Abstract
Cocaine-dependent (CD) subjects show attentional bias toward cocaine-related cues, and this form of cue-reactivity may be predictive of craving and relapse. Attentional bias has previously been assessed by models that present drug-relevant stimuli and measure physiological and behavioral reactivity (often reaction time). Studies of several CNS diseases outside of substance use disorders consistently report anti-saccade deficits, suggesting a compromise in the interplay between higher-order cortical processes in voluntary eye control (i.e., anti-saccades) and reflexive saccades driven more by involuntary midbrain perceptual input (i.e., pro-saccades). Here, we describe a novel attentional-bias task developed by using measurements of saccadic eye movements in the presence of cocaine-specific stimuli, combining previously unique research domains to capitalize on their respective experimental and conceptual strengths. CD subjects (N = 46) and healthy controls (N = 41) were tested on blocks of pro-saccade and anti-saccade trials featuring cocaine and neutral stimuli (pictures). Analyses of eye-movement data indicated (1) greater overall anti-saccade errors in the CD group; (2) greater attentional bias in CD subjects as measured by anti-saccade errors to cocaine-specific (relative to neutral) stimuli; and (3) no differences in pro-saccade error rates. Attentional bias was correlated with scores on the obsessive-compulsive cocaine scale. The results demonstrate increased saliency and differential attentional to cocaine cues by the CD group. The assay provides a sensitive index of saccadic (visual inhibitory) control, a specific index of attentional bias to drug-relevant cues, and preliminary insight into the visual circuitry that may contribute to drug-specific cue reactivity.
Keywords: Anti-saccades, Attentional Bias, Cocaine Dependence, Cue-Reactivity, Eye-tracking
1. Introduction
The high rate of relapse following abstinence remains a major hurdle in addiction treatment efforts (O’Brien & Gardner, 2005). Understanding the cognitive and physiological factors underlying relapse remains a challenge, however, studies that can identify key factors concerning relapse would provide utility for addiction treatment efforts (Vadhan et al., 2007; McKay, 1999; Donovan, 1996). In stimulant dependence, several relapse predictors have been examined including craving, demographics, length of substance use, neuroimaging, and baseline urine results (Farabee et al., 2013; Gowin et al., 2014; Paulus et al., 2005; Poling et al., 2007). Recently there has been increased interest in the role of neurocognitive measures, particularly attentional bias, as a sensitive index of relapse risk (Kosten et al., 2006; Franken, 2003; Kavanagh et al., 2004; Marlatt & Gordon, 1985; Robinson & Berridge, 1993).
The attentional bias phenomenon is well established in addiction research, where the presence of drug-related stimuli is posited to differentially capture attention, disrupt inhibitory control, and play a key role in drug cue reactivity, craving, and relapse (Field & Cox, 2008; Marhe et al., 2013a; Pike et al., 2013; Roberts et al., 2014). One widely used measure of attentional bias is the drug Stroop task (Cox et al., 2006; Wühr & Waszak, 2003). Studies using a cocaine-specific Stroop task have found that cocaine dependent (CD) subjects show attentional bias toward cocaine-related cues (Anastasio et al., 2014; Hester et al., 2006; Liu et al., 2011; Vadhan et al., 2007). Whereas some studies have reported that attentional bias predicts treatment outcome (Carpenter et al., 2006; Conklin et al., 2012; Marhe et al., 2013b), one recent study did not (Kennedy et al., 2014). Despite the replication of attentional bias effects using drug-Stroop and similar reaction-time based tasks, effects sizes for these attentional bias measures have generally been in the low – moderate range (Field & Cox, 2008; Kennedy et al., 2014; Leeman et al., 2014). Additionally, it has been noted that performance on the drug Stroop invokes a number of complex cognitive processes beyond attention (Cox et al., 2006; Field & Cox, 2008; Marks et al., 2014a). Accordingly, recent experimental procedures have utilized more direct observational methods by tracking rapid eye movements, specifically fixations and saccades (Marks et al., 2014a; Marks et al., 2014b; Miller & Fillmore, 2010).
Saccades are rapid eye movements that move from one fixation point to another (Holmqvist et al., 2011). With instruction, individuals can be trained to look in the opposite direction of a presented stimulus, e.g., the well-studied anti-saccade (Everling & Fischer, 1998). In order to accurately execute an anti-saccade, two processes must be intact and functional. First, the individual must detect the stimulus in the peripheral visual field and suppress a reflexive response to orient the eyes toward stimulus (e.g., a pro-saccade). Second, the individual must make a voluntary anti-saccade to the hemifield opposite the stimulus (Munoz & Everling, 2004). This two-stage process is primarily mediated by top down control via the DLPFC and supplementary eye fields (SEF) (Dyckman et al., 2011; Fukumoto-Motoshita et al., 2009). The DLPFC and the SEF are thought to be vital for inhibitory control (Guitton et al., 1985; Gaymard et al., 1998). Impairments to these regions cause difficulty in proper execution of the anti-saccade task resulting in increased error rates (Hasegawa et al., 2004; Coe et al., 2002; Stuphorn et al., 2000).
It has been widely reported that chronic stimulant use is related to dysregulation of DLPFC function, impulsive behavior, and inhibitory control deficits (Bechara 2005; Fillmore & Rush, 2002; Goldstein & Volkow, 2011). Because DLPFC dysfunction is related to increased anti-saccade error rates in patients with neurological disorders, including Huntington’s, Parkinson’s, Schizophrenia, and Alzheimer’s (Clementz et al., 1994; Chan et al., 2005; Sereno & Holzman, 1995), similar impairments in anti-saccade performance might be expected in CD patients. Furthermore, CNS-mediated inhibitory control deficits are unmasked via the anti-saccade task and have been suggested as early diagnostic indictors and endophenotypic markers for these diseases (Dyckman et al., 2011; Fukumoto-Motoshita et al., 2009; Vidailhet et al., 1994; Nilsson et al., 2013; Lasker et al., 1987; Levy, 1996; Pierrot-Deseilligny et al., 1991; Fukushima et al., 1990; Rosse et al., 1993b; Sereno & Holzman, 1995). Due to the hyper-salience of drug cues in the CD population (e.g., incentive salience, Robinson & Berridge, 1993) and the role of attentional bias in addiction, we reasoned that anti-saccade errors would be further exacerbated in the presence of cocaine-specific stimuli.
