Abstract
Background
Most studies with corticotropin releasing factor (CRF) and ethanol consumption have focused on CRF type 1 (CRF1) receptors; less is known about other components of the CRF system, such as the CRF type 2 (CRF2) receptors and the CRF binding protein (CRFBP). In humans, several nucleotide polymorphisms in the CRFBP gene have been associated with ethanol abuse.
Methods
The role of the CRFBP within the ventral tegmental area (VTA) and the central nucleus of the amygdala (CeA) was investigated in C57BL/6J mice exposed to an ethanol binge drinking paradigm (drinking-in-the-dark, DID), or to a dependence-producing drinking protocol (two-bottle choice, intermittent access to alcohol, IAA) for 4 weeks. Potential interactions between VTA CRFBP and CRF2 receptors on ethanol binge drinking were also assessed. Mice were microinjected with the CRFBP antagonist CRF6–33 into the VTA or CeA, or with the CRF2 antagonist Astressin2-B (A2B) alone or in combination with CRF6–33 into the VTA, and had access to 20% (w/v) ethanol for 4 h (DID). Separate cohorts of mice received vehicle and doses of CRF6–33 into the VTA or CeA and had access to ethanol/water for 24 h (IAA). Blood ethanol concentrations (BECs) were measured, and signs of withdrawal by handling-induced convulsion were determined.
Results
Intra-VTA CRF6–33 and A2B reduced ethanol intake dose-dependently in mice during DID. Furthermore, a combination of a sub-effective dose of CRF6–33 and a lower dose of A2B promoted additive effects in attenuating ethanol binge drinking. Intra-VTA CRF6–33 did not affect ethanol consumption in mice given IAA, and intra-CeA CRF6–33 did not change alcohol consumption in both models of drinking. DID and IAA promoted pharmacologically relevant BECs, however, only mice given IAA exhibited convulsive events during withdrawal.
Conclusions
These findings suggest that VTA CRFBP is involved in the initial stages of escalated ethanol drinking by mechanisms that may involve CRF2 receptors.
Keywords: Alcohol, CRF binding protein, CRF2 receptor, Ventral tegmental area, Drinking-in-the-dark
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines ethanol binge drinking as a pattern of drinking that elevates blood ethanol concentration (BEC) levels to 0.08 g/dl. This typically occurs after 4 drinks for women and 5 drinks for men in about 2 h (NIAAA, 2004). In mice, a history of binge-like ethanol dinking promotes subsequent increases of voluntary ethanol consumption (Cox et al., 2013). Moreover, long periods of heavy ethanol intake appear to be accompanied by neuroadaptative changes in the brain (Kasanetz et al., 2010; Koob and Volkow, 2010), including alterations in neuropeptide signaling, such as increases in corticotropin releasing factor (CRF) neurotransmission (Lowery-Gionta et al., 2012). Unfortunately, a comprehensive understanding of the neurobiology underlying the transition from binge drinking to ethanol dependence remains elusive.
The CRF system has been implicated in ethanol’s effect following both acute exposure and during procedures designed to model ethanol dependence (Hwa et al., 2013; Sparta et al., 2013; Zorrilla et al., 2013). The primary role of CRF is to activate the hypothalamic-pituitary-adrenal axis by increasing the release of glucocorticoids (Vale et al., 1981). However, CRF axons also project to extrahypothalamic areas, modulating the homeostatic balance involved in the mobilization of resources and behaviors for responses to stress (Bale and Vale, 2004). These projection areas include the amygdala, bed nucleus of the stria terminalis, and ventral tegmental area (VTA) (Sawchenko et al., 1993; Swanson et al., 1983),
The effects of CRF and the structurally related Urocortins are mediated by two CRF receptor subtypes, CRF type 1 (CRF1) and type 2 (CRF2) (Vale et al., 1981). In addition, CRF and Urocortin also bind to a CRF binding protein (CRFBP) with an affinity equal to or greater than the CRF receptors, suggesting a regulatory function (Behan et al., 1995a; Sutton et al., 1995). CRFBP is a 37 kDa secreted glycoprotein highly conserved across vertebrates (Enoch et al., 2008). Although its function in the brain is largely unknown, CRFBP is thought to bind extracellular CRF and thereby prevent receptor activation (Behan et al., 1995a). However, studies using the CRF fragment 6–33 (CRF6–33), a CRFBP ligand inhibitor, have suggested both inhibitory (Behan et al., 1995b) and facilitatory (Ungless et al., 2003) roles for CRFBP in CRF signaling. Moreover, intracerebroventricular injections of CRF6–33 lead to Fos protein expression in cells where CRF is not detected, suggesting that CRFBP may have distinctive functions (Chan et al., 2000).
Acute drug withdrawal increases CRF activity in the amygdala, including the central nucleus (CeA), and leads to a negative emotional state that motivates resumption and maintenance of drug taking (Funk et al., 2006; Roberto et al., 2010). In the VTA, however, the role of CRF remains unclear and most studies with CRF and ethanol consumption have focused on CRF1 receptors, although both CRF1 (Van Pett et al., 2000) and CRF2 (Korotkova et al., 2006; Ungless et al., 2003) subtypes are expressed in this area. The presence of CRFBP was demonstrated in a subset of VTA dopaminergic neurons (Wang and Morales, 2008). In turn, the release of dopamine from the VTA into projection areas is proposed to mediate rewarding processes and positive reinforcing effects of natural stimuli such as palatable food (Fields et al., 2007), and drugs of abuse (Di Chiara and Imperato, 1988), including alcohol (Di Chiara and Imperato, 1985).
A key issue of the present study is to investigate the mechanisms underlying the transition from binge to chronic ethanol use. Since recently studies have identified several single nucleotide polymorphisms in the human CRFBP gene associated with ethanol abuse (Ray, 2011), we investigated the role of CRFBP on alcohol excessive drinking in mice. We tested the hypothesis that VTA CRFBP modulates ethanol binge drinking exhibited by nondependent mice, whereas CeA CRFBP may be involved in ethanol intake exhibited by animals exposed to a long-term protocol to escalate drinking levels. Male C57BL/6J mice were microinjected with CRF6–33 into the VTA or CeA and were tested in a binge-like drinking procedure (drinking-in-the-dark, DID) or in an intermittent access to alcohol (IAA) protocol which implements voluntary, preferential, dependence-producing ethanol drinking. CRF6–33 is a selective CRFBP antagonist, characterized by high-affinity binding to the CRFBP and a much lower affinity than CRF to the CRF receptor subtypes (Behan et al., 1995a). Furthermore, we investigated the functional role of VTA CRFBP through potential interaction with CRF2 receptors. Signs of ethanol withdrawal were assessed by handling-induced convulsion (HIC), a measure of withdrawal severity.
