Abstract
Objective
This prospective study investigates the relationships between depressive symptoms, psychiatric medication use, and their interaction on risk of developing type 2 diabetes.
Method
Data come from the 1998 – 2010 waves of the Health and Retirement Study, a U.S. nationally-representative cohort of adults aged 51 and older. Analysis is restricted to participants <65 who did not have diabetes in 1998 (N=8,704). Depressive symptoms were assessed using the 8-item Center for Epidemiologic Studies–Depression scale. Risk of diabetes over the 12-year follow-up period was assessed using Cox proportional hazard models with time-varying covariates.
Results
After adjusting for covariates, both depressive symptoms (hazard ratio [HR]: 1.06, 95% Confidence Interval [CI]: 1.02 – 1.09) and psychiatric medication use (HR: 1.57, 95% CI: 1.25 – 1.96) were associated with development of diabetes. The interaction between depressive symptoms and medication use was significant (beta = −0.240, p = .049), indicating that the association between elevated depressive symptoms and diabetes was higher among respondents not taking medications. The associations between depressive symptoms and medication use were also attenuated by increasing BMI.
Conclusion
Findings highlight the complex relationship between depressive symptoms and psychiatric medications on diabetes risk, and the need for a nuanced understanding of these factors.
Keywords: depression, antidepressants, type 2 diabetes, longitudinal, epidemiology, aging
1. Introduction
Psychiatric medications (e.g., antidepressants, anxiolytics, tranquilizers, and neuroleptics) are among the most commonly prescribed classes of medications in the United States. For example, nearly three in ten adults over the age of 50 have used an antidepressant (e.g., selective serotonin reuptake inhibitors (SSRIs), tricyclics (TCAs), mono-amine oxidase inhibitors (MAOIs) and other medications such as bupropion) in the past year [1]. The prevalence of psychiatric medication use increases with age [2,3], although the prevalence of psychiatric disorders such as depression and anxiety declines in later life [4,5]. While psychiatric medication use is common, only a minority of cases of depression or anxiety receive adequate medical care, including pharmacotherapy or psychotherapy [6].
Prospective, population-based studies have consistently indicated a bi-directional relationship between depression and type 2 diabetes [7,8,9]. Depression is thought to increase risk of type 2 diabetes through a combination of behavioral (e.g., smoking, poor diet, sedentary behavior, weight gain, sleep disturbances [8,10]) and biological (e.g., hypercortisolemia, inflammation, sympathetic nervous system activation [11–13]) mechanisms that impair insulin sensitivity and glucose metabolism. Many, but not all, studies of depression and risk of type 2 diabetes have accounted for the influence of antidepressant medication use using multivariable regression modeling and found that the association between depression and diabetes persisted after accounting for medication use.
As summarized in a recent systematic review by Barnard, Peveler and Holts [14], several studies have reported statistically significant associations between antidepressant use and development of type 2 diabetes [15–20]. In one of the earliest analyses, Rubin et al. reported that in the Diabetes Prevention Program antidepressants, but not depression syndrome as measured by the Patient Health Questionnaire (PHQ), were associated with transition to diabetes in two of the three arms of this trial [17,18]. Similarly, in one of the largest studies to date, Pan et al. reported a modest (on the order of 10% to 15% increased risk) but statistically significant association between antidepressant use and risk of type 2 diabetes [14]. More recently, Vimalananda and colleagues reported that antidepressant medication use was associated with risk of type 2 diabetes over a 12-year period that persisted after accounting for depressive symptoms as indicated by the Center for Epidemiologic Studies – Depression scale [20]. Weight gain associated with some antidepressants may be a mediating mechanism linking depression and diabetes risk [16], and recent reviews indicate that some antidepressants impact glucose metabolism [13]. However, several randomized controlled studies have demonstrated that some classes of antidepressant medications are associated with weight loss and improved glycemic control among patients with both depression and diabetes [21–23]. Therefore, it remains unresolved as to whether antidepressant medication use, independent from depressive symptoms, is predictive of diabetes risk.
As summarized by Barnard and colleagues [13], the majority of prior studies of the relationship between psychiatric medication use and diabetes risk have been limited by infrequent, short follow-up periods which do not account for changes in medication use over time, or which fail to account for depressive symptoms which themselves have been associated with onset of type 2 diabetes. Also, few studies have examined whether psychiatric medications moderate the association between depressive symptoms and diabetes risk. It is possible that if depressive symptoms are well-controlled by medication that the subsequent risk of type 2 diabetes may be mitigated.
Building on this research, the objective of this study was to examine the prospective relationship between depressive symptoms, psychiatric medication use, and their interaction on the risk of incident type 2 diabetes in a population-based cohort over a 12-year period. We modeled both depressive symptoms and medication use as time-varying exposures to account for changes over follow-up. Finally, building on emerging findings regarding the potential modifying role of weight gain in this relationship, we assessed whether the relationship between depressive symptoms and diabetes risk was moderated by body mass index.
2. Methods
2.1 Sample
Data come from the 1998 – 2010 waves of the Health and Retirement Study (HRS). The HRS is an open, longitudinal, nationally-representative cohort study of adults over the age of 50 and was designed to examine relationships between health and economic factors during work transitions in later life. Follow-up surveys have been administered every two years since 1992, and the sample is periodically refreshed with new cohorts to maintain representativeness and a steady-state sample size of approximately 20,000 individuals. Further details of the HRS and its survey design are described elsewhere [24]. The 1998 wave was used as baseline for this study because it was the first year that the HRS merged with the Study of Assets and Health Dynamics Among the Oldest Old, and during this wave, the sample was refreshed with a new cohort of respondents.
