Abstract
Goals
We sought to determine whether a quantitative neurocheck biomarker could characterize the temporal pattern of early neurologic changes after intracerebral hemorrhage (ICH), and the impact of those changes on long-term functional outcomes.
Methods
We enrolled cases of spontaneous ICH in a prospective observational study. Patients underwent a baseline Glasgow Coma Scale (GCS) assessment, then hourly neurochecks utilizing the GCS in a neuroscience intensive care unit. We identified a period of heightened neurologic instability by analyzing the average hourly rate of GCS change over 5 days from symptom onset. We used a multivariate regression model to test whether those early GCS score changes were independently associated with 3 month outcome measured by the modified Rankin Scale (mRS).
Results
We studied 13,025 hours of monitoring from 132 cases. The average rate of neurologic change declined from 1.0 GCS points per hour initially to a stable baseline of 0.1 GCS points per hour beyond 12 hours from symptom onset (p<0.05 for time intervals before 12 hours). Change in GCS score within the initial 12 hours was an independent predictor of mRS at three months (odds ratio 0.81 [95% confidence interval 0.66–0.99], p=0.043) after adjustment for age, hematoma volume, hematoma location, initial GCS, and intraventricular hemorrhage.
Conclusions
Neurochecks are effective at detecting clinically important neurologic changes in the intensive care unit setting that are relevant to patients’ long-term outcomes. The initial 12 hours is a period of frequent and prognostically important neurologic changes in patients with ICH.
Introduction
Detecting and treating neurologic complications is an important component of critical care for severe neurologic and neurosurgical conditions. Neurochecks, which are brief neurologic assessments performed repeatedly to monitor neurologic status, are a key component of neurocritical care practice for ischemic and hemorrhagic stroke, and may contribute to the superior outcomes associated with the care of neurologic conditions in neuro-specialized units.(1–4) Despite their widespread use, there is very little research to validate the use of neurochecks as a monitoring modality and biomarker of brain function, although some data indicate they affect major care decisions.(5) Optimizing the practice of neurologic monitoring in the intensive care unit first requires validating the efficacy of a modality, then comparing different approaches. We sought to establish the practice of neurologic monitoring with neurochecks by evaluating two biomarker characteristics: the ability to detect real-time changes in physiologic function, and confirmation that the changes detected were clinically meaningful.
We chose the Glasgow Coma Scale (GCS) as our model neurocheck because of its familiarity, favorable inter-rater reliability, widespread use, and its adoption by the American Heart Association/American Stroke Association and the Joint Commission as a core data element for patients with brain hemorrhage.(6) We chose intracerebral hemorrhage (ICH) as a model disease for this study because it is known to be a dynamic process of brain injury with a high rate of early neurologic deterioration.(7, 8) Complications like hematoma growth and delayed intraventricular hemorrhage are common early in the disease course, are associated with worse long term outcomes, and may be amenable to emergent interventions.(9, 10) The objective of this study was to determine whether the GCS was useful as a monitoring biomarker to detect early neurologic changes after intracerebral hemorrhage, and to test whether the early neurologic changes detected were relevant to long-term functional outcomes.
Materials and Methods
Patients presenting to Northwestern Memorial Hospital with spontaneous ICH between January 2010 and August 2013 were prospectively enrolled in an observational cohort study. All cases were diagnosed by a board-certified vascular neurologist or neurointensivist utilizing CT and/or MR imaging. Patients with ICH attributed to trauma, hemorrhagic conversion of ischemic stroke, structural lesions or vascular malformations were excluded. All patients were admitted to a neuro/spine-intensive care unit (NSICU) with a standard order set in the electronic order entry system. The GCS score was prospectively recorded at the time of initial evaluation by a trained neurologist and/or neurosurgeon.
Demographic information, medical history, medication history, standardized clinical instruments (GCS, pre-ICH modified Rankin Scale [mRS]), pretreatment blood pressure, laboratory data, imaging data, medical management variables, surgical interventions and medical complications were prospectively recorded. A certified examiner independent of the primary clinical team recorded the mRS at 14 days or discharge, whichever came first, and ascertained the mRS prospectively at 28 days and 3 months with a validated questionnaire.(11, 12) Hematoma volumes were measured on industry standard DICOM images from both referring hospitals and ours using Analyze software (Mayo Clinic, Rochester, MN) with a semiautomated process, a technique with high reliability that has been used as an endpoint in other ICH studies, as we have previously described.(13)
Neurologic Examination Surveillance (Neurocheck Biomarker
The NSICU was staffed with registered nurses with additional training and certification in neuroscience nursing, and structured, specific training in the administration of standardized neurological examination instruments, including the GCS. Our NSICU surveillance protocol, as previously reported in detail, included serial neurologic examinations (neurochecks) using the complete GCS performed by a neuroscience nurse on admission to the NSICU and hourly thereafter throughout the entire stay in the intensive care unit.(5) Beginning in December 2009, the neurocheck results were populated into the electronic medical record in real time with other standard vital signs. To emphasize the requirement for complete and independent assessments, copying forward of assessments entered by nurses from a prior shift is not allowed in the electronic medical record.
