Abstract
Fractional flow reserve (FFR) is a physiological index of the severity of a stenosis in an epicardial coronary artery, based on the pressure differential across the stenosis. Clinicians are increasingly relying on this method because it is independent of baseline flow, relatively simple, and cost effective. The accurate measurement of FFR is predicated on maximal hyperemia being achieved by pharmacological dilation of the downstream resistance vessels (arterioles). When the stenosis causes FFR to be impaired by > 20%, it is considered to be significant and to justify revascularization. A diminished hyperemic response due to microvascular dysfunction can lead to a false normal FFR value, and a misguided clinical decision. The blunted vasodilation could be the result of defects in the signaling pathways modulated (activated or inhibited) by the drug. This might involve a downregulation or reduced number of vascular receptors, endothelial impairment, or an increased activity of an opposing vasoconstricting mechanism, such as the coronary sympathetic nerves or endothelin. There are data to suggest that microvascular dysfunction is more prevalent in post-menopausal women, perhaps due to reduced estrogen levels. The current review discusses the historical background and physiological basis for FFR, its advantages and limitations, and the phenomenon of microvascular dysfunction and its impact on FFR measurements. The question of whether it is warranted to apply gender-specific guidelines in interpreting FFR measurements is addressed.
Keywords: Angiography, coronary flow reserve, coronary stenosis, coronary vasodilators, fractional flow reserve, gender variation, microvascular dysfunction
INTRODUCTION
Coronary angiography is the traditional clinical method utilized to characterize the severity of an epicardial atherosclerotic coronary stenosis. A shortcoming of this imaging method is that it is strictly an anatomical assessment, and may be highly inaccurate when evaluating coronary lesions of intermediate severity, or when the severity is ambiguous, such as in the setting of diffuse atherosclerotic disease. These widely acknowledged limitations prompted the development of clinically accessible methods to assess the physiological significance of coronary stenoses. In the 1970s and 80s, several methods based on the concept of coronary flow reserve (CFR) were proposed [1, 2]. However, the conceptual limitations of CFR, including its dependence on baseline flow, as well as the need for expensive equipment and extensive off-line data processing, led to its abandonment as a measure of lesion severity in clinical practice. In 1993, Pijls et al. [3] presented the theoretical arguments and experimental findings for fractional flow reserve (FFR), a method based on measuring the pressure gradient across a stenosis (the ratio of distal intracoronary pressure to aortic pressure) during a maximal hyperemia achieved by pharmacological dilation of the downstream resistance vessels (arterioles). The implementation of FFR was facilitated by the development of a small pressure monitoring guidewire, which introduces minimal additional resistance to transstenotic flow [4]. Because FFR is independent of baseline flow, and is relatively simple and cost effective, it has become widely used in catheterization laboratories to provide a physiological assessment of stenosis severity.
A condition for accurate FFR measurements is that microvascular resistance is reduced maximally and remains stable during the measurement [3]. An impaired response of the coronary arterioles to the dilator drug can lead to a false normal FFR value, and a misguided clinical decision. The greater prevalence of microvascular dysfunction in women may make them more vulnerable to this error [5, 6].
The current review discusses the historical background and physiological basis for FFR, its advantages and limitations, and the phenomenon of microvascular dysfunction and its impact on FFR measurements. The question of whether it is warranted to apply gender-specific guidelines in interpreting FFR measurements is addressed.
CORONARY PHYSIOLOGY
Functional Anatomy of the Coronary Arterial Circulation
An appreciation of the anatomy and physiology of the coronary arterial circulation is important in understanding the hemodynamic consequences of an epicardial stenosis and the methods used to assess its severity. The coronary arterial circulation has three functionally distinct compartments [7, 8]. The proximal compartment is composed of the large epicardial coronary arteries (500 µm to 5 mm in diameter), which have capacitance and conductive functions and normally offer negligible resistance to blood flow. Since most coronary blood flow is during diastole, coronary perfusion pressure is estimated by diastolic aortic pressure minus left ventricular end-diastolic pressure (LVEDP) [9]. LVEDP is usually relatively small, and is often ignored. Aortic pressure is normally transmitted across the epicardial arteries without a significant pressure loss. However, pressure loss can be substantial when an obstruction due to an atherosclerotic plaque is present. The intermediate compartment is composed of the prearterioles (100 to 500 µm in diameter), which exhibit a moderate drop in pressure along their length and thus make a contribution, although limited, to the coronary vascular resistance. The prearterioles are not under the influence of vasoactive metabolites because of their extramyocardial location. Their principal function is to maintain pressure at the origin of the arterioles within a narrow range in the face of changes in coronary perfusion pressure and blood flow. This is accomplished by adjustments in vascular caliber via myogenic control mechanisms. The most distal compartment is composed of the intramural arterioles (<100 µm in diameter), which are the site of the most significant drop in pressure, and thus are termed “the resistance vessels.” The arterioles normally have a high resting tone and a substantial dilator reserve. These vessels are highly responsive to the vasoactive metabolites produced by the myocardium, i.e., local metabolic control, and to the effects of vasoactive drugs [8].
Mechanisms of Coronary Vasomotor Control
The high resting tone of the arterioles results in a level of myocardial blood flow that is low relative to myocardial oxygen demand; oxygen extraction is nearly maximal at rest (approximately 70-80%) and cannot increase appreciably. Thus, the myocardium is critically dependent on increases in blood flow to meet increases in cardiac work and oxygen demand [9]. In the normal coronary circulation, local metabolic control mechanisms ensure a tight coupling of coronary blood flow and myocardial oxygen demand, as reflected in an essentially constant value for coronary sinus PO2 of approximately 20 mmHg. The dilator reserve of the coronary arterioles can also be recruited in the face of decreases in perfusion pressure, e.g., an upstream epicardial stenosis [9]. This compensatory adjustment limits the capacity of the coronary circulation to respond to a superimposed increase in oxygen demand, thus enhancing the vulnerability to ischemia [10, 11].
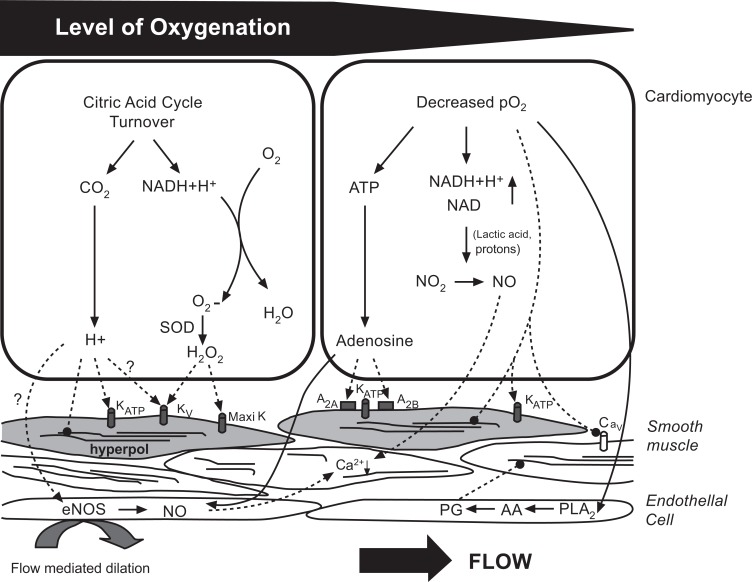
Current concepts of metabolic control suggest a role for a vasodilating metabolites produced by the myocardium in proportion to the level of cardiac work, i.e., a feed-forward mechanism. Metabolites that have been proposed are carbon dioxide, which is generated in decarboxylation reactions of the citric acid cycle, and reactive oxygen species, i.e., hydrogen peroxide, which are formed in the mitochondrial respiratory chain in proportion to oxygen consumption, as indicated in Fig. 1 [12]. The flow response initiated by these molecules is mediated by several secondary mechanisms, including the ATP-sensitive potassium channels and the voltage-gated potassium channels. Although endogenous adenosine was once considered the primary metabolite coupling coronary blood flow to myocardial oxygen demand, the current thought is that adenosine becomes involved in local coronary vasomotor regulation only when the stimulus for its production, low tissue PO2, is present [12]. Ultimately, vasodilation, whether in response to local metabolites or a vasodilating drug, requires a relaxation of vascular smooth muscle due to a decreased intracellular Ca2+ concentration and a increased myosin light chain phosphatase activity [13]. Ca2+ is removed from the cytosol by uptake into the sarcoplasmic reticulum and extrusion across the plasma membrane [13].
Fig. (1).
Current concepts of coronary metabolic control. The concepts are separated for physiological conditions (unchanged level of myocardial oxygenation) and pathological conditions (decreased oxygenation). These pathological conditions include coronary insufficiency. Biochemical reactions and metabolic interactions are indicated by solid arrows and links to effectors by dashed arrows. Pointed ends indicate activation and rounded ends inhibition. PLA2; phospholipase A2; AA: arachidonic acid; PG: prostaglandins [12].
