Abstract
Purpose
Determine if the histology of a breast malignancy influences the appearance of untreated osseous metastases on FDG PET/CT.
Methods
This retrospective study was performed under IRB waiver. Our Hospital Information System was screened for breast cancer patients who presented with osseous metastases, who underwent FDG PET/CT prior to systemic therapy or radiation from 2009–2012. Patients with invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), or mixed ductal/lobular (MDL) histology were included. Patients with history of other malignancies were excluded. PET/CT was evaluated, blinded to histology, to classify osseous metastases on a per patient basis as sclerotic, lytic, mixed lytic/sclerotic, or occult on CT, and record SUVmax for osseous metastases on PET.
Results
95 patients met inclusion criteria (74 IDC, 13 ILC, and 8 MDL). ILC osseous metastases were more commonly sclerotic and demonstrated lower SUVmax than IDC metastases. For all IDC and MDL patients with osseous metastases, at least one was FDG-avid. For ILC, all patients with lytic or mixed osseous metastases demonstrated at least one FDG-avid metastasis; however, only 3 of 7 patients with sclerotic osseous metastases were apparent on FDG PET.
Conclusions
The histologic subtype of breast cancer affects the appearance of untreated osseous metastases on FDG PET/CT. In particular, non-FDG-avid sclerotic osseous metastases were more common in patients with ILC, than in those with IDC. Breast cancer histology should be considered when interpreting non-FDG-avid sclerotic osseous lesions on PET/CT, which may be more suspicious for metastases (rather than benign lesions) in patients with ILC.
Keywords: breast cancer, ductal, lobular, PET/CT, osseous metastases
INTRODUCTION
Detection of osseous metastases by imaging is particularly important and can be challenging in patients with breast cancer. Bone is the most common site of distant metastasis in patients with breast cancer, as well as the first site of metastasis in 50% of patients [1]. Accurate assessment of bone metastases alters clinical management and may decrease patient morbidity [2, 3].
The most commonly employed strategies for the systemic staging of patients with breast cancer are 2-deoxy-2-(18F)fluoro-D-glucose (FDG) positron emission tomography (PET)/computed tomography (CT) or CT with technetium 99m (99mTc) methylene diphosphonate (MDP) bone scan. The choice between these two strategies is often determined by the perceived value of FDG PET and MDP bone scan for detecting osseous metastases. Multiple authors have concluded that FDG PET is more sensitive for the detection of lytic osseous metastases in patients with breast cancer, while MDP bone scan is more sensitive for the detection of sclerotic osseous metastases [4–6]. However, many patients in these studies had received prior systemic treatment, and effective systemic treatment results in both decreased FDG-avidity and increased sclerosis of osseous lesions [7–9]. Thus, non-avid sclerotic lesions may represent treated lesions, rather than active malignancy. In a study where patients with prior systemic treatment were excluded, FDG PET was more sensitive than MDP bone scan when including all patients, but still had lower visualization rate than MDP bone scan in detecting sclerotic bone metastases [10].
An important variable that has not been accounted for in these studies is the histology of the breast malignancy. Histology is determined at the time of breast cancer diagnosis, almost always prior to FDG PET/CT. Breast cancers are most commonly invasive ductal carcinomas (IDC), invasive lobular carcinomas (ILC), or mixed ductal and lobular (MDL) in histology, with IDC accounting for ~75–80% of primary breast malignancies and ILC accounting for ~10–15% [11–13]. Primary ILC lesions within the breast are more difficult to detect than IDC on imaging studies, including mammography, ultrasound, and MR [14, 15]. On FDG PET/CT, primary lobular breast cancers demonstrate lower FDG-avidity than comparable primary invasive ductal cancers [16–19], suggesting that patients with lobular breast cancers may be more apt to have osseous metastases that are less well visualized with FDG PET than ductal malignancies. Here we evaluate if the histology of a newly diagnosed breast malignancy influences the appearance of osseous metastases on FDG PET/CT.