Of the 21,384 respondents interviewed in 1998, 16,721 did not report a diagnosis of diabetes in 1998 or any previous (1992 – 1996) wave. To reduce possible bias from a survivor effect, this sample was further restricted to those under the age of 65 in 1998 (N=8,810). The final analytic sample included 8,704 respondents interviewed in 1998 who had complete data for diabetes status, depressive symptoms, psychiatric medication use, and all relevant covariates.
The Health and Retirement Study was approved by the Institutional Review Board at the University of Michigan and all participants provided informed consent.
2.2 Diabetes status
Diabetes status was determined by self-report at each wave. Respondents were asked if they have ever been told by a doctor that they have diabetes or high blood sugar. For all follow-up waves, respondents were asked, “Since we last talked to you, that is since [last interview dates], has a doctor told you that you have diabetes or high blood sugar?” If respondents had reported diabetes at a previous wave, they were asked to confirm this report at the present wave. Respondents were then asked the year that they were first told they had diabetes. Time since baseline to diagnosis of diabetes was calculated as the difference between 1998 and this reported year. If respondents were missing data on year of diagnosis we instead used the year of the follow-up interview that the respondent first reported diabetes. Those who were lost to follow-up, died, or who never reported diabetes during the follow-up period were right-censored, with time-to-event defined as the year of last completed interview minus 1998.
2.3 Depressive symptoms
Depressive symptoms were assessed using the 8-item Center for Epidemiological Studies – Depression (CES-D) scale. The CES-D has been widely used in studies of late-life depressive symptoms and has good psychometric properties for use with older populations [25,26]. Participants were asked to report whether or not eight specific symptoms (e.g., I felt depressed; I felt everything I did was an effort; My sleep was restless) were experienced for much of the past week. The number of endorsed symptoms (positive symptoms were reverse-coded) was summed to create a total depressive symptom score (range: zero to eight). Valid data on at least six of the eight symptom items were required in order to create the summary CES-D score. Depressive symptoms were treated as a continuous variable for the main analysis. For a supplemental analysis examining clinically-elevated levels of depressive symptoms, the CES-D score was dichotomized using cut-points of <4 vs. ≥4 symptoms. These cut-points have been established in earlier reports as indicative of clinically-relevant depressive symptoms [25,27,28].
2.4 Psychiatric medication use
Psychiatric medication use was assessed by self-report at each biennial interview wave. Respondents were asked, “Do you now take tranquilizers, antidepressants, or pills for nerves?” coded as a dichotomous (yes/no) variable. In the later years of the study (2006–2010), an additional question was added to the HRS asking whether or not the respondent regularly took prescription drugs to help relieve anxiety or depression. For these years, an affirmative response to either question was coded as a “yes”. Because of concerns regarding the quality of this measure of medication use, we first conducted a validation analysis using the HRS 2005 Prescription Drug Study (PDS).
The PDS is an off-year supplement to the HRS that details exact prescriptions and usage for drugs currently being taken. The 2005 PDS was administered to a subsample of 2004 HRS respondents who were born in 1942 or earlier or who were covered by Medicare or Medicaid between 2002 and 2004. These medications were then categorized into classes using publicly available databases. The response rate to the 2005 PDS was 88.1% (N=4684) [29]. For those listing a drug on the PDS within the “psychiatric” classification, 62.7% listed an antidepressant (e.g., SSRI, TCA) and 44.4% listed an anti-anxiety drug (e.g., benzodiazepine, hypnotic, anxiolytic). Among those who reported psychiatric medication use in the 2004 HRS and completed the 2005 PDS, 72.9% reported taking a specific psychiatric medication (i.e., antidepressant, anxiolytic) in the PDS drug list. Among those who did not report taking a psychiatric medication in 2004, 85.8% did not list any psychiatric drugs on the PDS. The total concordance between the 2004 HRS psychiatric medication use variable and the 2005 PDS psychiatric medications variable was 84.5%. Considering the one-year lag between these two surveys, this concordance was determined to be satisfactory to validate the self-report data. Because the PDS was not administered at each wave and its restricted sampling frame (i.e., respondents aged 65 and older in 2007 or on Medicare/Medicaid), we could not use this data in our longitudinal analysis.
2.5 Covariates
Demographic covariates included age (in 1998), race/ethnicity (categorized as White, Black, Hispanic, and Other), gender, education (categorized as high school or less vs. at least some college), and socioeconomic status indexed as net worth (total debt minus total assets, categorized into quintiles). Tobacco and alcohol use and physical activity were assessed by self-report at baseline. Smoking status was categorized as current, former, and never smoker. Alcohol use was measured by a dichotomous variable indicating “heavy drinker” (defined as consuming an average of >2 drinks per day for men and >1 drink per day for women, consistent with US Department of Health and Human Services guidelines [30]). Physical activity was assessed by a dichotomous variable of whether or not the respondent engaged in vigorous exercise three or more times per week vs. less. Two health characteristics that impact diabetes risk and which were expected to have significant variability over the follow-up period, body mass index (BMI) and hypertension status, were treated as time-varying covariates. BMI (kg/m2) was calculated from self-reported weight and height assessed at each wave. Physician diagnosis of hypertension (yes/no) was assessed by self-report at each wave.
2.6 Statistical Analysis
Initially, differences in cumulative incidence of diabetes over the follow-up period and average time-to-event were assessed using chi-square tests and F-tests. Next, Cox proportional hazard models were fit to estimate hazard ratios of type 2 diabetes over the 12-year period. Depressive symptoms, psychiatric medication use, BMI and hypertension status were treated as time-varying covariates. All other covariates were fixed at their baseline values because they did not violate the proportional hazard assumption or change substantially over time. In instances of time gaps between measurements for the time-varying covariates (i.e., missing data for a variable in one or more waves), values were imputed using the prior wave value; 12.7% of CES-D, 11.5% of psychiatric medication use, 12.2% of BMI, and 11.5% of high blood pressure values were imputed in this manner. The proportional hazard assumption was validated for baseline covariates by confirming that the interactions between the covariates and ln(time) were non-significant [31].
