Abstract
Nowadays, the worldwide number of left ventricular assist devices (LVADs) being implanted per year is higher than the number of cardiac transplantations. The rapid developments in the field of mechanical support are characterized by continuous miniaturization and enhanced performance of the pumps, providing increased device durability and a prolonged survival of the patients. The miniaturization process enabled minimally-invasive implantation methods, which are associated with generally benefitting the overall outcome of patients. Therefore, these new implantation strategies are considered the novel state of the art in LVAD surgery.
In this paper we provide a comprehensive review on the existing literature on minimally-invasive techniques with an emphasis on the different implantation approaches and their individual surgical challenges.
Keywords: Ventricular Assist Device, VAD, Left Ventricular Assist Device, LVAD, Minimal-invasive surgery, cardiac surgery, implantation techniques.
INTRODUCTION
According to today´s definition, the first left ventricular assist device was implanted minimally-invasively. In July of 1963, Stanley Crawford and Domingo Liotta implanted the first intrathoracic LVAD in a human bypassing the left ventricle from the left atrium to the descending thoracic aorta via left thoracotomy [1-4]. Mankind long dreamed of creating a total artificial heart (TAH) that could replace the failing human heart [5, 6]. Left ventricular assist devices (LVAD) evolved due to further clinical findings on terminal heart failure and its profound impact on the left ventricle, creating another milestone towards the development of the TAH. Three years after the first LVAD implantation in April 1966, DeBakey and Liotta implanted the first clinical LVAD in a paracorporal position, bypassing the left ventricle from the left atrium to the ascending aorta via full sternotomy. In both cases the patients died of perioperative complications [3, 4]. The first successful treatment by a LVAD took place in October 1966 when DeBakey and Liotta implanted a temporary support in a patient in postcardiotomy shock – again via full sternotomy [2-4].Eventually, the first TAH implantation in man was performed by Denton A. Cooley in April of 1969 – two years after Christian Barnard performed the first human heart transplantation and three months before Apollo 11 landed on the moon [5-9].
The following rapid developments in the field of mechanical support are characterized by continuous miniaturization and enhanced performance, providing increased device durability and a prolonged survival of the patients. Yet, despite all mechanical advances, heart transplantation is still the gold standard for the therapy of end stage heart failure [10-13].
However, transplantation is limited by donor shortage, which is causing mortality rates of approximately 30% on the waiting lists. Subsequently, the total amount of transplanted hearts has been continuously decreasing in the past years [14, 15].
Implanting LVADs “off the shelf” is an attractive alternative to transplantation. This applies especially to emergency cases as a bridge-to-transplant, or for patients who do not fulfill the transplantation requirements. Nowadays, the worldwide number of LVADs being implanted per year is higher than the number of cardiac transplantations.
LVADs clinical success is based on the shift from pulsatile to continuous flow pumps [16, 17]. With this change in paradigm, the durability of the pumps increased, while simultaneously the size and weight of the devices decreased. This has enabled minimally-invasive surgery for the implantation, explantation and exchange of ventricular assist devices [18-22]. Minimally-invasive cardio surgical procedures are known to be generally associated with many positive effects, e.g. reduction of trauma, blood loss and infection, decrease in intensive care unit and in-hospital stay as well as better outcomes [21-24]. The new developments and implantation techniques of LVADs equally contribute to the improvement of therapy outcomes by reducing important operative complication rates such as right ventricular failure by avoiding pericardial opening [18]. Since the miniaturization process of LVADs steadily continues, it is certainly possible that soon the majority of implantations will be performed using less invasive techniques.
This paper reviews the literature on minimal-invasive implantation techniques for the most common adult left ventricular assist devices (HVAD, HeartWare Inc., USA; HeartMate II (HMII), Thoratec Cooperation, USA).
METHOD
A systematic review of the literature was carried out using the Medline database and the search terms “left ventricular assist device”, “LVAD”, “minimal-invasive”, “less-invasive”, “HVAD”, “HeartWare”, “HeartMate II” and “new approach”. The literature search was carried out in October 2014. Only English language papers were incorporated in this review.
DEFINING MINIMAL-INVASIVENESS IN LVAD SURGERY
To this present day there is no common definition of minimal invasiveness existing. In this review, minimally-invasive LVAD surgery is defined as "non-sternotomy approach" which is associated with reduced trauma, thereby representing less invasive surgery compared to the conventional implantation technique via full sternotomy. Yet, miniaturization of the approach in LVAD surgery is limited due to the size of the devices.