Recent studies have shown that increased attentional bias to cocaine stimuli can be measured using eye-tracking methodologies. These studies utilized a visual probe task, which simultaneously presented a cocaine and neutral stimulus and assessed duration of gaze toward each stimulus as the measure of attentional bias. Under this eye-measurement paradigm, CD subjects spent significantly longer time gazing at pictures of cocaine stimuli compared to non-cocaine stimuli relative to control subjects (Marks et al., 2014a; Marks et al., 2014b). The visual probe task was useful in determining duration of gaze as an index of attentional bias, but does not measure deficits in visual control circuitry over anti-saccades that are prevalent in several of the above-noted CNS diseases.
The goal of the present study was to measure anti-saccade error rates during the presentation of cocaine and non-drug stimuli on a novel cocaine-specific attentional bias task, and compare performance of CD subjects to that of healthy controls. Examining both sensitivity and specificity, we hypothesized that (1) CD subjects would make more overall anti-saccade errors vs. control subjects, and (2) that errors rates of CD subjects would be differentially higher than controls on trials presenting cocaine vs. non-drug stimuli (i.e., attentional bias effect). Pro-saccades were examined to control for general visual-motor or attentional deficits. In exploratory analyses, we also examined correlations between attentional bias and measures of cocaine use, cocaine craving, and stress.
2. Materials and Methods
2.1 Subjects
The study was conducted at the University of Texas Health Science Center in Houston, where subjects were recruited by a variety of media advertisements (e.g., newspaper, flyers, public service announcements on television and radio) to participate in a study on cocaine dependence. This study was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston. Subjects provided written consent for their participation and were fully informed of the nature of the research. The study enrolled males and females ages 18–60 years old, designated as either control subjects (n=41) or active CD subjects (n=46) meeting current DSM-IV (SCID-1) criteria for cocaine dependence and reported cocaine use within the past 30 days (First, 1996). All subjects were urine tested for cocaine (benzoylecgonine), opiates, amphetamine, methamphetamine, benzodiazepines, and tetrahydrocannabinol using an E-Z split key cup II (Innovacon Company, San Diego, CA, USA) on each visit. To be eligible, CD subjects had to submit at least one positive urine toxicology screen for the cocaine metabolite benzoylecgonine (BE) > 300ng/mL during the two day screening period. Subjects who were currently dependent on any psychoactive substance other than cocaine or nicotine were excluded. Chronic marijuana smokers, defined as smoking marijuana ≥ 10 times in past 30 days (Lindsay, 2009), were excluded to eliminate the potentially confounding role of heavy cannabis on cognitive performance (Lundqvist, 2005). The Addiction Severity Index (ASI) and the Kreek–McHugh–Schluger– Kellogg (KMSK) questionnaire, which quantify self-exposure to opiates, cocaine, alcohol, and tobacco, were administered to all subjects in the study (Kellogg et al., 2003). Further exclusionary criteria included current or past medical disorders affecting the central nervous system, and any Axis I disorders other than substance abuse or dependence. CD subjects included both treatment seeking and non-treatment seeking individuals. Those seeking treatment participated in this study at baseline, prior to the initiation of any treatment intervention (e.g., medication or psychotherapy). Control subjects had drug-negative urine screens, no current or past DSM-IV axis I disorders (including substance dependence), and no medical disorder affecting the central nervous system. All subjects (CD and control) were free of alcohol at the time of testing as determined by a Breathalyzer test (Intoximeters, Inc., St. Louis, MO, USA). Female subjects were excluded if results from a urine pregnancy test were positive, however, no cases occurred during the study.
Of the 106 subjects enrolled in the study, 19 subjects were excluded from the data analyses (7 cocaine; 12 controls): 16 because the eye tracker was unable to detect and/or consistently track the subjects’ pupil, and three due to an excessive number of saccade errors (>80%), which indicated either a lack of motivation, inability to perform the task correctly, lack of instructional control, or some combination thereof. Two of these exclusions were further validated by the presence of low Shipley WAIS equivalent (IQ) scores below 80. The final sample consisted of 46 CD subjects and 41 controls subjects.
2.2 Eye-Tracking Cocaine Attentional Bias Task
Each subject was tested using eye-tracking technology (MiraMetrix S2 Eyetracker, Vancouver, BC, 16ms eye reacquisition, 60Hz data sampling rate) to measure performance on blocks of pro-saccade (look at stimulus) and anti-saccade (look away from stimulus) trials. The task began with a nine-point calibration procedure that was performed to map the eye-fixation position of each subject to designated screen coordinates. The calibration was considered valid if the maximum spatial error was less than 1 degree, and the average error was less than 0.5 degrees.
Subjects began with a brief training session (16 pro-, 16 anti-saccade trials), in which the image shown was a textured grey box. The instructions were summarized on the screen explicitly, stating whether the subject was to look at or away from the image. Subjects were asked to avoid blinking until after they made a saccade either toward or away from the image. Subjects were able to practice blinking between trials during the 32-trial training session. Following training, the experimental session began. Each trial used a gap paradigm, and had the following structure: (1) orienting stimulus (central cross hair; jittered 300–400ms to avoid anticipation effects); (2) cue = one of 6 unique cocaine images, 6 unique neutral images, or 6 shape (neutral/gray) images, counterbalanced either to the left or right; (3) image cue removed from screen after 800ms; (4) followed by an inter-trial interval (1600ms). For the pro-saccade trials, the subject was instructed to look at the image. Conversely for the anti-saccade trails the subject was told to look away from the image and to fixate on the blank screen on the opposite side. During each test session four blocks were administered (2 pro-saccade, 2 anti-saccade), with 36 pro- and 36 anti-saccade trials (144 trial total). Block order was fully counterbalanced across and within groups. Cocaine-related images were created from freely available online sources and matched as closely as possible to neutral images on visual characteristics such as color, background, complexity, and presence of people. Cocaine-related cues included powdered cocaine in isolation as well as close-ups of individuals’ hands as they smoked cocaine through pipes. Faces were not shown. Neutral cues included environmental scenes as well as close-ups of hands holding non-drug related objects. Shape cues were textured gray images. Each of the images (250 × 188 pixels) was presented on a 304 × 378mm screen, either 7° to the left or right of the centered fixation cross. Each session of this task lasted 8–10 min. Trials interrupted with blinks (which render an accurate measurement invalid) were captured, aborted, and then the trial was reinserted at the end of that (pro- or anti-) test block. Thus each subject completed the same number of trials (144) and no data were lost due to blinks (Patel et al., 2012).