MATERIALS AND METHODS
Breast cancer patients and histology
This HIPAA-compliant, retrospective study was performed under Institutional Review Board approval. Our Hospital Information System was screened for patients with breast cancer who presented with osseous metastases and underwent PET/CT prior to chemotherapy, hormonal therapy, or radiation from 2009 to 2012. Osseous metastases at presentation was defined as osseous lesions suspicious for metastases on any imaging studies prior to beginning therapy, and verification by histologic sampling or imaging followup as described below. Electronic medical records were used to define patients with IDC, ILC, or MDL histology. Patients with the following characteristics were excluded: Breast cancer histology other than IDC/ILC/MDL; those who received chemotherapy, hormonal therapy, or radiation therapy for the current breast malignancy; history of prior malignancy (except non-melanoma skin cancer); and synchronous second primary malignancy. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status was recorded for each patient as positive (+) or negative (−) according to the American Society of Clinical Oncology / College of American Pathologist guidelines [20]. Tumor grades were provided by a board certified pathologist with specialization in breast malignancies (AC).
FDG PET/CT imaging and analysis
All FDG PET/CTs were performed as an integrated study. FDG PET/CTs at our institution were performed on hybrid 18F-FDG PET/CT scanners, with acquisition of images from the mid skull to upper thigh approximately 60 minutes after intravenous administration of 12–15mCi of 18F-FDG. Patients fasted for six hours prior to imaging. Finger-stick blood glucose levels were less than 200mg/dL at time of injection. Of the total 95 FDG PET/CTs, 5 were performed at an outside institution. While the technique for FDG PET/CT acquisitions performed by outside institutions could not be controlled for, techniques and images were similar to those of our own studies. FDG PET, CT, and fused FDG PET/CT images were reviewed in multiplanar reconstructions on a GE AW Suite. All 18F-FDG PET/CT studies were reinterpreted by a radiologist (GU) with nine years of 18F-FDG PET/CT experience, blinded to histology, as well as other imaging and biopsy results.
Morphologic classification of osseous lesions
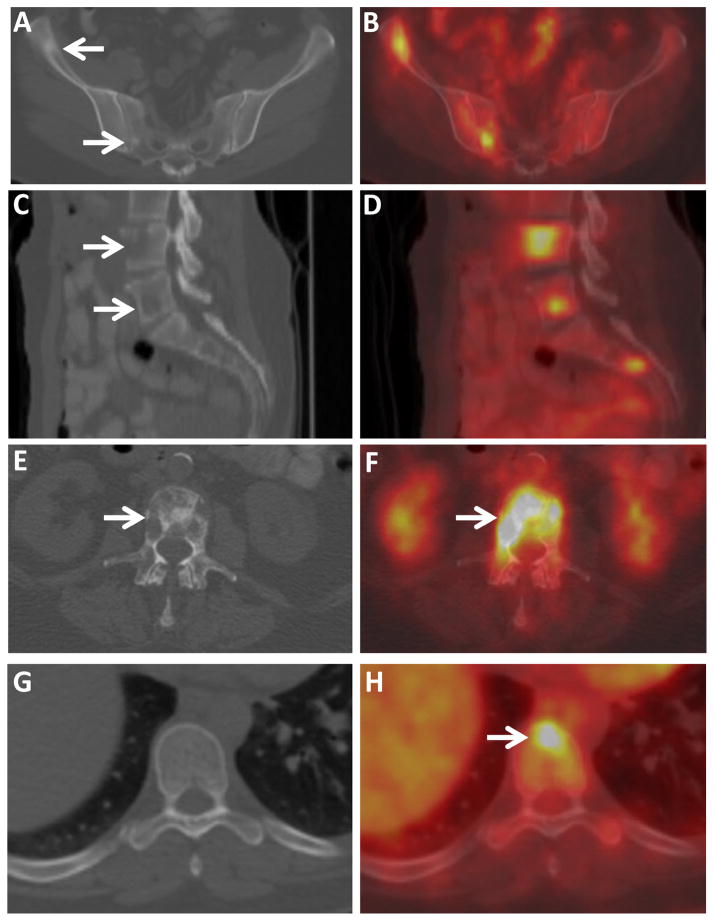
Osseous metastases were classified into morphologic subtypes based on their appearance on CT and FDG PET (Figure 1). High-attenuation osseous lesions on CT were classified as sclerotic (Figure 1), low-attenuation lucent osseous lesions on CT were classified as lytic, and osseous lesions with substantial sclerotic and lytic components on CT were classified as mixed lytic/sclerotic. FDG-avid osseous metastases without a correlate on CT were classified as CT occult.
Figure 1.