Nitric oxide is an endothelium-derived molecule, which is produced from the amino acid L-arginine in a reaction requiring the enzyme NO synthase (NOS) [14]. NOS activity is stimulated by increases in Ca2+ concentration within the endothelial cell, which occur in response to the interaction of a chemical agent, e.g., bradykinin and acetylcholine, with a specific membrane receptor and by increases in flow (shear stress). NO diffuses to the underlying vascular smooth muscle where it stimulates production of cyclic guanosine monophosphate (cGMP) leading to vascular relaxation. Endothelin-1 is a vasoconstricting molecule also produced in the vascular endothelium. The production of endothelin-1 is stimulated by angiotensin II, platelet-derived factors, thrombin, reactive oxygen species, and shear stress. Endothelin-1 acts by binding to ETA and ETB receptors on coronary vascular smooth muscle [15]. NO production may serve as a counter-regulatory mechanism to limit endothelin-dependent effects on coronary vessels.
The coronary arterioles express both α1- (constricting) and β2-(dilating) adrenergic receptors and muscarinic receptors, and are supplied by sympathetic and parasympathetic (vagus) fibers, respectively [9]. Stimulation of the muscarinic receptors associated with the vascular endothelium causes coronary vasodilation via the NO-cGMP pathway [16]. Autonomic pathways normally play a subordinate role to local metabolic mechanisms in coronary vascular regulation. However, there is evidence that α-adrenergic vasoconstriction may limit coronary blood flow during atherosclerosis [17] and percutaneous coronary intervention [18, 19].
ASSESSING SEVERITY OF A CORONARY STENOSIS
Angiography – Applications and Limitations
The clinical severity of a coronary stenosis is assessed by anatomic criteria using angiography [20]. Despite the well-documented shortcomings in interpretation relating to interobserver and intraobserver variability [21, 22], essentially all clinical trials of revascularization provide treatment guidance based on the presence, extent, and evolution of atherosclerotic coronary narrowings evaluated by angiography. However, the functional (physiological) consequences of an epicardial coronary narrowing cannot be accurately determined from anatomic information alone. Measurements of percent stenosis (% diameter reduction) do not take into account a variety of factors with potential influence on the adequacy of myocardial perfusion, including diffuse disease, multiple stenoses in proximity, and heterogeneous remodeling and endothelial dysfunction [23]. Moreover, when stenosis severity is assessed angiographically as a relative diameter reduction versus a normal reference segment, if the disease is diffuse, and there is no normal segment for comparison, inaccuracy is introduced. Finally, other factors, such as lesion foreshortening, angulations, calcification, eccentricity, vessel overlap, and streaming of contrast, can complicate angiographic assessment of lesion severity. Assessing the significance of a coronary stenosis from visual angiographic evidence alone has been shown to be an imperfect measure of functional significance [24], which can result in unnecessary procedures. The limitations of anatomic based techniques prompted calls for physiological approaches to assess the severity of a coronary stenosis [20, 23].
Coronary Flow Reserve
In 1974 the concept of CFR (defined as the ratio of maximal flow to resting flow) was proposed as a functional measure of stenosis severity [1]. Maximal vasodilation can be determined from an analysis of the reactive hyperemic response (the transient increase in flow that follows an interval of arterial occlusion) [25] or with an intracoronary or intravenous infusion of a vasodilating drug, such as adenosine, dipyridamole, or papaverine [26]. CFR (also referred to as absolute CFR) equals the ratio of maximal flow to baseline flow for a given artery with or without a stenosis or diffuse narrowing, whereas relative CFR references the peak flow in the diseased artery to the peak flow in the absence of disease, e.g., in an adjacent coronary artery [1]. In patients with large vessel coronary artery disease, the extent of the reduction in CFR is directly proportional to the severity of the stenosis, whereas in individuals with angiographically normal coronary arteries it is an indication of microvascular dysfunction [8].
A knowledge of the mechanism of a vasodilating drug, e.g., whether it is endothelium-independent or endothelium-dependent, is important in interpreting values for CFR (or FFR). If the response to an endothelium-dependent vasodilator is blunted, it could reflect endothelium or vascular smooth muscle dysfunction. Additional testing with an endothelium-independent vasodilator is necessary to distinguish between these possibilities.
Adenosine is used commonly to assess CFR in humans. The vasodilating effect of adenosine was originally thought to be due to the direct stimulation of A2 receptors on vascular smooth muscle cells, which mediate an increased cAMP production via stimulation of adenylyl cyclase [27]. Subsequent studies focusing on coronary arterioles from porcine hearts [28, 29] demonstrated that the coronary vasodilation by adenosine did not involve adenylyl cyclase/cAMP, and included activation of the A2A receptors on both the vascular smooth muscle and endothelium. These studies also demonstrated that the contribution of NO/endothelium was diminished with increasing adenosine concentrations. At lower concentrations of adenosine, an opening of the endothelial KATP channels caused production and release of NO which subsequently increased cGMP within the smooth muscle resulting in vasodilation. At higher concentrations of adenosine, an opening of the vascular smooth muscle KATP channels caused membrane hyperpolarization by shifting the membrane potential closer to the K+ reversal potential. Hyperpolarization inhibits calcium influx via voltage-dependent calcium channels leading to vasodilation [30]. The aforementioned in vitro findings are in keeping with studies demonstrating that the coronary flow responses to low doses of adenosine were attenuated by NOS inhibitors, e.g., NG-nitro-L-arginine methyl ester (L-NAME) [31-33], whereas those to high doses of adenosine were not [34-36]. A study of isolated coronary arterioles obtained during cardiopulmonary bypass surgery from diseased human hearts (coronary artery bypass graft, valve replacement, and repair of congenital heart disease) found that adenosine-induced coronary dilation was dependent upon an activation of the adenylyl cyclase/cAMP pathway and was independent of the vascular endothelium [37].
Dipyridamole acts by interfering with the degradation of the adenosine released from the cardiomyocytes, and thus its molecular pathways are the same as those described above for adenosine. Papaverine, another sometimes used coronary vasodilator [38, 39], is thought to have a phosphodiesterase inhibiting property and operate by increasing cAMP in the vascular smooth muscle [40]. At high doses, papaverine may also have a direct inhibitory effect on calcium mobilization in the vascular smooth muscle cell. Such an action has been shown to produce a negative inotropic effect in cardiomyocytes [41]. Acetylcholine and bradykinin are endothelium (NO)-dependent coronary vasodilators whereas sodium nitroprusside is an NO donor acting independently of the endothelium, which is used to assess vascular smooth muscle function per se [42].
The ability of CFR to provide an index of stenosis severity is limited by the wide variation in baseline coronary blood flow and by its dependency on the prevailing hemodynamic conditions, including myocardial oxygen demand (and its determinants, preload, heart rate, contractility, and left ventricular developed pressure), perfusion pressure, and the magnitude of coronary collateral flow [43-47].
Fractional Flow Reserve (FFR)
Utilizing principles of pressure-flow relations through coronary stenoses, the concept of FFR was developed as a means to assess the functional severity of an epicardial coronary stenosis while avoiding the limitations of CFR (Fig. 2) [3]. A narrowing of an epicardial vessel causes a drop in perfusion pressure. The pressure drop is due to viscous and expansion losses. The size of these losses can be estimated by Poiseuille’s law and Bernoulli’s equation, respectively. The pressure drop across the stenosis is directly related to the flow rate in an exponential manner (Fig. 3) [48]. A vasodilating drug (either intracoronary adenosine or papaverine, or intravenous administration of adenosine or dipyridamole) is administered to abolish vasomotor tone and, thus, to minimize microvascular resistance. Under this condition, blood flow across the stenosis is assumed to be maximal producing the maximal achievable pressure gradient. By measuring the ratio of the coronary pressure distal to the stenosis to aortic pressure, the percentage of normal coronary flow, or the fraction of normal flow (hence FFR), is calculated [3, 48-50]. FFR has a normal value of 1.0 for every patient and every coronary artery. FFR for a stenotic vessel is expressed as a decimal or fraction of this value. A fundamental assumption of FFR is that, at maximal vasodilation, the relationship between coronary perfusion pressure and flow is proportional and linear, i.e., that a stenosis affects distal coronary pressure to the same degree as it affects flow [48].
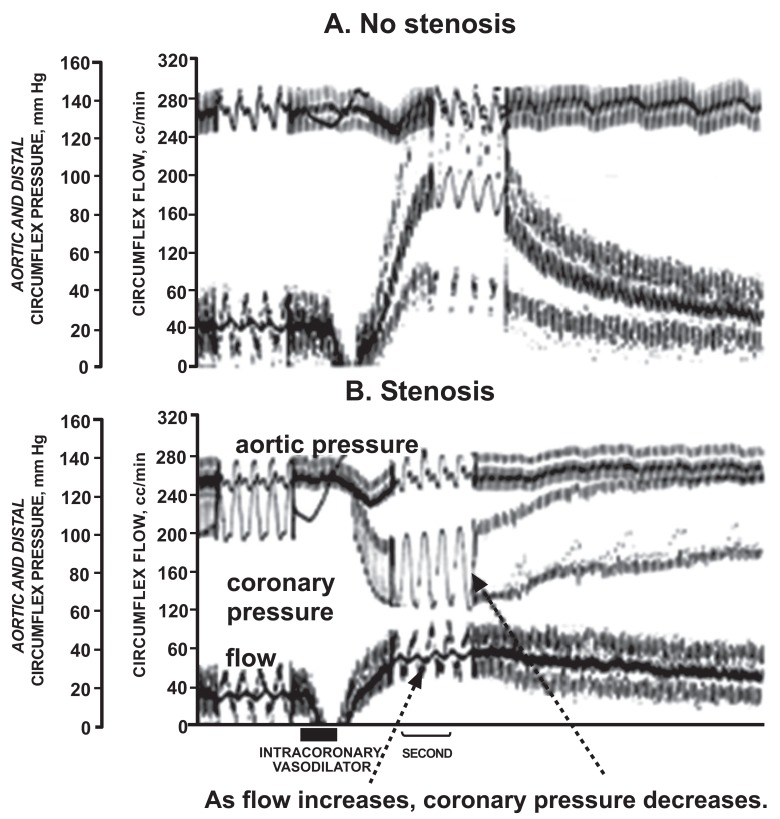
Fig. (2).