2.3 Dependent Measures
The two primary measurement variables were pro-saccade errors and anti-saccade errors. Pro-saccade errors were defined as any eye movement not directed toward or within the boundaries of the presented image. Anti-saccade errors were defined as the failure to inhibit a reflexive saccade towards the presented image and look in the opposite hemi-field. Each error was measured during the cue presentation (800ms), and each trial ended once a saccade was made or 800ms elapsed. A single saccade error was coded as an error on that trial (e.g., corrections and correction latencies were not recorded or scored).
Additional measures of cocaine use, craving, and stress were administered prior to beginning the attentional bias task. The Obsessive-Compulsive Cocaine Scale (OCCS) (Jardin, 2011; Vorspan, 2012) is a 14-item scale that measures both obsessive and compulsive aspects of cocaine use. The present analyses focused on the obsessive factor score, as it has shown better predictive power related to cocaine use severity (Vorspan, 2012). The Perceived Stress Scale (PSS) (Cohen et al., 1983; Cohen & Williamson, 1988) is 10-item scale widely used to measure the degree to which individuals appraise their life as stressful. The scale has a 5-point Likert-type response format. The Visual Analogue Scale - Cocaine Craving (VAS-CC) is a brief 3-item instrument in which subjects mark a point on a 100 mm line to indicate NOT AT ALL or VERY MUCH to three cocaine-related questions: (1) Right now, how much are you craving cocaine? (2) Over the last week on average how much have you been craving cocaine? (3) Over the last week how much did you crave cocaine when your craving was at its worst? (Sayette, 2000). The OCCS and VAS-CC was administered to CD subjects only. All subjects completed the PSS.
2.4 Data Analyses
The following comparisons were tested: (1) Error rates on anti-saccade trials in the CD group vs. controls across all stimuli (main effect); (2) Error rates during anti-saccade trials on drug vs. neutral and shape stimuli in the CD group vs. controls (interaction); (3) Error rates on pro-saccade trials in the CD group vs. controls across all stimuli (main effect), and between cocaine vs. neutral and shape stimuli (interaction). Note that comparison 1 examines the overall sensitivity of the anti-saccade assay in the CD group (rarely has this been researched); comparison 2 examines the specificity of the assay to cocaine-related stimuli (i.e., the cue-specific attentional bias); and comparison 3 examines experimental control related to inhibitory processes over visual circuitry, rather than general attentional, instructional, or visual deficits.
Initial linear effects mixed models using the R ‘lmer’ package examined the effects of group (CD, control), stimulus type (cocaine, neutral, shape), and the group x stimulus type interaction. Separate models were run for pro-saccade and anti-saccade error rates, as direct statistical comparison of performance on the two trial types was not of interest in this study and has already been well-established in many disease models (Patel et al., 2012; Bowling et al., 2012; Hutton et al., 2002; Reuter et al., 2007).
Demographic comparisons indicated that CD and control subjects differed on age, education, and gender. Therefore, these variables were identified as potential confounding variables and examined for association with the dependent variable (error rates) (Pocock et al., 2002). Pearson correlations (age p<0.01, education p<0.96, and gender p<0.51) indicated that only age was significantly correlated with anti-saccade error rates. The cocaine group was older than the control group, t (85) = 2.95, p < 0.01. This age difference is pervasive in studies of inner-city cocaine users, who are generally between 40 and 55 years old (Moeller et al., 2010; Haile et al., 2012, Kampman et al., 2013). Healthy control subjects in this age range without pathology are overwhelmingly employed and unable or unwilling to participate in research studies conducted during working hours. Because anti-saccade error rates increase with age (Shafiq-Antonacci, 1999), the final statistical models included age as a covariate to control for the age difference between groups. Correlations with pro-saccade errors did not suggest any significant confounders. Therefore, none of these three demographic variables were included in the pro-saccade statistical models.
The residuals from the initial linear model were examined for violations, with the Satterthwait approximation for degrees of freedom, of underlying assumptions that posed threats to stability and reliability, e.g., non-normality, heteroskedasticity, collinearity, and leverage. Any violations of normality of residuals were identified via Welch-Satterthwaite approximation, however, no violations were observed in this dataset. Post-hoc testing of significant main effects or interactions utilized testing of least-squared means using the R ‘difflsmeans’ package in order to establish factor-specific differences between and within groups, in which age was held constant. All post-hoc test outcomes were FDR corrected for multiple comparisons.
2.5 Questionnaires
Correlational analyses examined the relationships among anti-saccade errors and measures of cocaine use, craving, and stress. In the CD group, spearman correlations were conducted between OCCS score and an attentional bias difference score [(neutral + shape cue anti-saccade errors/2) – cocaine cue anti-saccade errors] as well as between the VAS-CC and the attentional bias difference score. In addition, spearman correlations were conducted between the attentional bias difference score and (a) the past 30 days of cocaine use (extracted from the ASI), and (b) the KMSK total cocaine use score. For the total sample (collapsed across groups), correlational analyses were conducted between anti-saccade errors across all stimuli and the PSS score, as well as between anti-saccade errors on cocaine stimuli and the PSS score.
3. Results
3.1 Demographics
Within the CD population, the majorities were African American (65%), male (85%), and employed at least part-time (91%). Within the control population, the majorities were African American (78%), male (51%), and employed (93%). All subjects had normal or corrected-to-normal vision and none were color-blind. Thirty-five of the 46 CD subjects were treatment seeking. Further demographics are shown in Table 1.
Table 1.
