Abstract
Background
While theorists have posited that adolescent depression is characterized by emotion processing biases (greater propensity to identify sad than happy facial expressions), findings have been mixed. Additionally, the neural correlates associated with putative emotion processing biases remain largely unknown. Our aim was to identify emotion processing biases in depressed adolescents and examine neural abnormalities related to these biases using high-density resting EEG and source localization.
Methods
Healthy (n = 36) and depressed (n = 23) female adolescents, aged 13–18 years, completed a facial recognition task in which they identified happy, sad, fear, and angry expressions across intensities from 10% (low) to 100% (high). Additionally, 128-channel resting (i.e., task-free) EEG was recorded and analyzed using a distributed source localization technique (LORETA). Given research implicating the dorsolateral prefrontal cortex (DLPFC) in depression and emotion processing, analyses focused on this region.
Results
Relative to healthy youth, depressed adolescents were more accurate for sad and less accurate for happy, particularly low-intensity happy faces. No differences emerged for fearful or angry facial expressions. Further, LORETA analyses revealed greater theta and alpha current density (i.e., reduced brain activity) in depressed versus healthy adolescents, particularly in the left DLPFC (BA9/BA46). Theta and alpha current density were positively correlated, and greater current density predicted reduced accuracy for happy faces.
Conclusion
Depressed female adolescents were characterized by emotion processing biases in favor of sad emotions and reduced recognition of happiness, especially when cues of happiness were subtle. Blunted recognition of happy was associated with left DLPFC resting hypoactivity.
Keywords: Adolescent Depression, Emotion Identification, Emotion Processing, Alpha Band, Theta Band
Introduction
Depression in adolescence is characterized by emotion processing biases,1 which lead depressed youth to more readily attend and perceive negative emotion while thwarting their capacity to detect positive emotion. Partly due to this dual deficit, depressed youth might experience diminished social support, and potentially contribute to greater interpersonal discord given their misinterpretation of salient social cues.2 In light of the fact that interpersonal stress is robustly associated with depression in adolescents,3 a better understanding of emotion processing biases may provide important information about the onset and maintenance of adolescent depressive symptoms.
Emotion Processing Biases
Emotion processing biases in major depressive disorder (MDD) has received a significant amount of attention in adults and to a lesser extent, adolescents. Results using emotion identification tasks have been, however, relatively mixed. Studies have shown that compared to healthy participants, depressed individuals display greater accuracy for sad faces,4 less accuracy for happy faces,5 a general deficit across all emotions,6,7 or no accuracy differences8,9 (for review see also 10,11). A potential confound in interpreting these data is the variability across experimental tasks (emotion identification vs. interpretation vs. attentional bias), stimuli (schematic faces vs. photographs), presentation mode (static vs. morphed emotion expressions), intensity (low vs. high intensity facial expressions), inclusion of other emotions besides happy and sad (e.g., fear, anger), and evaluated outcomes (accuracy vs. reaction time). Incorporating strengths of past research, Joorman and Gotlib (2007) used morphing emotions from photographs (in which the intensity of a facial expression changed continuously from neutral to a given emotion) and found that depressed adults required greater intensity to correctly identify happy facial expressions and less intensity to identify sad faces.1 Highlighting the potential specificity of this dual deficit among depressed adults, no differences emerged with angry faces. In related work using a facial morphing paradigm with a negative mood induction, however, remitted depressed adults demonstrated no group differences with sad faces but required greater intensity to accurately identify happy faces relative to never-depressed controls.12 Nevertheless, when using the same experimental design, relative to offspring of mothers without depression, children of depressed mothers required greater intensity to correctly identify sad but not happy facial expression.13 Clearly, more research is warranted to reconcile these inconsistent findings and evaluate emotion processing biases in depressed adolescents. Therefore, we tested whether emotion processing biases vary as a function of emotion (sad, happy, fearful, angry) and intensity (low, moderate, and high) in currently depressed adolescents; we were particularly interested in low intensity emotions which are more challenging to categorize and represent subtle social cues.
Resting EEG Activity
Dysfunction in the prefrontal cortex (PFC) has emerged as one of the most consistent neuroimaging findings in major depressive disorder (MDD).14 In addition to being associated with executive dysfunction,14 PFC abnormalities, particularly in dorsolateral PFC (DLPFC) regions, might be linked to emotion processing biases in MDD. Two independent lines of evidence support this assumption. First, neural models of emotional perception and processing have emphasized the role of the PFC within a dorsal stream critically implicated in regulating and processing emotional states in response to salient stimuli.15 Directly relevant to the current study, prior EEG research has typically probed PFC function within the theta (e.g., 6.5–8 Hz) and alpha (e.g., 8.5–12 Hz) band. In MDD, frontocentral theta activity has been linked with depression, but findings have varied as a function of region.16–19 One resting EEG study using low resolution electromagnetic tomography (LORETA) source localization reported greater theta current density in depressed versus healthy adults in the DLPFC,19 whereas decreased theta current density in MDD was observed in the anterior cingulate cortex, medial PFC, and orbital frontal cortex.20 Second, among healthy controls, frontal alpha asymmetry characterized by reduced left frontal activity has been repeatedly associated with reduced approach-related behavior and positivity biases (e.g., 21–23; for review, see 24,25), including avoidance of happy faces.26 Finally, synchronous alpha-theta oscillations have been implicated in a wide range of cognitive-affective processes (for review see 27). In spite of these advances, the majority of this research has been conducted in adults. Given important developmental differences, research is warranted to better understand the role of the DLPFC in MDD and putative emotion processing biases in depressed adolescents.