After fitting bivariate hazard models for each independent variable to estimate its crude relationship with diabetes risk, we fit a series of nested models adjusting for covariates. Model 1 included the two main effects (depressive symptoms and psychiatric medication use) plus their interaction (symptoms*medications). Model 2 included the covariates from Model 1 plus additional adjustment for demographic characteristics and socioeconomic status. Model 3 additionally adjusted for health behaviors (BMI, hypertension status, physical activity, alcohol use, and smoking status). We also examined whether the relationship between depressive symptoms, psychiatric medication use and diabetes risk was moderated by BMI by fitting two and three-way interactions between their main effects and BMI (i.e., symptoms*BMI, medications*BMI, and symptoms*medications*BMI). Further exploration of the interaction between depressive symptoms, psychiatric medication use, and BMI was conducted by calculating hazard ratios for various combinations of these covariates at different levels. Finally, we conducted a supplemental analysis stratifying the sample by baseline CES-D (i.e., CES-D<4 vs. CESD≥4) in order to examine whether these relationships varied by clinically-elevated depressive symptomology. Relative model fit was evaluated with the Akaike Information Criterion (AIC) where smaller values indicate better fit. All analyses were performed using SAS version 9.4, and all p-values represent two-tailed tests.
2.7 Sensitivity Analysis of Diabetes Status
Up to 25% of cases of type 2 diabetes are undiagnosed [32], and thus we conducted a sensitivity analyses to determine whether this measurement error materially impacted our results. In 2006, blood spots were collected from a random sample of 50% of the HRS cohort as part of the “Enhanced Face-to-Face Interviews;” blood spots were collected from the remaining 50% of the cohort in 2008 [33]. We used these blood spots to (1) evaluate the validity of self-reported diabetes status as compared to elevated blood glucose as indicated by hemoglobin A1c (HbA1c) ≥ 6.5% from the blood spots, using the latter as the “gold standard” [34]; and (2) assess whether the measurement error in self-report diabetes status was non-differential relative to both depression status and psychiatric medication use at those waves. If misclassification of diabetes status is not associated with either depressive symptoms or psychiatric medication use, the measures of association between the exposures and risk of diabetes will be biased towards, rather than away, from the null [35].
3. Results
3.1 Main Results
At baseline, 57.4% of respondents reported at least one depressive symptom and 6.7% were currently using a psychiatric medication. Respondents who reported taking a psychiatric medication had higher mean CES-D scores (3.36 for those taking a medication vs. 1.34 for those not) and higher prevalence of clinically-elevated depressive symptoms (45.3% vs 12.5%, respectively). The average follow-up time was 9.76 years (SD=3.44). The cumulative incidence of diabetes during the follow-up period was 18.5%. As shown by Table 1, respondents who developed diabetes were more likely to have elevated depressive symptoms (p<.0001), be using a psychiatric medication (p=.0034), be male (p=.0210), be obese (p<.0001), or have hypertension (p<.0001) at baseline. They were less likely to be White (p<.0001), highly educated (p<.0001), wealthy (p<.0001), engage in vigorously active (p<.0001), or be a heavy drinker (p<.0001). Also shown in Table 1, those with more depressive symptoms (p<.0001) and those using psychiatric medication (p=.0241) had significantly shorter average time to diabetes or censorship.
Table 1.