Subject demographics for cocaine dependent (CD) subjects and controls.
| CD | Control | |
|---|---|---|
| N | 46 | 41 |
| Age** | 46.3 (8.4) | 40.0 (11.3) |
| Gender N (%Male)** | 39 (84.8) | 21 (51.2) |
| Education (% College or Above)** | 24% | 63% |
| Shipley* | 87.4 (13.6) | 94.2 (15.1) |
| % Smokers** | 76.1 | 22.0 |
| Cigarettes (days smoked/wk)** | 6.6 (1.5) | 1.3 (2.7) |
| Alcohol (days/week)** | 3.4 (3.8) | 1.6 (1.9) |
| Marijuana (days smoked/wk)** | 3.9 (3.1) | 0.9 (1.7) |
All results are means (std. deviations)
p<0.05,
p<0.01
3.2 Errors during eye tracking
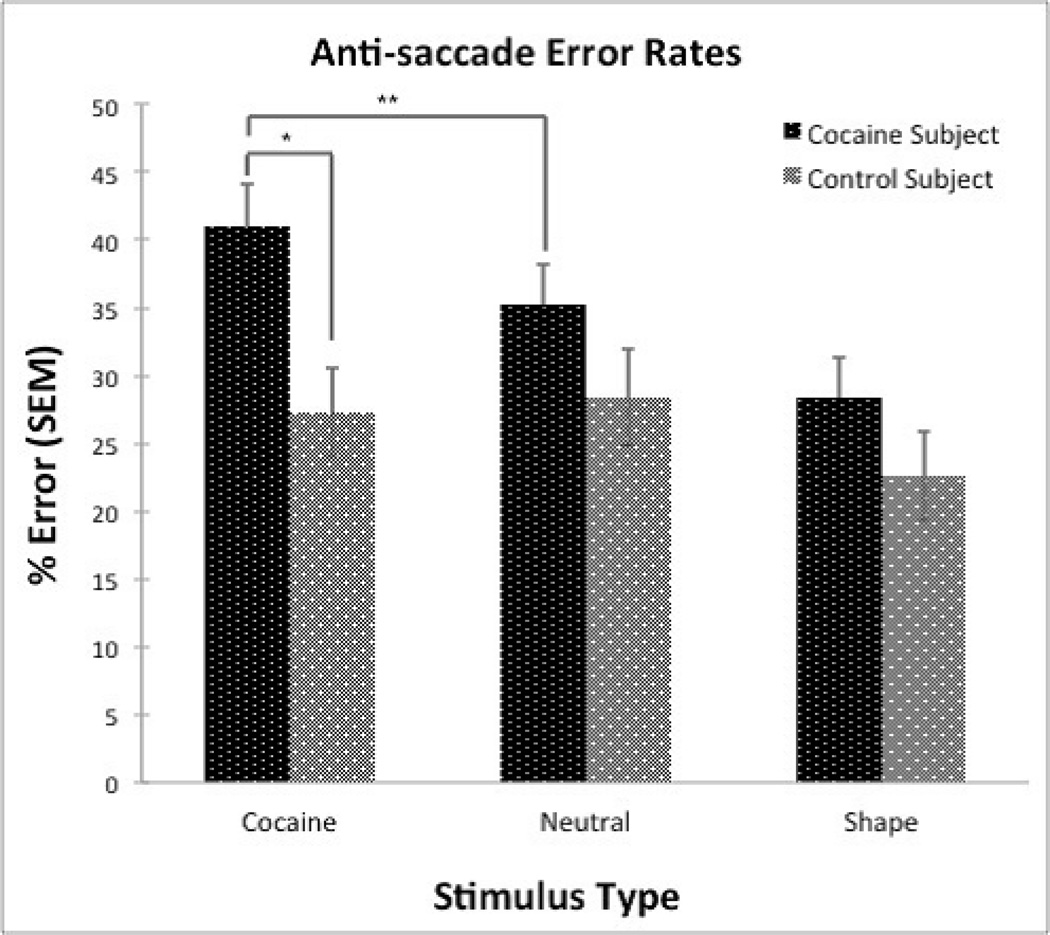
Analysis of anti-saccade errors including age as a covariate yielded a significant group x stimulus interaction (F [84, 167] = 4.81, p< 0.01), and a main effect of group, indicated by significant differences in error rates between the two groups (CD vs. control, t = 2.63, p<0.01) across all stimuli (Figure 1). CD subjects made more errors overall during anti-saccade trials across all stimuli. The Cohen’s d effect size for anti-saccade errors between groups across all stimuli was 0.40. Within the CD group, there were no significant differences in anti-saccade error rates between treatment seeking and non-treatment seeking individuals. Furthermore, split-half correlations for anti-saccade errors across all subjects were completed for each stimulus type: cocaine (r = 0.82), neutral (r = 0.75), shape (r = 0.81), and across all stimulus types (r = 0.91), establishing strong internal reliability within the task.
Figure 1.
Post hoc testing of specific interactions during anti-saccade trials utilized least-square mean comparison tests. The results are summarized in Figure 1. We found a significant difference between groups (p<0.03) on anti-saccade trials presenting cocaine stimuli (Cohen’s d effect size = 0.62). Significant between-group differences were not observed on trials with neutral stimuli or shape stimuli. In addition, significant within-group differences were observed for the CD group between cocaine and neutral stimuli (p<0.01) and shape stimuli (p<0.01), where significantly more errors were made on the cocaine-stimulus vs. neutral- and shape-stimulus trials. Within the control group, no statistically significant differences were observed between cocaine and neutral stimuli or between cocaine and shape stimuli. All significant least-square mean tests remained significant after FDR correction.