MATERIALS AND METHODS
Animals
Adult male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) were 8 weeks old upon arrival and were housed 6 per cage for 1 week to acclimate to the vivarium of Tufts University (Medford, MA, USA), which was maintained on a 12-h reversed light/dark cycle, 21 ± 2°C temperature and 30% humidity. Before starting the experiments, mice were housed individually in polycarbonate cages (28 × 17 × 12 cm) with pine shavings as bedding. Rodent laboratory chow (Purina LabDiet 5001, PMI Nutrition International, Brentwood, MO, USA) was continuously accessible through stainless steel wire mesh lids. Water bottles were continuously available, except when mice were exposed to the Rhodes et al., (2005) DID protocol. All procedures were approved by the Tufts University Institutional Animal Care and Use Committee and were in accordance with the National Research Council's “Guide for the Care and Use of Laboratory Animals” (2011).
Drugs
Ethanol (20% w/v) solutions were prepared in tap water from 95% ethyl alcohol (Pharmaco–AAPER, Brookfield, CT, USA). The selective CRFBP antagonist CRF6–33 (0.125, 0.25 and 0.5 µg; Tocris Bioscience, Bristol, United Kingdom) and the CRF2 antagonist Astressin2-B (A2B, 0.25 and 0.5 µg; Tocris Bioscience) were dissolved in artificial cerebrospinal fluid (aCSF).
Surgery and Infusion procedures
Mice were anesthetized with a combination of ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and surgically implanted with 26-gauge bilateral cannulae (Plastics One, Roanoke, VA, USA) aimed at the VTA or CeA. The stereotaxic coordinates, according to Paxinos and Franklin (2001) were: VTA, 3.20 mm posterior to bregma, ± 0.75 mm lateral to the midline, and 4.50 mm ventral to the dura; CeA, 0.94 mm posterior to bregma, ± 2.55 mm lateral to the midline, and 4.10 mm ventral to the dura (Lowery-Gionta et al., 2012). After surgery, mice were handled daily and recovered for 5–7 days. On the day before the test day, they received 1 sham injection, consisting of insertion of the injector needles into the cannulae for 3 min. On the test day, mice were given intra-VTA or -CeA injections of vehicle (aCSF) or drugs. Bilateral injections were conducted simultaneously in a volume of 0.2 µl per side over a period of 2 min. The injector needles (0.1 mm beyond the end of the guide cannulae) were left in place for 1 min after the end of the infusion to allow for diffusion.
Ethanol binge drinking
The DID protocol was used as originally described (Rhodes et al., 2005) or further modified, using two-bottle choice (Giardino and Ryabinin, 2013), 3 h after lights off in the animal room. In the original DID protocol, the single water bottle was replaced with a plastic 50 ml centrifuge bottle (Nalgene, Rochester, NY, USA) containing ethanol solution (20%) in the home cage. In the two-bottle choice DID, mice were given access to two bottles containing water in the home cage and, during the DID sessions, one water bottle was replaced with a bottle containing ethanol solution (20%). The position of the bottles presented through the lid of the cage was switched (left/right) in every session to avoid side preference. Centrifuge bottles had a rubber stopper (N° 5, Fisher Scientific, Agawam, MA, USA) with a sipper tube (Ancare Corp., Bellmore, NY, USA) constructed with two ball bearings to prevent fluid loss.
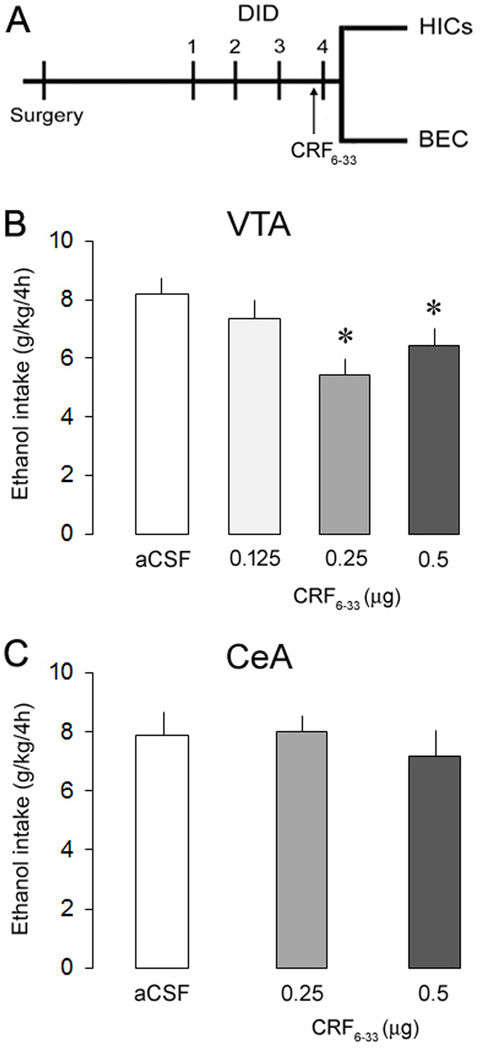
After recovery from surgery, ethanol-naïve mice were weighed and had access to ethanol or ethanol/water for 2 h. After this period, fluid intakes were recorded, and the ethanol bottles were replaced with water bottles. This procedure was repeated on days 2 and 3. On day 4, mice received intra-cranial injections of vehicle or drugs, and after 10 min, the procedure was again repeated except that the ethanol or ethanol/water bottles were left in place for 4 h (Fig. 2A). An empty “drip” cage served as a control for evaporation and spillage due to experimenter handling. Fluid loss in this control condition was deducted from each individual's intake value. Control animals for the original DID protocol had water bottles replaced 3 h into the dark cycle with another water bottle to mimic all the same parameters. Separate cohorts of mice were exposed to the original DID conditions and tested for HIC scores and BEC.
Fig. 2.
Experiment design (A) and ethanol consumption (g/kg/4h) in separate groups of mice exposed to the ethanol binge drinking procedure Drinking-in-the-Dark, 10 min after intra-VTA (B) or -CeA (C) infusions of aCSF or CRF6–33 (0.125, 0.25 and 0.5 µg). Data are mean ± SEM. *, versus aCSF. P < 0.05, n = 6–12 mice per group.
Dependence-producing drinking
This procedure used a voluntary, preferential, dependence-producing drinking paradigm, IAA, as described by Hwa et al. (2011). Mice were weighed, and given two bottles, one containing ethanol solution (20%) and the other tap water for 24 h 3 days per week separated by 24 – 48 h (i.e., Monday, Wednesday, Friday). The position of the bottles was switched (left/right) in every ethanol drinking session to avoid side preference. During the remaining days of the week, mice received two bottles of water. The same 50-ml water bottles and sipper tubes were used as previously described. A “drip” cage was maintained during this procedure to control for experimenter spillage and evaporation.