Goals of the Current Study
The aim of the study was to better understand emotion processing biases in depressed female adolescents. First, primary analyses tested whether relative to healthy youth, depressed adolescents exhibited a dual bias – greater identification (i.e., greater accuracy) of sad faces and worse identification of happy faces and whether these differences varied as a function of facial expression intensity. Then, secondary analyses tested the specificity of happy and sad effects relative to fear and angry facial expressions. Second, in line with adult research,19 we tested whether depressed youth, relative to healthy adolescents, exhibited greater theta and alpha current density in the DLPFC. Given prior studies comparing EEG with other neuroimaging techniques, we interpreted increased theta and alpha current density in the DLPFC as reflecting decreased resting brain activity.29,30 Last, we tested whether resting theta and alpha current density was associated with emotion identification accuracy. Specifically, we hypothesized that greater left DLPFC theta and alpha current density activity (i.e., lower resting brain activity) across subjects would be associated with lower accuracy to happy and greater accuracy to sad faces.
Materials and Methods
Participants
Female adolescents (n = 59) 13–18 years of age (healthy control (HC) = 36 and depressed adolescents (MDD) = 23) were recruited through online advertisement, posted fliers, and direct mailing. Inclusion required English fluency, right-handedness, and female gender. For the HC group, participants were excluded if they met diagnostic criteria for current or past depression, mania/hypomania, anxiety, eating disorders, substance use disorders, ADHD, psychosis, mental retardation, organic brain syndrome, and head injury resulting in loss of consciousness for 5 minutes or seizures. MDD participants had the same exclusion criteria with the exception of current depression. There were no between-group differences in age, ethnicity, or annual income (see Table 1). All participants were administered a diagnostic interview assessing psychopathology as well as a self-report depressive inventory. Additionally, resting EEG data were obtained from 51 participants (HC = 22, MDD = 29), and there were no demographic or symptom differences among participants with and without resting EEG data. Five depressed female participants were on antidepressant medication (SSRIs); as no differences emerged for medicated and unmedicated adolescents, data were pooled across depressed participants1. Power analyses were conducted with G*Power 3.1 (Dusseldorf, Germany), and using the most conservative sample size (HC = 22, MDD = 29) for testing between-group comparisons, there was >90% power to detect a moderate effect size (d = 0.50).
Table 1.
Demographic characteristics of depressed and healthy adolescents
| Demographic and Sample Characteristics | Depressed Adolescents (n = 23) | Healthy Adolescents (n = 36) |
|---|---|---|
| Depressive symptoms M (SD) | 33.65 (11.28) | 2.36 (3.39) |
| Age M (SD) | 15.70 (1.61) | 14.89 (1.74) |
| Ethnicitya | ||
| White n (%) | 32 (88.9) | 19 (86.4) |
| Asian n (%) | 2 (5.6) | 1 (4.5) |
| Black or African America n (%) | 1 (2.8) | 1 (4.5) |
| More than one Race n (%) | 1 (2.8) | 1 (4.5) |
| Family Incomea | ||
| Less than $10,000 n (%) | 1 (4.5) | 0 (0.0) |
| $10,000 – $25,000 n (%) | 1 (4.5) | 0 (0.0) |
| $25,000 – $50,000 n (%) | 0 (0.0) | 0 (0.0) |
| $50,000 – $75,000 | 4 (18.2) | 2 (5.6) |
| $75,000 – $100,000 | 4 (18.2) | 2 (5.6) |
| $100,000 or more | 9 (40.9) | 26 (72.2) |
| Unknown/Unreported | 3 (13.6) | 6 (16.7) |
Note. Significant between-group differences emerged for depressive symptoms (t(57) = 15.64, p < .001); No between-group differences emerged for age (t(57) = 1.79, p = .079), ethnicity (η2 (4) = 1.87, p = .759), or family income (η2 (5) = 9.78, p = .082).
One depressed adolescent had missing Ethnicity and Family Income data.
Procedure
The Institutional Review Board provided approval for all procedures. Adolescents under age 18 provided assent and their parents provided informed consent, and adolescents aged 18 years provided informed consent. During the initial session, adolescents were administered a clinical assessment. Additionally, participants completed an experimental task to examine emotion processing biases. Then, during the second study session, approximately 7–10 days later, resting EEG was recorded in 8 contiguous, 1-minute trials (counterbalanced: 4 eyes open, 4 eyes closed).
Instruments
The Schedule for Affective Disorders and Schizophrenia for School-Age Children - Present (K-SADS-PL)
The K-SADS-PL, which is the gold standard clinical interview for youth, assessed current and past DSM-IV diagnoses.31 All interviews were digitally recorded, and 20% of interviews were randomly selected to assess inter-rater reliability (κ = 1.00). Among the depressed adolescents, approximately half (n = 12, 54.6%) were experiencing a recurrent episode.