CONVENTIONAL LVAD IMPLANTATION TECHNIQUE
The conventional access for LVAD implantation is a full median sternotomy with Heart-Lung-Machine cannulation sites in the ascending aorta and the right atrium (RA). The prominent advantages of this approach are the exemplary anatomical overview and the basic access to the left ventricle (LV). Thus, disadvantages include risks for sternal instabilities and infection as well as postoperative bleedings. It is also prone to secondary right heart failure and especially challenging in re-operative cases [10, 18, 24].
New implantation methods derived from the conventional technique alternating the access paths to pump positioning, outflow graft anastomosis and cannulation site in order to avoid full sternotomy. The major variations will be described in the following passages.
VARIATIONS OF PUMP POSITIONING
The size restraining incision of LVAD implantation is the access path to the left ventricle due to the corpus size of the VADs.
Schmitto et al. use an anterolateral thoracotomy to place the sewing ring of the HVAD onto the left ventricle [Figure 1]. The pump is inserted and the outflow graft tunneled through the pleural cavity to the ascending aorta. Then, the outflow graft is anastomosed through an upper hemi-sternotomy [18]. The Viennese approach also uses the left lateral mini-thoracotomy to place the HVAD or a left subcostal incision for the placement of the HeartMate II [24]. Popov et al. describe an implantation technique via bilateral anterior thoracotomy incisions [25].
Fig. (1).
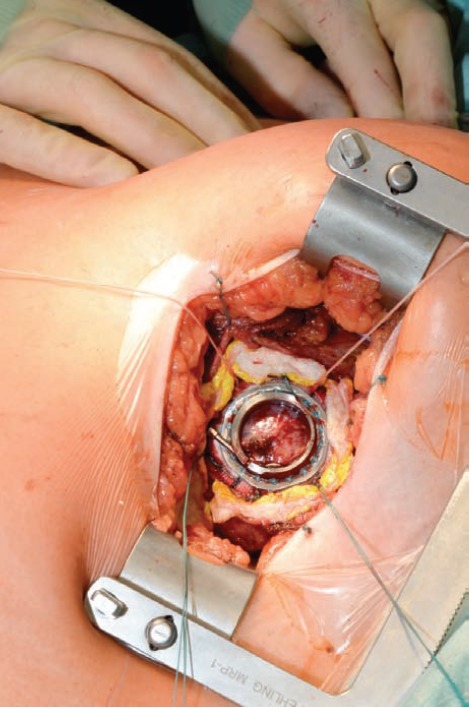
LVAD Pump placement in anterolateral thoracotomy.
The dominating minimal-invasive technique to implant the corpus of the HeartMate II (HMII) is a left subcostal incision [26]. Due to its size a subcostal pump pocket is needed to fit the pump into the thorax. Gregoric et al. perform a subcostal incision and separate the interfering muscles stopping extraperitoneally above the transverse muscle fascia. Then the pleura is opened in order o allow access to the pericardium and the left ventricular apex below [27]. LVAD exchange is also described via this technique [28]. Anyanwu et al. modified this technique and use it as their routine implantation strategy [29]. Samuels et al. also alternated the approach, combining a left-sided lateral thoracotomy and a partial midline upper abdominal pre-peritoneal laparotomy to form the pump pocket [30].
VARIATIONS OF OUTFLOW GRAFT ANASTOMOSIS
The positioning of the outflow-graft plays an important role in the long term outcome of the patient. False positioning leads to turbulences in the blood flow, resulting in a high risk of outflow graft thrombosis or kinking [31-33].
Schmitto et al. chose an upper mini sternotomy to perform the cannulation and the placement of the outflow graft by tunneling it through the right pleural cavity, leaving the pericardium closed and reducing the risk for right heart failure [18] (Figs. 2 and 3).
Fig. (2).
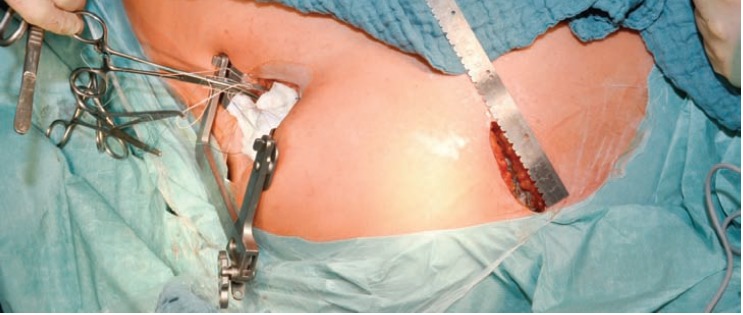
Hannover LVAD implantation technique combining an upper hemi sternotomy with an anterolateral thoracotomy [18].