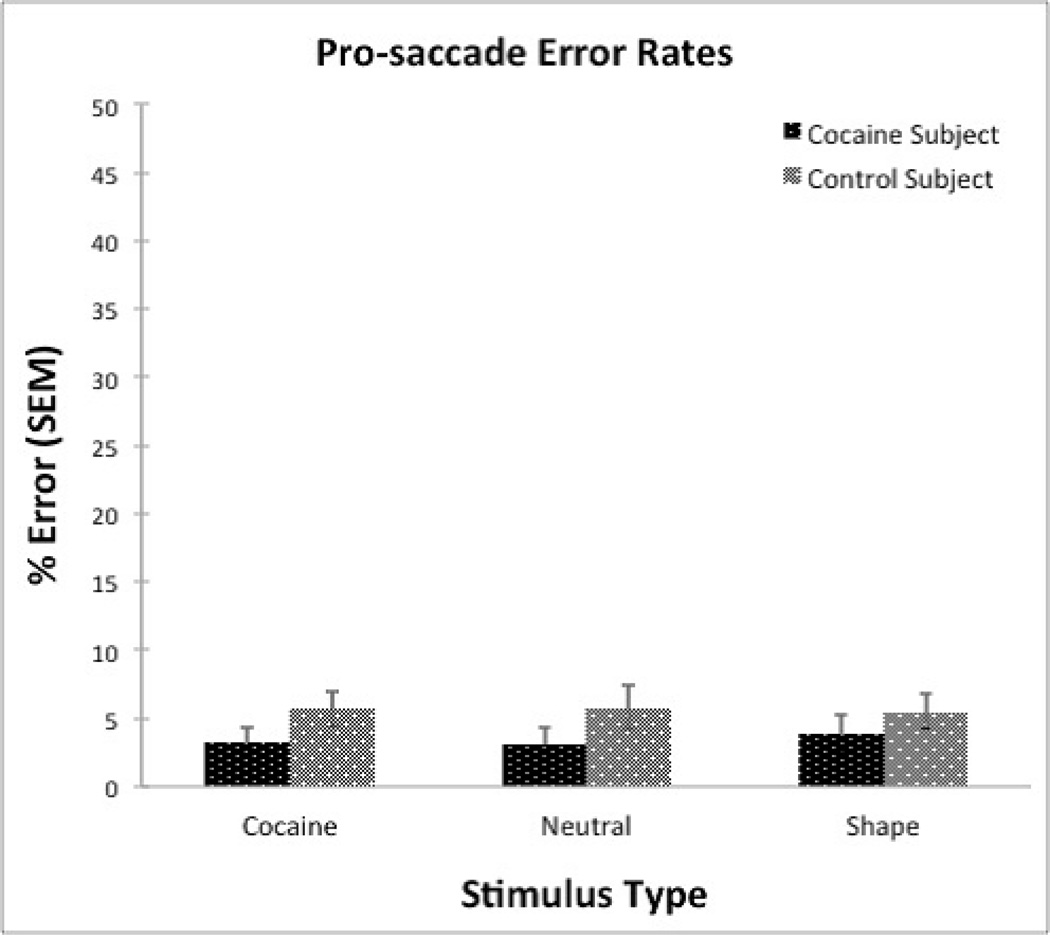
Pro-saccade trials did not reveal any main effect of group (F [84, 167] = 1.54, p< 0.22), stimulus (F [84, 167] = 0.09, p<0.91), or interaction of group x stimulus (F [84, 167] = 0.41, p< 0.66). These results are summarized in Figure 2. Error rates for both groups and all stimuli types are indicated in Table 2.
Figure 2.
Table 2.
Error rates on anti-saccade and pro-saccade trials for cocaine dependent (CD) and control subjects.
| Anti-saccade Trials | |||
|---|---|---|---|
| Cocaine Cue | Neutral Cue | Shape Cue | |
| Cocaine Subject | 40.94 ± 3.11 | 35.24 ± 2.96 | 28.35 ± 2.96 |
| Control Subject | 27.24 ± 3.3 | 28.35 ± 3.54 | 22.56 ± 3.24 |
| Pro-saccade Trials | |||
| Cocaine Cue | Neutral Cue | Shape Cue | |
| Cocaine Subject | 3.26 ± 1.09 | 3.08 ± 1.30 | 3.89 ± 1.38 |
| Control Subject | 5.69 ± 1.34 | 5.79 ± 1.61 | 5.49 ± 1.25 |
Data show mean ± SEM.
3.3 Association with Questionnaires
Spearman correlations between OCCS and the attentional bias difference score [cocaine cue anti-saccade errors – (neutral + shape cue anti-saccade errors / 2)] yielded a statistically reliable positive correlation (p <0.04). As the amount of obsessive and compulsive urges to use cocaine increased so did the number of errors toward cocaine related cues. Correlations between total anti-saccade errors across all stimuli and PSS score by group as well as anti-saccade errors on cocaine stimuli and PSS score by group were not significant. Likewise, correlations between VAS-CC, KMSK cocaine, and ASI past 30 days of use and the attentional bias difference score did not reveal any statistically significant correlations.
4. Discussion
The present study aimed to measure saccadic eye movements in CD individuals using an eye-tracking based attentional bias task. Consistent with the main hypotheses of the study, CD subjects made more overall anti-saccade errors indicating a deficit in visual inhibitory control. Importantly, CD subjects also made more errors specifically on trials with cocaine stimuli compared to neutral and shape stimuli, consistent with the operationally-defined definition of attentional bias toward drug cues.
CD subjects made more anti-saccade errors across all stimuli (cocaine, neutral, and shape), compared to control subjects. This provides evidence that cocaine-dependence subjects have poor inhibitory control over saccades, an index of prefrontal cortex dysfunction (Lane et al., 2007; Hasegawa et al., 2004; Coe et al., 2002; Stuphorn et al., 2000). Eye movement abnormalities, particularly a difficulty with voluntary saccades, are quite sensitive indicators of psychiatric and neurological disease, including Huntington’s (Lasker et al., 1987), Parkinson’s (Chan et al., 2005), and Schizophrenia (Sereno & Holzman, 1995). The anti-saccade error rates reported here for CD subjects are similar to those found in other disease models (Sereno & Holzman, 1995; Vidailhet et al., 1994; Nilsson et al., 2013). This may be due to the use of pictures with motivational salience (vs. simple shapes typically employed), and the use of a gap paradigm to enhance error rates. The results argue for the potential utility of this novel eye-tracking task to detect CNS deficits related to inhibitory control and attentional processing in substance-dependent populations. Subsequent experiments incorporating structural and functional MRI will help validate this assertion.
CD subjects made more anti-saccade errors than controls on trials presenting cocaine cues. This operationally-defined demonstration of attentional bias toward cocaine cues is consistent with findings in cocaine users on the picture and word emotional Stroop task (Hester et al., 2006), as well as recent attentional bias tasks measuring eye-gaze patterns (Marks et al., 2014a; Marks et al., 2014b). Importantly, results indicated a group x stimulus interaction, such that significantly greater errors towards cocaine-related vs. neutral stimuli (41% vs. 20% error rates, p<0.01) were shown in CD subjects only; no differences were observed between cocaine and neutral stimuli in the control group. This stimulus-dependent differential outcome between groups provides evidence of specificity of the attentional bias phenomena within this novel eye-tracking task; anti-saccade errors are greatest when cocaine users are viewing cocaine cues.