Baseline characteristics and cumulative incidence of diabetes over the 12-year follow-up period: The Health and Retirement Study
| Overall (N, %) |
Did not develop diabetes (N, %) |
Developed diabetes (N, %) |
χ2 p-value |
Time to Diabetes or Censorship (Mean, SD) |
F test p-value |
|
|---|---|---|---|---|---|---|
| Total sample (N) | 8810 | 7178 (81.5) | 1632 (18.5) | -- | 9.8 (3.4) | -- |
| CES-D | <.0001 | <.0001 | ||||
| 0 | 3506 (42.6) | 2978 (44.4) | 528 (34.6) | 10.1 (3.2) | ||
| 1–3 | 3497 (42.5) | 2790 (41.6) | 707 (46.4) | 9.7 (3.5) | ||
| 4+ | 1225 (14.9) | 936 (14.0) | 289 (19.0) | 9.3 (3.7) | ||
| Psychiatric Medication Use | 0.0034 | 0.0241 | ||||
| No | 8109 (93.3) | 6635 (93.7) | 1474 (91.7) | 9.8 (3.4) | ||
| Yes | 581 (6.7) | 447 (6.3) | 134 (8.3) | 9.4 (3.6) | ||
| Age | 0.3438 | <.0001 | ||||
| <55 | 2722 (30.9) | 2230 (31.1) | 492 (30.1) | 9.7 (3.5) | ||
| 55–59 | 3139 (35.6) | 2532 (35.3) | 607 (37.2) | 9.6 (3.6) | ||
| 60–64 | 2949 (33.5) | 2416 (33.7) | 533 (32.7) | 10.0 (3.3) | ||
| Race | <.0001 | <.0001 | ||||
| White | 6689 (75.9) | 5600 (78.0) | 1089 (66.7) | 9.3 (3.7) | ||
| Black | 1198 (13.6) | 895 (12.5) | 303 (18.6) | 9.3 (3.7) | ||
| Hispanic | 732 (8.3) | 539 (7.5) | 193 (11.8) | 9.4 (3.5) | ||
| Other | 191 (2.2) | 144 (2.0) | 47 (2.9) | 9.9 (3.4) | ||
| Gender | 0.0210 | <.0001 | ||||
| Male | 3572 (40.5) | 2869 (40.0) | 703 (43.1) | 10.0 (3.3) | ||
| Female | 5238 (59.5) | 4309 (60.0) | 929 (56.9) | 9.5 (3.6) | ||
| Education | <.0001 | <.0001 | ||||
| HS or less | 5018 (57.0) | 3969 (55.4) | 1049 (64.3) | 9.5 (3.5) | ||
| At least some college | 3780 (43.0) | 3198 (44.6) | 582 (35.7) | 10.0 (3.3) | ||
| Net Worth | <.0001 | <.0001 | ||||
| <$30000 | 1775 (20.1) | 1363 (19.0) | 412 (25.2) | 9.9 (3.4) | ||
| $30001–99999 | 1967 (22.3) | 1512 (21.1) | 455 (27.9) | 10.0 (3.3) | ||
| $100000–199999 | 1592 (18.1) | 1303 (18.2) | 289 (17.7) | 9.5 (3.6) | ||
| $200000–399999 | 1638 (18.6) | 1382 (19.3) | 256 (15.7) | 10.3 (3.1) | ||
| $400000+ | 1838 (20.9) | 1618 (22.5) | 220 (13.5) | 9.2 (3.7) | ||
| BMI | <.0001 | <.0001 | ||||
| <30 | 6436 (74.1) | 5585 (78.8) | 851 (53.2) | 10.0 (3.3) | ||
| 30+ | 2251 (25.9) | 1502 (21.2) | 749 (46.8) | 9.0 (3.8) | ||
| High Blood Pressure | <.0001 | <.0001 | ||||
| No | 5562 (64.0) | 4772 (67.3) | 790 (49.3) | 10.1 (3.2) | ||
| Yes | 3130 (36.0) | 2316 (32.7) | 814 (50.7) | 9.2 (3.7) | ||
| Vigorous Activity | <.0001 | <.0001 | ||||
| No | 4374 (49.7) | 3449 (48.1) | 925 (56.7) | 9.5 (3.6) | ||
| Yes | 4432 (50.3) | 3726 (51.9) | 706 (43.3) | 10.0 (3.3) | ||
| Smoking Status | 0.8788 | <.0001 | ||||
| Never | 3444 (39.4) | 2799 (39.3) | 645 (39.8) | 9.4 (3.5) | ||
| Former | 3260 (37.3) | 2665 (37.4) | 595 (36.7) | 9.8 (3.5) | ||
| Current | 2046 (23.4) | 1666 (23.4) | 380 (23.5) | 10.0 (3.3) | ||
| Heavy Drinker | <.0001 | 0.4741 | ||||
| No | 8194 (93.4) | 6634 (92.8) | 1560 (95.7) | 9.8 (3.4) | ||
| Yes | 583 (6.6) | 513 (7.2) | 70 (4.3) | 9.9 (3.4) | ||
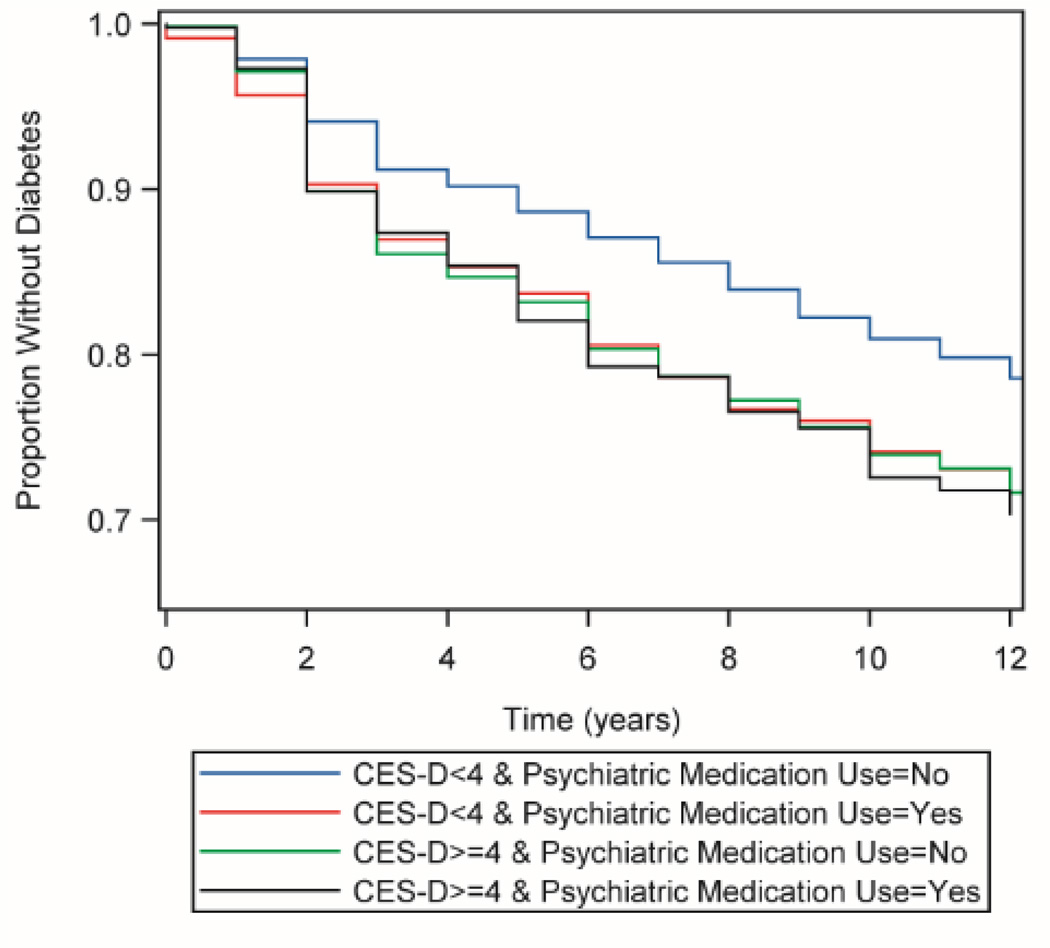
Results of Cox proportional hazard models are shown in Table 2. Both depressive symptoms and psychiatric medication use were significantly associated with elevated risk of developing diabetes. The interaction between CES-D and psychiatric medication use was significant (beta=−0.240, p=0.049) and including this term improved relative model fit as indicated by the AIC. Figure 1 illustrates the unadjusted baseline relationship between CES-D, psychiatric medication use, and their interaction on risk of diabetes over the follow-up period. The two-way interactions between CES-D and BMI (beta=−0.004, p=0.045), psychiatric medication use and BMI (beta=−0.041, p=0.001), and three-way interactions between CES-D, psychiatric medication use, and BMI (beta=0.006, p=0.121) also improved goodness-of-fit and were included in the final model.