CT and PET/CT images of osseous metastases categorized by their CT morphology (sclerotic, lytic, mixed, and CT occult). Axial CT (A) and fused FDG PET/CT (B) images through the pelvis of a 41 year old woman with IDC demonstrate FDG-avid sclerotic osseous lesions (arrows). Sagittal CT (C) and fused FDG PET/CT (D) images of the lumbosacral spine of a 45 year old woman with IDC demonstrate several FDG-avid lytic osseous lesions (arrows). Axial CT (E) and fused FDG PET/CT (F) images through the lumbar spine of a 68 year old woman with ILC demonstrate a FDG-avid mixed sclerotic-lytic osseous lesion. Axial CT (G) and fused FDG PET/CT (H) images through the thoracic spine of a 41 year old woman with IDC demonstrate an FDG-avid focus in a vertebral body (arrow), without CT correlate, classified as a CT occult lesion. Osseous metastases in all four patients were confirmed by biopsy.
As many patients had osseous metastases of more than one morphologic subtype, patients were then categorized according to the morphologic subtypes of osseous metastases on a per patient basis. We categorized as follows: 1) Sclerotic: Patients with only sclerotic metastases or sclerotic and occult metastases; 2) Lytic: Patients with only lytic metastases or lytic and occult metastases; 3) Mixed: Patients with both lytic and sclerotic metastases, as well as patients with mixed lytic/sclerotic metastases; and 4) Occult: Patients with only occult metastases (evidence of osseous metastases on FDG PET but not CT).
Measurement of FDG avidity
Osseous metastases were deemed FDG-avid when they demonstrated FDG avidity greater than the local background, which was not attributable to a benign cause, such as trauma or degenerative changes. For each patient, the maximum SUV (SUVmax) for the most FDG-avid osseous metastasis was recorded, with the SUVmax representing the single voxel with the greatest SUV adjusted for body weight.
Verification of osseous metastases
Tissue sampling (biopsy) was the preferred method to verify osseous metastases. When histology was not available, follow-up imaging was used. When follow-up imaging was used, lesions had to show typical features of osseous metastases on initial imaging and demonstrate either progression or response to treatment on follow-up.
Statistics
Clinical and imaging characteristics were descriptively summarized using medians and ranges for continuous variables and frequencies and percentages for categorical variables. Summary statistics were provided for the total population and also broken down by histology. For all statistical tests, CT appearance was dichotomized into sclerotic versus all others, and comparisons were made only between IDC and ILC histologic cohorts. The sample size of MDL patients was too small to perform meaningful analyses. The detection rates between IDC and ILC overall and for sclerotic lesions were compared using Fisher’s exact test, and the SUVmax values between groups were compared using the Wilcoxon Rank Sum test. Fisher’s exact test was also used to assess the relationship between CT morphology and histology and between receptor status and CT appearance. The Kruskal Wallis test was used to assess the relationship between receptor status and SUVmax. For statistical tests, ER and HER2 were grouped as follows: 1) ER+/HER2−, 2) HER2+, and 3) Triple Negative (ER−, PR−, HER2−). Receptor status statistical tests were only performed for the IDC cohort due to a lack of receptor status variation within the ILC and MDL cohorts. All tests were two sided and p values less than 0.05 were considered statistically significant. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Ninety-five patients with breast cancer and osseous metastases at initial presentation met our inclusion and exclusion criteria, including no prior systemic or radiation therapy (Table 1). The histologic subtypes of breast cancer were IDC (n=74/95, 78%), ILC (n=13/95, 14%) and MDL (n=8/95, 8%). The median age was 53 years, with age ranging from 29 to 90 years. Sixty-one percent (58/95) had biopsy confirmation of osseous metastases; the remaining patients were confirmed by follow-up imaging.
Table 1.