Analyses
For this analysis, we utilized the GCS neurochecks as our model for a simple neurocheck biomarker, given its widespread use in characterizing initial clinical severity in patients with acute intracranial hemorrhages. We queried all available GCS assessments and evaluated each patient for a change between the initial admission GCS and each subsequent hourly neurochecks performed in the NSICU for every hourly interval with available data between symptom onset and 168 hours (5 days) from onset.
Identifying a Period of Heightened Neurologic Instability
To test whether the neurologic changes detected were related to outcomes, we needed to select an observation interval. We sought to empirically identify a hyperacute period of neurologic fluctuations to define a meaningful observation interval. The goal of the first analysis was to determine whether the incidence of neurologic changes varied by time in such as way that an early period of increased instability could be identified. We defined time zero as the time of symptom onset, which was determined by patient interview, surrogate reporting, emergency medical services reporting, and any other reliable source of collateral information. We defined a neurologic change as any difference between the current hour GCS and the most recently previously recorded GCS. Thus, patients were not included in the analysis until they had undergone at least two GCS assessments. To account for different delays in hospital arrival and NSICU admission, and different lengths of ICU stay, we calculated a normalized rate of neurologic change during each time interval by dividing by the number of hours of monitoring completed within the time interval. We used the result of this analysis to identify the period of greatest symptomatic fluctuation by identifying the time point when the detected rate of neurologic change was no longer significantly greater than for subsequent time periods.
Assessing the Clinical Impact of Early Neurologic Changes
To address the main hypothesis that the GCS changes were markers of change relevant to patient outcomes, we calculated the total change in GCS score observed for each patient over the early instability period identified in the first analysis (which we found to be 12 hours). Thus, the early neurologic change was the GCS score at 12 hours after symptom onset minus the GCS score at the time of initial assessment. Patients who presented late and did not undergo at least two GCS assessments within 12 hours of symptom onset were necessarily excluded for lack of data. We evaluated the early change in GCS alongside the five variables that comprise the ICH Score (initial GCS, hematoma volume, age, infratentorial versus supratentorial hemorrhage location, and presence of intraventricular hemorrhage) as independent variables in an ordinal regression model, using the modified Rankin Scale (mRS) at three months as the dependent variable, to determine whether the early change in GCS was independently associated with long term outcomes. The ICH Score is a widely used measure of ICH severity based on admission characteristics that has been validated as a predictor of early mortality and long term outcomes after ICH.(14, 15) We used an ordinal regression model to analyze the mRS because of its superior sensitivity compared to dichotomized outcomes.(16) The test of parallel lines was used to confirm that the model conformed to the proportional odds assumption for a valid ordinal regression model. We also tested for associations between potential severity confounders (ICH Score and initial GCS), inclusion into the study cohort (by having at least two GCS measurements within the 12 hour initial period) using with Wilxocon rank sum test and duration of ICU monitoring using Spearman’s rho.
The study was approved by the Institutional Review Board (IRB). Written informed consent was obtained from the patient or their legally authorized representative. The IRB approved a waiver of consent for patients who died during initial hospitalization, or who were incapacitated and for whom a legal representative could not be located.
