Abstract
Objective
We determined if the epidermal growth factor receptor ligand HB-EGF is produced in cartilage and if it regulates chondrocyte anabolic or catabolic activity.
Methods
HB-EGF expression was measured by quantitative PCR using RNA isolated from mouse knee joint tissues and from normal and OA human chondrocytes.
Immunohistochemistry was performed on normal and OA human cartilage and meniscus sections. Cultured chondrocytes were treated with fibronectin fragments (FN-f) as a catabolic stimulus and osteogenic protein 1 (OP-1) as an anabolic stimulus. Effects of HB-EGF on cell signaling were analyzed by immunoblotting of selected signaling proteins. MMP-13 was measured in conditioned media, proteoglycan synthesis was measured by sulfate incorporation, and matrix gene expression by quantitative PCR.
Results
HB-EGF expression was increased in 12-month old mice at 8 weeks after surgery to induce OA and increased amounts of HB-EGF were noted in human articular cartilage from OA knees. FN-f stimulated chondrocyte HB-EGF expression and HB-EGF stimulated chondrocyte MMP-13 production. However, HB-EGF was not required for FN-f stimulation of MMP-13 production. HB-EGF activated the ERK and p38 MAP kinases and stimulated phosphorylation of Smad1 at an inhibitory serine site which was associated with inhibition of OP-1 mediated proteoglycan synthesis and reduced aggrecan (ACAN) but not COL2A1 expression.
Conclusion
HB-EGF is a new factor identified in OA cartilage that promotes chondrocyte catabolic activity while inhibiting anabolic activity suggesting it could contribute to the catabolic-anabolic imbalance seen in OA cartilage.
Keywords: cartilage, growth factors, metalloproteinases, integrins, bone morphogenetic proteins, chondrocytes
Introduction
The progressive degradation and loss of articular cartilage during the development of osteoarthritis (OA) is thought to be due to an imbalance in chondrocyte anabolic and catabolic activity1. An important goal of OA research has been to identify the factors that promote catabolic over anabolic activity with the hope that one or more of these mediators might serve as a therapeutic target. A host of soluble mediators capable of regulating chondrocyte activity have been found to be produced locally by the articular chondrocytes and act in an autocrine and paracrine fashion. These include various growth factors, cytokines, chemokines, Wnt family members, and toll-like receptor agonists, to name just a few. It is also well recognized that these and other factors produced by neighboring joint tissues, including the synovium, subchondral bone, and the menisci in the knee, can also regulate chondrocyte activity to promote catabolic over anabolic activity2. Despite the number of factors identified to date, it is not known which mediators would be the best targets for therapy and if other mediators not yet studied may also be involved.
In an attempt to discover novel biological mediators that could contribute to OA, we recently performed a computational analysis of gene expression microarray data generated using RNA isolated from knee joint tissues during a time course experiment in mice3. OA was induced using the destabilized medial meniscus (DMM) model and RNA was isolated from knee joint tissues at baseline, 2, 4, 8, and 16 weeks after surgery. We focused on signaling and metabolic pathways and found a subnetwork, significantly regulated during the development of OA, that included a potential protein-protein interaction link between matrix metalloproteinase (MMP)-2 and the epidermal growth factor receptor (EGFR) via heparin-binding epidermal growth factor-like growth factor (HB-EGF).
There is very little data on HB-EGF in joint tissues with one study showing a 2.76-fold difference in HBEGF gene expression in damaged relative to intact cartilage obtained at the time of joint replacement surgery for knee OA4. HB-EGF can serve as a ligand for the EGFR and activation of the chondrocyte EGFR by transforming growth-factorα (TGFα) has been shown to stimulate MMP expression and cartilage degradation as well as inhibit Sox-9 expression and anabolic activity5, 6. We postulated that HB-EGF could be another mediator that promotes catabolic over anabolic activity in cartilage. Therefore, the objective of the present study was to investigate HB-EGF expression and production in normal and OA cartilage and determine its effects on chondrocyte catabolic and anabolic activity.