Clinical and histopathologic characteristics of 95 untreated breast cancer patients with osseous metastases at presentation
| IDC | ILC | MDL | Total | |
|---|---|---|---|---|
|
| ||||
| Patients (N (%)) | 74 (78) | 13 (14) | 8 (8) | 95 |
|
| ||||
| Age (Median; Range) | 52; 29–90 | 59; 44–68 | 54; 37–62 | 53; 29–90 |
|
| ||||
| Biopsy proven (N (%)) | 44 (60) | 8 (62) | 6 (75) | 58 (61) |
|
| ||||
| Receptor Status (N (%)) | ||||
|
|
||||
| ER+/HER2− | 45 (61) | 12 (92) | 7 (88) | 64 (67) |
|
|
||||
| HER2+ | 16 (21) | 0 (0) | 0 (0) | 16 (21) |
|
|
||||
| Triple negative | 11 (15) | 1 (8) | 1 (12) | 13 (14) |
|
|
||||
| Indeterminate | 2 (3) | 0 (0) | 0 (0) | 2 (2) |
|
| ||||
| Tumor grade (N (%)) | ||||
|
| ||||
| 1 | 0 (0) | 3 (23) | 0 (0) | 3 (3) |
|
|
||||
| 2 | 29 (39) | 7 (54) | 3 (37) | 39 (41) |
|
|
||||
| 3 | 45 (61) | 3 (23) | 5 (63) | 53 (56) |
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; MDL, mixed ductal/lobular; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
Appearance of osseous metastases categorized by histologic subtype of breast cancer
For the 74 patients with IDC, the most common CT morphology of the osseous metastases was lytic (n = 29/74, 39%), followed by occult (n = 22/74, 30%), sclerotic (n = 14/74, 19%), and mixed (n = 9/74, 12%) (Table 2). Notably, all IDC patients with osseous metastases demonstrated at least one osseous metastasis that was FDG-avid, even among patients with osseous metastases classified as sclerotic (Figure 2). Thus, all 74 patients had osseous metastases that were apparent by FDG PET.
Table 2.
Number of patients with osseous metastases, number demonstrable on FDG PET, and SUVmax in 95 untreated breast cancer patients with osseous metastases at presentation. Patients are stratified by tumor histology and CT appearance of osseous metastases. Gray box highlights the ILC patients with sclerotic CT appearance, where only 3 of 7 patients had osseous metastases that were demonstrable on FDG PET.
| Patient CT Appearance | Ductal (IDC) | Lobular (ILC) | Mixed IDC + ILC (MDL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients with bone mets (N) | Patients with at least one bone met apparent on FDG PET (N, %) | SUVmax (median, range) | Patients with bone mets (N) | Patients with at least one bone met apparent on FDG PET (N, %) | SUVmax (median, range) | Patients with bone mets (N) | Patients with at least one bone met apparent on FDG PET (N, %) | SUVmax (median, range) | |
| Sclerotic | 14 | 14 (100) | 5.6 2.1 – 15.8 |
7 | 3 (43) | 2.0 1.6 – 7.2 |
0 | 0 (NA) | NA |
| Lytic | 29 | 29 (100) | 7.3 2.8 – 23.0 |
2 | 2 (100) | 3.5 2.8 – 4.2 |
1 | 1 (100) | 7.9 |
| Mixed | 9 | 9 (100) | 7.9 3.6–16.9 |
4 | 4 (100) | 9.5 3.4 – 12.4 |
4 | 4 (100) | 8.1 6.5 – 11.2 |
| Occult | 22 | 22 (100) | 6.1 2.1 – 22.5 |
0 | 0 (NA) | NA | 3 | 3 (100) | 5.8 4.0 – 7.7 |
| Total | 74 | 74 (100) | 6.6 2.1 – 23.0 |
13 | 9 (69) | 3.4 1.6 – 12.4 |
8 | 8 (100) | 6.5 4.0 – 11.2 |
Abbreviations: FDG, fluorodeoxyglucose; PET, positron emission tomography; SUVmax, maximum SUV; CT, computerized tomography; ILC, invasive lobular carcinoma; IDC, invasive ductal carcinoma; MDL, mixed ductal/lobular.
Figure 2.
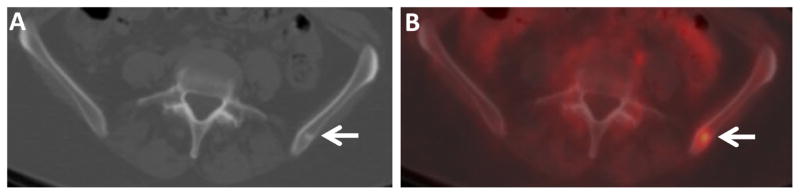
FDG-avid small sclerotic osseous metastasis in an IDC patient. Axial CT (A) and fused FDG PET/CT (B) images through the pelvis of a 56 year old woman with IDC demonstrate a small FDG-avid sclerotic osseous lesion in the left ilium (arrows). Similar small FDG-avid lesions were seen in the left scapula and left ischium (not shown). Osseous metastasis was confirmed by biopsy.
