Abstract
Importance
Individuals with schizophrenia (SZ) can encode item-specific information to support familiarity-based recognition, but are disproportionately impaired encoding inter-item relationships (relational encoding) and recollecting information. The Relational and Item-Specific Encoding (RiSE) paradigm has been used to disentangle these encoding and retrieval processes, which may be dependent on specific medial temporal lobe (MTL) and prefrontal cortex (PFC) subregions. Functional imaging during RiSE task performance could help to specify dysfunctional neural circuits in SZ that can be targeted for interventions to improve memory and functioning in the illness.
Objectives
To use functional magnetic resonance imaging (fMRI) to test the hypothesis that SZ disproportionately affects MTL and PFC subregions during relational encoding and retrieval, relative to item-specific memory processes. Imaging results from healthy comparison subjects (HC) will also be used to establish neural construct validity for RiSE.
Design, Setting, and Participants
This multi-site, case-control, cross-sectional fMRI study was conducted at five CNTRACS sites. The final sample included 52 clinically stable outpatients with SZ, and 57 demographically matched HC.
Main Outcomes and Measures
Behavioral performance speed and accuracy (d’) on item recognition and associative recognition tasks. Voxelwise statistical parametric maps for a priori MTL and PFC regions of interest (ROI), testing activation differences between relational and item-specific memory during encoding and retrieval.
Results
Item recognition was disproportionately impaired in SZ patients relative to controls following relational encoding. The differential deficit was accompanied by reduced dorsolateral prefrontal cortex (DLPFC) activation during relational encoding in SZ, relative to HC. Retrieval success (hits > misses) was associated with hippocampal (HI) activation in HC during relational item recognition and associative recognition conditions, and HI activation was specifically reduced in SZ for recognition of relational but not item-specific information.
Conclusions
In this unique, multi-site fMRI study, HC results supported RiSE construct validity by revealing expected memory effects in PFC and MTL subregions during encoding and retrieval. Comparison of SZ and HC revealed disproportionate memory deficits in SZ for relational versus item-specific information, accompanied by regionally and functionally specific deficits in DLPFC and HI activation.
Keywords: functional neuroimaging, episodic memory, schizophrenia
INTRODUCTION
Long-term memory (LTM) for episodic events can be facilitated by focusing on distinctive features of individual items (i.e., item-specific encoding) or by examining relationships between multiple items (i.e., relational encoding). These encoding processes are of scientific interest because they are mediated by distinct subregions in prefrontal cortex (PFC; [1, 2]) and they differentially impact representations formed in medial temporal lobe (MTL; [3–5]) subregions. Given evidence that schizophrenia (SZ) may disproportionately impact relational memory [6–10], an essential next step is to develop an efficient task to differentiate between relational and item-specific processing in individuals with psychiatric disorders.
The Relational and item-Specific Encoding task (RiSE) was created through the Cognitive Neuroscience Test Reliability and Clinical applications for Schizophrenia (CNTRaCS) Consortium (http://cntracs.ucdavis.edu). The original paradigm, developed in healthy undergraduates [2], was optimized to provide a valid and reliable measure of episodic LTM in SZ [11–13], to dissociate specific encoding and retrieval processes, and assist with identification of corresponding brain regions to facilitate translational research aimed at improving cognition and clinical and functional outcomes. The RiSE validation study [12] found individuals with SZ to be unimpaired using a sense of familiarity to retrieve information following item-specific encoding, but markedly impaired using familiarity following relational encoding, and when trying to recollect information - regardless of encoding process.
Work is underway with clinical high-risk and first-episode individuals to test whether relational encoding deficits represent a cognitive biomarker for psychosis. However, it is equally important to establish valid imaging biomarkers [14] of relational encoding deficits so that they can be used to identify candidate brain regions for treatment development and outcome assessment. In the current study, we adapted the RiSE for use in fMRI. Data were obtained during memory encoding and retrieval in a large sample of HC and individuals with SZ. Goals were to establish neural construct validity in HC by demonstrating functionally specific effects in PFC sub-regions during encoding and MTL sub-regions during retrieval and, more importantly, test the hypothesis that SZ specifically impairs functioning of MTL and PFC sub-regions associated with relational memory, but not with item-specific memory processes. By performing the study at multiple sites with multiple scanners we will also establish whether fMRI effects are sufficiently robust to survive increased variability associated with clinical trial settings.
METHODS
Participants
Complete details regarding CNTRACS recruitment and enrollment are found in Henderson et al. [15]. Briefly, participants were recruited nearly equally across five sites (Table 1, Supplementary Materials): University of California – Davis, Maryland Psychiatric Research Center at the University of Maryland, Rutgers University, University of Minnesota – Twin Cities, and Washington University in St. Louis.