Tracings demonstrating fractional flow reserve (FFR) as a measure of coronary flow reserve. Shown are coronary blood flow and aortic and coronary pressure tracings from a dog without a coronary stenosis (A) and a severe stenosis (B). A hyperemic response was induced by the coronary vasodilating effect of a contrast injection, indicated by the bar at the bottom of the figure. Without a stenosis (A), the contrast caused a marked increase in coronary blood flow, with little divergence of pressures. However, with a stenosis (B), the contrast increased coronary blood flow modestly with a marked increase in the the aortic-distal coronary pressure gradient. FFR is the ratio of coronary to aortic pressure at maximum hyperemia and reflects the flow reserve [23].
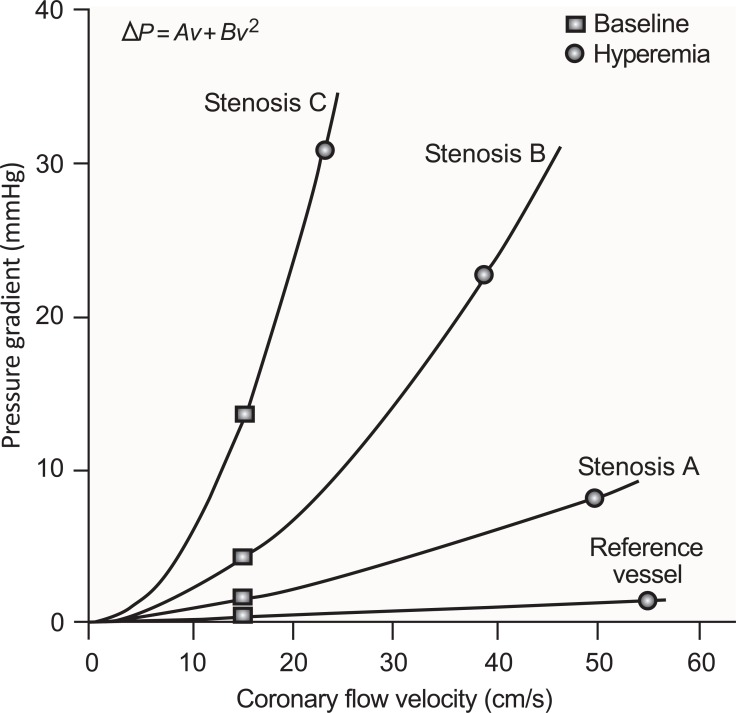
Fig. (3).
The pressure drop across a coronary stenosis (Δ P) is a direct function of coronary flow velocity (v). The steepness of this relationship increases with stenosis severity (from Stenosis A to C). For a given stenosis, the pressure gradient at baseline (square) is determined by resting microvascular resistance and that at maximal hyperemia (circle) is determined primarily by the vasodilator capability of the downstream resistance vessels, although the physical factors described in the text may pose a limitation. The relationship between Δ P and v is defined by the equation at the top of the figure. The first and second terms represent pressure loss caused by viscous friction and expansion losses at the exit of the stenosis, respectively. The coefficients A and B are determined by stenosis geometry and the rheological properties of the blood [48].
FFR was initially validated using a cut off value of 0.75. This value was determined from studies evaluating the relation between FFR and exercised-induced myocardial ischemia in patients with a single coronary artery lesion and normal ventricular function [51]. A nonischemic threshold value was prospectively confirmed [24] and compared with noninvasive stress testing. An FFR <0.75 was associated with inducible ischemia (specificity, 100%), whereas a value >0.80 indicated absence of inducible ischemia in the majority of patients (sensitivity, 90%) [49]. The data are sparse for patients with microvascular disease, acute or remote myocardial infarction, and unstable angina. Caution should be applied in extending the current physiological criteria to such patients [24]. With further experience, investigators appreciated that extending the cut off value could improve the sensitivity of FFR without appreciably compromising specificity. A cut off value of less than or equal to 0.80 is now recommended as the criterion for a hemodynamically significant lesion that may be treated with percutaneous intervention [24, 52].
The reproducibility of FFR measurements appears to depend on the severity of the stenosis [53]; at the extremes of the disease spectrum, the diagnostic agreement between repeated FFR measurements, made 10 min apart, was 100%, but within the range of physiologically intermediate values (0.77 to 0.83), measurement certainty was less than 80%, reaching a nadir of approximately 50% around the established clinical cut point of 0.80. Due to the inherent variability of FFR measurements, a significant percentage of lesions may fall within a “grey zone” [53]. When an FFR measurement is <0.75 or >0.85, clinicians can use a dichotomous approach based solely on the FFR result, being confident that a repeat FFR would result in the same strategic decision > 95% of the time. However, for an FFR measurement between 0.75 and 0.85, a repeat FFR might allocate the patient to the opposite treatment category, with the chance of change in strategy increasing as FFR approximates to 0.80. Between 0.77 and 0.83, the chance of this occurring is as high as 20%. Several clinical trials have shown that deferring revascularization for angiographically intermediate stenoses is a reasonable strategy. Within this grey zone, the decision to revascularize would be based on a broad clinical criteria, encompassing an evaluation of anatomical features, symptoms, results of noninvasive testing, and the risk-benefit profile of the patient [48].
Several limitations and potential sources of error in the FFR measurement warrant address. 1) Physiological studies have demonstrated that the pressure-flow relationship during maximal vasodilation is not linear and proportional, but is rather curvilinear, implying an increase in the hyperemic microvascular resistance at low perfusion pressures [11, 48], thus violating a key assumption of FFR. This deviation from linearity has been explained by a passive reduction in arteriolar caliber in response to reduced distending pressure [11]. The result is a progressive overestimation of FFR with increasing stenosis severity [48]. 2) Hemodynamic factors that increase extravascular compressive forces, such as tachycardia (reduced diastolic duration) and an increased LVEDP, can increase hyperemic microvascular resistance and raise FFR [11, 54]. 3) FFR is sensitive to the size of the perfusion territory, since this factor can influence the coronary flow rate and thus the pressure drop across the stenosis. 4) FFR is influenced by the minimal lumen diameter and lesion length [55]. 5) Microvascular dysfunction can impair drug-induced coronary vasodilation, resulting in a blunted flow response and an elevated FFR (Fig. 4) [48]. The clinical significance of microvascular dysfunction is discussed in detail below.
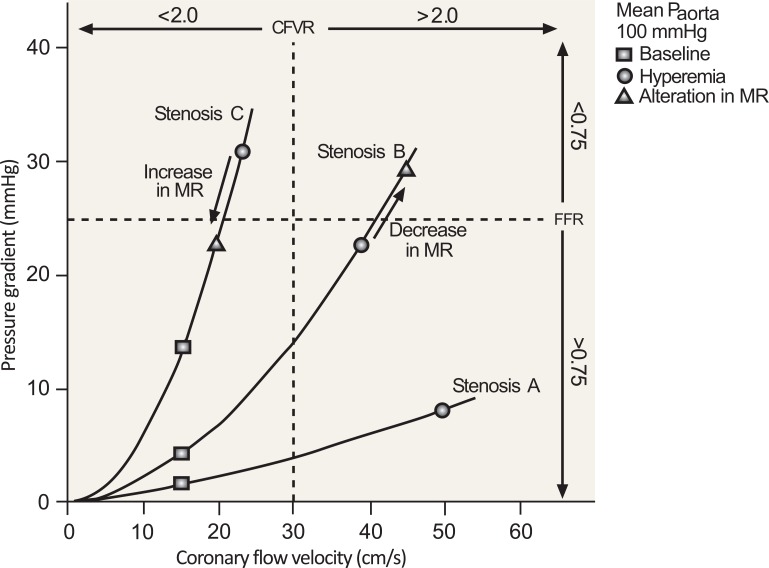
Fig. (4).
An increase in minimum microvascular resistance (MR) (as shown for Stenosis C) reduces hyperemic flow, which decreases the pressure gradient across the stenosis, thus increasing fractional flow reserve (FFR). This would be expected to occur with microvascular dysfunction. There is an opposite effect on coronary flow velocity reserve (CFVR). The dashed lines indicate clinically applicable cut-off values [48].
Minimal microvascular resistance has been shown to vary widely between patients and between adjacent perfusion territories within the same patient [48, 56, 57]. This is likely due to intrinsic morphological variations in vascular capacity and the influence of the hemodynamic factors alluded to above. It has been recommended that measurements of coronary flow velocity and pressure be made simultaneously so that calculations of microvascular resistance can be made and taken into account in FFR determinations [48]. Although a guidewire equipped with both a pressure and Doppler flow sensor is available, its complexity and lack of technical sophistication has deterred its adoption in daily practice [48].