For the 8 patients with MDL, the most common CT morphology of the osseous metastases was mixed (n = 4/8, 50%), followed by occult (n = 3/8, 38%), and lytic (n = 1/8, 12%). There were no patients with MDL classified as sclerotic. All MDL patients with osseous metastases demonstrated at least one osseous metastasis that was FDG-avid. Thus, like IDC patients, all MDL patients had at least one osseous metastasis that was apparent on FDG PET.
For the 13 patients with ILC, the most common CT morphology of the osseous metastases was sclerotic (n = 7/13, 54%), followed by mixed (n = 4/13, 31%), and lytic (n = 2/13, 15%). There were no patients with ILC classified as having only CT occult osseous metastases. All ILC patients with mixed and lytic CT morphology of their osseous metastases demonstrated at least one osseous metastasis that was FDG-avid, and thus were apparent on FDG PET (6/6, 100%). However, only 3 of 7 patients with ILC (43%) classified as having sclerotic osseous metastases demonstrated an FDG-avid osseous metastasis. Thus, 4 of the 7 patients with ILC (57%) classified as having sclerotic osseous metastases had osseous metastases that were not apparent on FDG PET. Of these 4 patients, 3 were diagnosed as having non-FDG-avid osseous metastases by biopsy, while 1 was diagnosed with osseous metastases at follow-up imaging, when growth of the sclerotic osseous metastases was apparent (Figure 3). The ability of FDG PET to detect sclerotic osseous metastases in patients with IDC was significantly greater than the ability to detect such metastases in patients with ILC (p=0.006).
Figure 3.
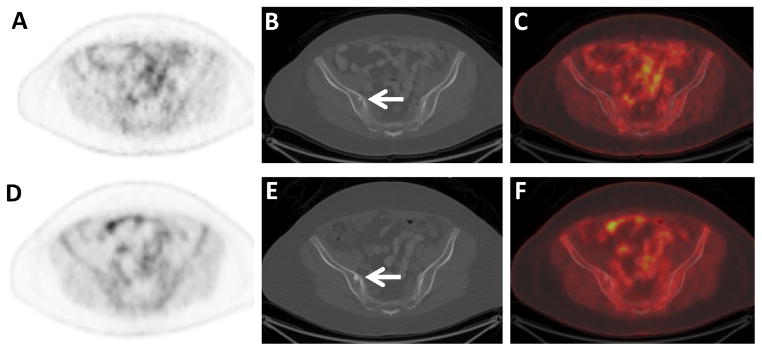
Non-FDG-avid sclerotic osseous metastases in an ILC patient. Axial FDG PET (A), CT (B), and fused FDG PET/CT (C) images through the pelvis of a 60 year old woman with ILC demonstrate a small non-FDG-avid sclerotic osseous lesion on CT (arrow). Additional small non-FDG-avid sclerotic osseous lesions were seen in the spine (not shown). Axial FDG PET (D), CT (E), and fused FDG PET/CT (F) images following adjuvant systemic therapy demonstrate increasing size of the osseous lesions, considered to be either flare response from treatment of osseous metastases or increasing metastases (arrow). Other sclerotic lesions had also increased in size (not shown).
The proportion of CT morphologies in patients with IDC versus ILC was significantly different, with the ILC cohort demonstrating a significantly higher proportion of patients classified as having sclerotic osseous metastases compared to IDC patients (7/13 vs. 14/74, p = 0.012).
Relationship between breast cancer histology and SUVmax of osseous metastases
The SUVmax for the patients with IDC (median 6.6, range: 2.1–23.0) was significantly higher than that of the patients with ILC (median 3.4, range 1.6–12.4, p = 0.008). This difference persisted when evaluating only patients with sclerotic osseous metastases. The SUVmax for IDC patients with sclerotic osseous metastases (median 5.6, range 2.1–15.8) was statistically greater than the SUVmax for ILC patients with sclerotic osseous metastases (median 2.0, range 1.6–7.2), p=0.019. When patients with sclerotic osseous metastases were excluded, the difference between SUVmax values according to histology (IDC median 6.9, range 2.1–23.0; ILC median 6.9, range 2.8–12.4) was not significant (p = 0.55). Though it could not be statistically tested, the SUVmax for the MDL patients appeared qualitatively similar to that of the IDC patients (median 6.5, range: 4.0–11.2).