Fig. (3).
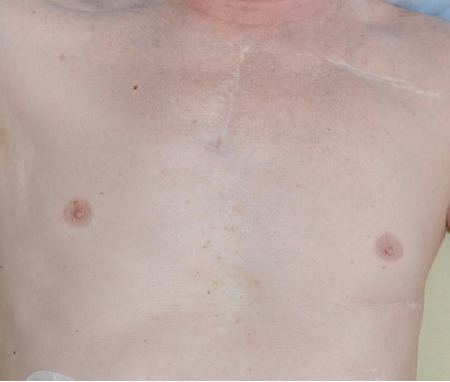
Cosmetic result three years after minimal-invasive LVAD implantation.
Other approaches avoid sternotomy completely and place the outflow graft to other major arteries. Possible options are the subclavian artery, the innominate artery or the descending aorta.
Strueber et al. use a left clavicular incision to suture the outflow graft onto the left sublavian artery [34]. The London Harefield group uses a combination of bilateral anterior thoracotomies to implant the HVAD, using the left subclavian artery for the graft placement [35].
The Viennese group uses a right mini-thoracotomy in the second intercostals space but if additional valve procedures are necessary they switch to mini-sternotomy [24]. Zimpfer et al. described a minimally-invasive HeartMate II implantation in the setting of severe aortic calcification in which the outflow graft is tunneled through the diaphragm, the right thoracic cavity, and the second intercostal space, and is anastomosed to the right subclavian artery [36]. A variation of this approach is the anastomosis of the graft to the bracheocephalical trunk. Endoscopic techniques to suture the outflow graft to the subclavian artery are currently in development to further increase the incision sites. Khalpey et al. introduced the technique of robotic-assisted LVAD implantation after pectoral muscle flap, thereby preserving the flap for transplantation [37].
Less prevalently used than the anastomosis to the ascending aorta is the alternate anastomosis to the descending aorta. Some studies suggest neither outflow-graft location to be inferior to the other. Yet, by the use of this technique the aortic arch remains a region without net flow and is prone to stasis resulting in thrombogenesis [38-40].
VARIATIONS OF CARDIOPULMONARY BYPASS
The conventional cannulation sites for cardiopulmonary bypass (CPB) are the ascending aorta and the right atrium (RA). Variations include the percutaneus or open cannulation of the femoral artery and vein as well as the combination of mini-sternotomy and cannulation of the ascending aorta and the femoral vein [18, 24]. Biventricular cannulation has been described in biventricular assist device implantation [42].
LVAD surgery can also be performed without the use of CPB. The “Off-pump” technique has been described by several groups in device implant, explant and exchange settings [20, 24, 43-50]. Positive assets of the “On-pump” technique are the possibility to inspect the left ventricle (LV) and the superior hemodynamic management options which permits combined cardiac procedures e.g. valve replacements or closing septal defects [24, 41, 51, 52].
VARIATION OF DRIVELINE EXIT SITES
The external driveline is the Achilles` heel of assist devices. It is prone to infection and mechanical stress, causing major complications in VAD therapy [53-56]. The conventional technique is the short and straight driveline tunnel. A modification of this approach is a double subfascial tunneling [57, 58]. Singh et al. describe advantages in externalization of only the silicone portion of the driveline regarding infections [59]. The Hannover group prefers their own double tunnel technique [58] and decides on left or right driveline exit side based on the patients´ history, e.g. sleeping side preferences as well as the handedness of the patient [60].
DISCUSSION
The described minimal-invasive approaches are the novel state of the art in LVAD surgery. Systemic literature review showed, that compared to the conventional approach they are associated with many positive effects, e.g. reduction of trauma, blood loss and infection, decrease in intensive care unit and in-hospital stay as well as better outcomes [19-24]. The new developments and implantation techniques of LVADs contribute to the improvement of therapy outcomes by reducing important operative complication rates such as right ventricular failure by avoiding pericardial opening [18]. Yet, long-term results are still missing.
Based on the literature review, the common denominator of the various minimal-invasive implantation techniques is the avoidance of full sternotomy, which represents a common trend in cardiac surgery. Minimal-invasive LVAD implantation is a combination of accessing the apex of the left ventricle via thoracotomy, creating a pump pocket [18, 24-26, 28-30] and a choice of possible outflow graft anastomosis positions [18, 24, 34-37]. The choice of procedure is contingent upon the anatomical situation of the patient.
Since the anatomy of re-operative cases is often dominated by adhesions, minimally-invasive techniques offer less traumatic access to the target regions. Furthermore, fewer adhesions are caused, benefitting the anatomical situation in bridge-to-transplant cases [18, 24].