Beck Depression Inventory-II (BDI-II)
The BDI-II is a 21-item self-report measure assessing depressive symptom severity over the past 2 weeks.32 Item scores range from 0–3, and higher scores indicate greater symptom severity. The Cronbach’s alpha for the BDI-II was .98, which suggests excellent internal consistency.
Experimental Task
The Facial Recognition Task (FRT), adapted from earlier versions,1,33 was used to examine emotion processing biases during the presentation of emotional faces. In the current task, 4 basic emotional expressions (happy, sad, fearful, angry) were presented in different intensity gradients ranging from 10% (low intensity) to 100% (high intensity). Standardized images from the Pictures of Facial Affect34 were presented in 10% increments, and four adult faces (2 male, 2 female) conveyed facial expressions at each intensity (10 total) of each emotion (4 total) for a total of 160 emotional facial pictures. Additionally, participants viewed 10 neutral stimuli. For each trial, facial stimuli were presented in a pseudorandom order and displayed for 500 ms. Participants were asked to identify, as quickly as possible, the correct emotional face displayed by pressing the key labeled with the corresponding emotion (i.e., happy, sad, fear, angry neutral). Following their response, an ITI fixation cross was displayed for 2000 ms. Facial intensities were binned in low (20–40%), moderate (50–70%), and high (80–100%), which resulted in each intensity bin including 12 trials for each emotion. Intensity at 10% for each emotion was not included given low accuracy rates across groups. Accuracy reflects the average correct response across a given bin (i.e., total correct/12 trials).
EEG Recording and Data Reduction
The EEG was recorded using an EGI 128-channel HydroCel GSN Electrical Geodesics, Inc. (EGI) net. Continuous EEG data were sampled at 250 Hz, referenced online to Cz, and impedances were kept below 75 kΩ. EEG data consisted of 8 contiguous, 1-minute segments (4 eyes open, 4 eyes closed), which were randomized and counterbalanced across participants. In the current study, only eyes closed data were utilized, which is consistent with past depression research.35 Prior to data processing, the four, 1-minute segments were concatenated using Matlab 8.1 (MathWorks, Natick, USA). Then, data were processed using BrainVision Analyzer 2.04 (Brain Products, Munich, Germany). EEG data were re-referenced to the average reference, and independent component analysis was conducted to identify and remove vertical and horizontal eye movement artifacts as well as eye blinks. Additionally, intervals for channels were rejected using a semiautomated procedure, using the following criteria: (a) a voltage step >50 μV between two consecutive samples, (b) a voltage difference >300 μV within a segment, and (c) a maximum voltage difference of <0.50 μV within a 100-ms interval. Critically, all segments also were visually inspected for manual artifact rejection. After processing was complete, non-overlapping 2.048 s segments were extracted for LORETA analyses.
Low-Resolution Electromagnetic Tomography
Low-resolution electromagnetic tomography (LORETA) was utilized to estimate the intracerebral current density of the theta (6.5–8.0 Hz) and low alpha (8.5–10 Hz) bands.36 For the theta and alpha bands, the frequency range was selected based on prior depression research.35,37,38 Current source density at each voxel (N = 2394; voxel resolution = 7 mm3) was computed as the linear weighted sum of the scalp cross spectra in the aforementioned frequency bands. The LORETA solution space is limited to cortical gray matter and hippocampi, as defined by the MNI305 (Montreal Neurological Institute) template. For each participant, LORETA values were normalized to a total power of 1 and then log-transformed (log 10) before analyses.
Data Analytic Overview
Facial Recognition Task
Prior to conducting omnibus analyses, outliers were removed on an analysis-by-analysis basis when overall accuracy and reaction time for each emotion (i.e., happy, sad, fearful, angry) exceeded mean ±3 standard deviations. In our primary analysis, a Group (HC, MD) x Intensity (Low, Moderate, High) x Emotion (Happy, Sad) repeated-measures Analysis of Covariance (RMANCOVA) was utilized to probe whether groups differed in emotion accuracy as a function of intensity. Then, for each emotion separately, Group x Intensity RMANCOVAs examined accuracy for happy and sad facial expressions. Secondary data analysis examined the specificity of the effects for happy and sad, and separate Group x Intensity RMANCOVAs examined accuracy as a function of fear and angry. Depressive symptoms were included as a covariate to test whether significant effects predict above and beyond current symptomology2.
LORETA
To test our a priori hypothesis, the left DLPFC region-of-interest (ROI) was anatomically defined using well-established landmarks (see Figure 1), and included Brodmann Area 9 (BA9; 94 voxels) and Brodmann Area 46 (BA46; 33 voxels) (see 39,40). To test for possible laterality effects, the homologous regions in the right DLPFC were defined. Additionally, secondary whole-brain analyses were performed to evaluate the regional specificity of ROI findings. To minimize Type II error in whole-brain analyses, a combination of a p < .05 threshold and a minimum cluster size of 5 voxels was utilized. Finally, Pearson correlations examined associations among theta, alpha, and FRT accuracy (i.e., average accuracy across trials).