MICROVASCULAR DYSFUNCTION
General Concepts
Approximately 10-20% of patients experiencing stress-induced angina have angiographically normal coronary arteries [58]. The accompanying hemodynamic and metabolic findings in these patients have suggested that this apparent paradox may be explained by an impaired responsiveness of arteriolar resistance vessels to the metabolic vasodilators linking oxygen supply (blood flow) to oxygen demand. These findings include: 1) a release of myocardial lactate and lipid peroxidation products, 2) a widening of the arterio-venous O2 difference, indicating increased myocardial oxygen extraction, 3) an ischemic shift of myocardial high-energy phosphate metabolism on magnetic resonance spectroscopy, 4) a marked increase in LVEDP, and 5) a blunted response to pharmacological vasodilators [59-63].
A blunted coronary hyperemic response during exercise in a patient with angiographically normal coronary arteries could involve a reduced production of a metabolic vasodilator(s), a decrease in receptor number or activity, and/or an impairment to distal signaling pathways within the arteriolar vascular smooth muscle. An abnormality in the mechanisms that lower intracellular Ca2+ and decrease myosin light chain phosphatase activity may also play a role.
Several molecular pathways have been implicated in the microvascular dysfunction. In some studies, a blunted flow response to acetylcholine (without effect on the response to an endothelium-independent vasodilator) provided evidence for a selective functional derangement within the endothelium [64-67]. However, in others, a blunted flow response to an endothelium-independent vasodilator (adenosine, dipyridamole, or papaverine) suggested that the impairment was located in the vascular smooth muscle [61, 64, 65, 68, 69]. Interestingly, Koglin and Scheidt [40] identified a sub-group of patients with normal epicardial angiograms in whom an isolated defect of adenosine-mediated coronary vasodilation existed. These patients demonstrated normal and comparable relative flow reserves, as assessed with the endothelium-dependent vasodilator, acetylcholine, and the endothelium-independent vasodilator, papaverine, but markedly impaired flow responses to two doses of adenosine. The impairment to adenosine-induced vasodilation could be attributable to a selective downregulation of the A2A receptor and/or a decrease in A2A receptor density, or to a defect in the relevant Gs protein. These findings suggest that in certain patients the use of adenosine for maximal vasodilation may result in deceptively high values for FFR.
An upregulation of the coronary sympathetic nerve/α-adrenergic receptor pathway may be involved in the pathogenesis of microvascular dysfunction [70, 71]. This mechanism could elevate vasomotor tone and oppose the action of vasodilating stimuli [72]. As evidence for this mechanism, both ergonovine injection and mental stress caused decreases in coronary blood flow in patients with documented microvascular dysfunction [61, 73]. Moreover, several studies have suggested that α1-adrenoreceptor-mediated coronary vasoconstriction can limit the maximal increase in blood flow induced pharmacologically. In canine studies, Vlahakes et al. [74] found that phentolamine (an α1- and α2-receptor blocker) increased myocardial blood flow during maximal adenosine-induced vasodilation, while Johannsen et al. [75] found that cardiac sympathetic nerve stimulation or phenylephrine (an α1-receptor agonist) had the opposite effect. In normal human volunteers, Lonenzoni et al. [76] demonstrated that dipyridamole caused a 40 % greater increase in myocardial blood flow following α 1-adrenergic blockade with doxazosin. In patients with coronary stenoses, the administration of phentolamine or urapidil (a selective α1- receptor blocker) caused a reduction in FFR values determined with adenosine (reflecting an unmasked residual microvascular tone), although these changes were small and did not affect clinical decision making [77, 78]. In patients with demonstrated microvascular dysfunction, endothelin-1 levels increased in the coronary sinus blood during atrial pacing [79], which may provide an additional mechanism opposing metabolic and pharmacologic coronary vasodilation.
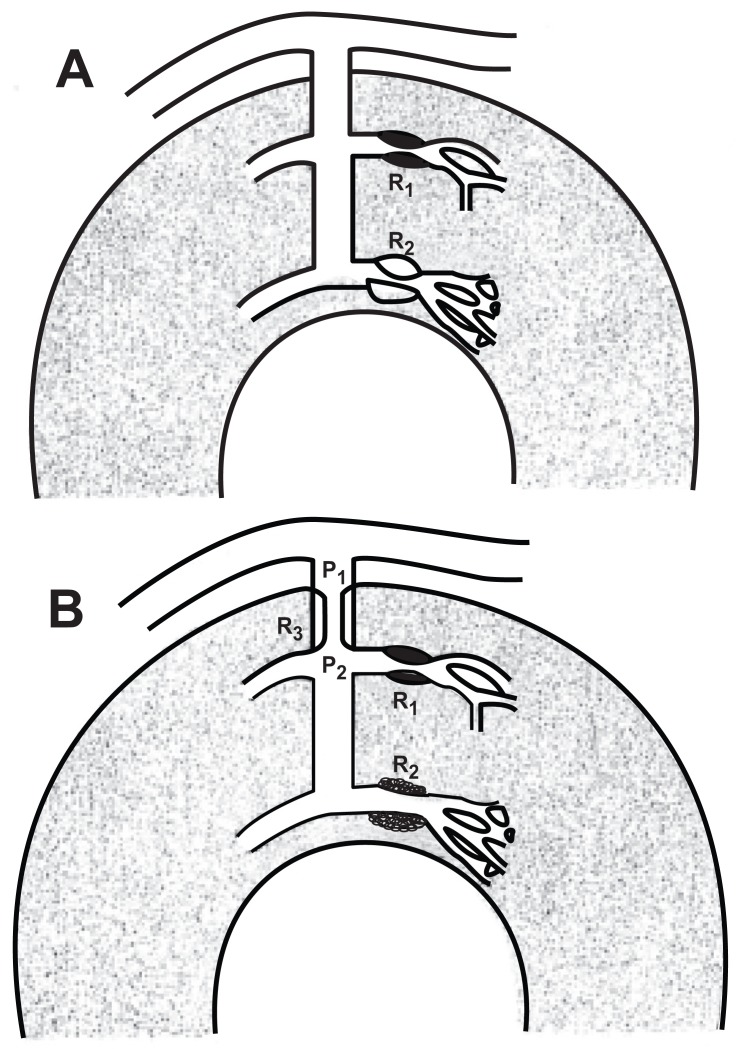
Epstein and Cannon [80] presented an alternative view to the assumption that the myocardial ischemia and angina in patients with normal epicardial coronary arteries is due to inadequate dilation of the coronary arterioles. These investigators found that dipyridamole infusion in these patients caused a marked increase in transmural myocardial blood flow, which was accompanied by severe chest pain. The authors suggested that these apparently paradoxical findings were consistent with a flow-impeding resistance located at the level of the upstream prearterioles (Fig. 5). Their argument is as follows: Because of a smaller autoregulatory capability (owing to a greater vulnerability to myocardial tissue pressure) and a higher metabolic demand, the accentuated pressure drop in the prearterioles results in exhaustion of the vasodilator reserve in the subendocardium, whereas reserve remains in the subepicardium. In this scenario, a vasodilator, such as dipyridamole, decreases vascular resistance selectively in the subepicardium, and increases blood flow there. This reduces the driving pressure for blood flow to the pressure-dependent subendocardium, thus reducing flow to that region (so-called transmural coronary steal), resulting in myocardial ischemia and angina. An involvement of the prearterioles is supported by findings of structural abnormalities of small coronary arteries and small vessel hypertrophy in patients with angina pectoris but normal coronary arteriograms [68].
Fig. (5).
Two models of increased coronary resistance with normal epicardial arteries. A. The increased resistance to flow (R2), which could be either a fixed anatomic or a functional defect, is at the arteriolar level. B. The increased resistance to flow (R3) is at the intramural prearteriole. P1 and P2 = pressures proximal and distal to the abnormal resistance, respectively; R1 = the normally responsive subepicardial arteriole [80]. Refer to text for a detailed explanation.
Microvascular Dysfunction in Women
The Women’s Ischemia Syndrome Evaluation (WISE) study was the first full-scale evaluation of gender-related differences in coronary artery disease [5, 6]. The WISE study found that women were more prone to microvascular dysfunction as the cause for angina pain and that the prevalence of this condition increased with the onset of menopause. The investigators used coronary velocity responses to adenosine in women with chest pain and no obstructive coronary disease to assess microvascular function. The results demonstrated that women with microvascular dysfunction, as evidenced by reduced CFR values, also had a loss of epicardial dilation during the adenosine-induced increase in blood flow. This impairment to endothelium-dependent, flow-mediated dilation suggested that derangement of endothelial function contributed to the blunted microcirculator reactivity.
