Abstract
For decades, it has been recognized that men have a higher age-adjusted risk of ischemic cardiovascular (CVD) events compared to women, thus generating hypotheses that sex steroids contribute to CVD risk. Potential mechanisms include genomic and non-genomic effects of sex steroids as well as mediation through classic CVD risk factors and obesity. However, results from randomized studies suggest that sex steroid supplementation in men and women do not result in improved CVD outcomes and may increase CVD risk. In contrast, prospective observations from endogenous sex steroid studies, i.e. among participants not using sex steroids, have suggested the opposite relationship. We reviewed the findings of prospective observational studies in men (17 studies) and women (8 studies) that examined endogenous sex steroids and CVD risk. These studies suggested a lack of association or that lower levels of testosterone or dihydrotestosterone are associated with higher CVD risk in both men and women. Higher, rather than lower, estradiol levels were associated with higher CVD risk in women. There were several significant gaps in the literature. First, it is unclear whether more sensitive measures of sex steroid levels might detect significant differences. Second, there are few prospective studies in women. Similarly, no studies report outcomes for high-risk groups such as African-Americans and Hispanics. Finally, few studies report upon ischemic coronary disease as opposed to ischemic stroke separately, although relationships between sex steroids and CVD may vary by vascular bed. Future investigations need to examine high risk groups and to distinguish between subtypes of CVD.
Keywords: Coronary disease, epidemiology, estradiol, ischemia, stroke, testosterone.
INTRODUCTION
For decades, it has been recognized that men have a higher age-adjusted risk of ischemic cardiovascular (CVD) events compared to women [1-4], thus generating hypotheses that sex steroids contribute to CVD risk. However, it is still unknown if and how sex steroids contribute to ischemic CVD risk for several reasons. First, the results from endogenous sex steroid studies, i.e. where participants are not using exogenous sex steroids, have conflicted with results from randomized trials of sex steroid therapy. Recent reports of endogenous sex steroids suggest that low endogenous testosterone (T) is associated with increased risk of CVD events in men [5-7], while randomized trials of T therapy suggest that higher T levels may increase CVD risk [8, 9]. Similarly, among women, low endogenous estradiol (E2) associated with the postmenopause has been thought to contribute to increased CVD risk in women [10], but estrogen supplementation trials suggest that higher E2 may be harmful [11]. Thus, exogenous sex steroid trials may not reflect naturally occurring physiology.
Second, few prospective endogenous sex steroid studies have reported upon both ischemic stroke events and ischemic coronary heart disease (CHD) events. Data from studies examining subclinical atherosclerosis [12-15] as well as events [16, 17] suggest that the relationships between endogenous sex steroids and CVD differ by vascular bed. Therefore, evaluation of risk factors would ideally examine CHD and ischemic stroke separately. As ischemic strokes may consist of strokes from thrombotic as well as atherogenic risk factors, hemostatic risk factors as well as classic CVD risk factors would be examined; however, such examinations are few.
In this report, we summarize the results from prospective studies of endogenous sex steroids and incident ischemic CVD events in men and women. Particular focus is placed upon how patterns differed between ischemic stroke and CHD events and how patterns differed between men and women. We discuss these findings in the context of purported mechanisms, randomized trial results, and sex steroid measurement issues. We conclude with a summary of major gaps in the existing literature and directions for future research.
PROSPECTIVE ENDOGENOUS SEX STEROID REPORTS
In May 2014, we performed a PubMed review using the following key words, limited to the English language: sex steroid AND (longitudinal OR prospective) AND (cardiovascular OR stroke OR coronary disease), which yielded 916 articles. In order to capture nested case-control studies, we performed another search substituting “case-control OR case-cohort” for “longitudinal OR prospective” which yielded 311 citations but only one additional citation not identified in the original search [18]. The publications retained for inclusion assessed endogenous serum levels of estrogens and/or androgens in relation to events attributed to ischemic CVD, ischemic stroke, or ischemic CHD. We focused upon prospective relationships between sex steroids and events. Reports that focused on only risk factors, subclinical atherosclerosis, or mortality were not included. Reports examining only effects of exogenous steroid therapy were not included.
Seventeen studies that met inclusion criteria examined men, and eight studies that met inclusion criteria examined women (Table 1). Studies in men spanned middle-age to older age. Most of the studies in women focused on mid-life and older women who were postmenopausal; the two studies that included premenopausal women only reported upon androgens, [18, 19] which have less variation with the menstrual cycle than estrogens [20]. Most studies examined the relationships between sex steroid levels and incident events before and after adjustment for conventional CVD risk factors. In general, as indicated in Table 1, studies that reported significant associations before adjustment also reported significant associations after adjustment, and studies that reported absence of significant associations before adjustment also reported lack of associations after adjustment. There were several exceptions [21-24] which are discussed in more detail below.
Table 1.
Relationships between sex steroids and ischemic cardiovascular disease (CVD), stroke, and coronary heart disease (CHD) events in prospective studies. (+) indicate that higher levels of sex steroid are associated with higher risk and (-) indicate that lower levels of sex steroid associated with higher risk in a linear manner. T=testosterone, DHT=dihydrotestosterone, E1=estrone, E2=estradiol, SHBG=sex hormone binding globulin, DHEAS = dehydroepiandrosterone, A=androstenedione
| Men | Ischemic CVD (Stroke or CHD) | Ischemic Stroke | Ischemic CHD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T/DHT | E1/E2 | Other | T/DHT | E1/E2 | Other | T/DHT | E1/E2 | Other | |
| Cauley 1987 [31] | T: none | E1, E2: none | A: none | ||||||
| Barrett-Connor 1988 [32] | T: none | E1, E2: none | A: none | ||||||
| Phillips 1988 [33] | T: none | E2: none | |||||||
| Yarnell 1993 [34] | T: none | E2: none | |||||||
| Hautanen 1994 [35] | T: none | DHEAS: (-)‡ | |||||||
| Mikulec 2004 [36] | T: none | E1, E2: none | |||||||
| Arnlov 2006 [27] | T: none | E2: (-) | DHEAS: none | ||||||
| Abbott 2007 [30] | none* | E2: (+)¶ | |||||||
| Akishita 2009 [25] | T: (-) | E2: none | DHEAS: none | ||||||
| Nilsson 2009 [37] | E2: none | ||||||||
| Vikan 2009 [38] | T: none | E2: none | SHBG: none | ||||||
| Yeap 2009 [29] | T: (-)* | ||||||||
| Ohlsson 2011 [16] | T: (-) | E2: none | SHBG: none | T: none# | E2: none | SHBG: none | T: (-) | E2: none | SHBG: none |
| Ruige 2011 [26]†‡ | T: (-) | E2: none | |||||||
| Vandenplas 2012 [39]† | E2: none | ||||||||
| Haring 2013 [28] | T: none | E2: none | DHEAS: none | ||||||
| Soisson 2013 [21] | T: J-shaped | E2: none | SHBG: none | T: J-shaped | E2: none | SHBG: none | T: J-shaped | E2: none | SHBG: none |
| Shores 2014 [5] | DHT: U-shaped, T none | ||||||||
| Shores 2014 [6] | DHT: U-shaped, T none | ||||||||
| Women | Ischemic CVD (Stroke or CHD) | Ischemic Stroke | Ischemic CHD | ||||||
| T/DHT | E1/E2 | Other | T/DHT | E1/E2 | Other | T/DHT | E1/E2 | Other | |
| Rexrode 2003 [22] | T: none§ | E2: none | SHBG: none | ||||||
| Nilsson 2009 [37] | E2: none | ||||||||
| Laughlin 2010 [7] | T: U-shaped | ||||||||
| Lee 2010 [23] | E2: (+)¶ | SHBG: none | |||||||
| Sievers 2010 [19] | T: (-) | ||||||||
| Chen 2011 [24] | E2: noneщ | ||||||||
| Scarabin 2012 [17] | T: none | E2: (+) | T: none | E2: none# | T: none | E2: (+) | |||
*Outcome = ischemic and hemorrhagic stroke, †Systematic review or meta-analysis; ‡Significant association in elderly but not middle-aged men; §Lower free androgen index associated with higher risk, but not after adjustment for CVD risk factors; # Higher bioavailable estradiol associated with higher risk, but not after adjustment for CVD risk factors; #Borderline, p<0.10.