Figure 1.
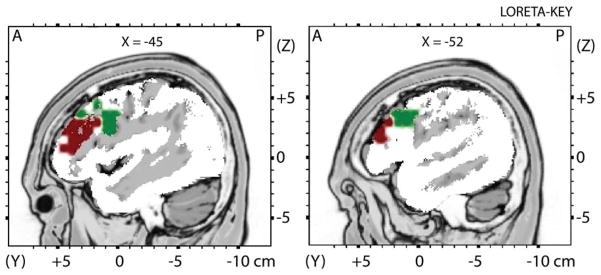
Anatomically defined region-of-interest (ROI) in the dorsolateral prefrontal cortex
Note. The DLPFC region-of-interest (ROI) was anatomically defined using well-established landmarks, and included Brodmann Area 9 (BA9; 94 voxels; Green) and Brodmann Area 46 (BA46; 33 voxels; Maroon).
Results
Behavioral Data
Emotion Comparisons for Happy and Sad
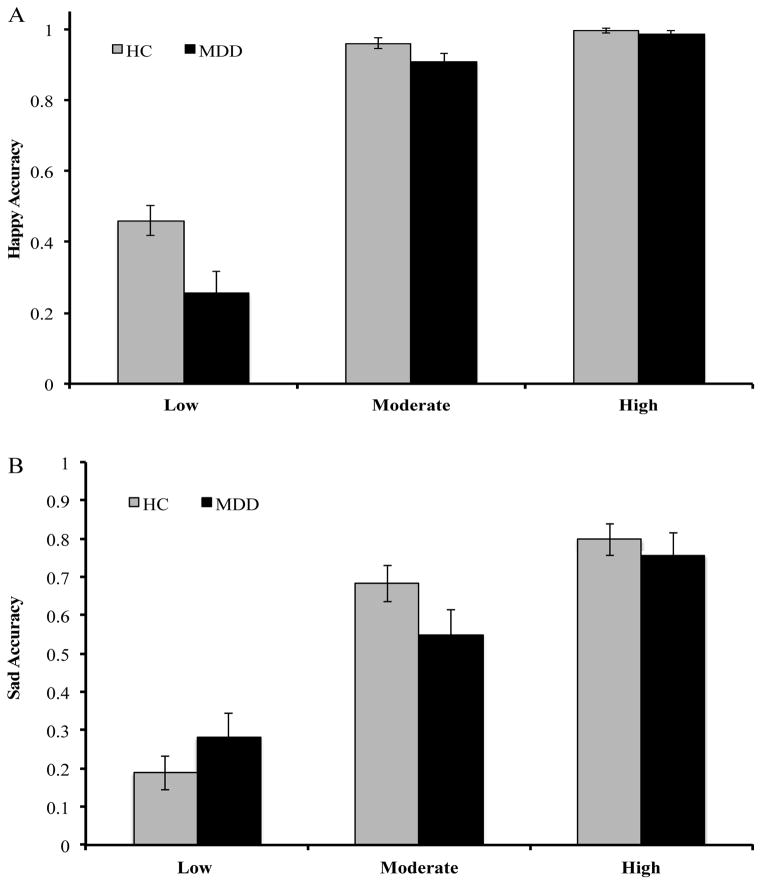
A Group x Intensity x Valence RMANCOVA was performed while controlling for depressive symptoms. Results showed a significant Group x Intensity x Valence interaction (F(1,54) = 4.95, p = .003, η2 = .084). Decomposing this 3-way interaction revealed a significant Group x Valence interaction at low (F(1,54) = 5.13, p = .028, η2 = .087) but not moderate (F(1,54) = 0.97, p = .33, η2 = .018) or high (F(1,54) = 0.22, p = .638, η2 = .004) intensity. At low intensity, relative to the HC group youth, depressed adolescents were less accurate for happy (p = .037) but not sad (p = .339) (see Figures 2A/B).
Figure 2.
Accuracy for Happy and Sad as a Function of Intensity
Note. Happy facial expression at low (20–40%), moderate (50–70%), and high (80–100%) intensity for (A) Happy Accuracy and (B) Sad Accuracy.
Happy
The main effect of Intensity was significant (F(1,55) = 163.95, p < .001; η2 = .75) as accuracy, irrespective of group, improved in a linear trend as intensity increased. Further, a main effect of Group emerged (F(1,55) = 5.55, p = .022; η2 = .09) as depressed female adolescents were overall less accurate at correctly identifying happy stimuli than healthy youth. Critically, these effects were qualified by a significant Group x Intensity interaction (linear trend: F(1,55) = 4.16, p = .046; η2 = .07) due to the fact that, relative to depressed youth, healthy female adolescents improved less across intensities (see Figure 2A).
Sad
The main effect of Intensity was significant (F(1,55) = 52.28, p < .001; η2 = .49), as adolescents were more accurate at higher intensity levels. The main effect of Group was not significant (F(1,55) = 0.13, p = .72; η2 = .002). The main effect of Intensity was qualified by a significant Group x Intensity interaction (quadratic trend: F(1,55) = 4.39, p = .04; η2 = .07) (see Figure 2B).