Methods
Reagents
Phospho-ERK, phospho-p38, phospho-Smad1ser206, phospho-Smad1ser463/465/Smad5 ser463/465/Smad8 ser465/467, total Smad1, total p38, and total ERK antibodies were from Cell Signaling (Beverly, MA). MMP-13 antibody was from Abcam (Cambridge, MA). HB-EGF antibody, HB-EGF ELISA duoset, MMP-13 ELISA, EGF receptor inhibitor AG1478, ERK inhibitor U0126, and recombinant HB-EGF were from R&D Systems (Minneapolis, MN). P38 inhibitor SB203580 and MMP-2 antibody were from EMD Millipore (Billerica, MA). Control siRNA and smartpool siRNA against HB-EGF were from Dharmacon (Lafayette, CO). Amaxa nucleofection reagents for transfection were from Lonza (Walkersville, MD). Predesigned MMP-13, HB-EGF, COL2A1, ID1, SMAD6, TGFα, and α5 integrin (ITGA5) real-time PCR primers were from SuperArray Biosciences (Frederick, MD). Primers for TBP, B2M, and ACAN were from the Wake Forest School of Medicine DNA laboratory. Sequences for these are provided in Table S1. AMV Reverse Transcriptase and RT2 SYBR® green ROX™ qPCR Mastermix were purchased from Promega and Qiagen, respectively. Recombinant fibronectin fragment containing the RGD cell binding domain was produced using an expression construct provided by Dr. Harold Erickson (Duke University, Durham, NC). Vectastain Elite ABC kit and Nova Red substrate were from Vector Labs (Burlingame, CA). PicoGreen DNA assay was from Invitrogen (Carlsbad, CA). Mayer’s Hematoxylin was from Sigma (St. Louis, MO).
Tissue acquisition and chondrocyte isolation
Normal human ankle articular cartilage was obtained from deceased tissue donors with no known history of arthritis from the Gift of Hope Organ and Tissue Donor Network (Itasca, IL) through the Department of Biochemistry at Rush University Medical Center (Chicago, IL). Tissue from a total of 35 individual donors with ages from 46–77 years (avg 64 years) was used for cell culture studies. Chondrocytes were isolated with sequential pronase and collagenase digestion and plated at high density monolayer as previously described7. All cells were used without passaging to ensure proper phenotype was retained.
Immunohistochemistry
Cartilage and medial meniscal sections used for immunohistochemistry were from young normal (n=4, ages 36–48), old normal (n=4, ages 68–76) and OA (n=4, ages 64–90) donors and were a kind gift of Dr. Martin Lotz (Scripps Research Institute, La Jolla, CA). Although the current study focused on HB-EGF in cartilage, we examined HB-EGF levels in the meniscus as a comparison to cartilage, in particular because the outer region of the meniscus contains blood vessels which would be expected to contain HB-EGF and could serve as a positive immunostaining control. The sections embedded in paraffin were deparaffinized followed by staining with Vectastain Elite Kit (Vector Labs) according to manufacturer’s instructions. Tissue was incubated overnight at 4°C with a 10μg/mL goat anti-HBEGF antibody. Tissue incubated with no primary antibody was used as a negative control. Staining was visualized with ImmPACT Nova Red substrate (Vector Labs). Nuclei were visualized by Mayer’s Hematoxylin staining. Images were captured using the indicated objectives.