A variation of outflow graft anastomosis positions, to major arteries of the aortic arch or the descending aorta, offer alternative approaches in challenging cases e.g. porcelain aorta [18, 24, 34-37]. Yet, placing the outflow graft to other structures than the ascending aorta may result in flow turbulences or stasis leading to thrombogenesis [38-40].
Robotic-assisted implantation of ventricular assist devices is another step towards further minimizing the surgical trauma of LVAD implantation [37].
Minimal-invasive LVAD implantation may be combined with other procedures e.g. aortic valve repair or closing septal defects [24, 41, 51, 52]. However, the extension of major surgery is limited by the reduced sight and access possibilities and requires highly experienced surgeons.
“On-” or “Off-pump” implantation offers another variation of the approach, providing the opportunity to reduce the operating time and the negative effects of the heart-lung-machine [20, 24, 34, 43-48] or increase the hemodynamic safety of the operation and the chance to fully inspect the heart e.g. for ventricular thrombus or perform combined procedures [24, 51, 52]. Recently, on June 25th, 2014, the worldwide first-in-man-Implantation of the novel centrifugal LVAD Heartmate III (Thoratec Corp., Pleasanton, CA, USA) was performed in Hannover, Germany [60]. It is expected to be possibly minimally-invasively impanted soon, too.
Despite the many benefits of this method, there is no gold standard in minimally-invasive LVAD surgery as long-term results of the outcomes are still missing. Consequently, the surgeon needs to combine the mentioned implantation strategies to fit individually to the patient in order to guarantee the best results. In summary, minimally-invasive LVAD implantation is the novel state of the art in LVAD surgery due to its association with multiple positive effects compared to the conventional implantation method.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest
Table 1.
Overview of Minimal-invasive Left Ventricular Assist Device Implantation techniques
| Reference | Device | Outflow graft position | Pump placement |
|---|---|---|---|
| Schmitto JD [18] J Thorac Cardiovasc Surg. 2012 |
Heart Ware ”HVAD” | Ascending aorta via mini sternotomy | Anterolateral thoracotomy |
| Haberl T [24] Eur J Cardiothorac Surg. 2014 |
Heart Ware ”HVAD” | Ascending aorta via mini sternotomy | Left lateral mini-thoracotomy or subcostal incision |
| Popov AF [25] Ann Thorac Surg. 2012 |
Heart Ware ”HVAD” | Ascending aorta via bilateral thoracotomy | Bilateral thoracotomy incisions |
| Strueber M [34] J Heart Lung Transplant. 2014 |
Heart Ware ”HVAD” | Left subclavian artery | Left lateral mini thoracotomy |
| Hanke JS, Schmitto JD [35] J Thorac Cardiovasc Surg |
Heart Ware ”HVAD” | Innominate artery | Anterolateral thoracotomy |
| Gregoric ID [27] J Heart Lung Transplant. 2008 |
Thoratec “Heart Mate II” | Ascending aorta via mini sternotomy | Left subcostal incision and separate the interfering muscles stopping extraperitoneally above the transverse muscle fascia |
| Anyanwu AC [29] Semin Thorac Cardiovasc Surg. 2011 |
Thoratec “Heart Mate II” | Ascending aorta via mini sternotomy | Left subcostal incision |
| Samuels LE [30] J Cardiothorac Surg 2012 | Thoratec “Heart Mate II” | Ascending aorta via mini-sternotomy | Left-sided lateral thoracotomy and partial midline upper abdominal pre-peritoneal laparotomy |
| Riebandt J [36] Ann Thorac Surg. 2013 |
Thoratec “Heart Mate II” | Right subclavian artery | Left subcostal incision with preperitoneal creation of the pump pocket |
| Khalpey Z, Schmitto JD [37] | Heart Ware ”HVAD” | Robotic anastomosis to the Ascending aorta | Anterolateral thoracotomy |