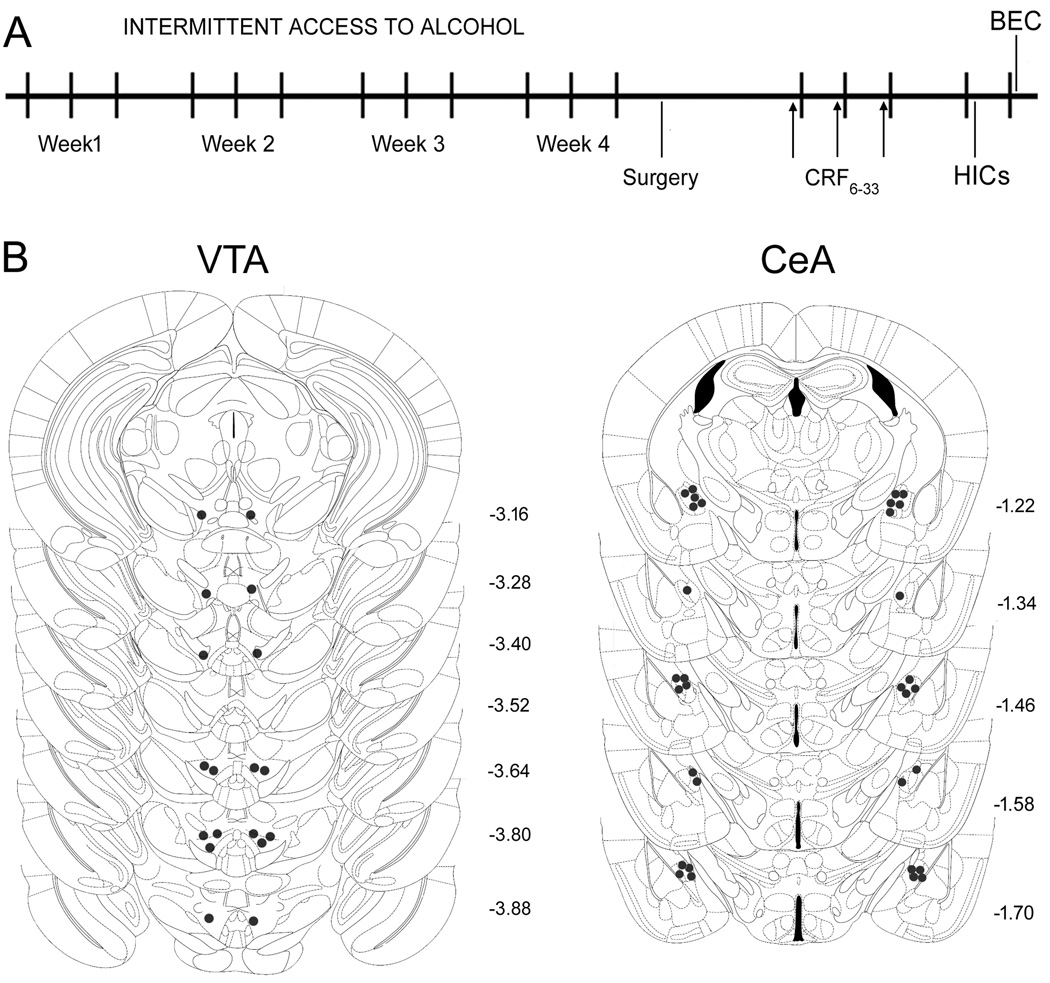
After 4 weeks of IAA, mice were surgically implanted with bilateral cannulae aimed at the VTA or CeA. After recovery, mice received bilateral injections of CRF6–33 in a design that counterbalanced aCSF and drug treatments. After 10 min they had access to the two-bottle choice. Fluid intake was evaluated 2 h, 4 h, and 24 h after drug injection. On the following two ethanol drinking sessions, mice were tested for HIC scores and BEC (Fig. 4A).
Fig. 4.
(A) Experiment design and (B) correct placements of intra-VTA and -CeA bilateral cannulae in mice exposed to the dependence-producing drinking procedure intermittent access to alcohol. Each figure corresponds to coronal sections of the mouse brain according to the bregma (Paxinos and Franklin, 2001).
Withdrawal measurements
Mice were tested for ethanol withdrawal by assessing convulsive events (Goldstein, 1972). The scoring system for convulsions elicited by handling a mouse by the tail, or HIC, is described using a 0 to 4 scale (0 = no withdrawal signs; 1 = tonic convulsion when the mouse is lifted and given a gentle 180° turn; 2 = tonic-clonic convulsion elicited by the gentle spin, or tonic convulsion when lifted without turning; 3 = tonic-clonic convulsion not requiring any spin; 4 = violent tonic-clonic convulsion, often continuing after release of the mouse). Ethanol bottles were removed at the end of the 4-h (DID) or 24-h exposure (IAA). Two blind experimenters observed the mice every 2 h after the ethanol bottles had been withdrawn for 4 h (4 h–10 h).
BEC analysis and Histology
After 4-h access to ethanol, mice were deeply anesthetized with an overdose of ketamine and xylazine combination. Blood samples were collected from a group of DID and IAA mice by cardiac puncture. The animals were perfused with 0.9% saline and 4% paraformaldehyde solution prior to removal of the brains. The fixed brains were sliced in 50-µg coronal section using a Cryostat (Leica CM 1900, Bannockburn, IL, USA). The brain slices were stained in cresyl violet, and the injector placements were verified by light microscopy, according to the mouse brain atlas (Paxinos and Franklin, 2001). Mice with injector tracks that did not terminate within the correct brain area were excluded from the analysis (n = 8 out of 170). Blood samples were centrifuged at 4°C for 10 min at 3000 rpm. Five µl of plasma were analyzed for BEC using the Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA, USA).
Statistical Analysis
Statistical analyses were performed using STATISTICA version 6.0. Descriptive statistics for all measurements, except for HIC scores, are reported as mean ± SEM. Graded HIC scores were considered as ordinal data and reported as median values and interquartile ranges.
To obtain a measure that corrected for individual differences in body weight, grams of ethanol consumed per kilogram of body weight were calculated. In the original and two-bottle choice DID protocols, fluid intake was analyzed with one-way analyses of variance (ANOVA), followed by Duncan multiple-range post hoc test. Water intake values (ml) from the original DID control groups (aCSF vs. CRF6–33 0.25 µg) were compared with Student's t test. In the IAA procedure, fluid intake was analyzed with two-way repeated measures ANOVAs.