Data were obtained on 60 HC and 60 SZ. Data were excluded for 2 patients with excess movement (i.e., > 0.37 mm of relative frame-to-frame movement), 4 patients and 2 controls with below-chance performance, and 2 patients and 1 control with image acquisition errors, leaving a final sample of 57 HC and 52 people with SZ. Groups were matched for age, sex, handedness, parental education, and estimated premorbid intelligence (WTAR; [16]; Table 1). SZ participants obtained fewer years of school than HC, likely reflecting disruption caused by illness onset. Patients were clinically stable, had remained on a fixed dose of medication for at least one month, and were experiencing mild symptoms (Table 1). All but 4 patients were receiving medication (2 first generation, 41 second generation, 4 first and second generation). After complete description of the study, written informed consent was obtained. The study was IRB approved at all participating research sites.
Table 1.
Study 1 Demographic Characteristics
| Controls (n=57) | Patients (n=52) | ||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p-value |
| Age (years) | 33.6 | 11.5 | 33.8 | 11.8 | ns |
| WTAR | 37.7 | 10.2 | 36.0 | 9.2 | ns |
| Subject Education (years) | 14.8 | 1.9 | 13.1 | 1.7 | <.0001 |
| Parental Education (years) | 14.9 | 3.9 | 15.2 | 3.2 | ns |
| Gender (% male) | 72 | 77 | ns | ||
| Handedness (% right) | 93 | 88 | ns | ||
| BPRS - Total | -- | -- | 42.4 | 10.9 | -- |
| BPRS - Positive | -- | -- | 10.3 | 5.2 | -- |
| BPRS - Disorganized | -- | -- | 6.6 | 2.3 | -- |
| BPRS - Negative | -- | -- | 4.9 | 1.8 | -- |
| UPSA-B | -- | -- | 79.6 | 9.6 | -- |
Note: WTAR = Wechsler Test of Adult Reading; BPRS = Brief Psychiatric Rating Scale; UPSA-B = Brief University of California San Diego Performance-based Skills Assessment; ns = not significant group difference at p<.05, two-tailed
Task Design
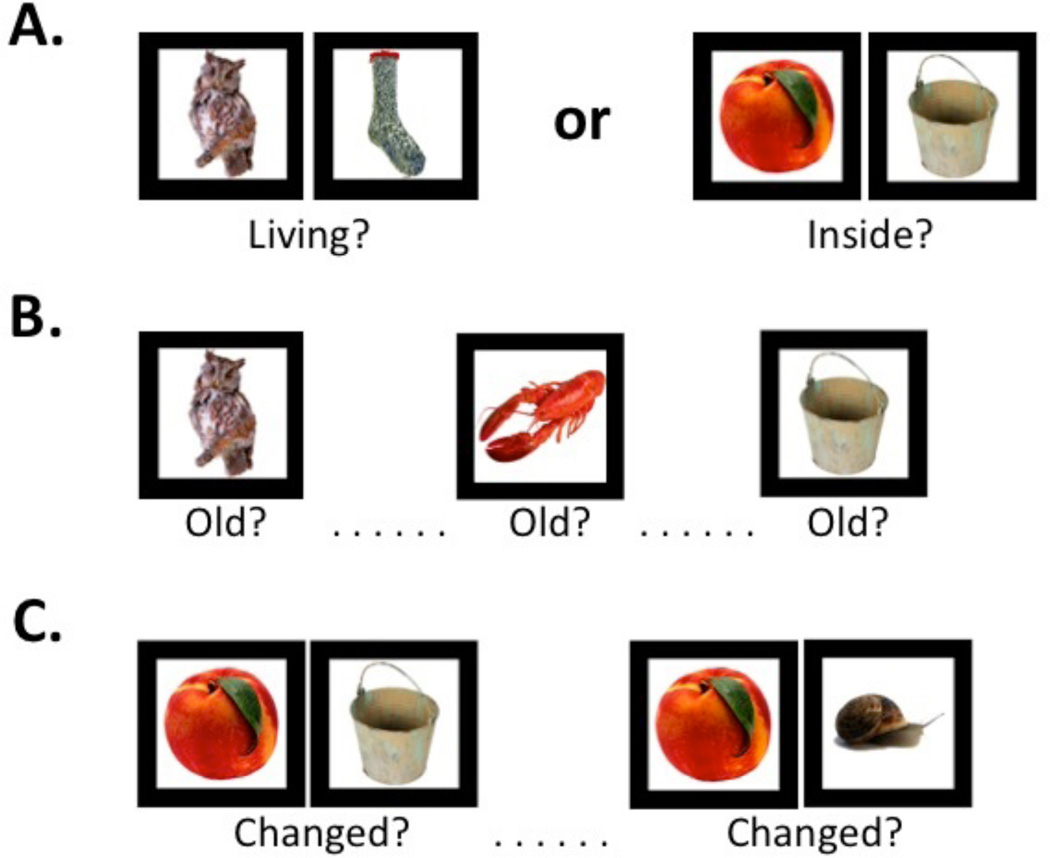
The design was identical to the original RiSE study [12], with the following exceptions: stimuli were presented in pairs during both encoding conditions (see below), and the item recognition task did not include confidence ratings. Participants completed one encoding and two retrieval fMRI runs. During encoding (Figure 1A), participants alternated between three item-specific (“Is either object living?”) blocks (9 trials each) and three relational (“Can one object fit inside the other?”) blocks (9 trials each) in a “jittered” event-related design. During item recognition (Figure 1B), participants made a two-button response to indicate whether objects were previously studied (“old”) or never studied (“new”). During associative recognition (Figure 1C), participants made a two-button response to indicate whether object pairs were “unchanged” (i.e., had been studied in the same relational encoding trial) or “changed” (i.e., had been studied in different relational encoding trials). Trials were presented for 3 s each, with a 0–10 s jittered ITI for both recognition tasks. Participants successfully completed practice versions of the encoding and retrieval tasks prior to scanning. During testing, they were encouraged to respond as quickly and accurately as possible, and guess if unsure. Total scanning duration was approximately 22 min.