SUMMARY OF PROSPECTIVE STUDIES IN MEN
Among men, six studies reported upon the prospective relationship between T or DHT (dihydrotestosterone) and incident ischemic CVD events. Three studies reported a linear relationship, [16, 25, 26], one study reported a J-shaped relationship [21], and one study reported a U-shaped relationship [6] between T or DHT [6] and incident events, i.e. extremes of T or DHT were associated with a higher risk of CVD events. Two studies reported no significant relationship [27, 28]. The single report examining DHT did not find an association between T and incident events [6].
Five studies reported upon the relationship between T or DHT and incident ischemic stroke, with one report noting a linear relationship [29] and another report noting a U-shaped relationship with DHT but not with T [5]. Three studies reported no significant relationship between total T level and incident stroke events [16, 21, 30], although the bioavailable fraction of T (the “active” portion not bound to sex hormone binding globulin or SHBG) was associated after but not before adjustment for CVD risk factors in one of these reports [21], and the association between T and incident stroke was of borderline significance in the other two reports [16, 30].
Nine studies reported upon the relationship between T and incident CHD events, with only two reports noting any relationship between T and incident CHD events [16, 21]. Of note, the majority of studies examining CHD in men were conducted prior to 2004 [31-36], in contrast to studies examining ischemic stroke or a composite of CVD events.
Among men, six studies reported upon the prospective relationship between estrone (E1) or E2 and incident ischemic CVD events. One study reported that a higher level of E2 was associated with incident events, [27] while the other studies reported no relationship [16, 21, 25, 28, 37]. Three studies reported upon the relationship between E2 and incident ischemic stroke, with one study noting a higher level of E2 was associated with incident ischemic stroke [30], and two other studies noting no relationship [16, 21]. Eight studies reported upon the relationship between E1 or E2 and incident CHD events; [16, 21, 31-34, 36, 38] no relationship was reported.
Among men, no prospective studies noted associations between other endogenous sex steroids and incident CVD events, with the exception of one study that reported a higher level of dehydroepiandrosterone (DHEAS) was associated with a higher risk of ischemic CHD [35].
One review attempted to pool risk estimates of T and incident CVD events [26]. Significant between-study heterogeneity was noted by year of publication, in that studies published after January 2007 noted stronger relationships. The relationship between low T and incident events was stronger in older men (hazard ratio 0.84, 95% CI 0.76, 0.92) than in younger men (HR 1.01, 95% CI 0.95, 1.08). Another report attempted to pool risk estimates of E2 and incident CVD events and noted that the quality of the E2 assay, presumably based upon coefficients of variation and detection limits, was a significant source of heterogeneity. E2 was not noted to have any association with CVD risk [39].
SUMMARY OF PROSPECTIVE STUDIES IN WOMEN
There were relatively few prospective studies of endogenous sex steroids and ischemic CVD events in women compared to men. As noted earlier, all of the studies focused exclusively upon postmenopausal women except for two reports [18, 19]. Only three prospective studies examined the relationship between endogenous T and ischemic CVD risk in women; one study reported that a lower level of T was associated with a higher risk of ischemic CVD, [19] and two other studies reported no association [17, 22]. One of these studies noted that lower free androgen index, representing the proportion of T not bound to SHBG, was associated with higher risk of CVD events before but not after adjustment for CVD risk factors [22]. The one study which examined the association between T and ischemic stroke in women reported no association [17]. Of the two studies that examined the relationship between T and ischemic CHD events, one report noted that a U-shaped relationship, i.e. lower and higher levels of T were associated with increased risk of incident events, [7] while the other reported no association [17].
Among women, one report noted that higher levels of DHEAS were associated with greater risk of ischemic stroke [18]. While SHBG is not a sex steroid but may influence active T and E2 levels through binding, one report examined SHBG levels but found no association with CVD risk [22].
POTENTIAL MECHANISMS
In this review, the sex steroids most frequently examined in relation to CVD included the androgen T and the estrogen E2. T and E2 are directly produced primarily by gonadal organs (Fig. 1). In both men and women, T is aromatized to E2, particularly in adipose tissue. T may also undergo 5 alpha-reduction in the gonads, the prostate gland in men, and hair follicles to form the more potent DHT compound; DHT cannot be converted to E2. Both albumin and SHBG bind to T and E2, but binding with albumin is relatively weak while binding to SHBG renders T and E2 inactive. DHEAS is secreted primarily by the adrenal gland and is the most abundant steroid in postmenopausal women. DHEAS is a precursor of both androgens and estrogens; [40] metabolites of DHEAS include androstenedione (A), which subsequently may be metabolized to T or estrone (E1), which is an E2 precursor. E1 and E2 are produced by the gonads and may also be produced when T is aromatized in adipose tissue. DHEAS is also the source of additional steroid hormone metabolites that may have different local effects [40] or have both androgenic and estrogenic activity [41].
Fig. (1).