The comparison of BECs (DID vs. IAA) was performed using Student's t test. A linear least-square regression was conducted to determine the relationship between ethanol intake (g/kg) and BEC (mg/dl). HIC scores over time within groups were assessed by Kruskal–Wallis one-way ANOVA by ranks followed by Mann–Whitney rank sum tests. Between group differences for HIC scores in each time point were analyzed with Mann–Whitney rank sum tests. Values of p < 0.05 were considered statistically significant.
RESULTS
Ethanol binge drinking
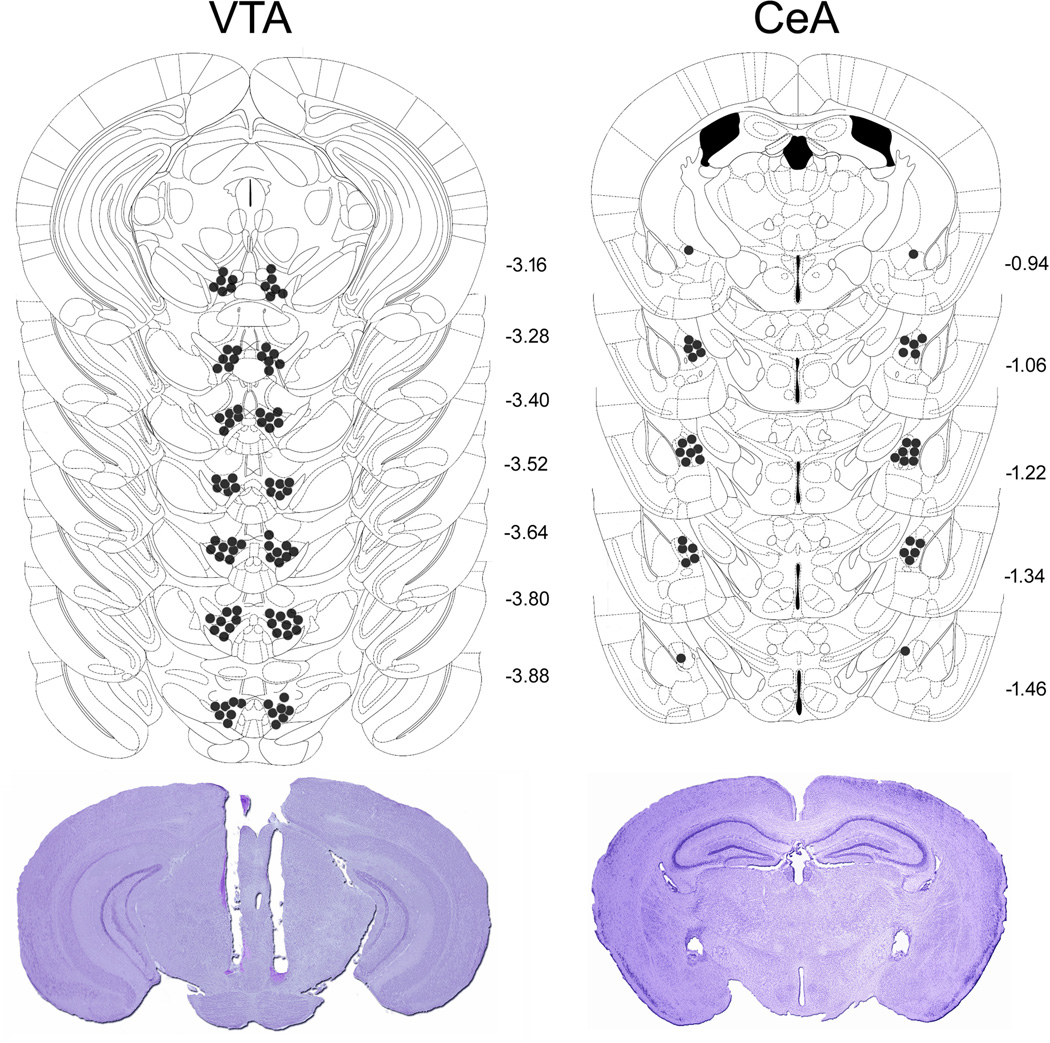
Diagrammatic representations of bilateral intra-VTA and -CeA injection sites, as well as representative photomicrographs are shown in Fig. 1. During the DID sessions 1 to 3 male C57BL/6J mice consumed on average 3.6 ± 0.19 g/kg of ethanol in 2 h, confirming previous reports (e.g., Rhodes et al., 2007). Intra-VTA injections of the selective CRFBP antagonist CRF6–33 decreased ethanol intake in mice subjected to the 4-h DID session (Fig. 2B). The one-way ANOVA revealed a significant main effect of the treatment (F(3, 42) = 4.02, p < 0.05), and a post hoc analysis indicated that 0.25 or 0.5, but not 0.125 µg of CRF6–33 significantly attenuated binge-like ethanol consumption in comparison to aCSF-treated animals, reducing alcohol intake by 33.6% and 21.4%, respectively. The most effective dose of CRF6–33 injected intra-VTA did not alter water intake in control animals (aCSF = 2.05 ml ± 0.13 (n = 8), 0.25µg = 1.77 ml ± 0.13 (n = 8); t = 1.51, p > 0.05). There is no difference in the body weight of the animals between groups (t = 0.89, p > 0.05). In contrast, CRF6–33 injected intra-CeA (0.25 and 0.5 µg) did not affect ethanol intake in DID animals (F(2, 17) = 0.39, p > 0.05) (Fig. 2C). These results point to a selective role of VTA CRFBP on the modulation of binge-like ethanol consumption.
Fig. 1.
Correct placements of intra-VTA and -CeA bilateral cannulae and representative photomicrographs after Nissl staining in mice exposed to the ethanol binge drinking procedure Drinking-in-the-Dark. Each figure corresponds to coronal sections of the mouse brain according to the bregma (Paxinos and Franklin, 2001). The number of points in the figures is less than the total number of animals because of overlapping injection sites.