Figure 1.
Illustration of item-specific and relational test procedures and task stimuli. (A) Fifty-four object pairs were visually presented while participants made either item410 specific encoding responses (Left Panel) or relational encoding responses (Right Panel). Conditions alternated (ABAB) between 6 blocks of 9 trials each, with 4 s.instruction screens between blocks to minimize alternation demands and maintain task set. (B) During item recognition, 54 individual objects from each encoding condition (54 item-specific, 54 relational) were randomly presented with 54 new items, and participants indicated whether each item was “old”. (C) During associative recognition, the 27 original relational encoding object pairs were randomly presented with 27 object pairs that had been changed by pairing items from different relational encoding trials (e.g., the left object from trial 6 and right object from trial 13), and participants indicated whether each object pair had “changed”.
Imaging Procedures (see Supplementary Materials for complete details)
Images were acquired in a single session on either a Siemens 3T TimTrio with a 12-channel phased array head coil (Univ. of California - Davis, Univ. of Minnesota, Washington Univ.), a Phillips 3T Achieva scanner with an 8-channel head coil (Univ. of Maryland), or a Siemens Allegra scanner with a circularly polarized (CP) transmit/receive head coil (Rutgers Univ.) using a consistent protocol across sites.
Pre-processing was accomplished with FMRI Expert Analysis Tool (FEAT) in the FMRIB Software Library (FSL version 4.1; www.fmrib.ox.ac.uk/fsl) using standard procedures, including fieldmap correction. Statistical analysis of subject-level fMRI data was performed using a general linear model (GLM) implemented in FEAT. Statistical analysis of group-level data was accomplished by entering parameter estimates from subject-level GLM analyses into group-level one-sample and two-sample t-tests in FEAT for the one encoding (relational minus item-specific), two item recognition (hits minus misses separately for item-specific and relational encoding), and one associative recognition (hits minus misses) contrast of interest, excluding any non-response trials. Because of site differences in scanner characteristics (Table 2, Supplementary Materials), research site was added as a co-variate in the group-level GLM designs.
At the group-level, we first examined a priori regions in PFC and MTL cortices, followed by exploratory whole brain analyses. Regional analysis goals were two-fold: 1) to establish neural construct validity and, 2) to identify group differences. To achieve these goals, voxelwise contrasts were performed within anatomically defined regions for the full sample (i.e., across HC and SZ). Anatomical ROIs for the PFC were identified for the relational minus item-specific encoding contrast with structural masks from the WFU_PickAtlas (Maldjian et al., 2003) restricting the mask to activated voxels within left and right DLPFC [Brodmann areas (BA) 9, 46, and 9/46] and VLPFC (BA 44, 45 and 47). MTL ROIs were identified for the hit minus miss contrast during item and associative recognition, and structural masks from the Harvard Oxford Atlas restricted these masks to activated voxels within left and right HI and PHG. Using these functionally and anatomically defined PFC and MTL ROIs, voxelwise one-sample t-tests identified activated voxel clusters separately for HC and SZ. Next, two-sample t-tests tested for between-group differences within the ROIs. For all analyses, resulting z (Gaussianized t) statistic images were subjected to a voxelwise threshold of z>2.3, and a corrected cluster mass significance threshold of p<0.05 based on Gaussian Random Field theory [17] as implemented in FEAT. Any effects outside these ROIs were explored using the FSL whole brain grey matter mask, with the same thresholding and cluster-correction procedures.
Pearson Product Moment correlations tested hypothesized relationships between fMRI activation (mean beta values in designated ROIs) and performance (d’) within both groups, and were used in exploratory analyses of relationships between clinical variables, task performance, and fMRI activation in SZ. Fisher’s Z transformation tested for differences in r-values. Significance criterion was set at p<.05, two-tailed.