Results
Early Instability Period Identified
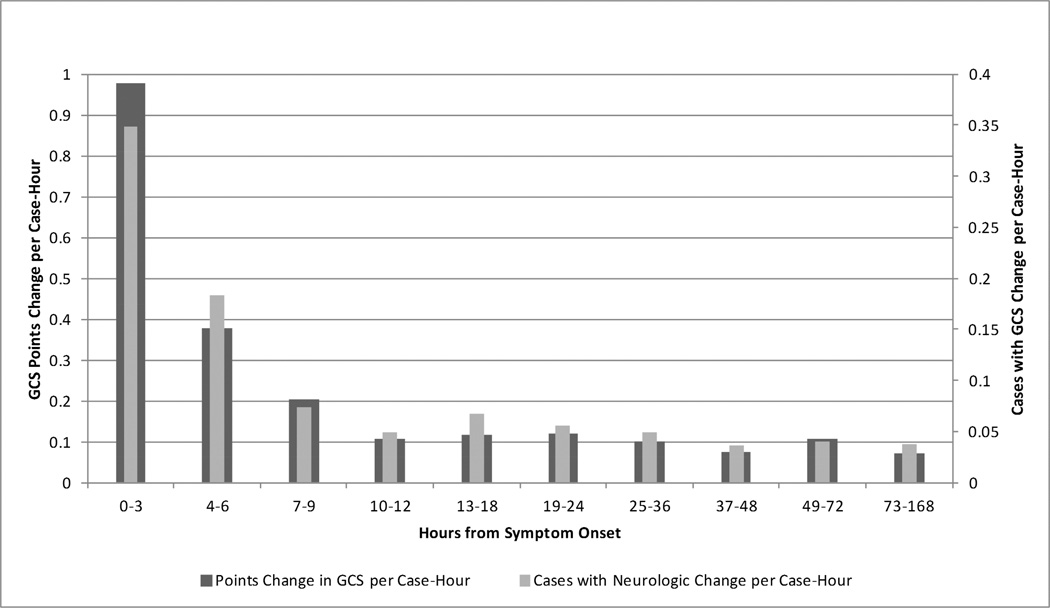
There were 132 patients in the cohort with 13,025 hours of monitoring available for analysis. Basic demographic and clinical characteristics are shown in Table 1. The rate of GCS changes detected per hour of monitoring and the number of cases found to have GCS changes per hour of monitoring are summarized graphically in Figure 1. The rate of clinical exam change detected by GCS was initially 1.0 GCS points per hour of monitoring, and diminished rapidly over the first hours to a baseline of approximately 0.1 GCS points per hour beyond 12 hours from symptom onset. From a case-based perspective, the rate of neurologic change was 0.35 cases with GCS changes per hour monitored within the first three hours, settling to a rate of 0.04 cases with neurologic change detected per hour beyond 12 hours. There was no significant difference in the rate of neurologic changes detected in the intervals beyond 12 hours, whereas the rate of change detection diminished significantly over every three hour interval in the first 12 hours (0– 3 hr vs 3–6 hr, 3–6 hr vs 6–9 hr, and 6–9 hr vs 9–12 hr all p<0.05). Thus, we defined the early instability period of ICH for this analysis as the time between symptom onset and 12 hours after onset.
Table 1.
Patient Demographic and Clinical Variables
| Number of patients | 132 |
|---|---|
| Gender (n male, %) | 70 (53.0%) |
| Age (years [mean, SD]) | 64 ± 14 |
| Race/Ethnicity (n, %) black | 62 (47.0%) |
| non-Hispanic white | 55 (41.7%) |
| Hispanic | 13 (9.8%) |
| other | 2 (1.5%) |
| Initial Glasgow Coma Scale (median, IQR) | 14 [9–15] |
| Initial hematoma volume (median, IQR) | 10.0 [4.0–24.9] |
| Intraventricular hemorrhage (n, %) | 56 (42.4%) |
| ICH Score (median, IQR) | 1 [0–3] |
| GCS change over first 12 hours (median, IQR) | 0 [−1–1] |
| mRS at discharge | 5 [4–5] |
| mRS at 3 months | 4 [2–6] |
SD: standard deviation, IQR: interquartile range, mRS: modified Rankin Scale score
Figure 1. Neurologic Change by Time from Symptom Onset.
The rate of neurologic change detection is shown by two different metrics in Figure 1. First, the rate of GCS point changes identified per case-hour of monitoring is shown, and second, the number of cases where a neurologic change is identified per case hour is displayed.
Clinical Impact of GCS Changes During the Early Instability Period
Some of the 132 patients arrived late, were transferred from other hospitals, or remained in the emergency department where neurochecks were not electronically recorded. There was an admission GCS and at least one follow up GCS assessment performed on 92 of the 132 patients (70.0%) within the 12 hour early instability period. These 92 patients were used to analyze the impact of early neurologic changes on outcome. We explored the possibility that there was a difference in important disease characteristics between the patient in our primary analysis and the patients without at least two data points during the initial 12 hours. There was no difference in baseline severity by ICH Score or initial GCS between the 92 patients who had at least two GCS measures within the first 12 hours and were included in the outcome analysis versus the 40 patients who were admitted late and therefore did not. Furthermore, there was no significant association between initial severity and the duration of ICU monitoring up through the first five days due to late arrival or early ICU discharge (all p>0.05).