ACKNOWLEDGEMENTS
Declared none
References
- 1.Liotta D., Hall C.W., Henly W.S., Cooley D.A., Crawford E.S., Debakey M.E. Prolonged assisted circulation during and after cardiac or aortic surgery. Prolonged partial left ventricular bypass by means of intracorporeal circulation. Am. J. Cardiol. 1963;12:399–405. doi: 10.1016/0002-9149(63)90235-2. [DOI] [PubMed] [Google Scholar]
- 2.Liotta D., Hall C.W., Maness J.H., Debakey M.E. The implantable intrathoracic circulatory pump: surgical technique. Cardiovasc. Res. Cent. Bull. 1964;92:54–61. [PubMed] [Google Scholar]
- 3.DeBakey M. Research in the Service of Man: Biomedical Knowledge. Development and Use. Committee of Government Operations. United States Senate, USA. Washington, DC: Government Printing Office; 1967. [Google Scholar]
- 4.Liotta D. Artificial heart left ventricular assist devices (LVADs): a bridge-to-recovery--the novel LVAD III-intrathoracic small blood pump with atriostomy drainage for combination therapies. Ann. Thorac. Cardiovasc. Surg. 2008;14(5):271–273. [Editorial]. [PubMed] [Google Scholar]
- 5.Cooley D.A., Liotta D., Hallman G.L., Bloodwell R.D., Leachman R.D., Milam J.D. Orthotopic cardiac prosthesis for two-staged cardiac replacement. Am. J. Cardiol. 1969;24(5):723–730. doi: 10.1016/0002-9149(69)90460-3. [DOI] [PubMed] [Google Scholar]
- 6.Cooley D.A. 100,000 hearts: a surgeon’s memoir. Austin, TX: Dolph Briscoe Center for American History; 2012. p. xi. [Google Scholar]
- 7.Cooley D.A. Some thoughts about the historic events that led to the first clinical implantation of a total artificial heart. Tex. Heart Inst. J. 2013;40(2):117–119. [PMC free article] [PubMed] [Google Scholar]
- 8.Barnard C.N. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S. Afr. Med. J. 1967;41(48):1271–1274. [PubMed] [Google Scholar]
- 9.Strueber M., Schmitto J.D., Kutschka I., Haverich A. Placement of 2 implantable centrifugal pumps to serve as a total artificial heart after cardiectomy. J. Thorac. Cardiovasc. Surg. 2012;143(2):507–509. doi: 10.1016/j.jtcvs.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Strueber M., O’Driscoll G., Jansz P., Khaghani A., Levy W.C., Wieselthaler G.M., HeartWare Investigators Multicenter evaluation of an intrapericardial left ventricular assist system. J. Am. Coll. Cardiol. 2011;57(12):1375–1382. doi: 10.1016/j.jacc.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Slaughter M.S., Rogers J.G., Milano C.A., Russell S.D., Conte J.V., Feldman D., Sun B., Tatooles A.J., Delgado R.M., III, Long J.W., Wozniak T.C., Ghumman W., Farrar D.J., Frazier O.H., HeartMate II Investigators Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 12.Mazzucotelli J-P., Leprince P., Litzler P-Y., Vincentelli A., Le Guyader A., Kirsch M., Camilleri L., Flecher E., reflection group on mechanical circulatory support (GRAM) Results of mechanical circulatory support in France. Eur. J. Cardiothorac. Surg. 2011;40(3):e112–e117. doi: 10.1016/j.ejcts.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Rose E.A., Gelijns A.C., Moskowitz A.J., Heitjan D.F., Stevenson L.W., Dembitsky W., Long J.W., Ascheim D.D., Tierney A.R., Levitan R.G., Watson J.T., Meier P., Ronan N.S., Shapiro P.A., Lazar R.M., Miller L.W., Gupta L., Frazier O.H., Desvigne-Nickens P., Oz M.C., Poirier V.L., Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 14.Stehlik J., Edwards L.B., Kucheryavaya A.Y., Benden C., Christie J.D., Dobbels F., Kirk R., Rahmel A.O., Hertz M.I. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J. Heart Lung Transplant. 2011;30(10):1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Schmid C, Schmitto JD, Scheld HH. Herztransplantation in Deutschland. Steinkopf Darmstadt. 2003. ISBN No. 3-7985-1390-2.