RESULTS
Behavior
Memory Encoding
Participants responded on most encoding trials, with no response rate differences between groups [HC = 99%, SZ = 98%; F(1,107)=1.73, p=.19], or any group by encoding interaction [F(1,107)=1.91, p=.17]. Median reaction times were longer for people with SZ versus HC [263.8 ms versus 232.6 ms; F(1,107)=9.3, p<.005], and during relational versus item-specific encoding [285.4 ms versus 221.8 ms; F(1,107)=343.8, p<.0001], but did not show any group by encoding interaction [F(1,107)=1.8, p=.18]. Accuracy of orienting responses remained high in both groups, but was slightly lower in people with SZ versus HC [75.0 % versus 79.2 %; F(1,107)=9.5, p<.005], and during relational versus item-specific encoding [72.9% versus 81.4%; F(1,107)=77.9, p<.0001]. There was no group by encoding interaction [F(1,107)=0.01, p=.98]. Thus, all participants appeared engaged during encoding, and RT and accuracy differences between conditions were consistent across groups. Because our interest was in engagement of encoding processes rather than accuracy of frequently equivocal responses (e.g., is an apple that is not on the tree living?), fMRI analysis utilized all trials in which participants responded.
Memory Retrieval
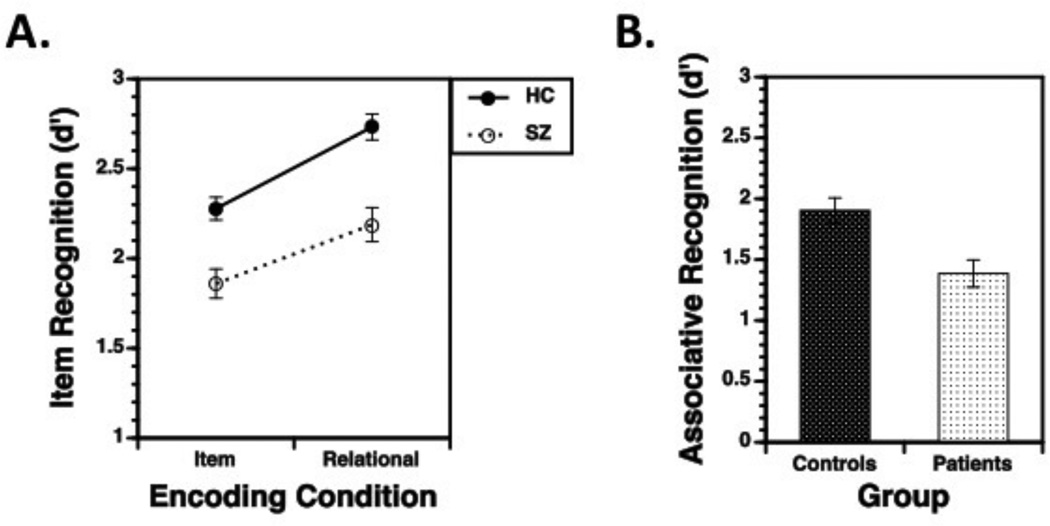
The group (SZ vs. HC) by encoding condition (item-specific vs. relational) mixed-effects ANOVA on item recognition (d’) revealed main effects for group [F(1,107)=20.4, p<.0001], encoding condition [F(1,107)=178.9, p<.0001], and a group by encoding interaction [F(1,107)=4.7, p<.05]. As illustrated in Figure 2A, recognition improved for relational versus item encoding in both HC [t(56)=11.8, p<.0001] and SZ [t(51)=7.4, p<.0001]. These effects were qualified, however, by a group by condition interaction [t(107)=2.2, p<.05], indicating more severe recognition impairments in SZ following relational encoding [Cohen’s d = .88; F(1,107)=21.2, p<.0001], versus item-specific encoding [Cohen’s d = .78; F(1,107)=16.7, p<.0001]. The Associative Recognition task (Figure 2B) also revealed a medium to large impairment in SZ relative to HC [Cohen’s d = 0.67; F(1,99)=9.5, p<.005]. These findings replicate our original study [12]. Examination of clinical variables in Table 1 did not reveal any significant clinical correlations with task performance in SZ.
Figure 2.
Performance accuracy (d’; mean + standard error of the mean) during (A) Item Recognition and (B) Associative Recognition tasks. Panel A reveals that item recognition was disproportionately impaired in patients, relative to controls, following relational encoding. Panel B reveals that associative recognition was significantly impaired in patients, relative to controls.
fMRI Image Quality
Examination of quality assurance metrics (Supplementary Materials, Table 1) revealed a main effect of site [F(4,99)=6.9, p<.0001], but no effect of group [F(1,99)=0.3, p=.57] or any group by site interactions [F(4,99)=2.0, p=.10], motivating the decision to include site as a covariate in group-level GLM analyses.
fMRI Relational Versus Item Encoding
Contrasts of relational against item-specific encoding across the entire SZ and HC sample revealed robust, bilateral activation in VLPFC and DLPFC. These regions were interrogated in voxelwise analyses to confirm reliable activation within-groups and test for between-group differences.