Quantitative real-time PCR
The RNA used for measuring HB-EGF expression in the DMM model of OA was from a previously published microarray study8. RNA from sham control and DMM knees joints was subjected to real-time PCR analysis using predesigned primers specific for mouse HB-EGF. HB-EGF levels were normalized to the expression of TATA box-binding protein measured in parallel samples. For studies using isolated human chondrocytes, RNA was isolated from confluent monolayers using TRIzol and primers specific for human HB-EGF were used. RNA isolation and real-time PCR were performed as previously described9. RNA was also isolated directly from frozen samples of articular cartilage obtained from normal tissue donors (n=7, mean age 53 years) and OA (n=5, mean age 57 years) patients who had undergone knee arthroplasty. For this, the tissue was first ground to a powder using the BioPulverizer system (BioSpec Products, Inc. #59012MS) which was pre-chilled in liquid nitrogen. 50–100 mg of cartilage powder from each sample was further homogenized in 2ml of TRIzol using a 3ml syringe with an 18G needle. RNA was isolated from the aqueous phase after Chloroform separation. Following isopropanol precipitation and 70% ethanol washes, the resulted RNA pellet was dissolved in 100 μl of dH2O followed by a cleanup step using the RNeasy Mini Kit (Qiagen). RNA concentration was measured using the NanoDrop 2000 (Thermo Scientific) and quality was verified using the Agilent Technologies 2200 TapeStation. 600 ng total RNA per sample was then converted to cDNA using AMV Reverse Transcriptase (Promega). The resulted cDNA was diluted 4 times and analyzed by real-time PCR using the Bio-Rad CFX96 Real-Time System and CFX manager analysis software.
Immunoblotting
Conditioned media and cell lysates were collected and used for immunoblotting as previously described7. Primary antibodies were diluted to 1:1000 and immunoreactive bands were detected using chemiluminescence. All immunoblotting experiments were repeated at least 3 times with cells from different donors. For analysis of HB-EGF levels in conditioned media in response to fibronectin fragment (FN-f), we stripped and probed for MMP-2 as a control which in previous studies we had found did not change with FN-f treatment10.
Chondrocyte transfection
Chondrocytes were transfected by the nucleofection method, using a human chondrocyte nucleofection kit (Lonza) as described previously11. Briefly, 2 million isolated cells were resuspended in transfection reagent and nucleofected with 1000 nm of siRNA for HB-EGF or control nontargeting siRNA. After the recovery period of 48 h, cells were made serum-free overnight before stimulation.
Proteoglycan synthesis assay
The [35S]sulfate incorporation assay was performed to measure proteoglycan synthesis. Chondrocytes were cultured in serum-free medium in monolayer for overnight stimulation using 100ng/mL OP-1 with or without 10ng/mL HB-EGF. The next day the medium was replaced with fresh serum-free medium 1 h prior to incubation with [35S]sulfate for another 4 h. [35S]sulfate incorporation was measured using the Alcian blue precipitation method and normalized to DNA content9. DNA was quantitated by PicoGreen double-stranded DNA assay according to the manufacturer’s protocol.
Statistical analysis
Each experiment was performed at least three times with cells from independent tissue donors for normal tissue and independent OA knee joint tissues for OA. The number of independent samples used for each experiment is provided in the figure legends and the total number of independent samples used for cell culture, immunohistochemistry, and RNA isolation are provided in the respective sections of the methods above. Statistical analysis was performed using Statview software (SAS Institute) or with Prism 6 (GraphPad). Results were analyzed by analysis of variance (ANOVA) and Fisher’s PLSD (Statview) or Tukey’s test (Prism) for post hoc testing.