Because microvascular dysfunction disproportionally affects menopausal women and because estrogen is a known coronary vasodilator, it has been hypothesized that microvascular dysfunction may be caused by estrogen deficiency [81]. Estrogen can dilate the coronary circulation via both endothelium-dependent and endothelium-independent mechanisms [82]. The endothelium-dependent mechanism involves the NO-cGMP pathway [83]. Estrogen may enhance endothelial NO production by increasing NOS expression or activity [82, 84], and by increasing the concentration of Ca2+ in the endothelial cell [84-86]. It has also been suggested that estrogen increases bioavailablity of NO via an anti-oxidant effect, which reduces the conversion of NO to the toxic molecule peroxynitrite [87]. Endothelium-independent coronary vasodilation is mediated by specific estrogen receptors on the coronary vascular smooth muscle cell, whose activation reduces intracellular Ca2+ concentration and Ca2+ influx from the extracellular space [82]. The chronic effect of endogenous estrogen is a reduction in the density and/or permeability of the Ca2+ channels [88]. The loss of this moderating influence of estrogen on vascular smooth muscle contraction may explain the blunted vasodilating effects of endothelium-independent vasodilators, such as dipyridamole, in post-menopausal women. Studies showed that 17-beta-estradiol cutaneous patches reduced the frequency of chest pain and improved exercise tolerance in women with microvascular dysfunction [81]. More than half of the women tested in the WISE study had coronary endothelial dysfunction when tested with acetylcholine. Besides estrogen deficiency, other factors have been suggested to explain microvascular dysfunction in post-menopausal women, including age, hypertension, cigarette smoking, dyslipidemia, visceral obesity, and a genetic predisposition.
A recent study evaluated the link between emotional stress and microvascular dysfunction in women [89]. The study was composed of sixteen women diagnosed with microvascular dysfunction and eight women of similar age and weight without microvascular dysfunction. Heart rate, arterial pressure, and heart rate variability were measured at rest and when the women were subjected to the emotional stress of anger. The results demonstrated that this stress increased sympathetic nerve activity only in the women with microvascular dysfunction, suggesting a neurogenic mechanism for this condition. It has been proposed that the sympathetic nerves may also play a role in the stress-induced transient hypokinesis of the left ventricular apex or midventricular segments found in post-menopausal women with angiographically normal coronary arteries, i.e., Takotsubo cardiomyopathy (TTC) [90-92]. Experimental support for this theory comes from a study demonstrating that combined α- and β-adrenergic blockade prevented apical ballooning in a rat model of emotional stress [93]. Post-menopausal changes in the sensitivity or density of local myocardial adrenergic receptors from base to apex may help to explain the regional pathophysiology of TTC patients [94]. The predominance of TTC in post-menopausal women has implicated estrogen deficiency in its pathogenesis [95]. This mechanism is consistent with experimental studies demonstrating that estradiol administration obtunded the emotional stress-induced cardiac dysfunction observed in ovariectomized rats [96, 97].
GENDER DIFFERENCES IN FFR VALUES
Recent studies consistently demonstrated that post-menopausal women have higher FFR values than men of a comparable age, although the difference between the sexes was not appreciable [98-101]. For example, in the FAME sub-study, Kim et al. [98] found that FFR values in women were 0.75±0.18 (SD) whereas those in men were 0.71±0.17 (P= 0.001). These investigators also reported that the proportion of functionally significant stenoses, defined as FFR<0.80, was lower in women compared to men. This was true for stenoses of 50 – 70 % severity (21.1% vs. 39.5%, p<0.001) and for stenoses of 70 – 90% severity (71.9% vs. 82.0%, p = 0.019). The most widely cited reason for the higher FFR values in women is microvascular dysfunction [102]. Another factor is that women generally have smaller hearts and myocardial perfusion territories [103, 104], which would result in a smaller hyperemic flow and a blunted pressure drop across a given sized stenosis. Because of a disproportionate impairment to microvascular reactivity in women, the question arises whether it is warranted to apply gender-specific guidelines in interpreting FFR measurements. In particular, would it be reasonable to apply a cut off value higher than 0.80 in women? Since the studies to date have found that the difference in FFR values between the sexes, although statistically significant, was relatively small, we must answer no. However, we would recommend that if a woman with a borderline or equivocal stenosis on an angiogram also has a borderline FFR, i.e., 0.75-0.83, that the test should be repeated. To increase the probability that a determination of FFR is obtained during the maximal hyperemic response, it would make sense, from a physiological perspective, to use a vasodilating drug with a different mechanism for the second test. However, this approach has not been studied, and is currently not standard practice. The well-documented differences in microvascular reactivity in women and men suggest that consideration should be given to making the vasodilating drug used during FFR measurements gender-specific. Further studies are necessary to ascertain the drug and the dose most likely to produce the maximal hyperemic response in each gender.
CONCLUSION
FFR is a useful tool for determining the physiological significance of a coronary stenosis, and has been shown to be of undeniable benefit in determining the need for revascularization. A methodological requirement of the technique is that maximal dilation of downstream resistance vessels be achieved pharmacologically. Microvascular dysfunction is a relatively common pathophysiological condition that may result in impaired vasodilator responses. The greater prevalence of microvascular dysfunction in women has been cited as an explanation for their higher FFR values compared with men when stenoses of similar severity are compared. Clinicians need to consider the impact of microvascular dysfunction, as well as other potential sources of error, in their interpretation of FFR findings. The appropriateness of applying a rigid FFR cut-off value for all patients requires further examination. The use of FFR necessitates an in-depth knowledge of the technique, including its limitations and sources of variability, an appreciation of the rheological and physical properties of coronary stenoses, and an understanding of the physiology, pathophysiology, and pharmacology of the coronary microvasculature.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors have no acknowledgements to report.
REFERENCES
- 1.Gould K.L., Lipscomb K., Hamilton G.W. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am. J. Cardiol. 1974;33(1):87–94. doi: 10.1016/0002-9149(74)90743-7. [DOI] [PubMed] [Google Scholar]
- 2.Vogel R.A., Bates E.R., O’Neill W.W., Aueron F.M., Meier B., Gruentzig A.R. Coronary flow reserve measured during cardiac catheterization. Arch. Intern. Med. 1984;144(9):1773–1776. doi: 10.1001/archinte.1984.00350210089016. [DOI] [PubMed] [Google Scholar]
- 3.Pijls N.H., van Son J.A., Kirkeeide R.L., De Bruyne B., Gould K.L. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354–1367. doi: 10.1161/01.CIR.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 4.De Bruyne B., Pijls N.H., Paulus W.J., Vantrimpont P.J., Sys S.U., Heyndrickx G.R. Transstenotic coronary pressure gradient measurement in humans: in vitro and in vivo evaluation of a new pressure monitoring angioplasty guide wire. J. Am. Coll. Cardiol. 1993;22(1):119–126. doi: 10.1016/0735-1097(93)90825-L. [DOI] [PubMed] [Google Scholar]