Healthy Control Results
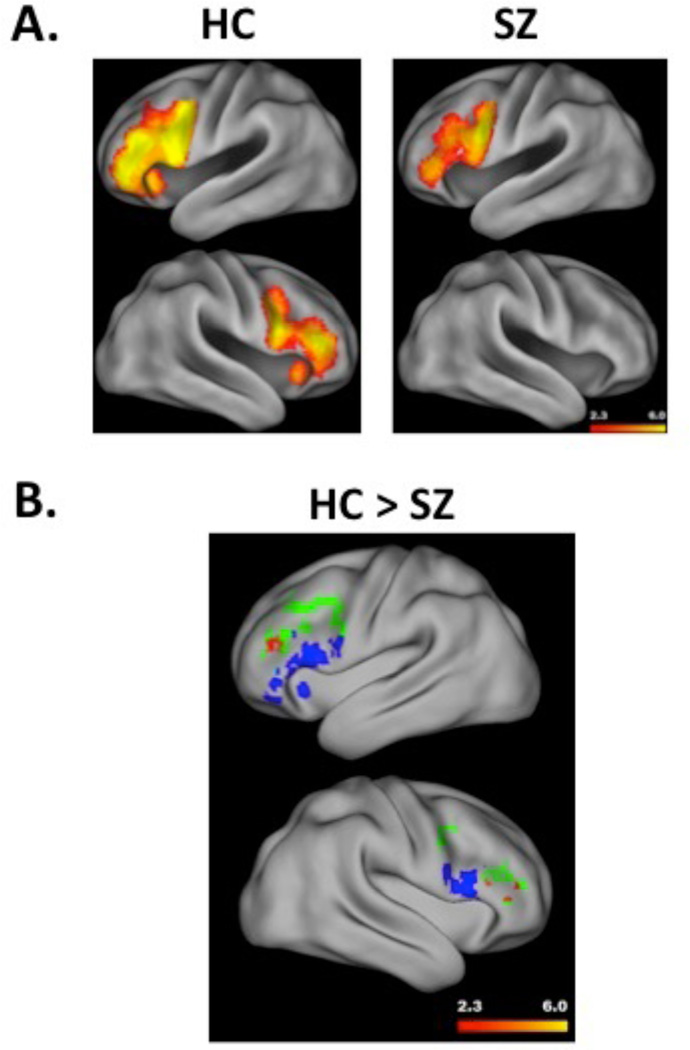
Consistent with [2] and full-sample results, regions in bilateral DLPFC and VLPFC (Figure 3A, left panel) showed increased activation during relational, relative to item-specific encoding trials. Whole brain analysis (Supplementary Materials, Table 2) revealed these bilateral PFC clusters, and a third cluster in parietal and occipital cortices.
Figure 3.
Panel A illustrates a surface rendering of left (top) and right hemisphere (bottom) PFC activation during relational versus item-specific encoding, separately for healthy comparison subjects (HC) and people with schizophrenia (SZ). Hotter colors reflect greater activation (range z = 2.3 to 6.0). Panel B illustrates significant group differences (HC – SZ) in DLPFC activation during relational versus item-specific encoding in the left (top) and right (bottom) hemisphere. Group differences are indicated in red, with hotter colors reflecting greater activation (range z = 2.3 to 6.0), and are overlaid on DLPFC (in green) and VLPFC (in blue) ROIs to illustrate the regional specificity of prefrontal dysfunction in SZ. Surface renderings performed with Caret (5.61) software (http://brainvis.wustl.edu/wiki/index.php/Caret:About)
Correlational analyses revealed that greater right DLPFC activity during relational encoding was associated with significantly better associative recognition [r(56) =.35, p<.05). This right DLPFC region did not correlate with item recognition [r(56)=.14, p=.30], however, the difference in these DLPFC correlations was not significant (Fisher’s z = 1.2, p=.24]. No correlations were obtained between VLPFC activity and performance in any condition (all r’s<.20).
Patient Results
The contrast between relational versus item-specific encoding revealed PFC activation in the left hemisphere only (Figure 3A, right panel). As illustrated, this activation was primarily in VLPFC, extending into ventral portions of DLPFC. Whole-brain analysis (Supplementary Materials, Table 3) revealed this left PFC cluster, and a second cluster in parietal and occipital cortices. Correlational analyses revealed that greater VLPFC activity during relational encoding was associated with significantly lower associative recognition [d(45) = −.46, p<.005]. No significant correlations were observed in DLPFC. Examination of clinical variables in Table 1 did not reveal any significant correlations in DLPFC. However, higher right hemisphere VLPFC activity during item-specific encoding correlated with less severe disorganization [r(46) = −.40, p<.01].
Group Differences
Between-group contrast of relational minus item-specific encoding revealed suprathreshold clusters in DLPFC, indicating reduced activation in SZ relative to HC (Fig. 3B). No group differences were observed in the VLPFC. Whole brain analysis revealed additional group differences in right cerebellum (Supplementary Materials, Table 4).
fMRI Retrieval Success
Analyses of activity differences between hits and misses during item recognition revealed bilateral suprathreshold activation in HI and PHG in the full sample. These regions were interrogated in further analyses described below.