Co-infusions of CRFBP and CRF2 receptor antagonists into the VTA
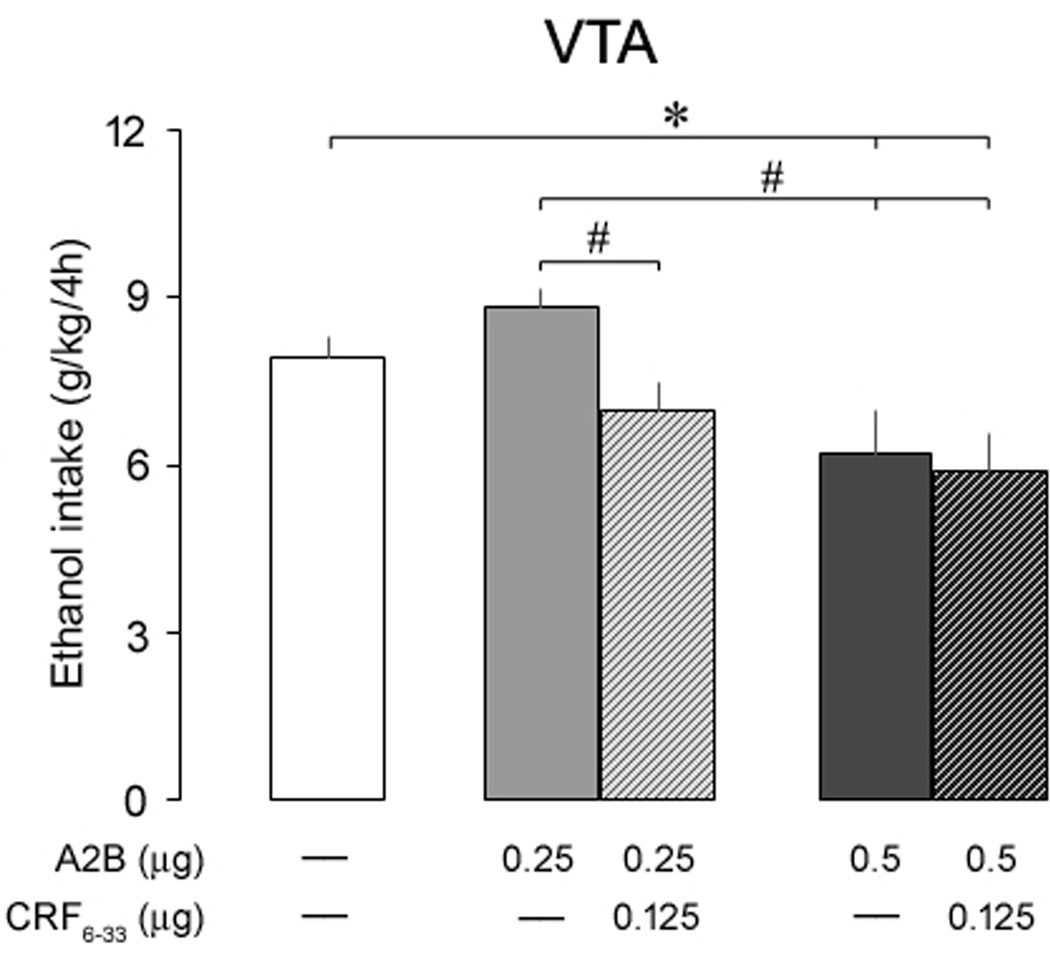
In order to test a potential role of CRFBP on CRF2 receptor activity in the VTA, mice were exposed to the two-bottle choice DID procedure, and received bilateral microinjections of aCSF, A2B (0.25 and 0.5 µg) or a combination of A2B (0.25 and 0.5 µg) and a sub-effective dose of CRF6–33 (0.125 µg), 10 min before the exposure to the 4-h DID test (Fig. 3). The one-way ANOVA revealed a significant main effect of the treatments (F(4, 43) = 4.89, p < 0.01) on ethanol consumption, and a post hoc analysis indicated that the higher dose of A2B (0.5 µg), regardless of CRF6–33, significantly attenuated binge-like ethanol intake in comparison to aCSF-treated animals. However, mice treated with 0.25 µg of A2B in combination with CRF6–33 presented a significant decrease in alcohol consumption in comparison to mice treated with 0.25 µg of A2B alone. In contrast, the treatment with aCSF, A2B, or a combination of A2B and CRF6–33 did not alter water intake in these animals (data not shown; F(4, 43) = 0.84, p > 0.05). There is no difference in the body weight of the animals between groups (F(4, 43) = 1.82, p > 0.05). These results indicate that VTA CRF2 receptors modulate ethanol binge drinking, and CRFBP may add to the anti-alcohol effect of A2B.
Fig. 3.
Ethanol consumption (g/kg/4h) in separate groups of mice exposed to the ethanol binge drinking procedure two-bottle choice Drinking-in-the-Dark, 10 min after intra-VTA infusions of aCSF, Astressin2-B (A2B, 0.25 and 0.5 µg) or a combination of A2B (0.25 and 0.5 µg) and CRF6–33 (0.125 µg). Control mice (white bar) were injected with aCSF. Data are mean ± SEM. *, versus aCSF; #, versus A2B 0.25 µg. P < 0.05, n = 8–15 mice per group.
Dependence-producing drinking
Diagrammatic representations of bilateral intra-VTA and -CeA injection sites are shown in Fig. 4B. Intra-VTA injections of CRF6–33 did not affect ethanol consumption in male C57BL/6J mice, as shown in Table 1. The two-way ANOVA with repeated measurements revealed a significant effect of time on cumulative ethanol intake (F(2, 48) = 213.76, p < 0.001), but failed to find a significant treatment effect (F(2, 48) = 0.09, p > 0.05), or an interaction effect (F(4, 48) = 0.72, p > 0.05). Similarly, the two-way ANOVA with repeated measurements revealed a significant effect of time on cumulative water intake (F(2, 48) = 38.17, p < 0.001), but failed to find a significant treatment effect (F(2, 48) = 0.38, p > 0.05), or an interaction effect (F(4, 48) = 0.08, p > 0.05).
Table 1.
Ethanol (g/kg) and water (ml) consumption in mice exposed to the intermittent access to alcohol protocol 2 h, 4 h and 24 h after infusion of aCSF or CRF6–33 (0.25 and 0.5 µg) into the ventral tegmental area (VTA) or central nucleus of the amygdala (CeA).
| VTA | CeA | |||||
|---|---|---|---|---|---|---|
| aCSF | 0.25 µg | 0.5 µg | aCSF | 0.25 µg | 0.5 µg | |
| Ethanol intake (g/kg) | ||||||
| 2h | 3.0 (0.22) | 4.1 (0.69) | 3.5 (0.38) | 3.3 (0.51) | 3.5 (0.40) | 2.8 (0.33) |
| 4h | 5.9 (0.42) | 6.3 (1.06) | 5.8 (0.54) | 6.1 (0.71) | 6.4 (0.37) | 6.3 (0.55) |
| 24 | 22.9 (1.44) | 20.6 (3.06) | 20.6 (2.46) | 21.3 (1.86) | 21.6 (1.68) | 19.8 (1.67) |
| Water intake (ml) | ||||||
| 2h | 0.3 (0.12) | 0.5 (0.10) | 0.7 (0.24) | 0.3 (0.08) | 0.3 (0.06) | 0.5 (0.20) |
| 4h | 0.8 (0.15) | 1.1 (0.26) | 1.2 (0.34) | 0.8 (0.15) | 0.8 (0.12) | 0.7 (0.14) |
| 24h | 1.9 (0.49) | 2.3 (0.50) | 2.4 (0.70) | 1.5 (0.29) | 1.7 (0.31) | 1.8 (0.34) |
Microinjections were performed in a design that counterbalanced vehicle and drug treatments. Each mice received three microinjections. Data shown as averages (± SEM), n = 9–16 mice per group.