Pro-saccade error rates between groups were non-significant and very low across all stimuli (3.3% cocaine subjects vs. 5.6% control subjects overall). Pro-saccade error rates were not of main interest in our hypotheses, but the uniformly low error rates argue against a non-specific global CNS dysfunction in the CD group, and provide evidence that anti-saccade error rates were not due to differences in motivation on the task or disruptions of simple sensory function. This outcome helps to further validate the sensitivity of the anti-saccade measure, in which the CD group performed significantly worse than controls. While we do not have corroborative fMRI data, the poor anti-saccade error rates suggest portions of the saccadic circuitry may be disrupted in cocaine dependence. Other studies have shown that when the FEF is lesioned, the suppression of the reflexive pro-saccade remains intact, however, the ability to generate anti-saccades becomes impaired (Gaymard et al., 1998; Davidson et al., 1999). Furthermore, imaging results in healthy elderly subjects – more prone to decline in executive function – indicated that with cognitive decline FEF activity was associated with poor anti-saccade performance (Pa et al., 2014).
Correlations between the attentional bias difference score and PSS, VAS-CC, KMSK, and ASI past 30 days of use did not yield any statistically reliable results. We did observe a statistically significant correlation between the OCCS score and the attentional bias difference score. Compulsive drug seeking behavior has been shown to contribute to chronic relapse of drug addiction and higher attentional bias toward cocaine in the presence of drug-related stimuli (Weiss et al., 2001). Additionally, inhibitory control deficits predict relapse in substance use disorders (Bechara, 2005). Consequently, cocaine-specific anti-saccade error rates could serve as a marker for the vulnerability to relapse. This possibility will require validation in the context of prospective studies and clinical trial outcomes.
Cocaine has been shown to dysregulate the stress system and affect executive function when high levels of stress are present (Fox et al., 2009). Therefore, it might be anticipated that higher PSS scores would be correlated with attentional bias in the cocaine group. However, we did not observe this relationship. The PSS measures past 30-day stress levels, and to evidence a direct relationship it may be necessary to experimentally manipulate stress levels prior to testing in this eye-movement protocol.
The VAS-CC, intended to measure subjective craving, was not significantly correlated with anti-saccade attentional bias. Previous visual scanning studies have reported that heavy cocaine users displayed a 90 second visual path pattern very similar to the entire cocaine-related picture they scanned (as opposed to a small segment), likely due to the associated reports of higher craving and greater interest in the cocaine image (Rosse et al., 1993a). The lack of association with craving in the present dataset may not be due to a lack of stimulus (i.e., cocaine picture) effect on craving, but rather a result of the short period of stimulus presentation; the on-screen stimulus time of 800ms may not be sufficient to elicit subjective craving. The lack of association with more temporally distinct craving reports remains undetermined.
4.1 Limitations
One limitation of this study was heterogeneity in the subject population. Greater control over factors such as age, gender balance, time since last use, and lifetime use patterns would likely aid in the prediction of anti-saccade error rates and attentional bias. It is well established that measures of cue-reactivity (including attention bias) and inhibitory control account for a moderate amount of variance in treatment outcome, across several drugs of abuse (Back et al., 2010; Kosten et al., 2006; Marhe et al., 2013a; Paulus et al., 2005; Sterling et al., 2004). In the present study, individuals were both treatment seeking (n=35, mean = 9.36 ± 5.97) and non-treatment seeking (n=11, mean = 9.97 ± 5.37), and the age range varied considerably within and across groups. Within the CD group, there were no differences on anti-saccade error performance between the treatment seeking and non-treatment seeking individuals. This may be due to the small sample sizes for each sub-group, as the groups were not balanced in size for a formal comparison of treatment related differences. Furthermore, other key measures of test validation were not undertaken in the present study, including error-rate stability over time, within-subject replication, and attempts to modify error rates via clinically relevant disruptors (e.g., stress, withdrawal). While such efforts were beyond the scope of this initial study, these factors will require careful consideration in future experiments. On the other hand, reaction times and latency data were collected for each subject, however, to maintain clarity and succinctness in this report, these reaction time data will be reported in a future manuscript.
Reports of subjective craving may well be associated with cue-reactivity and attentional bias, including cocaine-specific anti-saccade error rates. While we did not observe them using a VAS scale in this study, use of more extensive and validated scales, and direct experimental manipulations that promote craving (i.e., exposure to drug paraphernalia), might improve measurement sensitivity in future work and uncover this relationship.
We established both sensitivity (overall errors rates) and specificity (attentional bias, e.g., higher error rates to cocaine stimuli) using this eye-movement based attentional bias task. There is low internal reliability in some visual probe tasks and unblocked versions of the modified Stroop task (Ataya et al., 2012). Systematic replication and extension will be required to further examine issues of reliability and validity. Importantly, we do not yet have an index of whether this task has utility in predicting relapse. Accordingly, future investigations will focus on establishing reliability in the association between task performance and rates of relapse. Additionally, it will be useful to employ this task in other drug-using populations to establish generalizability across substances, and corroborate with previous eye-movement studies using alcohol and marijuana stimuli (Field et al., 2006; Roberts et al., 2014). Examination of test sensitivity to drug challenges will be important to establish sensitivity to acute manipulations.
The predictive utility of attentional bias toward drug-related cues has been documented in CD individuals (Carpenter et al., 2006) as well as in alcohol abusing subjects (Cox et al., 2007), smokers (Waters et al., 2003), and heroin users (Marissen et al., 2006). The consistency of this phenomenon is evident in predicting relapse in binge eating patients (Overduin et al., 1995), and symptom severity in individuals suffering from traumatic experiences, such as PTSD (Elsesser et al., 2005). Upon further validation, measurement of saccadic eye movements may provide specific and sensitive examination of attentional bias and inhibitory control in CD subjects, perhaps adding an additional marker for the prediction of relapse in those undergoing treatment.
5. Conclusions
Collectively, the results support the primary hypotheses, and confirm that this eye-tracking based measure of attentional bias is a quick, noninvasive, and valid assessment of attentional bias to drug cues. It has apparent sensitivity and specificity, and may prove useful in efforts toward further understanding the role of cue-reactivity in relapse, particularly related to stimulus control over visual activity.