No change was observed during the early instability period in 43 (47%) patients, 24 (26%) improved from baseline and 25 (27%) worsened. When the early change in GCS score was included in a multivariate model with the other five ICH Score variables, early neurologic change was found to be an independent predictor of functional outcomes at three months as measured by the modified Rankin Scale (adjusted odds ratio 0.81 [95% confidence interval 0.66–0.99], p=0.043). The results of the multivariate model are summarized in Table 2.
Table 2.
Clinical Factors Independently Associated with 3 Month Function Outcome by Modified Rankin Scale
| Variable | Odds Ratio [95% Confidence Interval] |
P-value |
|---|---|---|
| Hematoma volume | 1.01 [0.99–1.03] | 0.47 |
| Initial GCS | 0.70 [0.59–0.82] | <0.001 |
| GCS change over first 12 hours | 0.81 [0.66–0.99] | 0.043 |
| Age | 1.03 [1.00–1.07] | 0.085 |
| Intraventricular hemorrhage | 0.80 [0.27–2.33] | 0.68 |
| Infratentorial location | 0.33 [0.06–1.89] | 0.22 |
GCS: Glasgow Coma Scale.
Discussion
Recent research has shown that neuromonitoring with serial neurologic exams and neuroimaging in the ICU setting meaningfully impacts the care of patients with ICH once the period of initial emergency department evaluation has been completed.(5) These efforts are resource intensive, and optimizing our monitoring efforts requires knowing when the risk of neurologic change is greatest. In this study, we utilized trained critical care nurses to administer and record the GCS hourly on patients with acute ICH and found that the first 12 hours after symptom onset defines a period of significantly greater neurologic instability. Further, the neurologic changes that occur in those first hours are strongly associated with long term outcomes measured by the mRS. Thus, we demonstrate that simple bedside neurochecks are a monitoring technique that detects meaningful clinical changes relevant to functional outcomes.
There are few data describing early neurologic changes after severe neurologic injuries, regardless of how the early period is defined or described. Again using ICH as a representative example, while several studies have reporting some objective measure of early neurologic change or deterioration after ICH, only two described patient outcomes. One study of 46 patients reported a higher case fatality in those with neurologic deterioration, but did not assess whether the effect was independent of other admission variables, given that, for example, initial hematoma volumes were nearly triple in size among patients who deteriorated.(17) Another series of 61 cases reported that discharge scores on a modified GCS were worse for patients with neurologic deterioration, but likewise did not assess whether deterioration was independently associated with outcomes.(18) There is scant data to characterize the early course of most other acute neurologic conditions as well, likely hampered by the ability to capture frequent, quantified assessments of neurologic function. Likewise, there is little if any previously published data on the role of early improvement, although our data supports the intuitive conclusion a favorable response to early resuscitation is important and may have an enduring impact on outcome.
Despite the novelty of the data presented here, there are limitations worth noting. Confirming that the changes we detected meaningfully associated with functional outcomes required that we chose an observation interval. We attempted to make the time interval selection as objective as possible by studying the rates of neurologic fluctuation and choosing the interval of clear early instability. By using the total interval change within the early period as our analysis variable, fluctuations within the early period, like symptomatic hydrocephalus improved by ventriculostomy, would not be captured. Neurochecks performed in the emergency department were not recorded, which limits our ability to do such an analysis. Our approach, however, has the benefit of including less GCS change “noise” due to single point fluctuations that average out with a cross-sectional 12 hour approach. Our results compare accurately with other recent studies. For example, using the INTERACT2 definition of neurologic deterioration as a decline of 2 or more points on the GCS, our results are similar to the recently reported INTERACT2 trial (16.3% in our cohort within 12 hours, 14.8% in INTERACT2 within 24 hours), especially in light of the fact that the large majority of change takes place early in the instability period.(19) Finally, while the GCS has high inter-rater reliability and is thus well suited for a proof-of-concept study such as this, it is very limited in scope. In practice, more comprehensive neurochecks are needed to avoid missing important neurologic changes. At present, it is unknown what individual neurologic exam elements and techniques most reliably detect relevant changes when applied as a monitoring intervention in the intensive care setting.