Relationship between CT morphology and SUVmax of osseous metastases
SUVmax values were significantly lower for sclerotic lesions (median 4.2, range 1.6–15.8) when compared against all other lesions (median 6.9, range 2.1–23.0, p = 0.0003). Looking specifically within the IDC cohort, no difference was found between SUVmax for blastic lesions (median: 5.6, range: 2.1–15.8) versus all others (median: 6.9, range: 2.1–23.0, p=0.16); however, for the ILC cohort, the SUVmax for blastic lesions (median: 2.0, range: 1.6–7.2) was significantly lower compared to the other CT morphologies (median 6.9, range 2.8–12.4, p = 0.038).
Relationship between receptor status and SUVmax of osseous metastases
The majority of breast cancer patients with osseous metastases at initial presentation were ER+/HER2− (64/95, 67%), Table 1. While most IDC patients were ER+/HER2− (45/74, 61%), a substantial portion were HER2+ (16/74, 22%) or triple negative (11/74, 15%). Two IDC patients did not have adequate documentation of ER/HER2 status and were classified as indeterminate (3%). Within the IDC patients, no relationship was found between SUVmax and receptor status (p=0.48), nor between CT morphology and receptor status (p>0.95). Similar comparisons within the patients with ILC and MDL were not performed, as almost all patients with ILC and MDL were ER+/HER2− (Table 1).
Relationship between tumor grade and SUVmax of osseous metastases
Patients with IDC had a significantly higher proportion of grade 3 tumors as compared with patients with ILC patients (45/74 vs. 3/13, p=0.016). The SUVmax for grade 3 patients (median 6.1, range 2.2–22.5), was not found to significantly differ from the SUVmax for grade 1/2 patients (median 6.5, range: 1.6–23.0, p=0.96).
DISCUSSION
Previous reports suggest that FDG PET, while highly sensitive for detecting osseous metastases that are lytic on CT, has a lower detection rate for sclerotic osseous metastases [4–6, 10]. These reports considered all patients with breast cancer, independent of histologic subtype. Grouping all breast cancer patients together may be a result of the majority of breast malignancies being of a single histologic subtype: IDC. However, 20–25% of breast malignancies are histologic subtypes other than IDC, each with distinct molecular, pathologic, and imaging features. In particular, ILC, the second most common breast cancer histology, which accounts for 10–15% of primary breast malignancies [13], has very distinct imaging features [21], including the primary malignancy being less readily appreciable on FDG PET than IDC [16–19]. The question arises whether the histology of the primary breast cancer affects the appearance of osseous metastases on FDG PET/CT.
Our study suggests that histologic subtype does affect the appearance of osseous metastases on FDG PET/CT in untreated breast cancer patients. First, FDG-avidity of osseous metastases, as measured by SUVmax, was lower in patients with ILC than with IDC. Just as primary lobular breast cancers demonstrate lower FDG-avidity than comparable invasive ductal cancers [16–19], the osseous metastases from these malignancies follow a similar pattern. The lower FDG-avidity of ILC tumors may be explained by lower cellular density, proliferation rate, and number of GLUT transporters in this breast cancer histology than in the more common IDC [16, 18]. Second, osseous metastases were more commonly sclerotic on CT in patients with ILC as compared to IDC. Why there tends to be more attenuating bone in ILC osseous metastases, compared to IDC, is unclear. A possible explanation could be greater bone remodeling in ILC osseous metastases, owing to slower tumor growth or less dense packing of tumor cells, although this is only a hypothesis.
Distinguishing non-FDG-avid sclerotic osseous metastases from non-FDG-avid benign osseous lesions such as bone islands is of clinical importance. For example, the patient in Figure 2 demonstrates small sclerotic lesions that could be misinterpreted as benign were it not for the FDG-avidity seen on FDG PET. In this study, all IDC and MDL patients with sclerotic osseous metastases had appreciable metastases on FDG PET (14/14), while 4 out of 7 ILC patients with sclerotic osseous metastases did not have appreciable metastases on FDG PET. This difference was statistically significant (p=0.006). This is an important distinction, as knowledge of the histologic subtype of breast cancer can assist in the interpretation of non-FDG-avid sclerotic osseous lesions. When small non-FDG-avid sclerotic osseous lesions are seen on PET/CT of patients with untreated breast cancer, the histology of breast malignancy should be considered. For ILC patients, the propensity for sclerotic osseous metastases to be non-FDG-avid should be raised, and consideration given to performing a biopsy or obtaining follow-up imaging before excluding metastatic disease. For untreated IDC patients, our study suggests sclerotic osseous lesions, when none are FDG-avid, are probably benign. This distinction is relevant only for patients with breast cancer who have no received systemic treatment, since following systemic treatment, non-FDG-avid sclerotic lesions may represent treated metastases in patients with both ILC and IDC.