Acknowledgements
Funding was provided by NIH/NIDA P50 09262.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
Authors Contribution: ND, NR, and SL were responsible for study concept and design. ND and NR contributed to the acquisition of the human data. ND, NR, SR, AS, and SL contributed to data analysis and interpretation of findings. ND drafted the manuscript. JS, SR, AS, FM, SL provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Anastasio NC, Liu S, Maili L, Swinford SE, Lane SD, Fox RG, Hamon SC, Nielsen DA, Cunningham KA, Moeller FG. Variation within the serotonin (5-HT) 5-HTC receptor system aligns with vulnerability to cocaine cue reactivity. Transl Psychiatry. 2014;4:e369. doi: 10.1038/tp.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafò MR. Internal reliability of measures of substance-related cognitive bias. Drug Alcohol Depend. 2012;121(1–2):148–151. doi: 10.1016/j.drugalcdep.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Hindman EA, Donnelly JF. Pro-saccade errors in the anti-saccade task: differences between corrected and uncorrected errors and links to neuropsychological tests. Exp Brain Res. 2012;216:169–179. doi: 10.1007/s00221-011-2921-7. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol. 1994;103:277–287. [PubMed] [Google Scholar]
- Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia. 2005;43:784–796. doi: 10.1016/j.neuropsychologia.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J. Neurosci. 2002;22:5081–5090. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived Stress in a Probability Sample of the United States. Newbury Park, CA: The Social Psychology of Health; 1988. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:386–396. [PubMed] [Google Scholar]
- Conklin CA, Parzynski CS, Salkeld RP, Perkins KA, Fonte CA. Cue reactivity as a predictor of successful abstinence initiation among adult smokers. Exp Clin Psychopharmacol. 2012;20:473–478. doi: 10.1037/a0029599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: Theoretical considerations and procedural recommendations. Psychol Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Cox WM, Pothos EM, Hosier SG. Cognitive-motivational predictors of excessive drinkers’ success in changing. Psychopharmacology (Berl) 2007;192:499–510. doi: 10.1007/s00213-007-0736-9. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Everling SL, Munoz DP. Comparison of pro- and anti-saccades in primates. III. Reversible activation/inactivation of frontal eye field and superior colliculus. Soc. Neurosci. 1999;25:147. [Google Scholar]
- Donovan DM. Assessment issues and domains in the prediction of relapse. Addiction. 1996;91:S29–S36. [PubMed] [Google Scholar]
- Dyckman KA, Lee AK, Agam Y, Vangel M, Goff DC, Barton JJ, Manoach DS. Abnormally persistent fMRI activation during antisaccades in schizophrenia: a neural correlate of perseveration? Schizophr Res. 2011;132:62–68. doi: 10.1016/j.schres.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsesser K, Sartory G, Tackenberg A. Initial symptoms and reactions to trauma-related stimuli and the development of posttraumatic stress disorder. Depress Anxiety. 2005;21:61–70. doi: 10.1002/da.20047. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The anti-saccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Farabee D, McCann M, Brecht ML, Cousins SJ, Antonini VP, Lee AB, Hemberg J, Karno M, Rawson RA. An analysis of relapse prevention factors and their ability to predict sustained abstinence following treatment completion. Am J Addict. 2013;22:206–211. doi: 10.1111/j.1521-0391.2012.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Fox HC, Jackson ED, Sinha R. Elevated cortisol and learning and memory deficits in cocaine dependent individuals: relationship to relapse outcomes. Psychoneuroendocrinology. 2009;34:1198–1207. doi: 10.1016/j.psyneuen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Fukumoto-Motoshita M, Matsuura M, Ohkubo T, Ohkubo H, Kanaka N, Matsushima E, Taira M, Kojima T, Matsuda T. Hyperfrontality in patients with schizophrenia during saccade and antisaccade tasks: a study with fMRI. Psychiatry Clin Neurosci. 2009;63:209–217. doi: 10.1111/j.1440-1819.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Morita N, Yamashita I. Further analysis of the control of voluntary saccadic eye movements in schizophrenic patients. Biol Psychiatry. 1990;28:943–958. doi: 10.1016/0006-3223(90)90060-f. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud-Péchoux S, Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Exp Brain Res. 1999;129:288–301. doi: 10.1007/s002210050899. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;11:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Harlé KM, Stewart JL, Wittmann M, Tapert SF, Paulus MP. Attenuated insular processing during risk predicts relapse in early abstinent methamphetamine-dependent individuals. Neuropsychopharmacology. 2014;39:1379–1387. doi: 10.1038/npp.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Haile CN, De La Garza R, 2nd, Mahoney JJ, 3rd, Nielsen DA, Kosten TR, Newton TF. The Impact of Disulfiram Treatment on the Reinforcing Effects of Cocaine: A Randomized Clinical Trial. PLoS One. 2012;7:e47702. doi: 10.1371/journal.pone.0047702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa RP, Peterson BW, Goldberg ME. Prefrontal neurons coding suppression of specific saccades. Neuron. 2004;43:415–425. doi: 10.1016/j.neuron.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Holmqvist K, Nystrom M, Andersson R, Dewhurst R, Jarodzka H, Van de weijer J. Eye Tracking: A Comprehensive guide to methods and measures. Oxford: 2011. [Google Scholar]
- Hutton SB, Joyce EM, Barnes TR, Kennard C. Saccadic distractibility in first-episode schizophrenia. Neuropsychologia. 2002;40:1729–1736. doi: 10.1016/s0028-3932(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Jardin BF, Larowe SD, Hall BJ, Malcolm RJ. The Obsessive Compulsive Cocaine Scale: assessment of factor structure, reliability, and validity. Addict Behav. 2011;36:1223–1227. doi: 10.1016/j.addbeh.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O’Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133:94–99. doi: 10.1016/j.drugalcdep.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, May J. Beating the urge: implications of research into substance-related desires. Addict Behav. 2004;29:1359–1372. doi: 10.1016/j.addbeh.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kennedy AP, Gross RE, Ely T, Drexler KP, Kilts CD. Clinical correlates of attentional bias to drug cues associated with cocaine dependence. Am J Addict. 2014;23:478–484. doi: 10.1111/j.1521-0391.2014.12134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am. J. Drug Alcohol Abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS. Saccades in Huntington’s disease: initiation defects and distractibility. Neurology. 1987;37:364–370. doi: 10.1212/wnl.37.3.364. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Robinson CD, Waters AJ, Sofuoglu M. A critical review of the literature on attentional bias in cocaine use disorder and suggestions for future research. Exp Clin Psychopharmacol. 2014;22:469–483. doi: 10.1037/a0037806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DL. Psychopathology: The evolving science of mental disorder. New York: Cambridge University Press; 1996. Location, location, location: the pathway from behavior to brain locus in schizophrenia. [Google Scholar]