Results
HB-EGF is increased in mouse and human OA knee joints
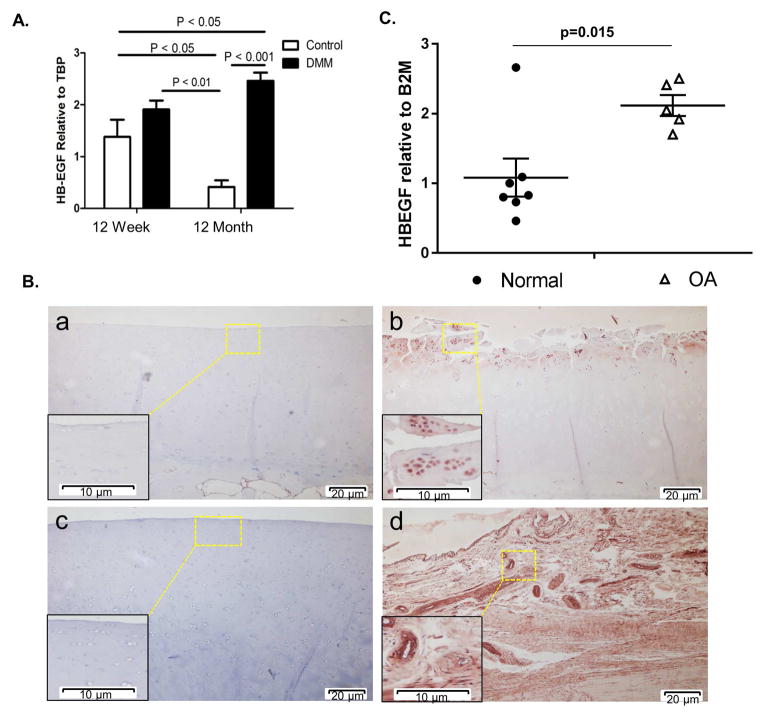
A recent gene microarray study from our group revealed that heparin-binding EGF-like growth factor (HB-EGF) was up-regulated following DMM surgery in knee joint tissue from both 12 week-old and 12 month-old mice8. In that study, the histological scores for OA in the older group of mice were twice that of the young which was accompanied by significant differences in gene expression related to both age and OA. To confirm the HB-EGF results on the gene array, we subjected samples from the same study to real-time PCR analysis for HB-EGF expression. HB-EGF was increased at 8 weeks after DMM surgery in the 12 week-old animals (+39%) relative to 12 week-old sham controls, although the difference was not significant (p = 0.11). However, HB-EGF expression was ~494% greater in the 12 month-old DMM animals relative to age-matched sham controls (p < 0.001; Fig. 1A).
Fig. 1.
HB-EGF is increased in a mouse model of OA and in human OA cartilage. (A) OA was surgically-induced by destabilization of the medial meniscus (DMM) in 12 week-old (n=9 sham controls and n=9 DMM) and 12 month-old mice (n=9 sham controls and n=9 DMM). RNA was isolated from the knee joint tissue at 8 weeks after surgery. Samples pooled from 3 animals per group were used for real-time PCR normalized to TBP as a housekeeping gene. Results are the mean±sem of three independent RNA pools per condition. (B) Immunohistochemical staining for HB-EGF on human cartilage sections from a young normal donor (a, 45 year-old), OA patient (b, 90 year-old), old normal donor (c, 70 year-old) and young normal donor meniscus (d, 48 year-old). Colorimetric detection was with NovaRed substrate. Results are representative of staining from four different donors for each group (young normal, old normal, and OA). (C) Real-time PCR analysis for HB-EGF expression using RNA isolated directly from normal (n=7, avg age 53.1 years) and OA (n=5, avg. age 57.4 years) cartilage samples normalized to B2M expression as a control.
We next determined if we could detect HB-EGF protein in adult human cartilage. Sections of human tibial plateaus from both normal donors and OA joints were subjected to immunohistochemical staining with anti-HB-EGF antibody. HB-EGF immunostaining was increased in OA cartilage relative to normal and was mainly localized to cells (Fig. 1B). We did not see any significant differences when comparing normal cartilage sections from young and older adults. We also examined meniscal tissue from the same joints and noted strong HB-EGF immunostaining in both cells, particularly in blood vessels (where HB-EGF would be expected), and diffusely in the matrix (Fig. 1B). There was no apparent difference between menisci from normal and OA joints (supplemental Fig. 1S). HB-EGF expression in cartilage was further investigated using RNA isolated directly from normal and OA human cartilage that was matched for age (avg age 53.1 years for normal and 57.4 years for OA). The levels of HB-EGF were on average 2-fold higher in RNA from OA cartilage (Fig. 1C).