- 16.Schima H., Schlöglhofer T., zu Dohna R., Drews T., Morshuis M., Roefe D., Schmitto J.D., Strüber M., Zimpfer D. Usability of ventricular assist devices in daily experience: a multicenter study. Artif. Organs. 2014;38(9):751–760. doi: 10.1111/aor.12394. [DOI] [PubMed] [Google Scholar]
- 17.Strueber M., Larbalestier R., Jansz P., Zimpfer D., Fiane A.E., Tsui S., Simon A., Schmitto J.D., Khaghani A., Wieselthaler G.M., Najarian K., Schueler S. Results of the post-market Registry to Evaluate the HeartWare Left Ventricular Assist System (ReVOLVE). J. Heart Lung Transplant. 2014;33(5):486–491. doi: 10.1016/j.healun.2014.01.856. [DOI] [PubMed] [Google Scholar]
- 18.Schmitto J.D., Molitoris U., Haverich A., Strueber M. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J. Thorac. Cardiovasc. Surg. 2012;143(2):511–513. doi: 10.1016/j.jtcvs.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Schmitto J.D., Rojas S.V., Hanke J.S., Avsar M., Haverich A. Minimally invasive left ventricular assist device explantation after cardiac recovery: surgical technical considerations. Artif. Organs. 2014;38(6):507–510. doi: 10.1111/aor.12201. [DOI] [PubMed] [Google Scholar]
- 20.Rojas S.V., Avsar M., Khalpey Z., Hanke J.S., Haverich A., Schmitto J.D. Minimally invasive off-pump left ventricular assist device exchange: anterolateral thoracotomy. Artif. Organs. 2014;38(7):539–542. doi: 10.1111/aor.12226. [DOI] [PubMed] [Google Scholar]
- 21.Schmitto J.D., Mohr F.W., Cohn L.H. Minimally invasive aortic valve replacement: how does this perform in high-risk patients? Curr. Opin. Cardiol. 2011;26(2):118–122. doi: 10.1097/HCO.0b013e328343983a. [DOI] [PubMed] [Google Scholar]
- 22.Schmitto J.D., Mokashi S.A., Cohn L.H. Minimally-invasive valve surgery. J. Am. Coll. Cardiol. 2010;56(6):455–462. doi: 10.1016/j.jacc.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 23.Schmitto J.D., Mokashi S.A., Cohn L.H. Past, present, and future of minimally invasive mitral valve surgery. J. Heart Valve Dis. 2011;20(5):493–498. [PubMed] [Google Scholar]
- 24.Haberl T., Riebandt J., Mahr S., Laufer G., Rajek A., Schima H., Zimpfer D. Viennese approach to minimize the invasiveness of ventricular assist device implantation. Eur. J. Cardiothorac. Surg. 2014;46(6):991–996. doi: 10.1093/ejcts/ezu051. [DOI] [PubMed] [Google Scholar]
- 25.Popov A.F., Hosseini M.T., Zych B., Simon A.R., Bahrami T. HeartWare left ventricular assist device implantation through bilateral anterior thoracotomy. Ann. Thorac. Surg. 2012;93(2):674–676. doi: 10.1016/j.athoracsur.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 26.Hill J.D., Avery G.J., Egrie G., Turley K., Reichenbach S. Less invasive Thoratec LVAD insertion: a surgical technique. Heart Surg. Forum. 2000;3(3):218–223. [PubMed] [Google Scholar]
- 27.Gregoric I.D., La Francesca S., Myers T., Cohn W., Loyalka P., Kar B., Gemmato C., Frazier O.H. A less invasive approach to axial flow pump insertion. J. Heart Lung Transplant. 2008;27(4):423–426. doi: 10.1016/j.healun.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Gregoric I.D., Bruckner B.A., Jacob L., Kar B., Cohn W.E., La Francesca S., Frazier O.H. Clinical experience with sternotomy versus subcostal approach for exchange of the HeartMate XVE to the HeartMate II ventricular assist device. Ann. Thorac. Surg. 2008;85(5):1646–1649. doi: 10.1016/j.athoracsur.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Anyanwu A.C. Technique for less invasive implantation of Heartmate II left ventricular assist device without median sternotomy. Semin. Thorac. Cardiovasc. Surg. 2011;23(3):241–244. doi: 10.1053/j.semtcvs.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Samuels L.E., Casanova-Ghosh E., Rodriguez R., Droogan C. Left ventricular assist device implantation in high risk destination therapy patients: an alternative surgical approach. J. Cardiothorac. Surg. 2012;7:21. doi: 10.1186/1749-8090-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kar B., Delgado R.M., III, Frazier O.H., Gregoric I.D., Harting M.T., Wadia Y., Myers T.J., Moser R.D., Freund J. The effect of LVAD aortic outflow-graft placement on hemodynamics and flow: Implantation technique and computer flow modeling. Tex. Heart Inst. J. 2005;32(3):294–298. [PMC free article] [PubMed] [Google Scholar]