Table 2.
Predictors of incident diabetes over the 12-year follow-up period: the Health and Retirement Study (N=8704)
| Effect | Unadjusted Crude HR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
|---|---|---|---|---|
| CES-D | 1.09 (1.07, 1.12) | 1.10 (1.07, 1.13) | 1.07 (1.04, 1.10) | 1.06 (1.02, 1.09) |
| Psychiatric Medication Use | 1.43 (1.25, 1.64) | 1.52 (1.23, 1.86) | 1.62 (1.32, 1.99) | 1.57 (1.25, 1.96) |
| Age | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | |
| Race (ref. White) | ||||
| Black | 1.63 (1.43, 1.85) | 1.37 (1.19, 1.58) | 1.11 (0.96, 1.28) | |
| Hispanic | 1.67 (1.43, 1.96) | 1.35 (1.15, 1.60) | 1.43 (1.21, 1.69) | |
| Other | 1.62 (1.21, 2.16) | 1.55 (1.15, 2.09) | 1.51 (1.12, 2.04) | |
| Female Gender | 0.85 (0.77, 0.94) | 0.79 (0.71, 0.88) | 0.74 (0.66, 0.82) | |
| At Least Some College Education | 0.71 (0.64, 0.78) | 0.86 (0.77, 0.96) | 0.89 (0.80, 1.00) | |
| Net Worth (ref. <$30,000) | ||||
| $30,001–99,999 | 0.96 (0.84, 1.10) | 1.09 (0.94, 1.25) | 1.10 (0.95, 1.26) | |
| $100,000–199,999 | 0.73 (0.63, 0.85) | 0.89 (0.76, 1.04) | 0.99 (0.84, 1.16) | |
| $200,000–399,999 | 0.62 (0.53, 0.73) | 0.78 (0.66, 0.93) | 0.91 (0.77, 1.08) | |
| $400,000+ | 0.47 (0.40, 0.55) | 0.62 (0.52, 0.75) | 0.79 (0.66, 0.96) | |
| BMI (kg/m2) | 1.09 (1.09, 1.10) | 1.09 (1.08, 1.10) | ||
| Hypertension | 2.27 (2.05, 2.52) | 1.76 (1.58, 1.97) | ||
| Vigorous Activity | 0.71 (0.64, 0.78) | 0.85 (0.77, 0.95) | ||
| Smoking Status (ref. Never) | ||||
| Former | 1.00 (0.89, 1.11) | 0.95 (0.84, 1.06) | ||
| Current | 1.07 (0.94, 1.22) | 1.18 (1.03, 1.36) | ||
| Heavy Drinker | 0.63 (0.50, 0.80) | 0.78 (0.61, 1.00) | ||
| AIC | - | 27,341 | 27,208 | 26,243 |
HR: Hazard ratio. 95% CI: 95% Confidence interval. AIC: Akaike’s information criterion.
Because of the interactions between these variables, the HR for CES-D, psychiatric medication use, and BMI are shown at set levels of these covariates. For Models 1 and 2, the HR for CES-D score are for psychiatric medication use=0, and HR for psychiatric medication use is for CES-D=0. For Model 3, the HR for CES-D is for psychiatric medication use=0 and mean BMI, the HR for psychiatric medication use is for CES-D=0 and mean BMI, and the HR for BMI is for psychiatric medication use=0 and mean CES-D.
Figure 1. Kaplan-Meier plot of time to diabetes onset by baseline CES-D and psychiatric medication use.
Kaplan-Meier plot of time to diabetes onset predicted by CES-D score and psychiatric medication use in the Health and Retirement Study (N=8,704); 1998 – 2010
As shown by Tables 2 and 3, in the fully-adjusted model, the relative hazard (HR) of incident diabetes increased by 6% for every one unit increase in CES-D (95% Confidence Interval (CI): 1.02–1.09) for respondents not taking psychiatric medications. However, for respondents who were taking a psychiatric medication, CES-D was not related to diabetes risk (HR: 0.98, 95% CI: 0.93 – 1.03). Psychiatric medication use was associated with 57% greater risk of diabetes (95% CI: 1.25–1.96) for respondents who did not endorse any depressive symptoms (CES-D=0). However, psychiatric medication use was not significantly associated with diabetes risk for respondents who endorsed four or more depressive symptoms (Table 3).
Table 3.