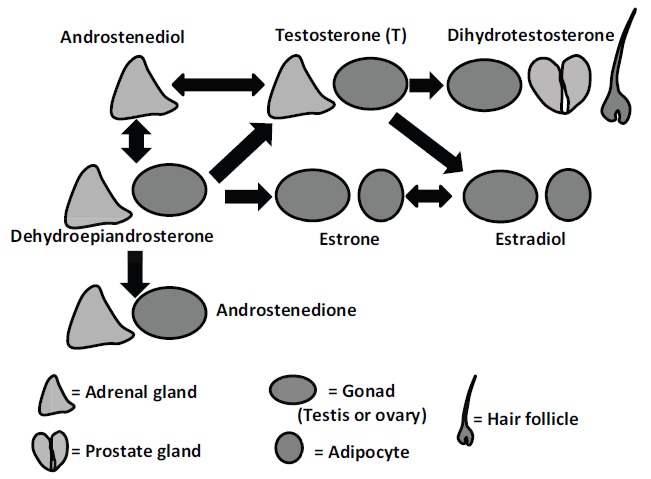
Androgen and estrogen production in men and women. Sex steroids in blue indicate sex steroids with greater androgenic activity, and sex steroids in red indicate sex steroids with greater estrogenic activity.
Sex steroid receptors are present throughout the cardiovasculature, immunoregulatory cells, and platelets. Sex steroids have genomic and non-genomic effects, [42-44] i.e. sex steroids affect gene transcription but also can have effects that are mediated through pathways other than protein or RNA synthesis (Fig. 1) [45]. Generally, animal and in-vitro studies suggest that androgens might be pro-inflammatory, thrombotic, and atherogenic while estrogens attenuate inflammation [46]. Potential mechanisms have been summarized in several recent reviews [42-45]. Briefly, in vascular endothelial cells, estrogens increase synthesis of nitric oxide synthase (NOS) and also increase NOS activity through phosphorylation, thus enabling NO-induced vasodilation. Estrogens enhance production of prostacyclin, another vasodilator, while androgens favor vasoconstriction through thromboxane. In addition, both androgens and estrogens have been identified as inflammatory regulators through the presence of sex steroid receptors on neutrophils, monocytes, macrophages, and platelets. Acute E2 infusion reduces secretion of interleukin-6 and fibrinogen and can reduce adenosine-diphosphate (ADP) induced platelet aggregation, while androgens may increase platelet sensitivity to ADP and cyclooxygenase. Finally, higher levels of E2 are associated with lower levels of plasminogen activator inhibitor-1 and tissue plasminogen activator levels, markers of fibrinolysis [47]. Androgen and estrogen receptors are also present in adipocytes with a greater proportion of androgen receptors in visceral fat, suggesting sex steroid effects could be further mediated by adipokines such as C-reactive protein, adiponectin, and leptinb [48-50]. The summary effect of these changes may increase the risk of thrombosis particularly in the cerebrovasculature, with or without underlying atherogenesis.
Both endogenous estrogen and androgen levels are associated with CVD risk factors in prospective studies. Thus, it is possible that sex steroid effects upon CVD risk may be mediated through classic CVD risk factors, in addition to or instead of the mechanisms noted above. Androgens have been noted to predict future levels of CVD risk factors; in general, higher androgen levels in men have been associated with more favorable CVD risk factor profiles in men, but less favorable profiles in women [51]. Specifically, lower levels of T in men are associated with diabetes and elevations in glucose, [51] blood pressure, [52] and lipids [53]. In contrast, higher levels of T in women are associated with diabetes, [51] elevations in blood pressure, [54, 55] and adverse lipid levels [56]. Although young women have higher E2 levels and more favorable CVD risk factor profiles than older women, higher endogenous E2 levels are associated with poorer classical CVD risk factors in postmenopausal women [51, 54]. These associations may explain why higher E2 levels are associated with greater CVD risk in women, even as higher E2 levels are thought to enable vasodilation, decreased platelet aggregation, and more favorable hemostatic profiles. Obesity likely has bidirectional relationships with sex steroids [51] and may influence both risk factor profiles as well as vascular reactivity and coagulation [57].
RANDOMIZED TRIALS
Randomized trials of T supplementation have not supported the hypothesis that supplementing with T can decrease CVD risk. A 2011 meta-analysis noted that androgen-deprivation therapy for prostate cancer was not associated with significant increases in CVD death [58]. Supplementation may actually increase risk of CVD events. Among men, a meta-analysis of T therapy conducted in 2013 including 27 trials noted that T therapy increased the risk of any CVD events (odds ratio [OR] 1.54, 95% confidence interval [CI] 1.09, 2.18), and the magnitude of effect did not vary by baseline T level, suggesting that T therapy was harmful regardless of whether men had low T [9].
Similarly, randomized trials of E2 supplementation among women with low levels of endogenous E2 (i.e. postmenopausal women) have not supported the hypothesis that supplementing with estrogen can decrease CVD risk. As in men, supplementation with exogenous sex steroids in women may actually increase risk of CVD outcomes. The Women’s Health Initiative (WHI) randomized postmenopausal women without known CVD to oral conjugated equine estrogen and medroxyprogesterone acetate vs. placebo and noted a significant increase in the risk of CHD events [59] and ischemic stroke [60]. Among participants in the WHI estrogen-alone trial, the risk of CHD events was similar in therapy vs. placebo groups [61] and the risk of ischemic stroke was higher among women randomized to estrogen [62]. A similar lack of benefit has been observed for estrogen supplementation in secondary prevention studies. In the Women’s Estrogen for Stroke Trial, [63] which randomized women with a recent mild stroke or transient ischemic attack to estrogen therapy vs. placebo, estrogen therapy did not reduce stroke risk. In the Heart Estrogen-Progestin Replacement Study, which randomized women with known CHD to estrogen/progestin vs. placebo, women randomized to estrogen had an increased risk of another CHD event, although a significant increased risk in stroke was not observed [64]. Younger postmenopausal women do not appear to benefit from estrogen therapy any more than older postmenopausal women: long-term follow-up in the WHI studies did not find that estrogen therapy was associated with benefit in younger as opposed to older women, although the magnitude of risk was smaller in younger women, probably due to their lower risk for CVD generally [59]. In randomized trials of younger women, preliminary presentations have noted that estrogen therapy is not associated with a significantly lower burden of coronary artery calcification [65].
Theoretically, exogenous sex steroid supplementation delivered transdermally rather than orally may more closely approximate a healthy hormonal milieu. Oral estrogen therapy leads to elevations in SHBG level and subsequently decreases bioavailable fractions of sex steroids, and these “first-pass” effects through the liver could influence CVD risk [66]. Oral, but not transdermal estrogen therapy, is associated with enhanced platelet reactivity [67]. Although analyses of the WHI Observational Study noted that transdermal E2 was not associated with lower risk of CHD compared with oral conjugated equine estrogen for CHD events or stroke, [68] women were not randomized to therapy route and thus an association may have been confounded by healthier women using transdermal rather than oral therapy. Different oral estrogen types may be lead to different risk, despite the fact that oral estrogen formulations are all metabolized by the liver. Although the mechanism is not understood, one case-control study noted that women using oral conjugated estrogen therapy had higher thrombin potential-based normalized activated protein C sensitivity ratio, indicating a stronger clotting propensity, than women using oral E2, and the former also had an increased risk of CVD events [69]. No trials or prospective studies assess the differential effects of estrogen formulation upon outcomes such as incident CVD. Among men, T therapy in randomized trials that have reported CVD outcomes have typically delivered T transdermally via gels or patches or intramuscularly, thus avoiding first-pass liver effects and the question of whether such effects might be contributing to increased risk [70].