Fear
The main effect of Intensity was reliable (F(1,55) = 99.93, p < .001; η2 = .65), as participants were more accurate at identifying fear at higher intensity levels. However, the main effect of Group (F(1,55) = 0.67, p = .42, η2 = .01) and the Group x Intensity interaction (quadratic trend: F(1,55) = 0.42, p = .52, η2 = .008) were not significant.
Angry
The main effect of Intensity was significant (F(1,56) = 101.50, p < .001; η2 = .64), as participants were more accurate at identifying angry at higher intensity levels, but the main effect of Group (F(1,56) = 0.74, p = .40, η2 = .01) and the Group x Intensity interaction (quadratic trend: F(1,56) = 0.08, p = .78 η2 = .01) were not significant.
ROI and Whole-Brain Analyses: Theta and Alpha Density
ROI analyses for theta and alpha current density were conducted in hypothesized areas of interest within the DLPFC (i.e., left BA9, left BA46). Relative to healthy adolescents, depressed youth showed greater theta current density in the left BA9 (t(49) = 2.33, p = .024) and left BA46 (t(49) = 2.98, p = .005). In the right DLPFC, there were no theta current density differences for BA9 (t(49) = 1.34, p = .186) or BA46 (t(49) = 1.33, p = .188). However, the Group (HC, MDD) x Hemisphere (Left, Right) interaction was not significant for BA9 (F(1,49) = 3.28, p = .076, η2 = .063) or BA46 (F(1,49) = 2.64, p = .111, η2 = .051).
Regarding alpha activity, groups did not differ in frontal asymmetry in BA9 (t(49) = 1.92, p = .056) or BA46 (t(49) = 0.87, p = .390). Despite null asymmetry findings, compared to healthy adolescents, depressed adolescents showed greater current density in the left BA9 (t(49) = 2.88, p = .006) and left BA46 (t(49) = 3.28, p = .002) but not the right BA9 (t(49) = 1.54, p = .12) or right BA46 (t(49) = 1.74, p = .089). In line with our hypothesis, across groups there were significant correlations between theta and alpha activity within the left BA9 (r = .86, p < .001) and left BA46 (r = .69, p < .001).
Whole-brain analyses tested the regional specificity of the ROI findings. Relative to healthy adolescents, depressed youth exhibited greater theta current density in the left superior frontal gyrus (t(49) = 3.12, p = .003), left middle temporal gyrus (t(49) = 3.06, p = .004), and left inferior frontal gyrus (t(49) = 2.456, p = .018) (see Table 2; Figure 3). Whole-brain analyses for alpha activity did not reveal any between group differences in the DLPFC or elsewhere.
Table 2.
LORETA Whole-Brain Analyses for Theta Activity
| Region | Brodmann Areas | MNI Coordinates | Voxels | t-value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left Superior Frontal Gyrus | 6 | −38 | 17 | 57 | 16 | 2.47 |
| Left Middle Temporal Gyrus | 9 | −31 | 31 | 36 | 7 | 2.27 |
| Left Inferior Frontal Gyrus | 46 | −45 | 48 | 29 | 10 | 2.61 |
Note. Positive t-values reflect greater current density in depressed versus healthy adolescents in contiguous voxels thresholded at p < .05; X = left (−) to right (+); Y = posterior (−) to anterior (+); Z = inferior (−) to superior (+).
Figure 3.
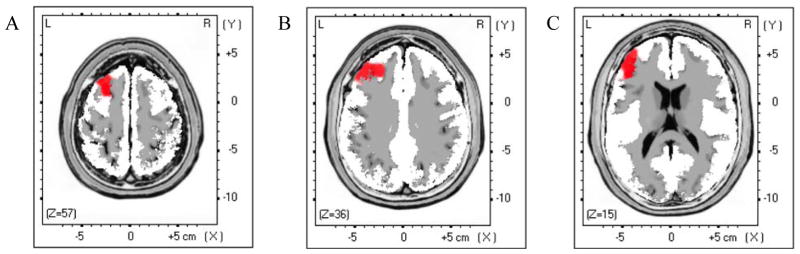
Theta activity LORETA whole-brain contrasts for healthy versus depressed adolescents
Note. Results of independent t-tests contrasting current density for theta activity in healthy versus depressed adolescents (Blue: HC > MDD; Red: MDD > HC). Statistical maps are thresholded at p < 0.05 (minimum cluster size: 5 voxels) displayed on the MNI template: (A) Left Superior Frontal Gyrus, (B) Left Middle Temporal Gyrus, and (C) Left Inferior Frontal Gyrus.