Healthy Control Results
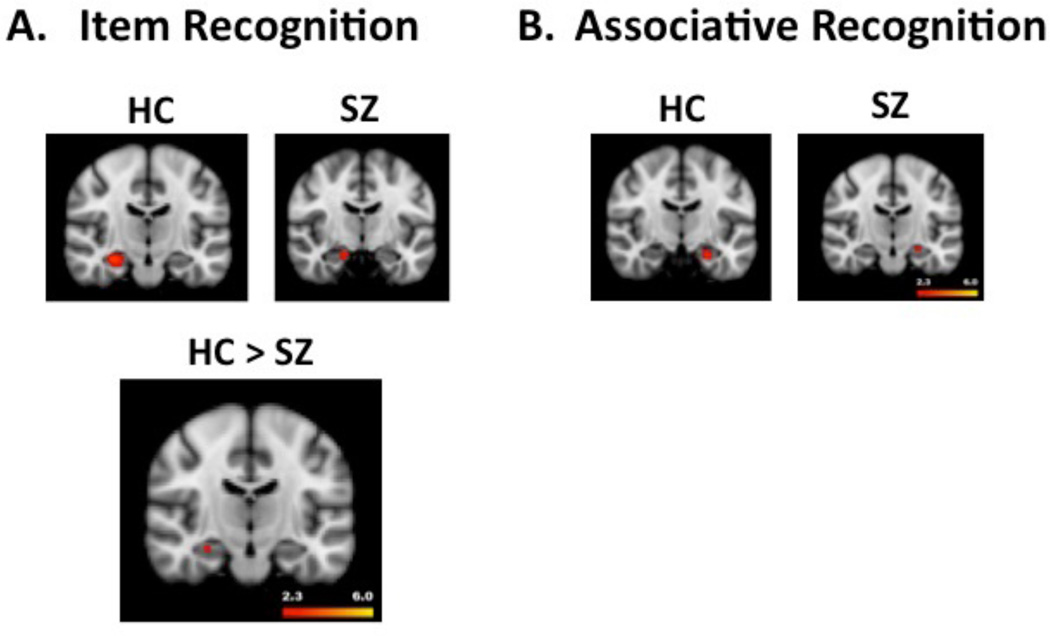
Left and right HI activation increased during item and associative recognition hits relative to misses, but only for objects encoded on relational trials (left panels, Figure 4A and 4B). Contrasts for item recognition success for objects encoded on item-specific trials revealed no suprathreshold MTL voxels. Whole brain analyses did not reveal any additional effects outside of HI for item or associative recognition following relational encoding. However, successful recognition following item-specific encoding revealed a significant cluster in left middle and superior frontal gyrus, a second cluster in parietal and occipital cortices, and a third cluster in right cuneus and precuneus (Supplementary Materials, Table 5).
Figure 4.
Panel A illustrates hippocampal activation during retrieval success (hits – misses) following relational encoding for the item recognition task. The top panel reveals results separately for HC (left) and SZ groups (right). The significant group difference (HC>SZ) is illustrated in the bottom figure. Panel B illustrates hippocampal activation during retrieval success (hits – misses) following relational encoding for the associative recognition task. The top panel reveals results separately for HC (left figure) and SZ group (right figure). For this task, there were no significant between-group differences in hippocampal activation. As in Figure 3, hotter colors reflect greater activation (range z = 2.3 to 6.0).
Patient Results
Patients showed increased left HI activation during successful item recognition following relational encoding and right HI activation during successful associative recognition (Figure 4A and 4B). No HI activation was observed following item-specific encoding. Whole brain analysis of successful item recognition following relational encoding (Table 6, Supplementary Materials) revealed bilateral basal ganglia clusters, including effects in amygdala and parahippocampal gyrus. A third cluster was observed in left cuneus and bilateral precuneus. Whole brain analysis of successful associative recognition did not reveal any additional activation. Whole brain analysis of item recognition success following item-specific encoding (Supplementary Materials, Table 7) revealed additional clusters in left and right posterior cortex, including bilateral cuneus and precuneus and inferior parietal cortex. Correlational analysis of clinical variables in Table 1 revealed that the greater left HI activity was associated with less severe positive symptom [r(44) = −.40, p<.01] and total BPRS scores [r(44) = −.30, p<.05].
Group Differences
Consistent with study hypotheses, HI activation was reduced in people with SZ relative to HC during successful item recognition following relational encoding (Figure 4A, bottom panel). The associative recognition task did not reveal suprathreshold group differences in HI activation. No group differences were seen in MTL regions during retrieval success for objects that had been encoded on item-specific trials. Exploratory whole brain analyses did not reveal any additional group differences.
DISCUSSION
Previous research [11–13] established the RiSE as a valid and reliable behavioral measure of episodic memory, capable of revealing differential deficits in relational encoding and recollection based retrieval associated with reduced functional capacity in SZ. The current study used functional neuroimaging to establish neural construct validity in HC, and identify sub-regions within PFC and MTL memory systems responsible for specific memory deficits in SZ.
Healthy participants exhibited increased DLPFC and VLPFC activation during relational versus item-specific encoding. DLPFC activity significantly correlated with associative recognition but not with item recognition performance – consistent with research findings from an earlier version of the task [2]. Patterns of HI activation in HC during retrieval provided further evidence of neural construct validity. HI activation increased during successful, compared with unsuccessful, item and associative recognition, but only for items studied during relational encoding. This finding is consistent with basic human and animal research demonstrating a specific role for the HI in relational memory (see [3, 4, 18, 19] for reviews).