Intra-CeA injections of CRF6–33 did not affect ethanol consumption either, as shown in Table 1. The two-way ANOVA with repeated measurements revealed a significant effect of time on cumulative ethanol intake (F(2, 88) = 361.38, p < 0.001), but failed to find a significant treatment effect (F(2, 88) = 0.40, p > 0.05), or an interaction effect (F(4, 88) = 0.21, p > 0.05). The two-way ANOVA with repeated measurements revealed also a significant effect of time on cumulative water intake (F(2, 90) = 52.08, p < 0.001), but failed to find a significant treatment effect (F(2, 90) = 0.21, p > 0.05), or an interaction effect (F(4, 90) = 0.51, p > 0.05).
Withdrawal measurements and BECs
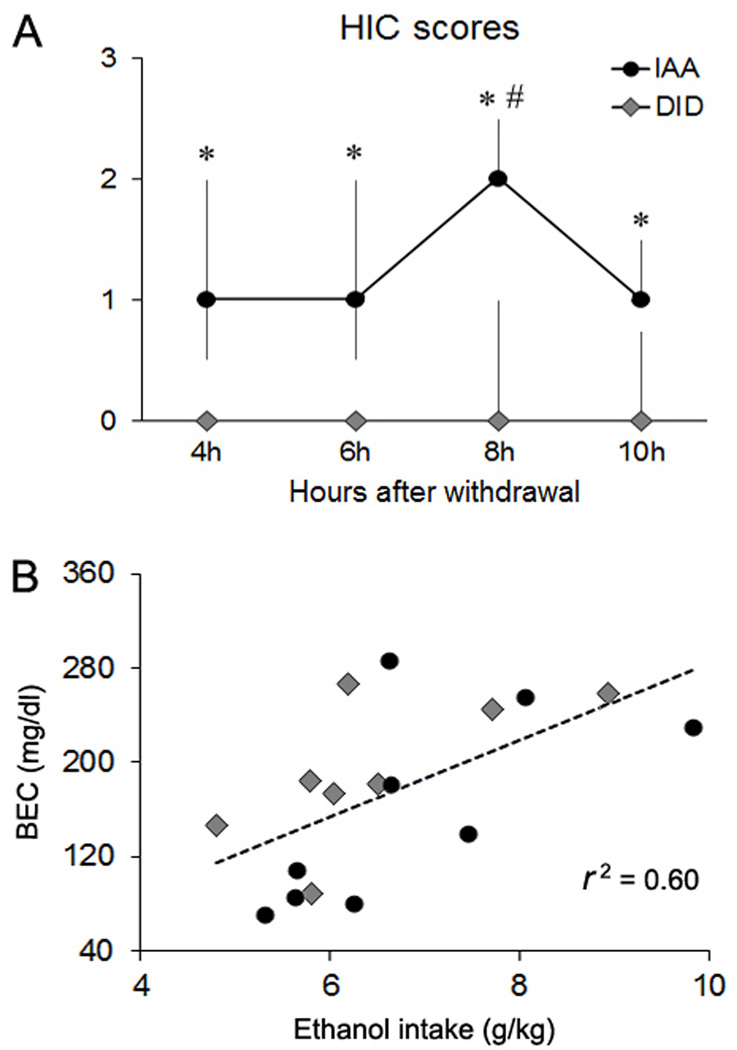
Severity of ethanol withdrawal was judged using the HIC rating scale described by Goldstein (1972) every 2 h after the ethanol bottles had been withdrawn for 4 h (4 h–10 h, Fig. 5A). A Kruskal–Wallis one-way ANOVA by ranks followed by Mann–Whitney rank sum tests indicated that IAA mice exhibited significant higher HIC reactions 8 h after withdrawal (H = 18.89, p < 0.01) compared to the other time points. A Mann–Whitney rank sum t-tests revealed that IAA animals presented higher HIC scores compared to DID mice at all time points (4h: T = 2.69; 6h: T = 2.45; 8h: T = 3.46; 10h: T = 2.79, p < 0.05 in all cases), indicating that the IAA procedure was able to increase seizure sensitivity, as index of ethanol withdrawal.
Fig. 5.
(A) Handling-induced convulsions (HIC) ratings of severity and (B) blood ethanol concentrations (BECs) exhibited by mice exposed to the ethanol binge drinking procedure Drinking-in-the-Dark (DID) or to the dependence-producing drinking procedure intermittent access to alcohol (IAA). HIC scores were taken every 2 h after ethanol withdrawal (4 h–10 h) and are shown as median and 1st to 3rd quartile range (error bars); *, versus DID; #, versus other time points. BECs are plotted as a function of ethanol intake and dotted line represents the linear fit (r2 = 0.60). P < 0.05, n = 8–9 mice per group.
Both DID and IAA protocols promoted similar pharmacologically relevant BECs (t = 0.92, p > 0.05). BECs in a subset of DID mice (n = 8) were 192.9 ± 21.38 mg/dl, ranging from 89.3 to 267.1 mg/dl after 4-h access to 20% ethanol. By comparison, BECs for IAA mice (n = 9) were 160.2 ± 27.25 mg/dl after 4-h access to two-bottle choice, ranging from 71.1 to 286.6 mg/dl. There was a significant linear relationship between ethanol intake and BEC (r2 = 0.60, p < 0.05, Fig. 5B).
DISCUSSION
Here, we present the first evidence that the CRFBP antagonist CRF6–33 and the CRF2 antagonist A2B microinjected into the VTA dose-dependently attenuated alcohol consumption in mice exposed to a binge drinking procedure (DID). Moreover, the co-infusion of CRF6–33 in a sub-effective dose potentiated the effect of the CRF2 antagonist in a lower dose. On the other hand, intra-VTA CRF6–33 did not affect alcohol consumption in mice exposed to a dependence-producing protocol of alcohol consumption (IAA). Intra-CeA CRF6–33 neither affected ethanol binge drinking nor escalated ethanol consumption in mice given IAA.
In order to investigate the role of the components of the CRF system in the modulation of alcohol binge drinking, we used the original DID protocol in the first set of experiments of the present study. This procedure promotes rapid intake of high levels of ethanol at pharmacologically relevant BECs (Rhodes et al., 2005). Adult C57BL/6J mice – a high ethanol drinking strain – exposed to DID exhibit signs of behavioral intoxication, such as reduced latency to fall during rotarod testing and increased frequency of foot-slips in a balance beam test, relative to water-drinking control mice (Rhodes et al., 2007). Recent variations of the original DID protocol include the use of an additional bottle of water during the 2 to 4-h test, giving the mice an alternate source of fluid, and the possibility of measuring concurrently water and alcohol consumption during the DID sessions (Giardino and Ryabinin, 2013). In this study, we implemented the two-bottle choice variation of the DID procedure to investigate the effect of co-infusions of CRFBP and CRF2 receptor antagonists into the VTA on ethanol/water intake.