HB-EGF expression and secretion are increased by catabolic stimulation of normal human chondrocytes
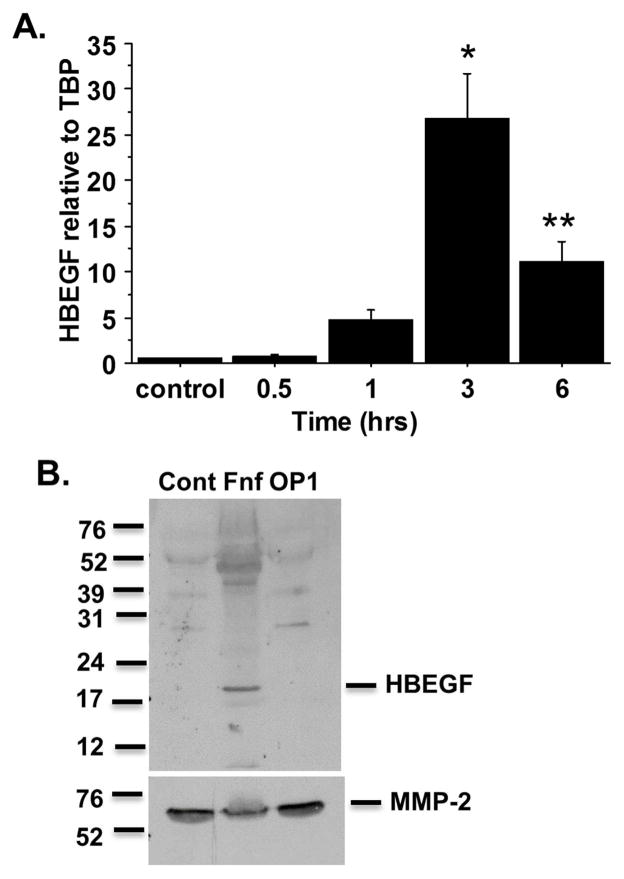
Since HB-EGF was found at increased levels in OA tissue, we examined if a catabolic stimulus would increase chondrocyte expression of HB-EGF. We chose to use recombinant fibronectin fragments (FN-f) as a model for catabolic stimulation of chondrocytes. FN-f are found at increased levels in OA tissue12, 13 and stimulate chondrocyte and meniscal cell catabolic signaling that results in increased production of cytokines, chemokines and matrix metalloproteinases (MMPs) that are relevant to OA11, 14, 15. In time course experiments from 30 minutes to 6 hours, FN-f increased chondrocyte HB-EGF expression that peaked at 3 hours (Fig. 2A). We also examined expression of TGFα which, like HB-EGF, serves as a ligand for the EGF receptor. We did not find a significant increase in TGFα in response to FN-f although we did detect a significant increase in expression of the α5 integrin subunit which, with the β1 integrin subunit, forms the α5β1 FN receptor (supplemental Fig. 2S). HB-EGF was increased in conditioned media from human chondrocytes cultured overnight in the presence of FN-f but not in cells treated with the anabolic growth factor OP-1 (Fig. 2B).
Fig. 2.
HB-EGF expression is increased by fibronectin fragment (FN-f) and is secreted by cells after FN-f treatment. (A) Chondrocytes were stimulated with 1μM FN-f for 30 minutes, 1 hour, 3 hours, and 6 hours. RNA was collected and real-time PCR was performed for HB-EGF expression. Results are the mean±sem of three experiments using cells from independent donors. *p<0.0001 vs control; **p=0.006 vs control (B) Chondrocytes were stimulated overnight with 1μM FN-f or 100ng/ml OP1. Conditioned media was collected and concentrated prior to SDS-PAGE and immunoblotting with anti-HBEGF antibody. Blots were stripped and reprobed for MMP-2 as a loading control. Results are representative of experiments with cells from 3 independent donors.