In this study we evaluated on a per patient, rather than a per lesion, basis. The clinically significant determination in newly diagnosed breast cancer is whether there are or are not osseous metastases, which will affect treatment algorithms far more than the number of osseous metastases. Indeed, in a single patient there may be populations of both FDG avid and non FDG-avid osseous metastases.
Our results indicate that the value of FDG PET may be greater for patients with IDC than with ILC, particularly when evaluating for osseous metastases. Of the 74 untreated IDC patients with osseous metastases, 30% (22/74) were occult on CT, and only detectable by PET. There were no untreated ILC patients with osseous metastases that were identified only on PET. Indeed, in 31% (4/13) of ILC patients with osseous metastases the metastases were occult on PET and identified only on the CT component of the PET/CT. Of course, FDG PET may still detect lesions outside of the bone [22–24], and FDG avidity may be used to predict survival in patients with metastatic breast cancer [25, 26].
It is difficult to establish a large cohort of breast cancer patients with FDG PET/CT performed prior to treatments that could alter the appearance of osseous metastases on FDG PET/CT. While still small, the size of our cohort is relatively large for our specific inclusion requirements. In addition, the large proportion of patients with biopsy proof of osseous malignancy is a strength of this analysis.
Although tumor grade could be proposed as a factor that could influence FDG avidity, our analysis did not find a difference in SUVmax of osseous metastases between grade 1/2 tumors and grade 3 tumors (p=0.96). This is similar to a prior study found which failed to find a significant correlation between breast cancer tumor grade and FDG-avidity of the primary breast malignancy [19].
Limitations of this study include its retrospective design, which introduces inherent biases. Additionally, some subgroups were small, especially the MDL subgroup (n = 8). We could not perform meaningful analyses on this subgroup. We also had only 7 cases of sclerotic metastases in ILC and 14 in IDC.
CONCLUSION
Our results demonstrate that the histologic subtype of breast cancer is significantly associated with the appearance of untreated osseous metastases on FDG PET/CT. Untreated osseous metastases were more often sclerotic in patients with ILC than with IDC. Osseous metastases of patients with ILC demonstrated lower FDG-avidity than those of IDC patients and non-FDG-avid sclerotic osseous metastases were significantly more common in untreated patients with ILC, than with IDC. Since the histology of breast cancer is usually known at the time of diagnosis, this information should be considered when interpreting osseous lesions on PET/CT. The value of FDG PET may be greater for patients with IDC than with ILC, particularly when evaluating for osseous metastases.
Acknowledgments
Financial Support: Susan G. Komen for the Cure Career Catalyst Research Grant KG110441 (GAU). We acknowledge the support of the MSKCC Biostatistics Core (P30 CA008748).