Correlations Among Emotion Accuracy, Theta, and Alpha Current Density
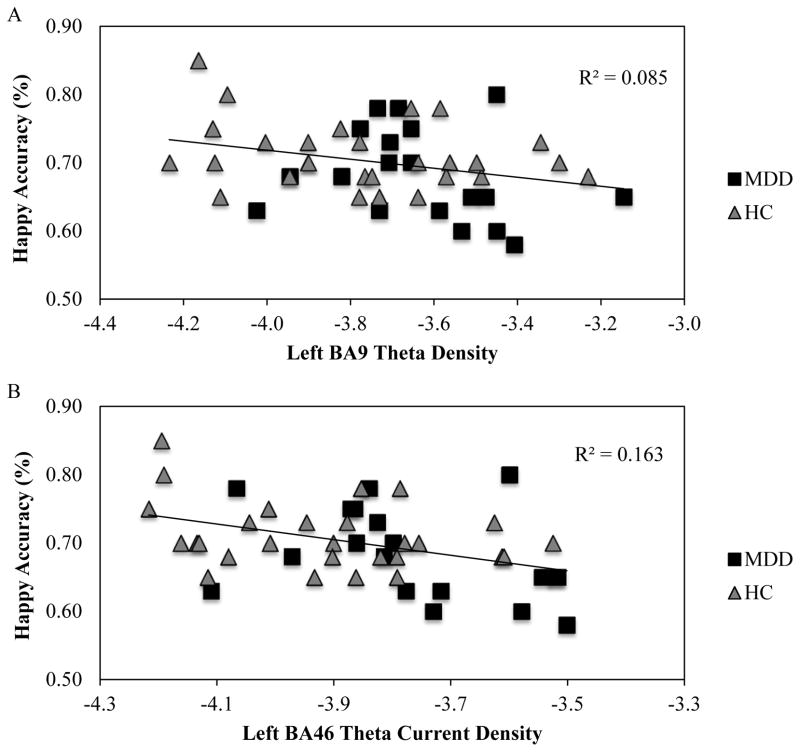
Bivariate correlations across groups examined the association with emotion (happy, sad) accuracy and current density in the left BA9 and left BA46 ROI. Greater left BA9 theta was significantly associated with less accuracy for happy (r = −.29, p = .045) but not sad (r = −.03, p = .87), and greater theta in the left BA46 showed significant associations for happy (r = −.41, p = .005) but not sad (r = −.05, p = .75) accuracy (see Figures 4A/B). Meng’s z-tests examined whether happy accuracy correlations in the left versus the right BA9 and BA46 were significantly different. We found that the left versus right correlation significantly differed for BA46 (Z = −1.81, p = .018 (one-tailed)) but not for BA9 (Z = −0.78, p = .079 (one-tailed)); highlighting hemispheric specificity for BA46.
Figure 4.
Association between Happy Accuracy and DLPFC Resting Theta Current Density
Note. Correlations across all participants for mean happy accuracy and (A) left BA9 theta current density (r = −.29, p = .045) and (B) left BA46 theta current density (r = −.41, p = .005).
In contrast to the theta findings, left BA9 alpha was not associated with happy (r = −.25, p = .092) or sad (r = −.09, p = .534). Consistent with theta, greater alpha current density in left BA46 was associated with less accuracy for happy (r = −.33, p = .024) but not sad (r = .14, p = .350).3 There was, however, no hemispheric specificity of the BA46 – happy accuracy association (Z = −0.39, p = 0.126 (one-tailed)). No differences emerged when comparing left BA46 alpha and theta associations with happy accuracy (Z = 0.05, p = .185 (one-tailed)).
Discussion
The current study investigated emotion processing biases and putative resting EEG abnormalities in depressed female adolescents. Three sets of findings emerged. First, analyses of behavioral data indicated that emotion identification deficits were specific to happy and sad (as opposed to fearful or angry) faces. Moreover, these biases emerged at low intensity such that depressed youth exhibited greater accuracy for sad and worse accuracy for happy faces. Second, depressed youth exhibited greater alpha and theta current density as compared to healthy adolescents in left BA9 and BA46, and whole-brain analyses confirmed the regional specificity of these findings. Third, alpha and theta current density was positively correlated, and critically, activity at both frequencies was negatively associated with happy accuracy. However, there were no associations between resting EEG activity and sad accuracy.