- Lindsay JA, Stotts AL, Green CE, Herin DV, Schmitz JM. Cocaine dependence and concurrent marijuana use: a comparison of clinical characteristics. Am J Drug Alcohol Abuse. 2009;35:193–198. doi: 10.1080/00952990902933860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Green CE, Cunningham KA, Moeller FG. Increased intra-individual reaction time variability in cocaine-dependent subjects: role of cocaine-related cues. Addict Behar. 2011;37:193–197. doi: 10.1016/j.addbeh.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Marhe R, Luijten M, van de Wetering BJ, Smits M, Franken IH. Individual differences in anterior cingulate activation associated with attentional bias predict cocaine use after treatment. Neuropsychopharmacology. 2013a;38:1085–1093. doi: 10.1038/npp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, Waters AJ, van de Wetering BJ, Franken IH. Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: an ecological momentary assessment study. J Consult Clin Psychol. 2013b;81:1–12. doi: 10.1037/a0030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- Marks KR, Pike E, Stoops WW, Rush CR. Test-retest reliability of eye tracking during the visual probe task in cocaine-using adults. Drug Alcohol Depend. 2014a;145:235–237. doi: 10.1016/j.drugalcdep.2014.09.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KR, Roberts W, Stoops WW, Pike E, Fillmore MT, Rush CR. Fixation time is a sensitive measure of cocaine cue attentional bias. Addiction. 2014b;109:1501–1508. doi: 10.1111/add.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: Guilford Press; 1985. [Google Scholar]
- McKay JR. Studies of factors in relapse to alcohol, drug and nicotine use: a critical review of methodologies and findings. J Stud Alcohol. 1999;60:566–576. doi: 10.15288/jsa.1999.60.566. [DOI] [PubMed] [Google Scholar]
- Miller MA, Fillmore MT. The effect of image complexity on attentional bias towards alcohol-related images in adult drinkers. Addiction. 2010;105:883–890. doi: 10.1111/j.1360-0443.2009.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. Review. [DOI] [PubMed] [Google Scholar]
- Nilsson MH, Patel M, Rehncrona S, Magnusson M, Fransson PA. Subthalamic deep brain stimulation improves smooth pursuit and saccade performance in patients with Parkinson’s disease. J Neuroeng Rehabil. 2013;10:33. doi: 10.1186/1743-0003-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Overduin J, Jansen A, Louwerse E. Stroop interference and food intake. Int J Eat Disord. 1995;18:277–285. doi: 10.1002/1098-108x(199511)18:3<277::aid-eat2260180310>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pa J, Dutt S, Mirsky JB, Heuer HW, Keselman P, Kong E, Trujillo A, Gazzaley A, Kramer JH, Seeley WW, Miller BL, Boxer AL. The functional oculomotor network and saccadic cognitive control in healthy elders. Neuroimage. 2014;95:61–68. doi: 10.1016/j.neuroimage.2014.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SS, Jankovic J, Hood AJ, Jeter CBAB. Reflexive and volitional saccades: biomarkers of Huntington disease severity and progression. J Neurol Sci. 2012;313:35–41. doi: 10.1016/j.jns.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114:1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Pike E, Stoops WW, Fillmore MT, Rush CR. Drug-related stimuli impair inhibitory control in cocaine abusers. Drug Alcohol Depend. 2013;133:768–771. doi: 10.1016/j.drugalcdep.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002:2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Reuter B, Jäger M, Bottlender R, Kathmann N. Impaired action control in schizophrenia: the role of volitional saccade initiation. Neuropsychologia. 2007;45:1840–1848. doi: 10.1016/j.neuropsychologia.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Roberts W, Miller MA, Weafer J, Fillmore MT. Heavy drinking and the role of inhibitory control of attention. Exp Clin Psychopharmacol. 2014;22:133–140. doi: 10.1037/a0035317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rosse RB, Miller MW, Hess AL, Alim TN, Deutsch SI. Measures of visual scanning as a predictor of cocaine cravings and urges. Biol Psychiatry. 1993a;33:554–556. doi: 10.1016/0006-3223(93)90013-4. [DOI] [PubMed] [Google Scholar]
- Rosse RB, Schwartz BL, Kim SY, Deutsch SI. Correlation between anti-saccade and Wisconsin Card Sorting Test performance in schizophrenia. Am J Psychiatry. 1993b;150:333–335. doi: 10.1176/ajp.150.2.333. [DOI] [PubMed] [Google Scholar]
- Sayette M, Shiffman S, Tiffany ST. The measurement of drug craving. Addiction. 2000:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Anti-saccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry. 1995;37:394–401. doi: 10.1016/0006-3223(94)00127-O. [DOI] [PubMed] [Google Scholar]
- Shafiq-Antonacci R, Maruff P, Whyte S, Tyler P, Dudgeon P, Currie J. The effects of age and mood on saccadic function in older individuals. J Gerontol B Psychol Sci Soc Sci. 1999;54:361–368. doi: 10.1093/geronb/54b.6.p361. [DOI] [PubMed] [Google Scholar]
- Sterling RC, Dean J, Weinstein SP, Murphy J, Gottheil E. Gender differences in cue exposure reactivity and 9-month outcome. Journal of substance abuse treatment. 2004;1:39–44. doi: 10.1016/j.jsat.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Carpenter KM, Copersino ML, Hart CL, Foltin RW, Nunes EV. Attentional bias towards cocaine-related stimuli: relationship to treatment-seeking for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:727–736. doi: 10.1080/00952990701523722. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Rivaud S, Gouider-Khouja N, Pillon B, Bonnet AM, Gaymard B, Agid Y, Pierrot-Deseilligny C. Eye movements in parkinsonian syndromes. Ann Neurol. 1994;35:420–426. doi: 10.1002/ana.410350408. [DOI] [PubMed] [Google Scholar]
- Vorspan F, Bellais L, Romo L, Bloch V, Neira R, Lépine JP. The Obsessive-Compulsive Cocaine Scale (OCCS): a pilot study of a new questionnaire for assessing cocaine craving. Am J Addict. 2012;21:313–319. doi: 10.1111/j.1521-0391.2012.00248.x. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wühr P, Waszak F. Object based attentional selection can modulate the Stroop effect. Mem Cognit. 2003;31:983–994. doi: 10.3758/bf03196450. [DOI] [PubMed] [Google Scholar]