Relative hazard of diabetes according to combined categories of CES-D, psychiatric medication use, and BMI: Health and Retirement Study 1998 – 2010
| Effect | HR (95% CI) |
|---|---|
| CES-D (1 unit increase) at psychiatric medication use=0 and: | |
| BMI = 20 | 1.09 (1.03, 1.15) |
| BMI = 25 | 1.07 (1.03, 1.11) |
| BMI = 28.20 (mean) | 1.06 (1.02, 1.09) |
| BMI = 30 | 1.05 (1.02, 1.08) |
| BMI = 35 | 1.03 (1.00, 1.06) |
| BMI = 40 | 1.01 (0.96, 1.05) |
| CES-D (1 unit increase) at psychiatric medication use=1 and: | |
| BMI = 20 | 0.96 (0.88, 1.05) |
| BMI = 25 | 0.97 (0.91, 1.04) |
| BMI = 28.20 (mean) | 0.98 (0.93, 1.03) |
| BMI = 30 | 0.98 (0.93, 1.03) |
| BMI = 35 | 0.99 (0.94, 1.05) |
| BMI = 40 | 1.00 (0.93, 1.07) |
| Psychiatric medication use at mean BMI=28.20 and: | |
| CES-D = 0 | 1.57 (1.25, 1.96) |
| CES-D = 1 | 1.45 (1.21, 1.75) |
| CES-D = 2 | 1.34 (1.14, 1.58) |
| CES-D = 3 | 1.24 (1.06, 1.47) |
| CES-D = 4 | 1.15 (0.96, 1.39) |
| CES-D = 5 | 1.07 (0.85, 1.34) |
| CES-D = 6 | 0.99 (0.75, 1.30) |
| CES-D = 7 | 0.92 (0.66, 1.27) |
| CES-D = 8 | 0.85 (0.58, 1.24) |
| Psychiatric medication use at mean CES-D=1.37 and: | |
| BMI = 20 | 1.84 (1.41, 2.41) |
| BMI = 25 | 1.57 (1.28, 1.92) |
| BMI = 30 | 1.33 (1.13, 1.57) |
| BMI = 35 | 1.13 (0.94, 1.35) |
| BMI = 40 | 0.96 (0.76, 1.21) |
HR: Hazard ratio. 95% CI: Confidence interval.
Table 3 displays the hazard ratios for incident diabetes according to combined categories of CES-D, psychiatric medication use, and BMI derived from the fully-adjusted model. The influence of both increasing depressive symptoms and psychiatric medication use on diabetes risk declined with increasing BMI. For example, a one-unit increase in CES-D was associated with elevated risk of diabetes for respondents who are normal weight (e.g., HR: 1.09 if BMI=20kg/m2) or overweight (e.g., HR: 1.05 if BMI=30kg/m2). However, CES-D was not significantly associated with diabetes risk for respondents who were obese (e.g., HR: 1.03 if BMI=35kg/m2). Similarly, psychiatric medication use was associated with elevated risk of diabetes for respondents who were normal weight (e.g., HR: 1.84 if BMI=20kg/m2) or overweight (e.g., HR: 1.33 if BMI=30kg/m2), but not for respondents who were obese (HR: 1.13 if BMI=35kg/m2).
Finally, Table 4 shows the results of the supplemental analysis stratifying the sample by clinically-elevated depressive symptoms at baseline. Among respondents with low levels of depressive symptoms at baseline, the interaction between psychiatric medication use and CES-D was marginally significant (beta=−0.347, p=0.050) and indicated that medication use was associated with diabetes risk only for those with consistently low levels of depressive symptomology over the follow-up period. Psychiatric medication use was not related to diabetes risk among respondents who had low levels of depressive symptomology at baseline but who developed clinically-elevated depressive symptomology (i.e., CES-D ≥4) over the follow-up period. Consistent with this finding, psychiatric medication use was not related to diabetes risk among respondents with clinically-elevated depressive symptoms at baseline.
Table 4.
Relative hazard of diabetes according to combined categories of CES-D, psychiatric medication use, and BMI stratified by baseline depressive symptoms
| Baseline CES-D<4 | Baseline CES-D≥4 | |
|---|---|---|
| Effect | HR (95% CI) | HR (95% CI) |
| Total N | 6,936 | 1,214 |
| CES-D (1 unit increase) at mean BMI=28.20 and: | ||
| Psychiatric medication use = No | 1.05 (1.00, 1.09) | 1.03 (0.97, 1.10) |
| Psychiatric medication use = Yes | 0.95 (0.88, 1.03) | 1.06 (0.95, 1.19) |
| Psychiatric medication use at mean BMI=28.20 and: | ||
| CES-D = 0 | 1.80 (1.41, 2.30) | 0.72 (0.37, 1.38) |
| CES-D = 1 | 1.64 (1.35, 2.00) | 0.74 (0.43, 1.28) |
| CES-D = 2 | 1.49 (1.24, 1.80) | 0.76 (0.48, 1.20) |
| CES-D = 3 | 1.36 (1.09, 1.70) | 0.79 (0.54, 1.15) |
| CES-D = 4 | 1.24 (0.93, 1.64) | 0.81 (0.58, 1.13) |
| CES-D = 5 | 1.13 (0.79, 1.61) | 0.84 (0.61, 1.16) |
| CES-D = 6 | 1.03 (0.66, 1.59) | 0.87 (0.60, 1.24) |
| CES-D = 7 | 0.93 (0.55, 1.57) | 0.89 (0.58, 1.38) |
| CES-D = 8 | 0.85 (0.46, 1.56) | 0.92 (0.55, 1.56) |
HR: Hazard ratio. 95% CI: Confidence interval.
3.2 Sensitivity Analysis of Diabetes Status
Combining the 2006 and 2008 blood spot collections and using HbA1c ≥ 6.5% as the gold standard, the sensitivity of self-report diabetes was 76.4% and the specificity was 86.5%. Limiting the sample to those who reported they did not have diabetes, the proportion of respondents with undiagnosed diabetes was 5.4% among those with CES-D≥4 and 4.2% among those with CES-D<4 (Chi2=3.0, p=0.081). The proportion of respondents with undiagnosed diabetes was 3.4% among those taking a psychiatric medication and 4.5% among those who were not (Chi2=3.3, p=0.697). Together these results indicate that the self-report assessment of diabetes status in this sample has acceptable sensitivity/specificity, and that the measurement error inherent in this assessment is non-differential relative to psychiatric medication use and depressive symptoms.