HB-EGF promotes MMP-13 production, but is not required for fibronectin fragment induced MMP-13 production
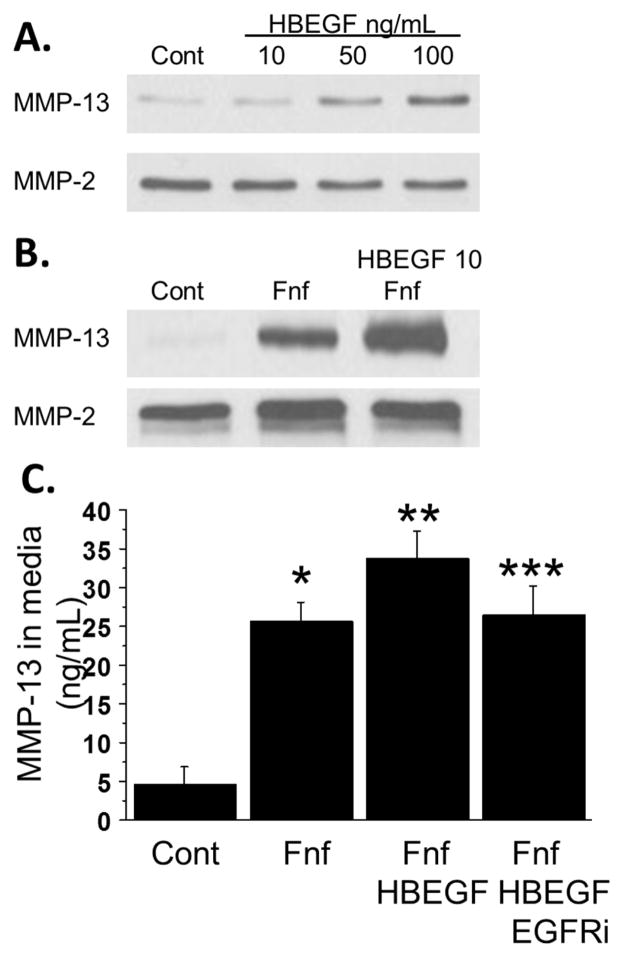
After showing that HB-EGF is increased in OA cartilage and that its expression and release by chondrocytes is stimulated by FN-f, we measured the effect of HB-EGF on production of MMP-13, a collagen degrading enzyme that plays a key role in cartilage destruction in OA16–18. We stimulated chondrocytes with 10–100 ng/ml HB-EGF and found that doses of 50 and 100ng/mL were able to stimulate chondrocyte MMP-13 production (Fig. 3A). HB-EGF signals through the EGF receptor and in a recent study we found that EGF receptor ligands enhanced FN-f stimulation of MMP-13 production19. We therefore tested the effect of low dose HB-EGF co-stimulation on FN-f mediated MMP-13 production. At 10ng/mL, HB-EGF increased FN-f mediated MMP-13 production (Fig. 3B). This effect was blocked when cells were pretreated with the EGF receptor inhibitor AG1478 (Fig. 3C). We had previously shown19 that AG1478 did not block FN-f induction of MMP-13 indicating that HB-EGF but not FN-f signals through the EGFR to stimulate MMP-13 production.
Fig. 3.
Role of HB-EGF in MMP-13 production. (A) Chondrocytes were stimulated overnight with increasing doses of HB-EGF. Media was collected and immunoblotted for MMP-13. Blots were stripped and reprobed for MMP-2 as a loading control. Results are representative of experiments performed with cells from 3 independent donors. (B) Chondrocytes were stimulated overnight with 1μM fibronectin fragment (FN-f) with or without the addition of 10ng/mL HB-EGF. Media was collected and immunoblotted for MMP-13 followed by MMP-2. Results are representative of experiments performed with cells from 3 independent donors. (C) Chondrocytes were treated with FN-f or the combination of FN-f and HB-EGF as in (B) with or without pretreated with 250nM AG1478, an EGF receptor inhibitor. Media was collected and analyzed for MMP-13 production by commercially available ELISA. Results are mean±sem from four independent donors. *p=0.0005 vs control; **p<0.0001 vs control; ***p=0.0003 vs control.