These results from randomized studies are consistent with the observation that exogenous therapy, presumably resulting in an increased serum level of a sex steroid, does not benefit CVD risk. This may be due to adverse effects of the therapy unrelated to serum sex steroid levels, supraphysiologic sex steroid levels that do not mimic endogenous levels, or absence of an association between sex steroids and CVD risk per se with confounding by other factors such as obesity or classic CVD risk factor levels. Rexrode et al noted that the relationship between sex steroid levels and incident CVD events among estrogen users and non-users differed, in that there were associations between bioavailable T and incident CVD among non-users but not among estrogen users before adjustment for CVD risk factors [22]. The majority of estrogen therapy trials have not measured sex steroid levels. However, comparisons of sex steroid levels in the Study of Women’s Health Across the Nation, a prospective observational study which measured endogenous sex steroid levels, noted that premenopausal women had E2 levels of 40.8 pg/ml (SD 4.3 pg/ml) vs. 41.5 pg/ml (SD 2.5 pg/ml) among women using estrogen and progestin therapy vs. 14.8 pg/ml (SD 0.7 pg/ml) among postmenopausal women not using any E2 therapy [71]. This result suggests that conventional oral conjugated estrogen therapy yielded similar serum E2 levels to those observed in premenopausal women, and therefore supraphysiologic sex steroid levels with supplementation are probably not the reason for the lack of agreement between trials and observational studies.
Of note, estrogen therapy randomized trials in women have targeted a particular serum E2 value, although oral and transdermal therapy approximately double total E2 concentrations.[66] Similarly, trials of T therapy have not noted that the risks of benefit of T therapy vary by baseline level of T, even as T levels after replacement are often not recorded continuously [9]. While the cutpoint for hypogonadism varied, approximately 10.4-16.4 nmol/l or 300 ng/dl to 475 ng/dl is a common range for hypogonadism in clinical trials [9, 72-75]. If doses exceeded about 1000 ng/dl or 34.7 nmol/l or were below about 250 ng/dl or 8.7 nmol/l, dose adjustments occurred in several [72, 73, 76] but not other studies [74, 75, 77]. Thus, there is not a consensus on what constitutes supraphysiologic sex steroid levels among older men and women.
ISSUES OF MEASUREMENT
There has been ongoing debate on how to best assess endogenous sex steroid levels, particularly in the older populations at greatest risk for ischemic CVD events but also with difficult to detect sex steroid levels. Newer assays may afford more sensitive detection levels [78, 79]. It is therefore possible that more recent studies may have greater power to detect an association between sex steroid levels and ischemic CVD risk. In support of this possibility, more recent prospective cohort studies [5, 6, 17, 19, 21, 24, 28] have tended to report the presence of significant relationships between endogenous sex steroids and ischemic CVD risk.
Currently, there is no single measurement standard for sex steroids, although there is a consensus that harmonization is needed [80, 81]. Older adults have low levels of sex steroids but are at highest risk of CVD events. The detection limit of the particular sex steroid assay used may also be a crucial factor, e.g. T levels in older women are very low. Direct assays, that is, assays performed without an extraction step, are considered unreliable, and thus measurement is generally done after extraction and chromatography [80, 81]. Direct measurement of unbound or free T and E2 can be done using dialysis or ultrafiltration. These methods require relatively large sample volume and are expensive. Therefore, equations that estimate free or bioavailable T and E2 are often substituted for direct measurement [82, 83]. These equations incorporate total T and E2 measurements using mass spectrometry or radioimmunoassay, SHBG measurements, and albumin measurements or estimations. In this review, the majority of the studies examining relationships between sex steroids and CVD used indirect radioimmunoassay with the exception of 2 cohorts of elderly men, [5, 6, 16] and it remains to be seen whether the use of mass spectrometric techniques currently endorsed by medical societies leads to a different pattern of results [78, 79].
Mass spectrometry relies on an extraction step, typically using serial liquid-liquid extraction with isolation of lipid fractions, reconstitution of the organic phase, followed by removal of phospholipids and other components [84]. Liquid chromatography-isotopic dilution mass spectrometry is then used to quantify total sex steroid amounts. Sample preparation is usually automatically conducted using mass through-put, although occasionally done by hand [84]. The minimal cross-reactivity of these assays with other sex steroids may make such methods more accurate than indirect radioimmunoassays as well as offering lower detection limits. The Centers for Disease Control and Prevention (CDC) has led a hormone standardization program designed to harmonize the measurement of T levels, with an emphasis on mass spectrometry particularly in low-androgen populations. Briefly, participating laboratories would send aliquots which were then tested independently in a blinded fashion by the CDC; the majority of assays relied upon liquid chromatography tandem mass spectrometry. A range of 2.50 to 1,000 ng/dl was tested. In order to meet CDC standards, the means needed to be less than 6.4% [85].
GAPS IN THE LITERATURE
Our report highlights the need for investigation in several areas. First, there is a paucity of prospective evaluations in women generally. Specific examinations of incident stroke vs. CHD events are needed for women; only a single European report examines the relationship between endogenous sex steroids for both ischemic stroke and CHD in women [17]. The two reports in men that examined both ischemic stroke and CHD noted statistically significant relationships between T and CHD, but not T and ischemic stroke [16, 21]. Examinations of both T and E2 are needed in women, rather than the traditional focus upon E2 levels only.
There is a lack of epidemiologic investigations regarding the differential mechanisms of endogenous sex steroids upon ischemic stroke vs. CHD, beyond classic CVD risk factors. Due in part to the lack of studies which directly compare relationships between sex steroids and stroke vs. CHD, it is unknown how particular factors might be influential for one vascular bed compared to another. Additional exploration of how sex steroids may interact with biomarkers, e.g. inflammatory measures or coagulation factors, to affect ischemic stroke risk vs. CHD risk are also needed. Such risk factors also include adiposity: examinations of the relationship between obesity and androgen levels need to include precise measures of the mass of fat depots in order to determine how changes in depot size affect sex steroid profiles and vice-versa.