In the last set of experiments, mice were exposed to a voluntary, preferential, dependence-producing drinking paradigm, IAA (Hwa et al., 2011). From a neurophysiological point of view, ethanol binge drinking is thought to be modulated by transient changes in brain systems as result of exposure to high BECs. Although these alterations may be initially transient, the neuroplastic changes may become permanent after repeated episodes of heavy drinking, contributing to the development of dependence (Cox et al., 2013; Cuzon Carlson et al., 2011; Wilcox et al., 2014). Evidence also suggests that brain regions controlling drug use extend from the VTA to subcortical and cortical areas (Koob and Volkow, 2010), and activation of dopamine neurotransmission has long been related to the reinforcing properties of ethanol and other drugs of abuse (Di Chiara and Imperato, 1985; 1988). Thus, repeated cycles of heavy drinking followed by periods of abstinence seem to be crucial to promote more compulsive drinking, and finally the development of alcohol dependence (Cuzon Carlson et al., 2011; Wilcox et al., 2014).
In agreement with this idea, adult C57BL/6J mice exposed to IAA escalate alcohol consumption and achieve high stable levels of ethanol drinking after three weeks, followed by initial signs of withdrawal (Hwa et al., 2011). In fact, intermittency appears to be a crucial feature of many conditions that promote ingestive behavior, including increasing amounts of alcohol (Avena et al., 2005; Falk and Tang, 1988; Sinclair and Senter, 1968). In the present study, two procedures of drinking promoted similarly high BECs after 4-h access to 20% ethanol. However, only mice exposed to IAA exhibited increased seizure susceptibility during withdrawal – i.e. higher HIC scores, as indices of convulsive events – compared to mice given DID. During long-term ethanol drinking, the depressant effects of alcohol on the central nervous system lead to compensatory excitatory mechanisms that emerge during withdrawal. Other symptoms include anxiety, delirium tremens, and insomnia (Saitz, 1998). According to the opponent-process theory of motivation (Solomon and Corbit, 1974), initial positive changes in mood such as euphoria produced by drugs of abuse decrease gradually following repeated exposure, while the dysphoric responses become more exacerbated.
The emergence of negative emotional states during acute withdrawal has been associated with dysregulation of neurochemical components involved in reward and stress, including increases in extracellular level of CRF in the CeA (Funk et al., 2006; Rassnick et al., 1993). This neuropeptide appears to be pivotal in the increased ethanol self-administration during withdrawal in dependent rats (Funk et al., 2006), and also plays a key role in excessive ethanol drinking in rats exposed to ethanol vapor, through activation of CRF1 receptors in the CeA (Roberto et al., 2010). Moreover, microinjections of a CRF1 antagonist into the CeA was shown to attenuate binge-like ethanol consumption in mice (Lowery-Gionta et al., 2012). In the VTA, however, the role of the CRF is less understood and most studies with CRF and ethanol consumption have focused on CRF1. These studies showed that increased CRF1 receptor-mediated signaling in the VTA promotes excessive ethanol consumption in mice (Hwa et al., 2013; Sparta et al., 2013) and rats (Hwa et al., 2013). Therefore, the present work extended the current comprehension of the CRF mechanisms involved on excessive ethanol consumption, focusing on the role of CRFBP in the VTA and CeA. Furthermore, we tested in vivo the hypothesis that CRFBP can modulate CRF signaling acting via CRF2 receptors (Ungless et al., 2003).
Early studies indicated that approximately 40–60% of the human CRF in brain is bound by CRFBP and support a role for CRFBP in limiting CRF and/or Urocortin-receptor activity (Behan et al., 1995b). However, the CRFBP may have different functions depending on the specific cell-type or context in which it is expressed. In the VTA, CRFBP was demonstrated to be expressed in a subset of dopaminergic neurons (Wang and Morales, 2008), and appears to be crucial in the modulation of neuronal activity (Ungless et al., 2003). In the present study, CRF6–33 microinjected into the VTA dose-dependently attenuated alcohol consumption in mice exposed to the DID procedure. These results corroborate the idea that the CRFBP actions in the VTA may be complex and extend beyond mere CRF or CRF-like ligands sequestration (Ungless et al., 2003). Accordingly, previous data showed that CRF6–33 produces central actions distinct from CRF-receptor agonists (Heinrichs et al., 1996; 1997; Ungless et al., 2003) and Fos expression in neurons that express the CRFBP itself, instead of CRF receptors (Chan et al., 2000), suggesting that CRFBP in the brain may act as an active participant in the CRF neurotransmission.
Based on a series of in vitro experiments using mice VTA, Ungless et al. (2003) proposed that CRF enhances postsynaptic N-methyl-D-aspartate (NMDA) receptor activity through a CRF2-phospholipase C (PLC)-protein kinase C (PKC) pathway. Interestingly, the activation of this pathway was demonstrated to require CRFBP. These findings were further supported by experiments showing that Urocortin, which binds to CRF2 and CRFBP with high affinity, is able to mimic the effects of CRF on NMDA receptor activity (Ungless et al., 2003). To test in vivo the hypothesis that the CRFBP can modulate the activity of CRF2 receptors within the VTA, we microinjected the CRF2 antagonist A2B alone, or in combination with a sub-effective dose of CRF6–33 (0.125 µg) in mice exposed to the two-bottle choice DID protocol. The higher dose of A2B (0.5 µg), regardless of CRF6–33, attenuated binge-like ethanol consumption in comparison to aCSF-treated animals. However, mice treated with A2B in a lower dose (0.25 µg) in combination with CRF6–33 drank less alcohol in comparison to mice treated with the CRF2 antagonist alone. These results demonstrate that, although the higher dose of A2B injected into the VTA is sufficient to attenuate ethanol binge drinking, the co-infusion of A2B in a lower dose in combination with a sub-effective dose of CRF6–33 exerts additive effects in attenuating ethanol binge drinking. This data support the idea that CRF may interact with CRFBP when signaling via CRF2 receptors.