Conclusions
In conclusion, our data showed that the first 12 hours after onset of ICH is a time of rapid changes that significantly impact patients’ long term functional outcomes. With respect to our understanding of ICH, these findings refine the ability of practicing clinicians to offer prognostic information to decision makers during the relevant timeframe, help clinical researchers select appropriate time-based enrollment criteria for ICH trials, and inform healthcare leaders about the optimal allocation of resource-intensive neuromonitoring efforts. More importantly, we have demonstrated that brief and reliable neurochecks are a practical biomarker of acute brain function and useful for monitoring patients with neurologic conditions requiring critical care. Neurochecks that use a quantitative scale are particularly appealing since they represent changes in the therapeutic endpoint (brain function) rather than changes in a surrogate marker of injury. However, the unfavorable aspect of the GCS’s simplicity is that it is a limited instrument for assessing neurologic function. Given the importance of early symptom changes on outcomes, further investigation should focus on identifying an optimal method of performing neurochecks and explore other modalities of neuromonitoring that can be validated against neurochecks. Incorporating intensive neuromonitoring throughout the early care period and defining time metrics for condition-specific interventions may lead to improved outcomes for patients with unstable neurologic injuries.
Acknowledgments
Funding:
Dr. Maas receives support from the National Institute of Neurologic Disorders and Stroke, US National Institutes of Health grant L30 NS080176 and the Northwestern Memorial Foundation. The infrastructure for automated data retrieval was funded in part by National Institutes of Health through a grant to Northwestern University’s Clinical and Translational Sciences UL1RR025741.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Kapinos G, Hemphill JC., 3rd Clinicoradiologic acute monitoring after intracerebral hemorrhage: Toward standards? Neurology. 2013;81:102–103. doi: 10.1212/WNL.0b013e31829a3564. [DOI] [PubMed] [Google Scholar]
- 2.Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635–640. doi: 10.1097/00003246-200103000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Berman MF, Solomon RA, Mayer SA, Johnston SC, Yung PP. Impact of hospital-related factors on outcome after treatment of cerebral aneurysms. Stroke. 2003;34:2200–2207. doi: 10.1161/01.STR.0000086528.32334.06. [DOI] [PubMed] [Google Scholar]
- 4.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373–380. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology. 2013;81:107–112. doi: 10.1212/WNL.0b013e31829a33e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Joint Commission. [Accessed 10/15, 2014];Disease-specific care certification program comprehensive stroke performance measurement implementation guide [online] Available at: www.jointcommission.org/assets/1/6/CSTK_Manual.pdf.
- 7.Smith EE, Shobha N, Dai D, et al. A risk score for in-hospital death in patients admitted with ischemic or hemorrhagic stroke. J Am Heart Assoc. 2013;2:e005207. doi: 10.1161/JAHA.112.005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leira R, Dávalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: Predictors and associated factors. Neurology. 2004;63:461–467. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 9.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE VISTA Collaboration. Defining hematoma expansion in intracerebral hemorrhage: Relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maas MB, Nemeth AJ, Rosenberg NF, Kosteva AR, Prabhakaran S, Naidech AM. Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology. 2013;80:1295–1299. doi: 10.1212/WNL.0b013e31828ab2a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, Bone I. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified rankin scale. Stroke. 2002;33:2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 13.Naidech AM, Jovanovic B, Liebling S, et al. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40:2398–2401. doi: 10.1161/STROKEAHA.109.550939. [DOI] [PubMed] [Google Scholar]
- 14.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 15.Hemphill JC, 3rd, Farrant M, Neill TA., Jr Prospective validation of the ICH score for 12-month functional outcome. Neurology. 2009;73:1088–1094. doi: 10.1212/WNL.0b013e3181b8b332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke. 2007;38:3055–3062. doi: 10.1161/STROKEAHA.107.488536. [DOI] [PubMed] [Google Scholar]
- 17.Mayer SA, Sacco RL, Shi T, Mohr JP. Neurologic deterioration in noncomatose patients with supratentorial intracerebral hemorrhage. Neurology. 1994;44:1379–1384. doi: 10.1212/wnl.44.8.1379. [DOI] [PubMed] [Google Scholar]
- 18.Flemming KD, Wijdicks EF, St Louis EK, Li H. Predicting deterioration in patients with lobar haemorrhages. J Neurol Neurosurg Psychiatry. 1999;66:600–605. doi: 10.1136/jnnp.66.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]