Finally, there are no studies that report relationships of sex steroids among blacks and whites separately or non-Hispanics and Hispanics, which is particularly important given the higher risk of stroke among blacks and Hispanics [86]. Among men and women in the Atherosclerosis Risk in Communities cohort (ARIC), age-adjusted incidence of stroke was 2.4 in middle-aged white men and women, but 9.7 in black men and 7.2 in black women per 1000 person years [87]. Among Mexican-Americans, these risk ratios are roughly similar [88]. These disparities persisted in older age groups. Age-adjusted incidence of coronary events were 3.6 in white men and 2.1 in white women, but 5.6 in black men and 3.8 in black women per 1000 person-years [87]. Table 1 shows relationships between sex steroids and CVD events in whites, and Asians, and mixed populations in which the number of CVD events was too small to perform race or ethnicity-specific analyses. However, it is well-recognized that the risk for incident CHD events in black women exceeds that in white women and (to a lesser extent) in black men compared to white men, [89] and that the risk for incident ischemic stroke in black women exceeds than in white women and in black men compared to white men at younger ages, before survival biases comparisons [3]. Moreover, a 2014 meta-analysis reported that black men have slightly but significantly higher endogenous free T (the active portion not bound to sex hormone binding globulin or SHBG) levels than white men [90]. E2 is also higher in black men than white men, even after adjustment for adiposity [91, 92]. Some, [93] although not all, [94] studies suggest that postmenopausal black women have higher endogenous E2 levels than white women even after consideration of adiposity.
Whether or not these differences contribute to racial-ethnic differences in CVD events is not known. Genomewide Association Studies (GWAS) have identified hundreds of loci associated with atherosclerosis and cardiovascular disease, but each of these loci account for a small part of the variance of disease and are present to different extents across populations [95]. In addition, the implicated variants are linked to disease, but not thought to be truly causal in most instances [95]. Targeted and genomewide sequencing can identify rare genetic variants, although these are unlikely to explain atherosclerosis on a population-wide basis. To date, these genes have focused upon classic cardiovascular and inflammatory risk factors, but have not examined shared loci with sex steroids.
CONCLUSIONS
Although sex comparisons and comparisons of pre- and postmenopausal women have suggested a role for sex steroids in the pathophysiology of incident CVD events, it is unclear if and how sex steroids affect CVD risk. Prospective studies of endogenous sex steroid levels suggest that simplistic models of elevated androgens explaining greater CVD risk in men and decreased estrogens explaining decreased CVD risk in women are incorrect. These studies also suggest that randomized trials of sex steroid therapy may not extend naturally occurring pathophysiology, as the pattern of endogenous associations is opposite to the risks observed in randomized trials. To determine the contribution of sex steroids to CVD risk, studies are needed that examine men with measurement tools sensitive enough to detect lower androgen levels in older populations with separate assessment of stroke and CHD outcomes. Studies in women are few, and further investigations need to extend beyond investigation of estrogen levels only. Due to significant risk differences in ischemic CVD events by race/ethnicity, reports are needed that examine relationships separately for blacks and whites. Exploration of shared lock between sex steroids and cardiovascular events across populations might also be explored.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by DK083297, DK098129, HL080477, and K24 HL111154.
References
- 1.Appelros P., Stegmayr B., Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-Connor E. The Rancho Bernardo Study: 40 years studying why women have less heart disease than men and how diabetes modifies women’s usual cardiac protection. Glob. Heart. 2013;8(2):95–104. doi: 10.1016/j.gheart.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go A.S., Mozaffarian D., Roger V.L., et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 4.Bushnell C., McCullough L.D., Awad I.A., et al. American Heart Association Stroke Council. Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Council on Epidemiology and Prevention. Council for High Blood Pressure Research Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shores M.M., Arnold A.M., Biggs M.L., et al. Testosterone and dihydrotestosterone and incident ischaemic stroke in men in the Cardiovascular Health Study. Clin Endocrinol (Oxf) 2014;81(5):746–753. doi: 10.1111/cen.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shores M.M., Biggs M.L., Arnold A.M., et al. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J. Clin. Endocrinol. Metab. 2014;99(6):2061–2068. doi: 10.1210/jc.2013-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laughlin G.A., Goodell V., Barrett-Connor E., et al. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J. Clin. Endocrinol. Metab. 2010;95(2):740–747. doi: 10.1210/jc.2009-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigen R., O’Donnell C.I., Barón A.E., et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 9.Xu L., Freeman G., Cowling B.J., Schooling C.M. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crandall C.J., Barrett-Connor E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: a systematic review. Endocrinol. Metab. Clin. North Am. 2013;42(2):227–253. doi: 10.1016/j.ecl.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Marjoribanks J., Farquhar C., Roberts H., Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2012;7:CD004143. doi: 10.1002/14651858.CD004143.pub4. [DOI] [PubMed] [Google Scholar]
- 12.Golden S.H., Maguire A., Ding J., Crouse J.R., Cauley J.A., Zacur H., Szklo M., et al. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am. J. Epidemiol. 2002;155(5):437–445. doi: 10.1093/aje/155.5.437. [DOI] [PubMed] [Google Scholar]
- 13.El Khoudary S.R., Wildman R.P., Matthews K., Thurston R.C., Bromberger J.T., Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis. 2012;225(1):180–186. doi: 10.1016/j.atherosclerosis.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurston R.C. Getting to the heart of things: is endogenous estradiol associated with coronary artery calcification? Menopause. 2010;17(5):887–888. doi: 10.1097/gme.0b013e3181eb9d3c. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang P., Vaidya D., Dobs A., et al. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;204(1):255–261. doi: 10.1016/j.atherosclerosis.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohlsson C., Barrett-Connor E., Bhasin S., Orwoll E., Labrie F., Karlsson M.K., Ljunggren O., Vandenput L., Mellström D., Tivesten A., et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J. Am. Coll. Cardiol. 2011;58(16):1674–1681. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Scarabin-Carré V., Canonico M., Brailly-Tabard S., et al. High level of plasma estradiol as a new predictor of ischemic arterial disease in older postmenopausal women: the three-city cohort study. J. Am. Heart Assoc. 2012;1(3):e001388. doi: 10.1161/JAHA.112.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiménez M.C., Sun Q., Schürks M., et al. Low dehydroepiandrosterone sulfate is associated with increased risk of ischemic stroke among women. Stroke. 2013;44(7):1784–1789. doi: 10.1161/STROKEAHA.111.000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sievers C., Klotsche J., Pieper L., et al. Low testosterone levels predict all-cause mortality and cardiovascular events in women: a prospective cohort study in German primary care patients. Eur. J. Endocrinol. 2010;163(4):699–708. doi: 10.1530/EJE-10-0307. [DOI] [PubMed] [Google Scholar]
