; this alteration can be acutely corrected by stimulating resolution with RvD1, restoring diabetic defects in macrophage phagocytosis, which decreases the accumulation of apoptotic/necrotic cells and microbes present in chronically inflamed tissues [29]. These studies demonstrate the potential use of SPMs as therapeutics to improve wound outcomes.
In another in vivo study using two different mouse burn injury models involving significant partial thickness injuries, Bohr et al. [37] show that a systemically administered single dose of RvD2 (25 pg/g) effectively prevented thrombosis of the deep dermal vascular network, subsequently preventing dermal necrosis. In addition, RvD2 enhances neutrophil access to the dermis. RvD2 also inhibits TNF-α, IL-1β, and neutrophil platelet-endothelial cell adhesion molecule-1 [37].
Our data show that SPMs, in addition to known anti-inflammatory and proresolving actions, modulate fibroblast proliferation and migration directly to limit fibrosis at early time points without interfering with myofibroblast differentiation allowing wound healing and collagen deposition in the extracellular matrix to proceed.
Supplementary Material
Supplemental Figure 1: Resting fibroblasts do not respond to SPM. A: Western blot analysis of SMA, Collagen I and Collagen III protein expression by resting fibroblasts stimulated with LXA4 and RvD2. B-actin was used as the reference protein. These proteins are not changed after SPM stimulation compared to unstimulated control. B: The impact of LXA4 and RvD2 on fibroblast migration is represented in the fibroblast scratch assay. Quantification of the results are plotted in panel C.
Highlights.
TGF-β1 up-regulates LXA4 receptor (ALX/FPR2) expression on fibroblast.
LXA4 regulates fibroblast migration and proliferation induced by TGF-β1.
SPMs have no impact on α-SMA, collagen type-I and III expression by fibroblast.
RvD2 regulates TGF-β1-induced fibroblast proliferation and scratch wound closure.
Acknowledgments
This work was supported in part by the US Army Medical Research and Material Command Combat Casualty Care (RAD II), Clinical Rehabilitative Medicine (RAD V) Research Directorates and the National Institute of Dental and Craniofacial Research grants DE15566 and DE19938. This research was performed while the first author held a National Research Council Research Associateship Award at U.S. Army Institute of Surgical Research.
Footnotes
DoD disclaimer
KPL is an employee of the U.S. Government. The work presented is part of his official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defined a U.S. Government work as a work prepared by a military service member or employees of the U.S. Government as part of that person’s official duties. The opinions or assertions contained herein are the private views of these authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mulla F, Leibovich SJ, Francis IM, Bitar MS. Impaired TGF-beta signaling and a defect in resolution of inflammation contribute to delayed wound healing in a female rat model of type 2 diabetes. Molecular bioSystems. 2011;7:3006–3020. doi: 10.1039/c0mb00317d. [DOI] [PubMed] [Google Scholar]
- 4.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. The American journal of pathology. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature reviews Molecular cell biology. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 6.Mak K, Manji A, Gallant-Behm C, Wiebe C, Hart DA, Larjava H, Hakkinen L. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. Journal of dermatological science. 2009;56:168–180. doi: 10.1016/j.jdermsci.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson MW, O’Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention, Philosophical transactions of the Royal Society of London. Series B. Biological sciences. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nall AV, Brownlee RE, Colvin CP, Schultz G, Fein D, Cassisi NJ, Nguyen T, Kalra A. Transforming growth factor beta 1 improves wound healing and random flap survival in normal and irradiated rats. Archives of otolaryngology--head & neck surgery. 1996;122:171–177. doi: 10.1001/archotol.1996.01890140057011. [DOI] [PubMed] [Google Scholar]
- 10.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. International journal of burns and trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annual review of immunology. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN, Chiang N. Novel endogenous small molecules as the checkpoint controllers in inflammation and resolution: entree for resoleomics. Rheumatic diseases clinics of North America. 2004;30:69–95. doi: 10.1016/S0889-857X(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 13.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. Journal of dental research. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 14.Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. The Biochemical journal. 2011;437:185–197. doi: 10.1042/BJ20110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara MN, Serhan CN, Van Dyke TE, Gyurko R. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. British journal of pharmacology. 2008;155:1214–1223. doi: 10.1038/bjp.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasturk H, Kantarci A, Ebrahimi N, Andry C, Holick M, Jones VL, Van Dyke TE. Topical H2 antagonist prevents periodontitis in a rabbit model. Infection and immunity. 2006;74:2402–2414. doi: 10.1128/IAI.74.4.2402-2414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nature immunology. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochemical and biophysical research communications. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annual review of pathology. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. Journal of immunology. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 28.Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, Serhan CN. Proresolving nanomedicines activate bone regeneration in periodontitis. Journal of dental research. 2015;94:148–156. doi: 10.1177/0022034514557331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oates TW, Mumford JH, Carnes DL, Cochran DL. Characterization of proliferation and cellular wound fill in periodontal cells using an in vitro wound model. Journal of periodontology. 2001;72:324–330. doi: 10.1902/jop.2001.72.3.324. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacological reviews. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 33.Rieger GM, Hein R, Adelmann-Grill BC, Ruzicka T, Krieg T. Influence of eicosanoids on fibroblast chemotaxis and protein synthesis in vitro. Journal of dermatological science. 1990;1:347–354. doi: 10.1016/0923-1811(90)90591-z. [DOI] [PubMed] [Google Scholar]
- 34.Trackman PC. Diverse biological functions of extracellular collagen processing enzymes. Journal of cellular biochemistry. 2005;96:927–937. doi: 10.1002/jcb.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: contribution of interleukin-1beta and nuclear factor-kappaB. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1080–1086. doi: 10.1161/ATVBAHA.108.180760. [DOI] [PubMed] [Google Scholar]
- 36.Mustafa M, Zarrough A, Bolstad AI, Lygre H, Mustafa K, Hasturk H, Serhan C, Kantarci A, Van Dyke TE. Resolvin D1 protects periodontal ligament. American journal of physiology Cell physiology. 2013;305:C673–679. doi: 10.1152/ajpcell.00242.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Resting fibroblasts do not respond to SPM. A: Western blot analysis of SMA, Collagen I and Collagen III protein expression by resting fibroblasts stimulated with LXA4 and RvD2. B-actin was used as the reference protein. These proteins are not changed after SPM stimulation compared to unstimulated control. B: The impact of LXA4 and RvD2 on fibroblast migration is represented in the fibroblast scratch assay. Quantification of the results are plotted in panel C.