- 5.Reis S.E., Holubkov R., Lee J.S., Sharaf B., Reichek N., Rogers W.J., Walsh E.G., Fuisz A.R., Kerensky R., Detre K.M., Sopko G., Pepine C.J. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J. Am. Coll. Cardiol. 1999;33(6):1469–1475. doi: 10.1016/S0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 6.Merz C.N., Kelsey S.F., Pepine C.J., Reichek N., Reis S.E., Rogers W.J., Sharaf B.L., Sopko G. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J. Am. Coll. Cardiol. 1999;33(6):1453–1461. doi: 10.1016/S0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 7.Chilian W.M. Coronary microcirculation in health and disease. Summary of an NHLBI workshop. Circulation. 1997;95(2):522–528. doi: 10.1161/01.CIR.95.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camici P.G., Crea F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007;356(8):830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 9.Feigl E.O. Coronary physiology. Physiol. Rev. 1983;63(1):1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Kern M.J. Coronary physiology revisited : practical insights from the cardiac catheterization laboratory. Circulation. 2000;101(11):1344–1351. doi: 10.1161/01.CIR.101.11.1344. [DOI] [PubMed] [Google Scholar]
- 11.Spaan J.A., Piek J.J., Hoffman J.I., Siebes M. Physiological basis of clinically used coronary hemodynamic indices. Circulation. 2006;113(3):446–455. doi: 10.1161/CIRCULATIONAHA.105.587196. [DOI] [PubMed] [Google Scholar]
- 12.Deussen A., Ohanyan V., Jannasch A., Yin L., Chilian W. Mechanisms of metabolic coronary flow regulation. J. Mol. Cell. Cardiol. 2012;52(4):794–801. doi: 10.1016/j.yjmcc.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Webb R.C. Smooth muscle contraction and relaxation. Adv. Physiol. Educ. 2003;27(1-4):201–206. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- 14.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 15.Heerdt P.M., Crystal G.J. Cardiovascular physiology: Cellular and molecular regulation. In: Hemmings H.C., Egan T.D., editors. Pharmacology and physiology for anesthesia: Foundations and clinical applications. Philadelphia: Elsevier; 2013. pp. 351–365. [DOI] [Google Scholar]
- 16.Crystal G.J., Zhou X., Alam S., Piotrowski A., Hu G. Lack of role for nitric oxide in cholinergic modulation of myocardial contractility in vivo. Am. J. Physiol. Heart Circ. Physiol. 2001;281(1):H198–H206. doi: 10.1152/ajpheart.2001.281.1.H198. [DOI] [PubMed] [Google Scholar]
- 17.Baumgart D., Haude M., Görge G., Liu F., Ge J., Grosse-Eggebrecht C., Erbel R., Heusch G. Augmented alpha-adrenergic constriction of atherosclerotic human coronary arteries. Circulation. 1999;99(16):2090–2097. doi: 10.1161/01.CIR.99.16.2090. [DOI] [PubMed] [Google Scholar]
- 18.Gregorini L., Marco J., Kozàkovà M., Palombo C., Anguissola G.B., Marco I., Bernies M., Cassagneau B., Distante A., Bossi I.M., Fajadet J., Heusch G. Alpha-adrenergic blockade improves recovery of myocardial perfusion and function after coronary stenting in patients with acute myocardial infarction. Circulation. 1999;99(4):482–490. doi: 10.1161/01.CIR.99.4.482. [DOI] [PubMed] [Google Scholar]
- 19.Gregorini L., Marco J., Farah B., Bernies M., Palombo C., Kozàkovà M., Bossi I.M., Cassagneau B., Fajadet J., Di Mario C., Albiero R., Cugno M., Grossi A., Heusch G. Effects of selective alpha1- and alpha2-adrenergic blockade on coronary flow reserve after coronary stenting. Circulation. 2002;106(23):2901–2907. doi: 10.1161/01.CIR.0000040998.88272.A7. [DOI] [PubMed] [Google Scholar]
- 20.White C.W., Wright C.B., Doty D.B., Hiratza L.F., Eastham C.L., Harrison D.G., Marcus M.L. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis? N. Engl. J. Med. 1984;310(13):819–824. doi: 10.1056/NEJM198403293101304. [DOI] [PubMed] [Google Scholar]
- 21.Zir L.M., Miller S.W., Dinsmore R.E., Gilbert J.P., Harthorne J.W. Interobserver variability in coronary angiography. Circulation. 1976;53(4):627–632. doi: 10.1161/01.CIR.53.4.627. [DOI] [PubMed] [Google Scholar]
- 22.DeRouen T.A., Murray J.A., Owen W. Variability in the analysis of coronary arteriograms. Circulation. 1977;55(2):324–328. doi: 10.1161/01.CIR.55.2.324. [DOI] [PubMed] [Google Scholar]
- 23.Gould K.L., Johnson N.P., Bateman T.M., Beanlands R.S., Bengel F.M., Bober R., Camici P.G., Cerqueira M.D., Chow B.J., Di Carli M.F., Dorbala S., Gewirtz H., Gropler R.J., Kaufmann P.A., Knaapen P., Knuuti J., Merhige M.E., Rentrop K.P., Ruddy T.D., Schelbert H.R., Schindler T.H., Schwaiger M., Sdringola S., Vitarello J., Williams K.A., Sr, Gordon D., Dilsizian V., Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J. Am. Coll. Cardiol. 2013;62(18):1639–1653. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 24.Lotfi A., Jeremias A., Fearon W.F., Feldman M.D., Mehran R., Messenger J.C., Grines C.L., Dean L.S., Kern M.J., Klein L.W., Society of Cardiovascular Angiography and Interventions Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the Society of Cardiovascular Angiography and Interventions. Catheter. Cardiovasc. Interv. 2014;83(4):509–518. doi: 10.1002/ccd.25222. [DOI] [PubMed] [Google Scholar]
- 25.Olsson R.A., Gregg D.E. Myocardial reactive hyperemia in the unanesthetized dog. Am. J. Physiol. 1965;208:224–230. doi: 10.1152/ajplegacy.1965.208.2.224. [DOI] [PubMed] [Google Scholar]
- 26.Bradley A.J., Alpert J.S. Coronary flow reserve. Am. Heart J. 1991;122(4 Pt 1):1116–1128. doi: 10.1016/0002-8703(91)90480-6. [DOI] [PubMed] [Google Scholar]
- 27.Silver P.J., Walus K., DiSalvo J. Adenosine-mediated relaxation and activation of cyclic AMP-dependent protein kinase in coronary arterial smooth muscle. J. Pharmacol. Exp. Ther. 1984;228(2):342–347. [PubMed] [Google Scholar]
- 28.Hein T.W., Kuo L. cAMP-independent dilation of coronary arterioles to adenosine : role of nitric oxide, G proteins, and K(ATP) channels. Circ. Res. 1999;85(7):634–642. doi: 10.1161/01.RES.85.7.634. [DOI] [PubMed] [Google Scholar]
- 29.Hein T.W., Belardinelli L., Kuo L. Adenosine A(2A) receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 1999;291(2):655–664. [PubMed] [Google Scholar]
- 30.Nelson M.T., Patlak J.B., Worley J.F., Standen N.B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 1990;259(1 Pt 1):C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 31.Parent R., Paré R., Lavallée M. Contribution of nitric oxide to dilation of resistance coronary vessels in conscious dogs. Am. J. Physiol. 1992;262(1 Pt 2):H10–H16. doi: 10.1152/ajpheart.1992.262.1.H10. [DOI] [PubMed] [Google Scholar]
- 32.Davis C.A., III, Sherman A.J., Yaroshenko Y., Harris K.R., Hedjbeli S., Parker M.A., Klocke F.J. Coronary vascular responsiveness to adenosine is impaired additively by blockade of nitric oxide synthesis and a sulfonylurea. J. Am. Coll. Cardiol. 1998;31(4):816–822. doi: 10.1016/S0735-1097(97)00561-5. [DOI] [PubMed] [Google Scholar]
- 33.Buus N.H., Bøttcher M., Hermansen F., Sander M., Nielsen T.T., Mulvany M.J. Influence of nitric oxide synthase and adrenergic inhibition on adenosine-induced myocardial hyperemia. Circulation. 2001;104(19):2305–2310. doi: 10.1161/hc4401.098293. [DOI] [PubMed] [Google Scholar]
- 34.Canty J.M., Jr, Schwartz J.S. Nitric oxide mediates flow-dependent epicardial coronary vasodilation to changes in pulse frequency but not mean flow in conscious dogs. Circulation. 1994;89(1):375–384. doi: 10.1161/01.CIR.89.1.375. [DOI] [PubMed] [Google Scholar]
- 35.Quyyumi A.A., Dakak N., Andrews N.P., Gilligan D.M., Panza J.A., Cannon R.O., III Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92(3):320–326. doi: 10.1161/01.CIR.92.3.320. [DOI] [PubMed] [Google Scholar]
- 36.Gurevicius J., Salem M.R., Metwally A.A., Silver J.M., Crystal G.J. Contribution of nitric oxide to coronary vasodilation during hypercapnic acidosis. Am. J. Physiol. 1995;268(1 Pt 2):H39–H47. doi: 10.1152/ajpheart.1995.268.1.H39. [DOI] [PubMed] [Google Scholar]
- 37.Sato A., Terata K., Miura H., Toyama K., Loberiza F.R., Jr, Hatoum O.A., Saito T., Sakuma I., Gutterman D.D. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am. J. Physiol. Heart Circ. Physiol. 2005;288(4):H1633–H1640. doi: 10.1152/ajpheart.00575.2004. [DOI] [PubMed] [Google Scholar]
- 38.Christensen C.W., Rosen L.B., Gal R.A., Haseeb M., Lassar T.A., Port S.C. Coronary vasodilator reserve. Comparison of the effects of papaverine and adenosine on coronary flow, ventricular function, and myocardial metabolism. Circulation. 1991;83(1):294–303. doi: 10.1161/01.CIR.83.1.294. [DOI] [PubMed] [Google Scholar]
- 39.De Bruyne B., Pijls N.H., Barbato E., Bartunek J., Bech J.W., Wijns W., Heyndrickx G.R. Intracoronary and intravenous adenosine 5′-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation. 2003;107(14):1877–1883. doi: 10.1161/01.CIR.0000061950.24940.88. [DOI] [PubMed] [Google Scholar]
- 40.Koglin J., von Scheidt W. Isolated defect of adenosine-mediated coronary vasodilation: functional evidence for a new microangiopathic entity. J. Am. Coll. Cardiol. 1997;30(1):103–107. doi: 10.1016/S0735-1097(97)00131-9. [DOI] [PubMed] [Google Scholar]
- 41.Endoh M., Schümann H.J. Effects of papaverine on isolated rabbit papillary muscle. Eur. J. Pharmacol. 1975;30(2):213–220. doi: 10.1016/0014-2999(75)90102-8. [DOI] [PubMed] [Google Scholar]