Emotion Processing and Neural Oscillations
Despite receiving significant attention, research examining emotion processing biases in depression has been mixed. The current study sought to overcome limitations of past research by utilizing an experimental design that tested intensity and specificity differences in depressed and healthy adolescents, potentially providing a more granular exploration of emotion processing biases in adolescents. Findings from the current study suggest that emotion identification biases are particularly pronounced at low intensity of facial expression. Moreover, whereas depressed adolescents were more accurate at identifying sad and less effective at identifying happy, there were no differences in the context of fear, suggesting specificity of emotional processing deficits among depressed youth. Importantly, our findings raise the possibility that biases related to identifying happy may play a particularly pernicious role for the course and maintenance of depressive symptoms. Specifically, depressed adolescents’ inability to recognize subtle cues of happiness may limit their ability to perceive positive social reinforcement and validation.2 Coupled with an inability to decode happy facial expressions at low intensity, depressed youth may also have difficulties reasoning about the mental states of others – especially as this relates to integrating contextual and historical information about the behaviors of people in their life.41 Collectively, this misinterpretation of key social cues may further exacerbate depressogenic views of the self (and associated stress), resulting in further isolation, avoidance of social situations, and potentiation of depressive symptoms.42
In line with our hypothesis and past research in adults, ROI and whole-brain analyses revealed greater DLPFC theta current density – likely indexing decreased levels of brain activity – for depressed versus healthy adolescents.19 We also found that greater left DLPFC theta and alpha activity were negatively correlated with accuracy for happy but not sad emotion identification. Although resting EEG and emotion identification were not obtained concurrently, we believe these findings have important implications. Specifically, we found that individual differences in resting left DLPFC EEG activity predicted ability to categorize happy facial expression, which is consistent with current neural models of emotion identification and regulation emphasizing the role of the DLPFC,15 as well as theories highlighting the role of left prefrontal cortex regions in approach-related behavior24,25 and greater frontal midline theta in focused attention.43
Limitations
Our results should be considered in light of the following limitations. First, emotion identification and EEG data were not concurrently assessed, which limits our capacity to intuit direct links between resting activity and emotion processing. Therefore, future research would benefit from examining alpha and theta current density during an emotion processing task to better understand neural oscillations associated with emotion identification. Second, the study examined currently depressed female adolescents, and consequently, we cannot determine whether the emotion identification and resting EEG results are a cause or consequence of depression or whether these effects are state-dependent. Taken together, future research would benefit from exploring putative differences among healthy, currently depressed, and remitted depressed adolescents. Third, the present study did not include an assessment of IQ or pubertal status, which may impact the generalizability of our findings. Fourth, emotion identification and emotion processing are related constructs. The current study assessed whether or not differences emerged in accurately identifying emotions across different intensities. An equally important empirical question to address in future research is whether depressed adolescents also exhibit deficits in processing emotions (e.g., interpreting happy emotions as neutral). Fifth, LORETA is particularly well suited to probe intracortical sources of scalp effects. At the same time, there are assumptions and limitations to this technique, which are important to consider (see 44,45). Last, the study assessed only female adolescents. Although this choice stemmed from the fact that females are at higher risk to experience depression during adolescence,28 future studies should test the current effects in male adolescents.
Conclusion
Depressed female adolescents demonstrated a dual emotion processing bias (greater identification of sad and worse identification of happy emotions), and these deficits were associated with neural abnormalities in the left DLPFC. Future studies are warranted to test whether these abnormalities contribute to increased risk for and persistence of MDD.
Acknowledgments
We wish to thank Alex Shackman, Ph.D. for providing regions-of-interest definition for LORETA regions-of-interest based on anatomical atlases and landmarks. Randy P. Auerbach was partially supported through funding from: NIMH K23MH097786, the Kaplen Fellowship on Depression awarded by Harvard Medical School, the Jewett Foundation, and the Tommy Fuss Fund. Diego A. Pizzagalli was partially supported through NIMH R01MH68376 and R01MH101521 grants.
Footnotes
We found no evidence of differences between medicated and non-medicated participants with respect to symptom severity, emotion identification biases, or alpha and theta current source density. Moreover, results across analyses remained the same when including or excluding medicated participants.
All results remained the same whether depressive symptoms were included or excluded as a covariate.
The correlation of happy accuracy and EEG activity also was explored as a function of group. Among healthy adolescents, happy accuracy was not correlated with theta (left BA9: r = −.28, p = .143; left BA46: r = −.34, p = .081) or alpha (left BA9: r = −.28, p = .146; left BA46: r = −.30, p = .116) current density. Similarly, when correlational analyses were conducted among depressed adolescents, no significant correlations emerged for theta (left BA9: r = −.21, p = .385; left BA46: r = −.38, p = .106) or alpha (left BA9: r = −.06, p = .820; left BA46: r = −.23, p = .354) current density.
Financial Disclosures: Over the past three years, Dr. Pizzagalli has received consulting fees from Otsuka Pharmaceutical, Pfizer and Servier for activities unrelated to the current research.