We acknowledge the Susan G. Komen for the Cure Career Catalyst Research Grant KG110441 (GAU and MG) for financial support. We acknowledge the support of the MSKCC Biostatistics Core (P30 CA008748). We thank both Jane Howard and Jonathan Wills for patient database assistance.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
This study was partially funded by Susan G. Komen for the Cure Career Catalyst Research Grant KG110441 (GAU and MG). The authors declare they have no other conflicts of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–53. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN, Theriault RL, Lipton A, Porter L, Blayney D, Sinoff C, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. J Clin Oncol. 1998;16:2038–44. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 3.Rubens RD. Bone metastases--the clinical problem. Eur J Cancer. 1998;34:210–3. doi: 10.1016/s0959-8049(97)10128-9. [DOI] [PubMed] [Google Scholar]
- 4.Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–9. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 5.Huyge V, Garcia C, Vanderstappen A, Alexiou J, Gil T, Flamen P. Progressive osteoblastic bone metastases in breast cancer negative on FDG-PET. Clin Nucl Med. 2009;34:417–20. doi: 10.1097/RLU.0b013e3181a7d03c. [DOI] [PubMed] [Google Scholar]
- 6.Uematsu T, Yuen S, Yukisawa S, Aramaki T, Morimoto N, Endo M, et al. Comparison of FDG PET and SPECT for detection of bone metastases in breast cancer. AJR Am J Roentgenol. 2005;184:1266–73. doi: 10.2214/ajr.184.4.01841266. [DOI] [PubMed] [Google Scholar]
- 7.Israel O, Goldberg A, Nachtigal A, Militianu D, Bar-Shalom R, Keidar Z, et al. FDG-PET and CT patterns of bone metastases and their relationship to previously administered anti-cancer therapy. Eur J Nucl Med Mol Imaging. 2006;33:1280–4. doi: 10.1007/s00259-006-0141-3. [DOI] [PubMed] [Google Scholar]
- 8.Du Y, Cullum I, Illidge TM, Ell PJ. Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25:3440–7. doi: 10.1200/JCO.2007.11.2854. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Macapinlac HA. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247:189–96. doi: 10.1148/radiol.2471070567. [DOI] [PubMed] [Google Scholar]
- 10.Nakai T, Okuyama C, Kubota T, Yamada K, Ushijima Y, Taniike K, et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging. 2005;32:1253–8. doi: 10.1007/s00259-005-1842-8. [DOI] [PubMed] [Google Scholar]
- 11.Tulinius H, Bjarnason O, Sigvaldason H, Bjarnadottir G, Olafsdottir G. Tumours in Iceland. 10. Malignant tumours of the female breast. A histological classification, laterality, survival and epidemiological considerations. APMIS. 1988;96:229–38. doi: 10.1111/j.1699-0463.1988.tb05296.x. [DOI] [PubMed] [Google Scholar]
- 12.Caldarella A, Buzzoni C, Crocetti E, Bianchi S, Vezzosi V, Apicella P, et al. Invasive breast cancer: a significant correlation between histological types and molecular subgroups. J Cancer Res Clin Oncol. 2013;139:617–23. doi: 10.1007/s00432-012-1365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–52. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez JK, Bassett LW. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics. 2009;29:165–76. doi: 10.1148/rg.291085100. [DOI] [PubMed] [Google Scholar]
- 15.Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–49. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 16.Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495–502. doi: 10.1200/JCO.2000.18.20.3495. [DOI] [PubMed] [Google Scholar]
- 17.Avril N, Menzel M, Dose J, Schelling M, Weber W, Janicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16. [PubMed] [Google Scholar]
- 18.Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–87. doi: 10.1200/JCO.2002.20.2.379. [DOI] [PubMed] [Google Scholar]
- 19.Buck A, Schirrmeister H, Kuhn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29:1317–23. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 21.He H, Gonzalez A, Robinson E, Yang WT. Distant metastatic disease manifestations in infiltrating lobular carcinoma of the breast. AJR Am J Roentgenol. 2014;202:1140–8. doi: 10.2214/AJR.13.11156. [DOI] [PubMed] [Google Scholar]
- 22.Morris PG, Lynch C, Feeney JN, Patil S, Howard J, Larson SM, et al. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol. 2010;28:3154–9. doi: 10.1200/JCO.2009.27.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuster D, Duch J, Paredes P, Velasco M, Munoz M, Santamaria G, et al. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol. 2008;26:4746–51. doi: 10.1200/JCO.2008.17.1496. [DOI] [PubMed] [Google Scholar]
- 24.Groheux D, Giacchetti S, Espie M, Vercellino L, Hamy AS, Delord M, et al. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011;52:1526–34. doi: 10.2967/jnumed.111.093864. [DOI] [PubMed] [Google Scholar]
- 25.Ulaner GA, Eaton A, Morris PG, Lilienstein J, Jhaveri K, Patil S, et al. Prognostic value of quantitative fluorodeoxyglucose measurements in newly diagnosed metastatic breast cancer. Cancer Med. 2013;2:725–33. doi: 10.1002/cam4.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris PG, Ulaner GA, Eaton A, Fazio M, Jhaveri K, Patil S, et al. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer. 2012;118:5454–62. doi: 10.1002/cncr.27579. [DOI] [PubMed] [Google Scholar]