Our data also show that intra-VTA CRF6–33 did not affect ethanol consumption in mice given IAA, indicating that CRFBP, and potentially the CRFBP/CRF2 receptor system, can be modified by ethanol exposure. Actually, previous studies have shown that alcohol exposure can alter CRF2 receptor levels (Weitemier and Ryabinin, 2005). Besides, the CRFBP antagonist microinjected into the CeA did not affect alcohol intake in both models of consumption used in the present study either, suggesting that the activity of CRF in the CeA may occur independently of CRFBP modulation, and probably via CRF1 receptors (Lowery-Gionta et al., 2012; Rassnick et al., 1993). Corroborating these results, CRFBP mRNA expression in the CeA, as well as in the basolateral and basomedial nuclei of amygdala, was shown to be unaltered after repeated cycles of DID (Ketchesin et al., 2014).
Further investigation is required to determine the mechanism through which CRFBP interacts with CRF2 receptors. One possible explanation is that CRFBP plays a permissive role and facilitates the binding of CRF to the CRF2 receptor (Ungless et al., 2003). Accordingly, it has been demonstrated that CRFBP and CRF receptors use a distinct molecular interaction to bind CRF (Cortright et al., 1995). Another possibility is that CRFBP may extend the CRF half-life, increasing the time that CRF spends in proximity to the CRF2 receptor. Gel filtration studies have shown that when CRF binds to CRFBP the complex forms a stable dimer (Woods et al., 1994). This change, associated with post-translational modifications, could signal specifically though CRF2 receptors (Kemp et al., 1998; Ungless et al., 2003).
Overall, the present results extend our current knowledge about the involvement of the CRF system on excessive ethanol intake, implicating the VTA CRFBP and CRF2 receptors in the first stages of escalated ethanol drinking. Moreover, CRFBP appear to affect the activation of CRF2 receptors in the VTA, possibly modulating NMDA receptors in dopaminergic neurons (Ungless et al., 2003). Excitatory glutamatergic projections in the VTA exert regulatory control of dopamine neurons and appear to be involved in short- and long-term changes in dopaminergic activity (Bonci and Malenka, 1999; Overton and Clark, 1997). Our findings corroborate the idea that transition to addiction involves different brain areas and neurocircuitries (Koob and Volkow, 2010), and ultimately, may open new perspectives of therapeutic intervention to prevent the development of alcohol-use disorders, and to treat other neuropsychiatric conditions associated with abnormal CRF levels.
ACKNOWLEDGMENTS
This research was supported by NIH grants AA0211622 (LS Hwa), AA013983 and DA031734 (KA Miczek) and CNPq–Brazil 202392/2012-0 (L Albrechet-Souza). The authors would like to thank Dr. Carolina Haass-Koffler for providing helpful comments during the development of this study.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84:359–362. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995a;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer's disease. Nature. 1995b;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Vale WW, Sawchenko PE. Paradoxical activational effects of a corticotropin-releasing factor-binding protein "ligand inhibitor" in rat brain. Neurosci. 2000;101:115–129. doi: 10.1016/s0306-4522(00)00322-5. [DOI] [PubMed] [Google Scholar]
- Cortright DN, Nicoletti A, Seasholtz AF. Molecular and biochemical characterization of the mouse brain corticotropin-releasing hormone-binding protein. Mol Cell Endocrinol. 1995;111:147–157. doi: 10.1016/0303-7207(95)03558-o. [DOI] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, Thiele TE. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013;37:1688–1695. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–132. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, Albaugh B, Hodgkinson CA, Goldman D. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One. 2008;3:e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL, Tang M. What schedule-induced polydipsia can tell us about alcoholism. Alcohol Clin Exp Res. 1988;12:577–585. doi: 10.1111/j.1530-0277.1988.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Ryabinin AE. CRF1 receptor signaling regulates food and fluid intake in the drinking-in-the-dark model of binge alcohol consumption. Alcohol Clin Exp Res. 2013;37:1161–1170. doi: 10.1111/acer.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB. Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther. 1972;180:203–215. [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Behan DP, Chan RK, Sawchenko PE, Lorang M, Ling N, Vale WW, De Souza EB. Corticotropin-releasing factor-binding protein ligand inhibitor blunts excessive weight gain in genetically obese Zucker rats and rats during nicotine withdrawal. Proc Natl Acad Sci USA. 1996;93:1547–1580. doi: 10.1073/pnas.93.26.15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Vale EA, Lapsansky J, Behan DP, McClure LV, Ling N, De Souza EB, Schulteis G. Enhancement of performance in multiple learning tasks by corticotropin-releasing factor-binding protein ligand inhibitors. Peptides. 1997;18:711–716. doi: 10.1016/s0196-9781(97)00120-4. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Debold JF, Miczek KA. Alcohol in excess: CRF1 receptors in the rat and mouse VTA and DRN. Psychopharmacology (Berl) 2013;225:313–327. doi: 10.1007/s00213-012-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kemp CF, Woods RJ, Lowry PJ. The corticotrophin-releasing factor-binding protein: an act of several parts. Peptides. 1998;19:1119–1128. doi: 10.1016/s0196-9781(98)00057-6. [DOI] [PubMed] [Google Scholar]
- Ketchesin KD, Stinnett GS, Seasholtz AF. Regulation of CRH, CRH receptor 1, and CRH-binding protein expression in the amygdala and paraventricular nucleus during binge drinking in mice. RSA/ISBRA. 2014 abstract. [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAA. NIAAA council approves definition of binge drinking. NIAAA Newslett Winter. 2004;3:3. [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Elsevier Academic; 2001. [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: Preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin Exp Res. 2011;35:166–174. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R. Introduction to alcohol withdrawal. Alcohol Health Res World. 1998;22:5–12. [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovács K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Hopf FW, Gibb SL, Cho SL, Stuber GD, Messing RO, Ron D, Bonci A. Binge ethanol-drinking potentiates corticotropin releasing factor R1 receptor activity in the ventral tegmental area. Alcohol Clin Exp Res. 2013;37:1680–1687. doi: 10.1111/acer.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SW, Behan DP, Lahrichi SL, Kaiser R, Corrigan A, Lowry P, Potter E, Perrin MH, Rivier J, Vale WW. Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology. 1995;136:1097–1102. doi: 10.1210/endo.136.3.7867564. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J Comp Neurol. 2008;509:302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Brain region-specific regulation of urocortin 1 innervation and corticotropin-releasing factor receptor type 2 binding by ethanol exposure. Alcohol Clin Exp Res. 2005;29:1610–1620. doi: 10.1097/01.alc.0000179363.44542.05. [DOI] [PubMed] [Google Scholar]
- Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci. 2006;11:1878–1891. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology. 2014;39:579–594. doi: 10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RJ, Kennedy KM, Gibbins JM, Behan D, Vale W, Lowry PJ. Corticotropin-releasing factor binding protein dimerizes after association with ligand. Endocrinology. 1994;135:768–773. doi: 10.1210/endo.135.2.8033825. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Heilig M, de Wit H, Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128:175–186. doi: 10.1016/j.drugalcdep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]