- 20.Bui H.N., Sluss P.M., Blincko S., Knol D.L., Blankenstein M.A., Heijboer A.C. Dynamics of serum testosterone during the menstrual cycle evaluated by daily measurements with an ID-LC-MS/MS method and a 2nd generation automated immunoassay. Steroids. 2013;78(1):96–101. doi: 10.1016/j.steroids.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Soisson V., Brailly-Tabard S., Helmer C., et al. A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: the French 3C cohort study. Maturitas. 2013;75(3):282–288. doi: 10.1016/j.maturitas.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Rexrode K.M., Manson J.E., Lee I.M., et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108(14):1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.S., Yaffe K., Lui L.Y., et al. Study of Osteoporotic Fractures Group Prospective study of endogenous circulating estradiol and risk of stroke in older women. Arch. Neurol. 2010;67(2):195–201. doi: 10.1001/archneurol.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Zeleniuch-Jacquotte A., Arslan A.A., et al. Endogenous hormones and coronary heart disease in postmenopausal women. Atherosclerosis. 2011;216(2):414–419. doi: 10.1016/j.atherosclerosis.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akishita M., Hashimoto M., Ohike Y. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210(1):232–236. doi: 10.1016/j.atherosclerosis.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Ruige J.B., Mahmoud A.M., De Bacquer D., Kaufman J.M. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97(11):870–875. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 27.Arnlöv J., Pencina M.J., Amin S. Endogenous sex hormones and cardiovascular disease incidence in men. Ann. Intern. Med. 2006;145(3):176–184. doi: 10.7326/0003-4819-145-3-200608010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Haring R., Teng Z., Xanthakis V., Coviello A., Sullivan L., Bhasin S., Murabito J.M., Wallaschofski H., Vasan R.S. Association of sex steroids, gonadotrophins, and their trajectories with clinical cardiovascular disease and all-cause mortality in elderly men from the Framingham Heart Study. Clin. Endocrinol. (Oxf) 2013;78(4):629–634. doi: 10.1111/cen.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeap B.B., Hyde Z., Almeida O.P., Norman P.E., Chubb S.A., Jamrozik K., Flicker L., Hankey G.J. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J. Clin. Endocrinol. Metab. 2009;94(7):2353–2359. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- 30.Abbott R.D., Launer L.J., Rodriguez B.L., Ross G.W., Wilson P.W., Masaki K.H., Strozyk D., Curb J.D., Yano K., Popper J.S., Petrovitch H. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68(8):563–568. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- 31.Cauley J.A., Gutai J.P., Kuller L.H., Dai W.S. Usefulness of sex steroid hormone levels in predicting coronary artery disease in men. Am. J. Cardiol. 1987;60(10):771–777. doi: 10.1016/0002-9149(87)91021-6. [DOI] [PubMed] [Google Scholar]
- 32.Barrett-Connor E., Khaw K.T. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78(3):539–545. doi: 10.1161/01.CIR.78.3.539. [DOI] [PubMed] [Google Scholar]
- 33.Phillips G.B., Yano K., Stemmermann G.N. Serum sex hormone levels and myocardial infarction in the Honolulu Heart Program. Pitfalls in prospective studies on sex hormones. J. Clin. Epidemiol. 1988;41(12):1151–1156. doi: 10.1016/0895-4356(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 34.Yarnell J.W., Beswick A.D., Sweetnam P.M., Riad-Fahmy D. Endogenous sex hormones and ischemic heart disease in men. The Caerphilly prospective study. Arterioscler. Thromb. 1993;13(4):517–520. doi: 10.1161/01.ATV.13.4.517. [DOI] [PubMed] [Google Scholar]
- 35.Hautanen A., Mänttäri M., Manninen V., Tenkanen L., Huttunen J.K., Frick M.H., Adlercreutz H. Adrenal androgens and testosterone as coronary risk factors in the Helsinki Heart Study. Atherosclerosis. 1994;105(2):191–200. doi: 10.1016/0021-9150(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 36.Mikulec K.H., Holloway L., Krasnow R.E., Javitz H., Swan G.E., Reed T., Marcus R., Carmelli D. Relationship of endogenous sex hormones to coronary heart disease: a twin study. J. Clin. Endocrinol. Metab. 2004;89(3):1240–1245. doi: 10.1210/jc.2003-031333. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson S.E., Fransson E., Brismar K. Relationship between serum progesterone concentrations and cardiovascular disease, diabetes, and mortality in elderly Swedish men and women: An 8-year prospective study. Gend. Med. 2009;6(3):433–443. doi: 10.1016/j.genm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Vikan T., Schirmer H., Njølstad I., Svartberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromsø Study. Eur. J. Endocrinol. 2009;161(3):435–442. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- 39.Vandenplas G., De Bacquer D., Calders P., Fiers T., Kaufman J.M., Ouwens D.M., Ruige J.B. Endogenous oestradiol and cardiovascular disease in healthy men: a systematic review and meta-analysis of prospective studies. Heart. 2012;98(20):1478–1482. doi: 10.1136/heartjnl-2011-301587. [DOI] [PubMed] [Google Scholar]
- 40.Labrie F., Bélanger A., Bélanger P., Bérubé R., Martel C., Cusan L., Gomez J., Candas B., Castiel I., Chaussade V., Deloche C., Leclaire J. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J. Steroid Biochem. Mol. Biol. 2006;99(4-5):182–188. doi: 10.1016/j.jsbmb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Davis S.R., Panjari M., Stanczyk F.Z. Clinical review: DHEA replacement for postmenopausal women. J. Clin. Endocrinol. Metab. 2011;96(6):1642–1653. doi: 10.1210/jc.2010-2888. [DOI] [PubMed] [Google Scholar]
- 42.Roy-O’Reilly M., McCullough L.D. Sex differences in stroke: the contribution of coagulation. Exp. Neurol. 2014;259:16–27. doi: 10.1016/j.expneurol.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzales R.J. Androgens and the cerebrovasculature: modulation of vascular function during normal and pathophysiological conditions. Pflugers Arch. 2013;465(5):627–642. doi: 10.1007/s00424-013-1267-3. [DOI] [PubMed] [Google Scholar]