References
- 1.Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol. 2006;115:705–14. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- 2.Yoon KL, Joormann J, Gotlib IH. Judging the intensity of facial expressions of emotion: depression-related biases in the processing of positive affect. J Abnorm Psychol. 2009;118:223–8. doi: 10.1037/a0014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbach RP, Ho MH, Kim JC. Identifying cognitive and interpersonal predictors of adolescent depression. J Abnorm Child Psychol. 2014 doi: 10.1007/s10802-013-9845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandal MK, Bhattacharya BB. Recognition of facial affect in depression. Percept Mot Skills. 1985;61:13–4. doi: 10.2466/pms.1985.61.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Mandal MK, Palchoudhury S. Responses to facial expression of emotion in depression. Psychol Rep. 1985;56:653–4. doi: 10.2466/pr0.1985.56.2.653. [DOI] [PubMed] [Google Scholar]
- 6.Mikhailova ES, Vladimirova TV, Iznak AF, et al. Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biol Psychiat. 1996;40:697–705. doi: 10.1016/0006-3223(96)00032-7. [DOI] [PubMed] [Google Scholar]
- 7.Cooley EL, Nowicki S., Jr Discrimination of facial expressions of emotion by depressed subjects. Genet Soc Gen Psych. 1989;115:449–65. [PubMed] [Google Scholar]
- 8.Gur RC, Erwin RJ, Gur RE, et al. Facial emotion discrimination: II. Behavioral findings in depression. Psychiat Res. 1992;42:241–51. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- 9.Murphy FC, Sahakian BJ, Rubinsztein JS, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–21. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 10.Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: A review. Aust NZ J Psychiat. 2010;44:681–96. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- 11.Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: A systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1:10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeMoult J, Joormann J, Sherdell L, et al. Identification of emotional facial expressions following recovery from depression. J Abnorm Psychol. 2009;118:828–33. doi: 10.1037/a0016944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. J Child Psychol Psyc. 2010;51:575–82. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzagalli DA. Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacol. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiat. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 16.Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neurosci Lett. 2001;310:57–60. doi: 10.1016/s0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- 17.Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: An EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40:939–49. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- 18.Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev. 2007;31:377–95. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Korb AS, Cook IA, Hunter AM, Leuchter AF. Brain electrical source differences between depressed subjects and healthy controls. Brain Topogr. 2008;21:138–46. doi: 10.1007/s10548-008-0070-5. [DOI] [PubMed] [Google Scholar]
- 20.Mientus S, Gallinat J, Wuebben Y, et al. Cortical hypoactivation during resting EEG in schizophrenics but not in depressives and schizotypal subjects as revealed by low resolution electromagnetic tomography (LORETA) Psychiat Res. 2002;116:95–111. doi: 10.1016/s0925-4927(02)00043-4. [DOI] [PubMed] [Google Scholar]
- 21.Diaz A, Bell MA. Frontal EEG asymmetry and fear reactivity in different contexts at 10 months. Dev Psychobiol. 2012;54:536–45. doi: 10.1002/dev.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: A source-localization study. Psychol Sci. 2005;16:805–13. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- 23.Sutton SK, Davidson RJ. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia. 2000;38:1723–33. doi: 10.1016/s0028-3932(00)00076-2. [DOI] [PubMed] [Google Scholar]
- 24.Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol Psychol. 2004;67:7–50. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biol Psychol. 2004;67:219–33. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Edgar K, Kujawa A, Nelson SK, et al. The relation between electroencephalogram asymmetry and attention biases to threat at baseline and under stress. Brain Cogn. 2013;82:337–43. doi: 10.1016/j.bandc.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Rev. 1999;29:169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 28.Merikangas KR, He J-P, Burstein M, et al. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication, Adolescent Supplement (NCS-A) J Am Acad Child Psy. 2010;49:980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oakes TR, Pizzagalli DA, Hendrick AM, et al. Functional coupling of simultaneous electrical and metabolic activity in the human brain. Hum Brain Mapp. 2004;21:257–70. doi: 10.1002/hbm.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pizzagalli DA. Electroencephalography and high-density electrophysiological source Localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge, U.K: Cambridge University Press; 2004. pp. 56–84. [Google Scholar]
- 31.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Psy. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. 1996. [Google Scholar]
- 33.Young A, Perrett D, Calder A, et al. Facial expressions of emotion - Stimuli and tests (FEEST) Edmunds, UK: Thomas Valley Test Company; 2002. [Google Scholar]
- 34.Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists; 1976. [Google Scholar]
- 35.Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: A 128-channel EEG study. Hum Brain Mapp. 2006;27:185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- 37.Kubicki S, Herrmann WM, Fichte K, Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsych Neuro. 1979;12:237–45. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- 38.Fingelkurts AA, Fingelkurts AA, Rytsala H, et al. Impaired functional connectivity at EEG alpha and theta frequency bands in major depression. Hum Brain Mapp. 2007;28:247–61. doi: 10.1002/hbm.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5:323–37. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 40.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–22. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 41.Lee L, Harkness KL, Sabbagh MA, Jacobson JA. Mental state decoding abilities in clinical depression. J Affect Disord. 2005;86:247–58. doi: 10.1016/j.jad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Auerbach RP, Ho MH. A cognitive-interpersonal model of adolescent depression: the impact of family conflict and depressogenic cognitive styles. J Clin Child Adolesc Psychol. 2012;41:792–802. doi: 10.1080/15374416.2012.727760. [DOI] [PubMed] [Google Scholar]
- 43.Ishii R, Canuet L, Ishihara T, Ikeda S, et al. Frontal midline theta rhythm and gamma power changes during focused attention on mental calculation: An MEG beamformer analysis. Front Hum Neurosci. 2014;8:406. doi: 10.3389/fnhum.2014.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thatcher RW, North D, Biver C. Parametric vs. non-parametric statistics of low resolution electromagnetic tomography (LORETA) Clin EEG Neuroscience. 2005;36:1–8. doi: 10.1177/155005940503600103. [DOI] [PubMed] [Google Scholar]
- 45.Thatcher RW, North D, Biver C. Evaluation and validity of a LORETA normative EEG database. Clin EEG Neuroscience. 2005;36:116–22. doi: 10.1177/155005940503600211. [DOI] [PubMed] [Google Scholar]