Between-group comparisons replicated previous behavioral findings [12] of disproportionate memory impairments in SZ following relational versus item-specific encoding. Most importantly, the fMRI data showed that these memory deficits were linked to regionally-specific reductions in DLPFC activation during relational encoding, and to functionally specific reductions in HI activation during successful recognition following relational encoding.
Results in HC reflect important anatomical and functional dissociations. Within the PFC, cognitive neuroscience research demonstrates that DLPFC (BA 9 and 46) and VLPFC (BA 44, 45, and 47) [20, 21] support distinct cognitive control processes that facilitate encoding of different, yet complementary, aspects of a given item or event [1]. VLPFC increases when one must activate or inhibit goal-relevant features of items, or “item-specific” WM, to support successful item recognition [22]. In contrast, DLPFC increases during processing of relationships amongst items that are active in memory, which, in turn, promotes formation of representations that support retrieval of relational information and associative recognition [23–29]. Within the MTL, several lines of evidence suggest that HI supports recollection and associative memory [4, 5, 30], possibly by binding item and context information [30]. Current results fit this model and substantiate RiSE fMRI neural construct validity, demonstrating that it can be used to dissociate PFC memory control and HI relational binding processes in healthy and clinical populations.
Rather than solely attributing episodic memory deficits in SZ to failed memory consolidation and retrieval in HI or to disrupted strategic memory control in the PFC, current results suggest that distinct PFC and HI sub-regions and mnemonic processes may be disrupted. Patients were most impaired following relational encoding, which demanded recruitment of DLPFC during encoding and HI during retrieval. In contrast, patients showed less prominent memory impairments when required to engage the VLPFC to encode item-specific information. This richer and more integrated account of episodic memory in SZ emphasizes the importance of dissociating discrete encoding and retrieval processes, and may also help explain variability in the literature and arguments about presence or absence of recognition impairments, and consistency of DLPFC and HI dysfunction.
Based on these results, we speculate that interventions to improve memory in patients might adopt a two-step approach. The first is to increase compensatory recruitment of VLPFC through training in the use of item-specific semantic encoding strategies [31]. However, strategy training alone is unlikely to restore more persistent deficits in relational processing and recollection. Therefore, we also suggest that training in relational processing, possibly in combination with neurostimulation, pharmacological or other mechanistic interventions, could improve patients’ ability to recruit DLPFC and HI [31].
Although the most prominent SZ impairments were observed during relational and associative memory, it is important to note that patients showed a medium to large sized deficit in item recognition discriminability following item-specific encoding. A similar magnitude deficit was observed in the original RiSE study [12], but use of confidence ratings in that study allowed us to separately estimate contributions of recollection and familiarity to recognition performance (see [32]). Those analyses revealed two effects – patients showed a global recollection impairment, and a specific effect of relational encoding on familiarity-based recognition. Collectively, we believe that these two effects can account for many observed memory deficits in SZ. Thus, it is likely that the current recognition discriminability deficit following item-specific encoding was due to patient difficulties in recollection rather than familiarity-based retrieval. This can be tested in future fMRI studies by including “high”, “medium” and “low” confidence ratings during recognition testing, which would allow use of dual process signal detection models (DPSD; [33]) to obtain familiarity and recollection parameter estimates.
The study had other limitations worth noting. Because of the multi-site nature of the project, the patient sample was quite heterogeneous. However, a potential benefit of this heterogeneity is that it increases generalizability of results to the larger population of individuals with schizophrenia, and demonstrates that individuals with different demographic and clinical characteristics are capable of completing RiSE fMRI. In addition, the majority of patients were medicated. RiSE studies are underway with clinical high risk and first episode patients, a number of which are un-medicated or never medicated, which will allow investigation of medication and treatment effects. Finally, the associative recognition task was less successful than the item recognition task in revealing significant group differences in HI activation following relational encoding. We believe that this was because the task had half as many trials, which reduced sensitivity to detect between-group differences. Future studies may, therefore, benefit from doubling associative recognition trials.
In summary, this multi-site fMRI study of episodic encoding and retrieval establishes the neural construct validity of the RiSE paradigm, and suggests that it can successfully detect functionally and neuroanatomically specific deficits in relational memory processes and related DLPFC and HI function in people with SZ across multiple sites, employing different investigators and imaging environments, similar to what would be encountered in a clinical trials setting.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants: 5R01MH084840-03 (DMB), 5R01MH084826-03 (CSC), 5R01MH084828-03 (SMS), 5R01MH084821-03 (JMG), 5R01MH084861-03 (AWM), 4 R01MH084895 (JDR), 5R01MH059352 (APY). Preliminary data from this manuscript was presented on December 2013, at the American College of Neuropsychopharmacology. The funding organization did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The authors have no financial disclosures or conflicts of interest to report. Drs. Ragland and Harms performed the statistical analysis in this study, and Dr. Ragland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank the staff at each of the CNTRACs sites for their hard work, and our participants for their time, energy and cooperation. We also thank Sam Lockhart for assistance with piloting early versions of the memory task.