- 42.Crystal G.J., Gurevicius J. Nitric oxide does not modulate myocardial contractility acutely in in situ canine hearts. Am. J. Physiol. 1996;270(5 Pt 2):H1568–H1576. doi: 10.1152/ajpheart.1996.270.5.H1568. [DOI] [PubMed] [Google Scholar]
- 43.McGinn A.L., White C.W., Wilson R.F. Interstudy variability of coronary flow reserve. Influence of heart rate, arterial pressure, and ventricular preload. Circulation. 1990;81(4):1319–1330. doi: 10.1161/01.CIR.81.4.1319. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman J.I. A critical view of coronary reserve. Circulation. 1987;75(1 Pt 2):I6–I11. [PubMed] [Google Scholar]
- 45.Rossen J.D., Winniford M.D. Effect of increases in heart rate and arterial pressure on coronary flow reserve in humans. J. Am. Coll. Cardiol. 1993;21(2):343–348. doi: 10.1016/0735-1097(93)90673-O. [DOI] [PubMed] [Google Scholar]
- 46.Gould K.L., Kirkeeide R.L., Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J. Am. Coll. Cardiol. 1990;15(2):459–474. doi: 10.1016/S0735-1097(10)80078-6. [DOI] [PubMed] [Google Scholar]
- 47.Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res. Cardiol. 2010;105(1):1–5. doi: 10.1007/s00395-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 48.van de Hoef T.P., Meuwissen M., Escaned J., Davies J.E., Siebes M., Spaan J.A., Piek J.J. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat. Rev. Cardiol. 2013;10(8):439–452. doi: 10.1038/nrcardio.2013.86. [DOI] [PubMed] [Google Scholar]
- 49.Pijls N.H., De Bruyne B., Peels K., Van Der Voort P.H., Bonnier H.J., Bartunek J Koolen J.J., Koolen J.J. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med. 1996;334(26):1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 50.de Bruyne B., Bartunek J., Sys S.U., Pijls N.H., Heyndrickx G.R., Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94(8):1842–1849. doi: 10.1161/01.CIR.94.8.1842. [DOI] [PubMed] [Google Scholar]
- 51.De Bruyne B., Bartunek J., Sys S.U., Heyndrickx G.R. Relation between myocardial fractional flow reserve calculated from coronary pressure measurements and exercise-induced myocardial ischemia. Circulation. 1995;92(1):39–46. doi: 10.1161/01.CIR.92.1.39. [DOI] [PubMed] [Google Scholar]
- 52.Melikian N., De Bondt P., Tonino P., De Winter O., Wyffels E., Bartunek J., Heyndrickx G.R., Fearon W.F., Pijls N.H., Wijns W., De Bruyne B. Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc. Interv. 2010;3(3):307–314. doi: 10.1016/j.jcin.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Petraco R., Sen S., Nijjer S., Echavarria-Pinto M., Escaned J., Francis D.P., Davies J.E. Fractional flow reserve-guided revascularization: practical implications of a diagnostic gray zone and measurement variability on clinical decisions. JACC Cardiovasc. Interv. 2013;6(3):222–225. doi: 10.1016/j.jcin.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 54.van de Hoef T.P., Nolte F., Rolandi M.C., Piek J.J., van den Wijngaard J.P., Spaan J.A., Siebes M. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J. Mol. Cell. Cardiol. 2012;52(4):786–793. doi: 10.1016/j.yjmcc.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 55.Shiono Y., Kubo T., Tanaka A., Kitabata H., Ino Y., Tanimoto T., Wada T., Ota S., Ozaki Y., Orii M., Shimamura K., Ishibashi K., Yamano T., Yamaguchi T., Hirata K., Imanishi T., Akasaka T. Impact of myocardial supply area on the transstenotic hemodynamics as determined by fractional flow reserve. Catheter. Cardiovasc. Interv. 2014;84(3):406–413. doi: 10.1002/ccd.25300. [DOI] [PubMed] [Google Scholar]
- 56.Meuwissen M., Chamuleau S.A., Siebes M., Schotborgh C.E., Koch K.T., de Winter R.J., Bax M., de Jong A., Spaan J.A., Piek J.J. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation. 2001;103(2):184–187. doi: 10.1161/01.CIR.103.2.184. [DOI] [PubMed] [Google Scholar]
- 57.Chamuleau S.A., Siebes M., Meuwissen M., Koch K.T., Spaan J.A., Piek J.J. Association between coronary lesion severity and distal microvascular resistance in patients with coronary artery disease. Am. J. Physiol. Heart Circ. Physiol. 2003;285(5):H2194–H2200. doi: 10.1152/ajpheart.01021.2002. [DOI] [PubMed] [Google Scholar]
- 58.Cannon R.O., III, Camici P.G., Epstein S.E. Pathophysiological dilemma of syndrome X. Circulation. 1992;85(3):883–892. doi: 10.1161/01.CIR.85.3.883. [DOI] [PubMed] [Google Scholar]
- 59.Greenberg M.A., Grose R.M., Neuburger N., Silverman R., Strain J.E., Cohen M.V. Impaired coronary vasodilator responsiveness as a cause of lactate production during pacing-induced ischemia in patients with angina pectoris and normal coronary arteries. J. Am. Coll. Cardiol. 1987;9(4):743–751. doi: 10.1016/S0735-1097(87)80227-9. [DOI] [PubMed] [Google Scholar]
- 60.Crake T., Canepa-Anson R., Shapiro L., Poole-Wilson P.A. Continuous recording of coronary sinus oxygen saturation during atrial pacing in patients with coronary artery disease or with syndrome X. Br. Heart J. 1988;59(1):31–38. doi: 10.1136/hrt.59.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cannon R.O., III, Epstein S.E. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am. J. Cardiol. 1988;61(15):1338–1343. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 62.Buffon A., Rigattieri S., Santini S.A., Ramazzotti V., Crea F., Giardina B., Maseri A. Myocardial ischemia-reperfusion damage after pacing-induced tachycardia in patients with cardiac syndrome X. Am. J. Physiol. Heart Circ. Physiol. 2000;279(6):H2627–H2633. doi: 10.1152/ajpheart.2000.279.6.H2627. [DOI] [PubMed] [Google Scholar]
- 63.Buchthal S.D., den Hollander J.A., Merz C.N., Rogers W.J., Pepine C.J., Reichek N., Sharaf B.L., Reis S., Kelsey S.F., Pohost G.M. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N. Engl. J. Med. 2000;342(12):829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 64.Motz W., Vogt M., Rabenau O., Scheler S., Lückhoff A., Strauer B.E. Evidence of endothelial dysfunction in coronary resistance vessels in patients with angina pectoris and normal coronary angiograms. Am. J. Cardiol. 1991;68(10):996–1003. doi: 10.1016/0002-9149(91)90485-4. [DOI] [PubMed] [Google Scholar]
- 65.Chauhan A., Mullins P.A., Taylor G., Petch M.C., Schofield P.M. Both endothelium-dependent and endothelium-independent function is impaired in patients with angina pectoris and normal coronary angiograms. Eur. Heart J. 1997;18(1):60–68. doi: 10.1093/oxfordjournals.eurheartj.a015119. [DOI] [PubMed] [Google Scholar]
- 66.Egashira K., Inou T., Hirooka Y., Yamada A., Urabe Y., Takeshita A. Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. N. Engl. J. Med. 1993;328(23):1659–1664. doi: 10.1056/NEJM199306103282302. [DOI] [PubMed] [Google Scholar]
- 67.Quyyumi A.A., Cannon R.O., III, Panza J.A., Diodati J.G., Epstein S.E. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation. 1992;86(6):1864–1871. doi: 10.1161/01.CIR.86.6.1864. [DOI] [PubMed] [Google Scholar]
- 68.Opherk D., Zebe H., Weihe E., Mall G., Dürr C., Gravert B., Mehmel H.C., Schwarz F., Kübler W. Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation. 1981;63(4):817–825. doi: 10.1161/01.CIR.63.4.817. [DOI] [PubMed] [Google Scholar]
- 69.Bøttcher M., Botker H.E., Sonne H., Nielsen T.T., Czernin J. Endothelium-dependent and -independent perfusion reserve and the effect of L-arginine on myocardial perfusion in patients with syndrome X. Circulation. 1999;99(14):1795–1801. doi: 10.1161/01.CIR.99.14.1795. [DOI] [PubMed] [Google Scholar]
- 70.Adamopoulos S., Rosano G.M., Ponikowski P., Cerquetani E., Piepoli M., Panagiota F., Collins P., Poole-Wilson P., Kremastinos D., Coats A.J. Impaired baroreflex sensitivity and sympathovagal balance in syndrome X. Am. J. Cardiol. 1998;82(7):862–868. doi: 10.1016/S0002-9149(98)00493-7. [DOI] [PubMed] [Google Scholar]
- 71.Gulli G., Cemin R., Pancera P., Menegatti G., Vassanelli C., Cevese A. Evidence of parasympathetic impairment in some patients with cardiac syndrome X. Cardiovasc. Res. 2001;52(2):208–216. doi: 10.1016/S0008-6363(01)00369-8. [DOI] [PubMed] [Google Scholar]
- 72.Lanza G.A., Giordano A., Pristipino C., Calcagni M.L., Meduri G., Trani C., Franceschini R., Crea F., Troncone L., Maseri A. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy. Circulation. 1997;96(3):821–826. doi: 10.1161/01.CIR.96.3.821. [DOI] [PubMed] [Google Scholar]
- 73.Chauhan A., Mullins P.A., Taylor G., Petch M.C., Schofield P.M. Effect of hyperventilation and mental stress on coronary blood flow in syndrome X. Br. Heart J. 1993;69(6):516–524. doi: 10.1136/hrt.69.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vlahakes G.J., Baer R.W., Uhlig P.N., Verrier E.D., Bristow J.D., Hoffmann J.I. Adrenergic influence in the coronary circulation of conscious dogs during maximal vasodilation with adenosine. Circ. Res. 1982;51(3):371–384. doi: 10.1161/01.RES.51.3.371. [DOI] [PubMed] [Google Scholar]