4. Discussion
The three main findings of this study are: First, both depressive symptoms and psychiatric medication use are related to diabetes risk in mid- and late-life, and he association between depressive symptoms and diabetes risk is moderated by psychiatric medication use. Specifically, our data are consistent with a threshold effect whereby the relative increase in diabetes risk only occurs at the lower end of depressive symptomology, and once a threshold of “clinically significant” elevated symptomology is met (indicated by either CESD≥4 or use of medications), there is no additional risk of diabetes associated with more severe symptomology. Second, the association between both depressive symptoms and psychiatric medication use is attenuated by increasing BMI. Third, the results of our sensitivity analysis are consistent with the hypothesis that misclassification of diabetes status was not differentially associated with these two exposures (indicating our findings are conservative), and that the association between psychiatric medication use and risk of diabetes is not solely an artifact of clinical ascertainment bias.
Our interpretation of the moderating impact of psychiatric medication use on the association between depressive symptoms and diabetes risk is that this result indicates a threshold (or ceiling) effect: that is, a relative increase in diabetes risk only occurs at the low-end of depressive symptomology. This is consistent with the findings of Campayo and colleagues (2010) who reported that depression severity was not related to diabetes risk once diagnostic criteria were met [36], and those of Pan et al. who reported that low levels of depressive symptomology were predictive of diabetes risk [9]. The corollary to this pertains to the association between psychiatric medications and diabetes risk: respondents taking medications had consistently higher depressive symptoms throughout the follow-up period, and thus a ceiling/threshold effect of depressive symptoms would explain why relative increases in CES-D was not related to diabetes risk among this group. The supplemental analysis which stratified by sample by clinically-elevated depressive symptoms at baseline is consistent with this explanation because it shows that neither increasing depressive symptoms nor psychiatric medication use were related to diabetes risk for those with elevated depressive symptoms at baseline. In short, our interpretation is that once a threshold level of “clinically significant” depressive symptomology has been met (indicated either by CES-D≥4 or clinical detection of depressive symptoms as proxied by use of psychiatric medications), a relative increase in depressive symptoms does not predict increased diabetes risk.
Two of our findings contrast with recent reports. First, in this study the association between psychiatric medication use and risk of diabetes was only observed among those with less severe depressive symptomology. Although this finding needs to be replicated in samples with more detailed data regarding specific medications, these findings contrast with a recent analysis of the Black Women’s Health Study (BWHS) that indicated the association between antidepressant medication use and diabetes risk was strongest among those with elevated depressive symptoms, suggesting a synergistic effect [20]. Second, our findings indicate that the association between both depressive symptoms and psychiatric medication use is attenuated by increasing BMI. It is possible that BMI mediates the association between these exposures and diabetes risk, or that the relatively modest effects of depression and medication use are simply overwhelmed by the influence of such a potent diabetes risk factor like obesity. However, we note that the analysis of the BWHS found that both depressive symptoms and antidepressant use were associated with diabetes risk even among obese respondents [20]. While differences in sample composition may contribute to these contrasting findings, together these studies indicate that future research should explicitly investigate potential interrelationships between psychiatric medications, depressive symptoms, and BMI on diabetes risk.
Results should be interpreted in light of study limitations. First, all variables were derived by self-report, and thus may be subject to reporting bias. This was of particular concern for the self-report of psychiatric medication use and diabetes status. However, our validation analysis using the 2005 PDS showed high concordance between the self-reported questionnaire and specific psychotropic drugs from the PDS. Additionally, our sensitivity analysis showed that misclassification of diabetes status in the 2006 and 2008 waves, indicated by HbA1c ≥ 6.5%, was not significantly associated with either depressive symptoms or psychiatric medication use, and thus associations would be biased toward the null [35]. The CES-D is also not a diagnostic instrument, and thus we may not have been able to capture all clinically-relevant components of depressive symptomology. Finally, while over 95% of diabetes in the adult population are type 2, without additional biological data we cannot confirm that no cases were type 1.
The study also has several strengths. This is among the first studies of diabetes risk to explicitly focus on the interaction between depressive symptoms and psychiatric medications using a nationally-representative cohort. The long follow-up period and relatively frequent (every 2 years) assessments allowed us to model depressive symptoms and psychiatric medication use as time-varying covariates to account for change in status over time. Finally, we were able to account for important confounders, such as physical activity, smoking status, and alcohol use, and explicitly investigated effect modification by BMI.
5. Conclusions
Our findings add to the growing body of research on how depressive symptoms, psychiatric medication use, and diabetes risk interrelate in mid- and late-life. In particular, our results highlight the complex relationship between depressive symptoms and psychiatric medications on diabetes risk, and the need for a nuanced understanding of these factors. Examining the potential interactions between these variables can inform the development of targeted intervention strategies, as well as open the door to examining novel hypotheses regarding the mechanisms linking depressive symptoms and diabetes risk for older adults.
Acknowledgements
The Health and Retirement Study is supported by the National Institute on Aging (NIA U01AG009740) and the Social Security Administration. B. Mezuk is supported by the National Institute of Mental Health (grant number K01-MH093642) and the National Institute of Diabetes and Digestive and Kidney Diseases (grant number R21-DK8356430). The sponsors had no role in the design, analysis, or interpretation of the findings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
Contributor Information
Scott Ratliff, Email: smratliff@vcu.edu.
Briana Mezuk, Email: bmezuk@vcu.edu.