- 32.Benk C., Mauch A., Beyersdorf F., Klemm R., Russe M., Blanke P., Korvink J.G., Markl M., Jung B. Effect of cannula position in the thoracic aorta with continuous left ventricular support: four-dimensional flow-sensitive magnetic resonance imaging in an in vitro model. Eur. J. Cardiothorac. Surg. 2013;44(3):551–558. doi: 10.1093/ejcts/ezt095. [DOI] [PubMed] [Google Scholar]
- 33.Schmitto J.D., Avsar M., Haverich A. Increase in left ventricular assist device thrombosis. N. Engl. J. Med. 2014;370(15):1463–1464. doi: 10.1056/NEJMc1401768. [DOI] [PubMed] [Google Scholar]
- 34.Strueber M., Meyer A.L., Feussner M., Ender J., Correia J.C., Mohr F.W. A minimally invasive off-pump implantation technique for continuous-flow left ventricular assist devices: early experience. J. Heart Lung Transplant. 2014;33(8):851–856. doi: 10.1016/j.healun.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Hanke J.S., Rojas S.V., Martens A., Schmitto J.D. Minimally invasive left ventricular assist device implantation with outflow graft anastomosis to the innominate artery. J. Thorac. Cardiovasc. Surg. 2015;149(4):e69–e70. doi: 10.1016/j.jtcvs.2014.12.066. [DOI] [PubMed] [Google Scholar]
- 36.Riebandt J., Sandner S., Mahr S., Haberl T., Rajek A., Laufer G., Schima H., Zimpfer D. Minimally invasive thoratec Heartmate II implantation in the setting of severe thoracic aortic calcification. Ann. Thorac. Surg. 2013;96(3):1094–1096. doi: 10.1016/j.athoracsur.2013.04.114. [DOI] [PubMed] [Google Scholar]
- 37.Khalpey Z., Riaz I.B., Marsh K.M., Ansari M.Z., Bilal J., Cooper A., Paidy S., Schmitto J.D., Smith R., Friedman M., Slepian M.J., Poston R. Robotic Left Ventricular Assist Device (LVAD) Implantation Using Left Thoracotomy Approach In Patients With Previous Sternotomies. ASAIO J; 2015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Nawata K., Nishimura T., Kyo S., Hisagi M., Kinoshita O., Saito A., Motomura N., Takamoto S., Ono M. Outcomes of midterm circulatory support by left ventricular assist device implantation with descending aortic anastomosis. J. Artif. Organs. 2010;13(4):197–201. doi: 10.1007/s10047-010-0521-0. [DOI] [PubMed] [Google Scholar]
- 39.Tuzun E., Narin C., Gregoric I.D., Cohn W.E., Frazier O.H. Ventricular assist device outflow-graft site: effect on myocardial blood flow. J. Surg. Res. 2011;171(1):71–75. doi: 10.1016/j.jss.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Kar B., Delgado R.M., III, Frazier O.H., Gregoric I.D., Harting M.T., Wadia Y., Myers T.J., Moser R.D., Freund J. The effect of LVAD aortic outflow-graft placement on hemodynamics and flow: Implantation technique and computer flow modeling. Tex. Heart Inst. J. 2005;32(3):294–298. [PMC free article] [PubMed] [Google Scholar]
- 41.Mokashi S.A., Schmitto J.D., Lee L.S., Rawn J.D., Bolman R.M., III, Shekar P.S., Couper G.S., Chen F.Y. Ventricular assist device in patients with prosthetic heart valves. Artif. Organs. 2010;34(11):1030–1034. doi: 10.1111/j.1525-1594.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- 42.Schlensak C., Schibilsky D., Siepe M., Brehm K., Klemm R., von Wattenwyl R., Berchthold-Herz M., Benk C., Beyersdorf F. Biventricular cannulation is superior regarding hemodynamics and organ recovery in patients on biventricular assist device support. J. Heart Lung Transplant. 2011;30(9):1011–1017. doi: 10.1016/j.healun.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Cohn W.E., Frazier O.H. Off-pump insertion of an extracorporeal LVAD through a left upper-quadrant incision. Tex. Heart Inst. J. 2006;33(1):48–50. [PMC free article] [PubMed] [Google Scholar]
- 44.Strueber M., Meyer A.L., Feussner M., Ender J., Correia J.C., Mohr F.W. A minimally invasive off-pump implantation technique for continuous-flow left ventricular assist devices: early experience. J. Heart Lung Transplant. 2014;33(8):851–856. doi: 10.1016/j.healun.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Cohn W.E., Mallidi H.R., Frazier O.H. Safe, effective off-pump sternal sparing approach for HeartMate II exchange. Ann. Thorac. Surg. 2013;96(6):2259–2261. doi: 10.1016/j.athoracsur.2013.06.068. [DOI] [PubMed] [Google Scholar]