- 75.Johannsen U.J., Mark A.L., Marcus M.L. Responsiveness to cardiac sympathetic nerve stimulation during maximal coronary dilation produced by adenosine. Circ. Res. 1982;50(4):510–517. doi: 10.1161/01.RES.50.4.510. [DOI] [PubMed] [Google Scholar]
- 76.Lorenzoni R., Rosen S.D., Camici P.G. Effect of alpha 1-adrenoceptor blockade on resting and hyperemic myocardial blood flow in normal humans. Am. J. Physiol. 1996;271(4 Pt 2):H1302–H1306. doi: 10.1152/ajpheart.1996.271.4.H1302. [DOI] [PubMed] [Google Scholar]
- 77.Barbato E., Bartunek J., Aarnoudse W., Vanderheyden M., Staelens F., Wijns W., Heyndrickx G.R., Pijls N.H., De Bruyne B. Alpha-adrenergic receptor blockade and hyperaemic response in patients with intermediate coronary stenoses. Eur. Heart J. 2004;25(22):2034–2039. doi: 10.1016/j.ehj.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Aarnoudse W., Geven M., Barbato E., Botman K.J., De Bruyne B., Pijls N.H. Effect of phentolamine on the hyperemic response to adenosine in patients with microvascular disease. Am. J. Cardiol. 2005;96(12):1627–1630. doi: 10.1016/j.amjcard.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 79.Lanza G.A., Lüscher T.F., Pasceri V., Shaw S.G., Buffon A., Montenero A.S., Crea F., Maseri A. Effects of atrial pacing on arterial and coronary sinus endothelin-1 levels in syndrome X. Am. J. Cardiol. 1999;84(10):1187–1191. doi: 10.1016/S0002-9149(99)00532-9. [DOI] [PubMed] [Google Scholar]
- 80.Epstein S.E., Cannon R.O., III Site of increased resistance to coronary flow in patients with angina pectoris and normal epicardial coronary arteries. J. Am. Coll. Cardiol. 1986;8(2):459–461. doi: 10.1016/S0735-1097(86)80067-5. [DOI] [PubMed] [Google Scholar]
- 81.Rosano G.M., Peters N.S., Lefroy D., Lindsay D.C., Sarrel P.M., Collins P., Poole-Wilson P.A. 17-beta-Estradiol therapy lessens angina in postmenopausal women with syndrome X. J. Am. Coll. Cardiol. 1996;28(6):1500–1505. doi: 10.1016/S0735-1097(96)00348-8. [DOI] [PubMed] [Google Scholar]
- 82.Thompson J., Khalil R.A. Gender differences in the regulation of vascular tone. Clin. Exp. Pharmacol. Physiol. 2003;30(1-2):1–15. doi: 10.1046/j.1440-1681.2003.03790.x. [DOI] [PubMed] [Google Scholar]
- 83.Darkow D.J., Lu L., White R.E. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am. J. Physiol. 1997;272(6 Pt 2):H2765–H2773. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 84.Knot H.J., Lounsbury K.M., Brayden J.E., Nelson M.T. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. Am. J. Physiol. 1999;276(3 Pt 2):H961–H969. doi: 10.1152/ajpheart.1999.276.3.H961. [DOI] [PubMed] [Google Scholar]
- 85.Wellman G.C., Bonev A.D., Nelson M.T., Brayden J.E. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ. Res. 1996;79(5):1024–1030. doi: 10.1161/01.RES.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 86.Rahimian R., Wang X., van Breemen C. Gender difference in the basal intracellular Ca2+ concentration in rat valvular endothelial cells. Biochem. Biophys. Res. Commun. 1998;248(3):916–919. doi: 10.1006/bbrc.1998.9068. [DOI] [PubMed] [Google Scholar]
- 87.Barbacanne M.A., Rami J., Michel J.B., Souchard J.P., Philippe M., Besombes J.P., Bayard F., Arnal J.F. Estradiol increases rat aorta endothelium-derived relaxing factor (EDRF) activity without changes in endothelial NO synthase gene expression: possible role of decreased endothelium-derived superoxide anion production. Cardiovasc. Res. 1999;41(3):672–681. doi: 10.1016/S0008-6363(98)00254-5. [DOI] [PubMed] [Google Scholar]
- 88.Johnson B.D., Zheng W., Korach K.S., Scheuer T., Catterall W.A., Rubanyi G.M. Increased expression of the cardiac L-type calcium channel in estrogen receptor-deficient mice. J. Gen. Physiol. 1997;110(2):135–140. doi: 10.1085/jgp.110.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehta P.K., Milic M., Bharadwaj M. Cardiac autonomic function in response to emotional mental stress in women with microvascular coronary dysfunction.; 2014. [Google Scholar]
- 90.Bybee K.A., Prasad A., Barsness G.W., Lerman A., Jaffe A.S., Murphy J.G., Wright R.S., Rihal C.S. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am. J. Cardiol. 2004;94(3):343–346. doi: 10.1016/j.amjcard.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 91.Cecchi E., Parodi G., Giglioli C., Passantino S., Bandinelli B., Liotta A.A., Bellandi B., Cioni G., Costanzo M., Abbate R., Gensini G.F., Antoniucci D., Mannini L. Stress-induced hyperviscosity in the pathophysiology of takotsubo cardiomyopathy. Am. J. Cardiol. 2013;111(10):1523–1529. doi: 10.1016/j.amjcard.2013.01.304. [DOI] [PubMed] [Google Scholar]
- 92.Sadamatsu K., Tashiro H., Maehira N., Yamamoto K. Coronary microvascular abnormality in the reversible systolic dysfunction observed after noncardiac disease. Jpn. Circ. J. 2000;64(10):789–792. doi: 10.1253/jcj.64.789. [DOI] [PubMed] [Google Scholar]
- 93.Ueyama T., Kasamatsu K., Hano T., Yamamoto K., Tsuruo Y., Nishio I. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ‘tako-tsubo’ cardiomyopathy. Circ. J. 2002;66(7):712–713. doi: 10.1253/circj.66.712. [DOI] [PubMed] [Google Scholar]
- 94.Lyon A.R., Rees P.S., Prasad S., Poole-Wilson P.A., Harding S.E. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(1):22–29. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 95.Bielecka-Dabrowa A., Mikhailidis D.P., Hannam S., Rysz J., Michalska M., Akashi Y.J., Banach M. Takotsubo cardiomyopathy--the current state of knowledge. Int. J. Cardiol. 2010;142(2):120–125. doi: 10.1016/j.ijcard.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 96.Ueyama T., Hano T., Kasamatsu K., Yamamoto K., Tsuruo Y., Nishio I. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J. Cardiovasc. Pharmacol. 2003;42(Suppl. 1):S117–S119. doi: 10.1097/00005344-200312001-00024. [DOI] [PubMed] [Google Scholar]
- 97.Ueyama T., Ishikura F., Matsuda A., Asanuma T., Ueda K., Ichinose M., Kasamatsu K., Hano T., Akasaka T., Tsuruo Y., Morimoto K., Beppu S. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ. J. 2007;71(4):565–573. doi: 10.1253/circj.71.565. [DOI] [PubMed] [Google Scholar]
- 98.Kim H.S., Tonino P.A., De Bruyne B., Yong A.S., Tremmel J.A., Pijls N.H., Fearon W.F., FAME Study Investigators The impact of sex differences on fractional flow reserve-guided percutaneous coronary intervention: a FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) substudy. JACC Cardiovasc. Interv. 2012;5(10):1037–1042. doi: 10.1016/j.jcin.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 99.Kang S.J., Ahn J.M., Han S., Lee J.Y., Kim W.J., Park D.W., Lee S.W., Kim Y.H., Lee C.W., Park S.W., Mintz G.S., Park S.J. Sex differences in the visual-functional mismatch between coronary angiography or intravascular ultrasound versus fractional flow reserve. JACC Cardiovasc. Interv. 2013;6(6):562–568. doi: 10.1016/j.jcin.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 100.Li J., Rihal C.S., Matsuo Y., Elrashidi M.Y., Flammer A.J., Lee M.S., Cassar A., Lennon R.J., Herrmann J., Bell M.R., Holmes D.R., Bresnahan J.F., Hua Q., Lerman L.O., Lerman A. Sex-related differences in fractional flow reserve-guided treatment. Circ. Cardiovasc. Interv. 2013;6(6):662–670. doi: 10.1161/CIRCINTERVENTIONS.113.000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fineschi M., Guerrieri G., Orphal D., Palmerini E., Münzel T., Warnholtz A., Pierli C., Gori T. The impact of gender on fractional flow reserve measurements. EuroIntervention. 2013;9(3):360–366. doi: 10.4244/EIJV9I3A58. [DOI] [PubMed] [Google Scholar]
- 102.Bairey Merz C.N., Shaw L.J., Reis S.E., Bittner V., Kelsey S.F., Olson M., Johnson B.D., Pepine C.J., Mankad S., Sharaf B.L., Rogers W.J., Pohost G.M., Lerman A., Quyyumi A.A., Sopko G., WISE Investigators Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J. Am. Coll. Cardiol. 2006;47(3) Suppl.:S21–S29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 103.Lin F.Y., Devereux R.B., Roman M.J., Meng J., Jow V.M., Jacobs A., Weinsaft J.W., Shaw L.J., Berman D.S., Callister T.Q., Min J.K. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc. Imaging. 2008;1(6):782–786. doi: 10.1016/j.jcmg.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 104.Iqbal M.B., Shah N., Khan M., Wallis W. Reduction in myocardial perfusion territory and its effect on the physiological severity of a coronary stenosis. Circ. Cardiovasc. Interv. 2010;3(1):89–90. doi: 10.1161/CIRCINTERVENTIONS.109.904193. [DOI] [PubMed] [Google Scholar]