- 44.Gilliver S.C. Sex steroids as inflammatory regulators. J. Steroid Biochem. Mol. Biol. 2010;120(2-3):105–115. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Oskui P.M., French W.J., Herring M.J., Mayeda G.S., Burstein S., Kloner R.A. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J. Am. Heart Assoc. 2013;2(6):e000272. doi: 10.1161/JAHA.113.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quillinan N., Deng G., Grewal H., Herson P.S. Androgens and stroke: good, bad or indifferent? Exp. Neurol. 2014;259:10–15. doi: 10.1016/j.expneurol.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sowers M.R., Matthews K.A., Jannausch M., Randolph J.F., McConnell D., Sutton-Tyrrell K., Little R., Lasley B., Pasternak R. Hemostatic factors and estrogen during the menopausal transition. J. Clin. Endocrinol. Metab. 2005;90(11):5942–5948. doi: 10.1210/jc.2005-0591. [DOI] [PubMed] [Google Scholar]
- 48.Dieudonné M.N., Leneveu M.C., Giudicelli Y., Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am. J. Physiol. Cell Physiol. 2004;286(3):C655–C661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 49.Dieudonne M.N., Pecquery R., Boumediene A., Leneveu M.C., Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am. J. Physiol. 1998;274(6 Pt 1):C1645–C1652. doi: 10.1152/ajpcell.1998.274.6.C1645. [DOI] [PubMed] [Google Scholar]
- 50.Dieudonne M.N., Pecquery R., Leneveu M.C., Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141(2):649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 51.Kim C., Halter J.B. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr. Cardiol. Rep. 2014;16(4):467. doi: 10.1007/s11886-014-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torkler S., Wallaschofski H., Baumeister S.E., Völzke H., Dörr M., Felix S., Rettig R., Nauck M., Haring R. Inverse association between total testosterone concentrations, incident hypertension and blood pressure. Aging Male. 2011;14(3):176–182. doi: 10.3109/13685538.2010.529194. [DOI] [PubMed] [Google Scholar]
- 53.Haring R., Baumeister S.E., Völzke H., Dörr M., Felix S.B., Kroemer H.K., Nauck M., Wallaschofski H. Prospective association of low total testosterone concentrations with an adverse lipid profile and increased incident dyslipidemia. Eur. J. Cardiovasc. Prev. Rehabil. 2011;18(1):86–96. doi: 10.1097/HJR.0b013e32833c1a8d. [DOI] [PubMed] [Google Scholar]
- 54.Wang L., Szklo M., Folsom A.R., Cook N.R., Gapstur S.M., Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224(1):228–234. doi: 10.1016/j.atherosclerosis.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziemens B., Wallaschofski H., Völzke H., Rettig R., Dörr M., Nauck M., Keevil B.G., Brabant G., Haring R. Positive association between testosterone, blood pressure, and hypertension in women: longitudinal findings from the Study of Health in Pomerania. J. Hypertens. 2013;31(6):1106–1113. doi: 10.1097/HJH.0b013e3283603eb1. [DOI] [PubMed] [Google Scholar]
- 56.Sutton-Tyrrell K., Wildman R.P., Matthews K.A., Chae C., Lasley B.L., Brockwell S., Pasternak R.C., Lloyd-Jones D., Sowers M.F., Torréns J.I., SWAN Investigators Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 2005;111(10):1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 57.Santilli F., Vazzana N., Liani R., Guagnano M.T., Davì G. Platelet activation in obesity and metabolic syndrome. Obes. Rev. 2012;13(1):27–42. doi: 10.1111/j.1467-789X.2011.00930.x. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen P.L., Je Y., Schutz F.A., Hoffman K.E., Hu J.C., Parekh A., Beckman J.A., Choueiri T.K. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306(21):2359–2366. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 59.Manson J.E., Chlebowski R.T., Stefanick M.L., Aragaki A.K., Rossouw J.E., Prentice R.L., Anderson G., Howard B.V., Thomson C.A., LaCroix A.Z., Wactawski-Wende J., Jackson R.D., Limacher M., Margolis K.L., Wassertheil-Smoller S., Beresford S.A., Cauley J.A., Eaton C.B., Gass M., Hsia J., Johnson K.C., Kooperberg C., Kuller L.H., Lewis C.E., Liu S., Martin L.W., Ockene J.K., O’Sullivan M.J., Powell L.H., Simon M.S., Van Horn L., Vitolins M.Z., Wallace R.B. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wassertheil-Smoller S., Hendrix S.L., Limacher M., Heiss G., Kooperberg C., Baird A., Kotchen T., Curb J.D., Black H., Rossouw J.E., Aragaki A., Safford M., Stein E., Laowattana S., Mysiw W.J., WHI Investigators Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 61.Anderson G.L., Limacher M., Assaf A.R., Bassford T., Beresford S.A., Black H., Bonds D., Brunner R., Brzyski R., Caan B., Chlebowski R., Curb D., Gass M., Hays J., Heiss G., Hendrix S., Howard B.V., Hsia J., Hubbell A., Jackson R., Johnson K.C., Judd H., Kotchen J.M., Kuller L., LaCroix A.Z., Lane D., Langer R.D., Lasser N., Lewis C.E., Manson J., Margolis K., Ockene J., O’Sullivan M.J., Phillips L., Prentice R.L., Ritenbaugh C., Robbins J., Rossouw J.E., Sarto G., Stefanick M.L., Van Horn L., Wactawski-Wende J., Wallace R., Wassertheil-Smoller S., Women’s Health Initiative Steering Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 62.Hendrix S.L., Wassertheil-Smoller S., Johnson K.C., Howard B.V., Kooperberg C., Rossouw J.E., Trevisan M., Aragaki A., Baird A.E., Bray P.F., Buring J.E., Criqui M.H., Herrington D., Lynch J.K., Rapp S.R., Torner J., WHI Investigators Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113(20):2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 63.Viscoli C.M., Brass L.M., Kernan W.N., Sarrel P.M., Suissa S., Horwitz R.I. A clinical trial of estrogen-replacement therapy after ischemic stroke. N. Engl. J. Med. 2001;345(17):1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 64.Grady D., Herrington D., Bittner V., Blumenthal R., Davidson M., Hlatky M., Hsia J., Hulley S., Herd A., Khan S., Newby L.K., Waters D., Vittinghoff E., Wenger N., HERS Research Group Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288(1):49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 65.Manson J.E. The Kronos Early Estrogen Prevention Study by Charlotte Barker. Womens Health (Lond. Engl) 2013;9(1):9–11. doi: 10.2217/whe.12.69. [DOI] [PubMed] [Google Scholar]
- 66.Nachtigall L.E., Raju U., Banerjee S., Wan L., Levitz M. Serum estradiol-binding profiles in postmenopausal women undergoing three common estrogen replacement therapies: associations with sex hormone-binding globulin, estradiol, and estrone levels. Menopause. 2000;7(4):243–250. doi: 10.1097/00042192-200007040-00006. [DOI] [PubMed] [Google Scholar]
- 67.Flaumenhaft R., Nachtigall M., Lowenstein J., Nachtigall L., Nachtigall R., Nachtigall L. Association of oral but not transdermal estrogen therapy with enhanced platelet reactivity in a subset of postmenopausal women. Menopause. 2009;16(2):407–412. doi: 10.1097/gme.0b013e3181833886. [DOI] [PubMed] [Google Scholar]
- 68.Shufelt C.L., Merz C.N., Prentice R.L., Pettinger M.B., Rossouw J.E., Aroda V.R., Kaunitz A.M., Lakshminarayan K., Martin L.W., Phillips L.S., Manson J.E. Hormone therapy dose, formulation, route of delivery, and risk of cardiovascular events in women: findings from the Women’s Health Initiative Observational Study. Menopause. 2014;21(3):260–266. doi: 10.1097/GME.0b013e31829a64f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith N.L., Blondon M., Wiggins K.L., Harrington L.B., van Hylckama Vlieg A., Floyd J.S., Hwang M., Bis J.C., McKnight B., Rice K.M., Lumley T., Rosendaal F.R., Heckbert S.R., Psaty B.M. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern. Med. 2014;174(1):25–31. doi: 10.1001/jamainternmed.2013.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunningham G.R., Toma S.M. Clinical review: Why is androgen replacement in males controversial? J. Clin. Endocrinol. Metab. 2011;96(1):38–52. doi: 10.1210/jc.2010-0266. [DOI] [PubMed] [Google Scholar]