- 46.Cheung A., Bashir J., Kaan A., Kealy J., Moss R., Shayan H. Minimally invasive, off-pump explant of a continuous-flow left ventricular assist device. J. Heart Lung Transplant. 2010;29(7):808–810. doi: 10.1016/j.healun.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Rojas S.V., Avsar M., Hanke J.S., Khalpey Z., Maltais S., Haverich A., Schmitto J.D. Minimally Invasive Ventricular Assist Device Surgery. Artif. Organs. 2015 doi: 10.1111/aor.12422. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Rojas S.V., Haverich A., Schmitto J.D. Off-pump versus on-pump left ventricular assist device exchange. Artif. Organs. 2014;38(11):992. doi: 10.1111/aor.12397. [DOI] [PubMed] [Google Scholar]
- 49.Rojas S.V., Avsar M., Uribarri A., Hanke J.S., Haverich A., Schmitto J.D. A new era of ventricular assist device surgery: less invasive procedures. Minerva Chir. 2015;70(1):63–68. [PubMed] [Google Scholar]
- 50.Collart F., Feier H., Metras D., Mesana T.G. A safe, alternative technique for off-pump left ventricular assist device implantation in high-risk reoperative cases. Interact. Cardiovasc. Thorac. Surg. 2004;3(2):286–288. doi: 10.1016/j.icvts.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Robertson J.O., Grau-Sepulveda M.V., Okada S., O’Brien S.M., Matthew Brennan J., Shah A.S., Itoh A., Damiano R.J., Prasad S., Silvestry S.C. Concomitant tricuspid valve surgery during implantation of continuous-flow left ventricular assist devices: a Society of Thoracic Surgeons database analysis. J. Heart Lung Transplant. 2014;33(6):609–617. doi: 10.1016/j.healun.2014.01.861. [DOI] [PubMed] [Google Scholar]
- 52.Fujita T., Kobayashi J., Hata H., Seguchi O., Murata Y., Yanase M., Nakatani T. Right heart failure and benefits of adjuvant tricuspid valve repair in patients undergoing left ventricular assist device implantation. Eur. J. Cardiothorac. Surg. 2014;46(5):802–807. doi: 10.1093/ejcts/ezu040. [DOI] [PubMed] [Google Scholar]
- 53.Walter V., Stock U.A., Soriano-Romero M., Schnitzbauer A., Moritz A., Beiras-Fernandez A. Eradication of a chronic wound and driveline infection after redo-LVAD implantation. J. Cardiothorac. Surg. 2014;9:63. doi: 10.1186/1749-8090-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baronetto A., Centofanti P., Attisani M., Ricci D., Mussa B., Devotini R., Simonato E., Rinaldi M. A simple device to secure ventricular assist device driveline and prevent exit-site infection. Interact. Cardiovasc. Thorac. Surg. 2014;18(4):415–417. doi: 10.1093/icvts/ivt549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nienaber J.J., Kusne S., Riaz T., Walker R.C., Baddour L.M., Wright A.J., Park S.J., Vikram H.R., Keating M.R., Arabia F.A., Lahr B.D., Sohail M.R., Mayo Cardiovascular Infections Study Group Clinical manifestations and management of left ventricular assist device-associated infections. Clin. Infect. Dis. 2013;57(10):1438–1448. doi: 10.1093/cid/cit536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldstein D.J., Naftel D., Holman W., Bellumkonda L., Pamboukian S.V., Pagani F.D., Kirklin J. Continuous-flow devices and percutaneous site infections: clinical outcomes. J. Heart Lung Transplant. 2012;31(11):1151–1157. doi: 10.1016/j.healun.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Schibilsky D., Benk C., Haller C., Berchtold-Herz M., Siepe M., Beyersdorf F., Schlensak C. Double tunnel technique for the LVAD driveline: improved management regarding driveline infections. J. Artif. Organs. 2012;15(1):44–48. doi: 10.1007/s10047-011-0607-3. [DOI] [PubMed] [Google Scholar]
- 58.Fleissner F., Avsar M., Malehsa D., Strueber M., Haverich A., Schmitto J.D. Reduction of driveline infections through doubled driveline tunneling of left ventricular assist devices. Artif. Organs. 2013;37(1):102–107. doi: 10.1111/aor.12036. [DOI] [PubMed] [Google Scholar]
- 59.Singh A., Russo M.J., Valeroso T.B., Anderson A.S., Rich J.D., Jeevanandam V., Akhter S.A. Modified HeartMate II driveline externalization technique significantly decreases incidence of infection and improves long-term survival. ASAIO J. 2014;60(6):613–616. doi: 10.1097/MAT.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 60.Schmitto J.D., Hanke J.S., Rojas S.V., Avsar M., Haverich A. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J. Heart Lung Transplant. 2015 doi: 10.1016/j.healun.2015.03.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