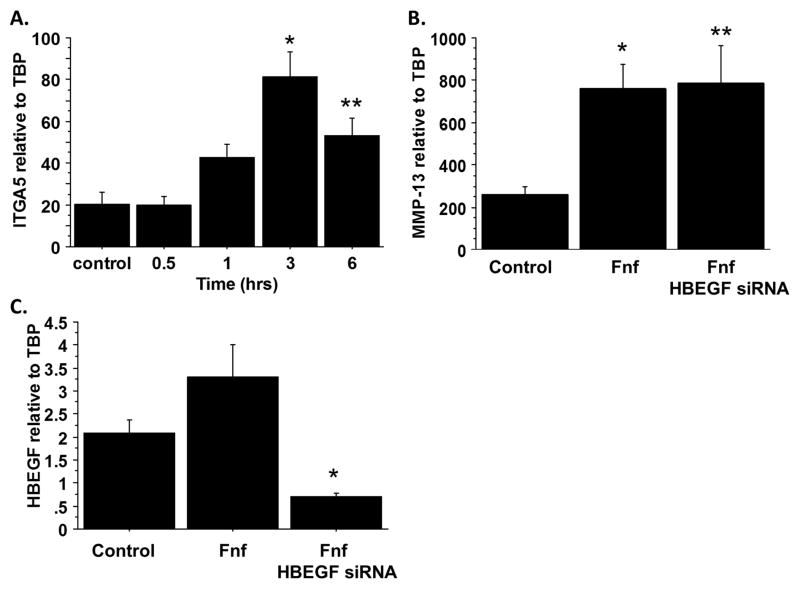
A recent report has indicated that HB-EGF can up-regulate α5β1 integrin expression in intestinal epithelial cells20 and this integrin is required for matrix degrading effects of FN-f21. We treated chondrocytes with HB-EGF for 0.5, 1, 3, and 6 hours and found that α5 integrin expression was significantly increased at 3 and 6 hours (Fig. 4A). We next determined if HB-EGF was required for FN-f mediated MMP-13 production. We transfected chondrocytes with siRNA to silence HB-EGF expression and then stimulated with FN-f. Silencing of HB-EGF had no effect on FN-f mediated MMP-13 expression (Fig. 4B) despite a significant reduction in HB-EGF expression (Fig. 4C). Together, these results suggest that HB-EGF can promote FN-f mediated MMP-13 production and α5β1 expression but HB-EGF is not required for FN-f stimulation of MMP-13 expression.
Fig. 4.
Effects of HB-EGF on α5 integrin expression and fibronectin fragment-induced MMP-13 expression. (A) Chondrocytes were stimulated for 0 (control), 0.5, 1, 3, and 6 hours with 100ng/mL HB-EGF. RNA was isolated and analyzed for expression of α5 integrin (ITGA5) by real-time PCR. Results are mean±sem from three independent donors. *p=0.002 vs control; **p=0.0134 vs control. (B) Chondrocytes were stimulated overnight with 1 μM fibronectin fragments (FN-f) with or without inhibition of HB-EGF expression using small interfering RNA. RNA was isolated and analyzed for expression of MMP-13 and HB-EGF (C) by real-time PCR. Results are mean±sem from four independent donors. *p=0.013 vs control; **p=0.0097 vs control; ***p=0.001 vs control.