Financial Disclosures
Dr. Barch has received grants from the NIMH, NIA, NARSAD, Allon, Novartis, and the McDonnell Center for Systems Neuroscience.
Dr. Carter has received research grants from the NIMH, NIDA, and the Robert Wood Johnson Foundation and from Glaxo Smith Kline and has been an external consultant for Roche, Servier, Lilly, Merck and Pfizer.
Dr. Gold has received grants from NIMH, receives royalty payments from the BACS, and has consulted with Amgen, Astra Zenaca, Glaxo Smith Kline, Merck, Pfizer, Hoffman LaRoche, and Solvay.
Dr. MacDonald has received research grants from the NIH and NARSAD.
Dr. Ranganath has received research grants from the NIH, the Guggenheim Foundation, and the Office of Naval Research, and he has been an external consultant for Helicon Pharmaceuticals.
Dr. Ragland has received research grants from the NIH, NARSAD, the EJLB Foundation, and the Robert Wood Johnson Foundation.
Dr. Silverstein has received research grants from NIMH, Pfizer, and AstraZeneca.
Dr. Yonelinas has received research grants from the NIH.
Dr. Lesh and Mr. Layher and Mr. Phillips have no financial disclosures.
Reference List
- 1.Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13(3):280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- 2.Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 2007;27(20):5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in Schizophrenia? A meta-analysis. Brain Cogn. 2003;53(2):121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong K, et al. Impaired Associative Inference in Patients With Schizophrenia. Schizophrenia bulletin. 2010 doi: 10.1093/schbul/sbq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannula DE, et al. Use of eye movement monitoring to examine item and relational memory in schizophrenia. Biol Psychiatry. 2010;68(7):610–616. doi: 10.1016/j.biopsych.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ongur D, et al. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63(4):356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 10.Williams LE, et al. Eye-movement behavior reveals relational memory impairment in schizophrenia. Biological psychiatry. 2010;68(7):617–624. doi: 10.1016/j.biopsych.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold JM, et al. Clinical, functional, and intertask correlations of measures developed by the Cognitive Neuroscience Test Reliability and Clinical Applications for Schizophrenia Consortium. Schizophr Bull. 2012;38(1):144–152. doi: 10.1093/schbul/sbr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ragland JD, et al. Relational and Item-Specific Encoding (RISE): task development and psychometric characteristics. Schizophr Bull. 2012;38(1):114–124. doi: 10.1093/schbul/sbr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss ME, et al. Temporal stability and moderating effects of age and sex on CNTRaCS task performance. Schizophr Bull. 2014;40(4):835–844. doi: 10.1093/schbul/sbt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragland JD, et al. CNTRICS imaging biomarkers final task selection: Long-term memory and reinforcement learning. Schizophr Bull. 2012;38(1):62–72. doi: 10.1093/schbul/sbr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson D, et al. Optimization of a goal maintenance task for use in clinical applications. Schizophr Bull. 2012;38(1):104–113. doi: 10.1093/schbul/sbr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Test of Adult Reading (WTAR) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 17.Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. New York: OUP; 2001. [Google Scholar]
- 18.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13(10):713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 19.Eichenbaum H, et al. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36(7):1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F GJ, editor. Handbook of Neuropsychology. Amersterdam: Elsevier Science BV; 1994. [Google Scholar]
- 21.Fuster J. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobes. 3rd ed. New York: Raven Press; 1997. [Google Scholar]
- 22.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 23.D'Esposito M, et al. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41(1):66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- 24.Postle BR, D'Esposito M. "What"-Then-Where" in visual working memory: an event-related fMRI study. J Cogn Neurosci. 1999;11(6):585–597. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- 25.Wagner AD, et al. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral Prefrontal cortex. Neuroimage. 2001;14(6):1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- 26.Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41(3):473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- 27.Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26(3):916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crone EA, et al. Brain regions mediating flexible rule use during development. J Neurosci. 2006;26(43):11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohr HM, Goebel R, Linden DE. Content- and task-specific dissociations of frontal activity during maintenance and manipulation in visual working memory. J Neurosci. 2006;26(17):4465–4471. doi: 10.1523/JNEUROSCI.5232-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranganath C. Binding items and contexts: The cognitive neuroscience of episodic memory. Current Directions in Psychological Science. 2010;19(3):131–137. [Google Scholar]
- 31.Flegal KE, Ragland JD, Ranganath C. Cognitive Neuroscience Society. San Francisco, CA: 2015. Adaptive task difficulty influences functional neuroplasticity in cognitive training. [Google Scholar]
- 32.Libby LA, et al. Recollection and Familiarity in Schizophrenia: A Quantitative Review. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yonelinas AP. Receiver-Operating Characteristics in Recognition Memory - Evidence for a Dual-Process Model. Journal of Experimental Psychology-Learning Memory and Cognition. 1994;20(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.