References
- 1.Olfson M, Marcus S. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 2.Middleton N, Gunnell D, Whitley E, Dorling D, Frankel S. Secular trends in antidepressant prescribing in the UK, 1975–1998. J Public Health Med. 2001;23:262–267. doi: 10.1093/pubmed/23.4.262. [DOI] [PubMed] [Google Scholar]
- 3.Moore M, Yuen HM, Dunn N, Mullee MA, Maskell J, Kendrick T. Explaining the rise in antidepressant prescribing: A descriptive study using the general practice research database. BMJ. 2009;339 doi: 10.1136/bmj.b3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzlez H, Tarraf W, Whitfield K, Vega W. The epidemiology of major depression and ethnicity in the united states. J Psychiatr Res. 2010;44:1043–1051. doi: 10.1016/j.jpsychires.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: Results from the national comorbidity survey replication (NCS-R) Psychol Med. 2010;40:225–237. doi: 10.1017/S0033291709990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration. Results from the 2010 national survey on drug use and health: Mental health findings. 2012:11–4667. NSDUH Series H-42, HHS Publication No.(SMA); [Google Scholar]
- 7.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan A, Lucas M, Sun Q, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170:1884–1891. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strine T, Mokdad A, Dube S, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30:127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Champaneri S, Wand GS, Malhotra SS, Casagrande SS, Golden SH. Biological basis of depression in adults with diabetes. Curr Diab Rep. 2010;10:396–405. doi: 10.1007/s11892-010-0148-9. [DOI] [PubMed] [Google Scholar]
- 12.Golden S. A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev. 2007;3:252–259. doi: 10.2174/157339907782330021. [DOI] [PubMed] [Google Scholar]
- 13.DeSantis AS, DiezRoux AV, Hajat A, et al. Associations of salivary cortisol levels with metabolic syndrome and its components: The multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2011;96:3483–3492. doi: 10.1210/jc.2011-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnard K, Peveler RC, Holt RI. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care. 2013;36:3337–3345. doi: 10.2337/dc13-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan A, Sun Q, Okereke OI, et al. Use of antidepressant medication and risk of type 2 diabetes: Results from three cohorts of US adults. Diabetologia. 2012;55:63–72. doi: 10.1007/s00125-011-2268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kivimaki M, Tabak AG, Batty GD, et al. Hyperglycemia, type 2 diabetes, and depressive symptoms: The British Whitehall II Study. Diabetes Care. 2009;32:1867–1869. doi: 10.2337/dc09-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin RR, Ma Y, Marrero DG, et al. Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care. 2008;31:420–426. doi: 10.2337/dc07-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin RR, Ma Y, Peyrot M, et al. Antidepressant medicine use and risk of developing diabetes during the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. 2010;33:2549–2551. doi: 10.2337/dc10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Balasubramanian R, Pagoto S, et al. Elevated depressive symptoms, antidepressant use, and diabetes in a large multiethnic national sample of postmenopausal women. Diabetes Care. 2011;34:2390–2392. doi: 10.2337/dc11-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vimalananda VG, Palmer JR, Gerlovin H, et al. Depressive symptoms, antidepressant use, and the incidence of diabetes in the Black Women’s Health Study. Diabetes Care. 2014;37:2211–2217. doi: 10.2337/dc13-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustman P, Freedland K, Griffith LS, Clouse R. Fluoxetine for depression in diabetes: A randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23:618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 22.Lustman PJ, Clouse RE, Nix BD, et al. Sertraline for prevention of depression recurrence in diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:521–529. doi: 10.1001/archpsyc.63.5.521. [DOI] [PubMed] [Google Scholar]
- 23.Ye Z, Chen L, Yang Z, et al. Metabolic effects of fluoxetine in adults with type 2 diabetes mellitus: A meta-analysis of randomized placebo-controlled trials. PLoS ONE. 2011;6:e21551–e21551. doi: 10.1371/journal.pone.0021551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juster FT, Suzman R. An overview of the Health and Retirement Study. Journal of Human Resources. 1995;30:S7–S56. [Google Scholar]
- 25.Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study. [accessed 17 June 2014];2000 Available from http://hrsonline.isr.umich.edu/index.php. [Google Scholar]
- 26.Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: its use in a community sample. Am J Psychiatry. 1983;140:41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- 27.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 28.Mojtabai R, Olfson M. Major depression in community-dwelling middle-aged and older adults: prevalence and 2- and 4-year follow-up symptoms. Psychol Med. 2004;34:623–634. doi: 10.1017/S0033291703001764. [DOI] [PubMed] [Google Scholar]
- 29.Health and Retirement Study, 2005 Prescription Drug Study. Ann Arbor, MI: 2006. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740) [Google Scholar]
- 30.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans. 7th Edition. Washington, DC: US Government Printing Office; 2010. Chapter 3 – Foods and Food Components to Reduce; pp. 30–32. 2010. [Google Scholar]
- 31.Selvin S. Statistical analysis of epidemiologic data. 3rd ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 32.Centers for Disease Control and Prevention. Atlanta, GA: U.S. Department of Health and Human Services; 2014. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. [Google Scholar]
- 33.Crimmins E, Guyer H, Langa K, Ofstedal MB, Wallace R, Weir D. Documentation of biomarkers in the Health and Retirement Study. [accessed 29 May 2014];HRS Documentation Report. 2008 Available from http://hrsonline.isr.umich.edu/sitedocs/userg/HRS2006BiomarkerDescription.pdf. [Google Scholar]
- 34.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wacholder S, Hartge P, Lubin JH, Dosemeci M. Non-differential misclassification and bias towards the null: A clarification. Occup Environ Med. 1995;52:557–558. doi: 10.1136/oem.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campayo A, de Jonge P, Roy JF, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167:580–588. doi: 10.1176/appi.ajp.2009.09010038. [DOI] [PubMed] [Google Scholar]