- 71.Sowers M.R., Randolph J., Jr, Jannausch M., Lasley B., Jackson E., McConnell D. Levels of sex steroid and cardiovascular disease measures in premenopausal and hormone-treated women at midlife: implications for the “timing hypothesis”. Arch. Intern. Med. 2008;168(19):2146–2153. doi: 10.1001/archinte.168.19.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snyder P.J., Peachey H., Berlin J.A., Rader D., Usher D., Loh L., Hannoush P., Dlewati A., Holmes J.H., Santanna J., Strom B.L. Effect of transdermal testosterone treatment on serum lipid and apolipoprotein levels in men more than 65 years of age. Am. J. Med. 2001;111(4):255–260. doi: 10.1016/S0002-9343(01)00813-0. [DOI] [PubMed] [Google Scholar]
- 73.Spitzer M., Basaria S., Travison T.G., Davda M.N., Paley A., Cohen B., Mazer N.A., Knapp P.E., Hanka S., Lakshman K.M., Ulloor J., Zhang A., Orwoll K., Eder R., Collins L., Mohammed N., Rosen R.C., DeRogatis L., Bhasin S. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann. Intern. Med. 2012;157(10):681–691. doi: 10.7326/0003-4819-157-10-201211200-00004. [DOI] [PubMed] [Google Scholar]
- 74.Svartberg J., Agledahl I., Figenschau Y., Sildnes T., Waterloo K., Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int. J. Impot. Res. 2008;20(4):378–387. doi: 10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- 75.Legros J.J., Meuleman E.J., Elbers J.M., Geurts T.B., Kaspers M.J., Bouloux P.M., Study 43203 Investigators Oral testosterone replacement in symptomatic late-onset hypogonadism: effects on rating scales and general safety in a randomized, placebo-controlled study. Eur. J. Endocrinol. 2009;160(5):821–831. doi: 10.1530/EJE-08-0634. [DOI] [PubMed] [Google Scholar]
- 76.Kaufman J.M., Miller M.G., Garwin J.L., Fitzpatrick S., McWhirter C., Brennan J.J. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. J. Sex. Med. 2011;8(7):2079–2089. doi: 10.1111/j.1743-6109.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- 77.Kalinchenko S.Y., Tishova Y.A., Mskhalaya G.J., Gooren L.J., Giltay E.J., Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin. Endocrinol. (Oxf) 2010;73(5):602–612. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 78.Wartofsky L., Handelsman D.J. Standardization of hormonal assays for the 21st century. J. Clin. Endocrinol. Metab. 2010;95(12):5141–5143. doi: 10.1210/jc.2010-2369. [DOI] [PubMed] [Google Scholar]
- 79.Handelsman D.J., Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J. Clin. Endocrinol. Metab. 2013;98(10):3971–3973. doi: 10.1210/jc.2013-3375. [DOI] [PubMed] [Google Scholar]
- 80.Rosner W., Hankinson S.E., Sluss P.M., Vesper H.W., Wierman M.E. Challenges to the measurement of estradiol: an endocrine society position statement. J. Clin. Endocrinol. Metab. 2013;98(4):1376–1387. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosner W., Auchus R.J., Azziz R., Sluss P.M., Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J. Clin. Endocrinol. Metab. 2007;92(2):405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 82.Vermeulen A., Verdonck L., Kaufman J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 83.Södergård R., Bäckström T., Shanbhag V., Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J. Steroid Biochem. 1982;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y., Gay G.D., Botelho J.C., Caudill S.P., Vesper H.W. Total testosterone quantitative measurement in serum by LC-MS/MS. Clin. Chim. Acta. 2014;436:263–267. doi: 10.1016/j.cca.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prevention CfDCa CDC HoSt-Testosterone Certified Procedures. Available from: http://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Testosterone_Procedures.pdf . 2014. [cited 2014].
- 86.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Franco S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Huffman M.D., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Magid D., Marcus G.M., Marelli A., Matchar D.B., McGuire D.K., Mohler E.R., Moy C.S., Mussolino M.E., Nichol G., Paynter N.P., Schreiner P.J., Sorlie P.D., Stein J., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Association A.H. African-Americans and cardiovascular disease. 2014.
- 88.Cruz-Flores S., Rabinstein A., Biller J., Elkind M.S., Griffith P., Gorelick P.B., Howard G., Leira E.C., Morgenstern L.B., Ovbiagele B., Peterson E., Rosamond W., Trimble B., Valderrama A.L., American Heart Association Stroke Council. Council on Cardiovascular Nursing. Council on Epidemiology and Prevention. Council on Quality of Care and Outcomes Research Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(7):2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 89.Safford M.M., Brown T.M., Muntner P.M., Durant R.W., Glasser S., Halanych J.H., Shikany J.M., Prineas R.J., Samdarshi T., Bittner V.A., Lewis C.E., Gamboa C., Cushman M., Howard V., Howard G., REGARDS Investigators Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richard A., Rohrmann S., Zhang L., Eichholzer M., Basaria S., Selvin E., Dobs A.S., Kanarek N., Menke A., Nelson W.G., Platz E.A. Racial variation in sex steroid hormone concentration in black and white men: a meta-analysis. Andrology. 2014;2(3):428–435. doi: 10.1111/j.2047-2927.2014.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rohrmann S., Nelson W.G., Rifai N., Brown T.R., Dobs A., Kanarek N., Yager J.D., Platz E.A. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J. Clin. Endocrinol. Metab. 2007;92(7):2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 92.Orwoll E.S., Nielson C.M., Labrie F., Barrett-Connor E., Cauley J.A., Cummings S.R., Ensrud K., Karlsson M., Lau E., Leung P.C., Lunggren O., Mellström D., Patrick A.L., Stefanick M.L., Nakamura K., Yoshimura N., Zmuda J., Vandenput L., Ohlsson C., Osteoporotic Fractures in Men (MrOS) Research Group Evidence for geographical and racial variation in serum sex steroid levels in older men. J. Clin. Endocrinol. Metab. 2010;95(10):E151–E160. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Setiawan V.W., Haiman C.A., Stanczyk F.Z., Le Marchand L., Henderson B.E. Racial/ethnic differences in postmenopausal endogenous hormones: the multiethnic cohort study. Cancer Epidemiol. Biomarkers Prev. 2006;15(10):1849–1855. doi: 10.1158/1055-9965.EPI-06-0307. [DOI] [PubMed] [Google Scholar]
- 94.Randolph J., Jr, Sowers M., Bondarenko I., Harlow S., Luborsky J., Little R. Change in estradiol and FSH across the early menopausal transition: effects of ethnicity and age. J. Clin. Endocrinol. Metab. 2004;89:1555–1561. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 95.O’Donnell C.J., Nabel E.G. Genomics of cardiovascular disease. N. Engl. J. Med. 2011;365(22):2098–2109. doi: 10.1056/NEJMra1105239. [DOI] [PubMed] [Google Scholar]