HB-EGF inhibits anabolic signaling mediated by OP-1
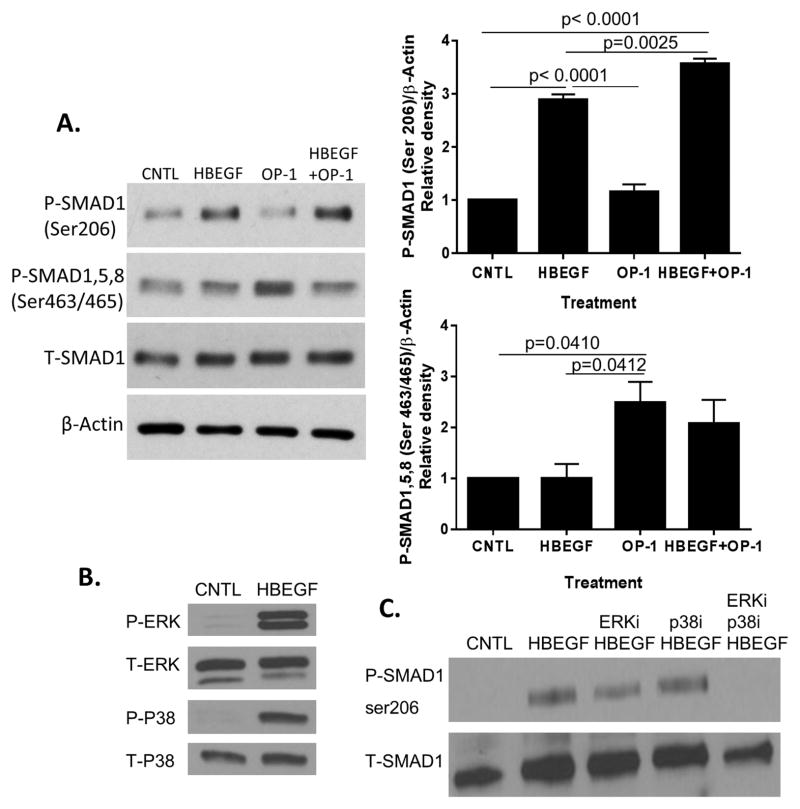
We also tested for a potential anti-anabolic activity of HB-EGF. Reports have indicated that EGF signaling can inhibit bone morphogenetic protein (BMP) signaling through mitogen activated protein (MAP) kinase-mediated phosphorylation of Smad1 at inhibitory serine residues, including serine 206, present in the Smad1 linker region22. We therefore sought to determine if HB-EGF could lead to phosphorylation of this inhibitory region on Smad1 and to determine the role of MAP kinases in this process. Smad1 phosphorylation at the inhibitory serine 206 was seen with HB-EGF and HB-EGF plus OP-1 but not OP-1 alone (Fig. 5A). HB-EGF did not stimulate phosphorylation of the activating site, which is at serine 463/465 present in Smads1 and 5, and 465/467 in Smad8, and did not alter the ability of OP-1 to stimulate the active site serines. Treatment of chondrocytes for 30 minutes with 10ng/mL HB-EGF stimulated phosphorylation of both the ERK and p38 MAP kinases (Fig. 5B). The inhibitory Smad1 serine 206 phosphorylation noted with HB-EGF was modestly reduced with inhibition of ERK but not p38, and was completely blocked when both p38 and ERK were inhibited at the same time, suggesting that both MAP kinases contributed to HB-EGF induced Smad1 serine 206 phosphorylation (Fig. 5C).
Fig. 5.
HB-EGF induces inhibitory Smad1 phosphorylation through MAP kinases. (A) Chondrocytes were stimulated for 30 minutes with control media, 10 ng/ml HB-EGF, 100 ng/ml OP-1, or the same doses of HB-EGF+OP-1. Cell lysates were immunoblotted with antibodies against Smad1 phosphorylated on Ser206 or Ser463/465 present in Smad1,5,8. Blots were stripped and reprobed for total (T) Smad1 or β-actin. Densitometric results using cells from 4 independent donors are shown to the right of the blots (mean±sem). (B) Chondrocytes were stimulated for 30 minutes with 10ng/mL HB-EGF. Cell lysates were immunoblotted with antibodies against phosphorylated (P) and total (T) ERK and p38. Results are representative of experiments with cells from four independent donors. (C) Chondrocytes were pretreated for 30 minutes with either 10μM U0126 (ERK inhibitor), 10μM SB203580 (p38 inhibitor), or the combination of the two prior to stimulation with 10ng/mL HB-EGF for 30 minutes. Cell lysates were immunoblotted with antibodies against Smad1 phosphorylated on serine206 and total Smad1. Results are representative of three independent donors.
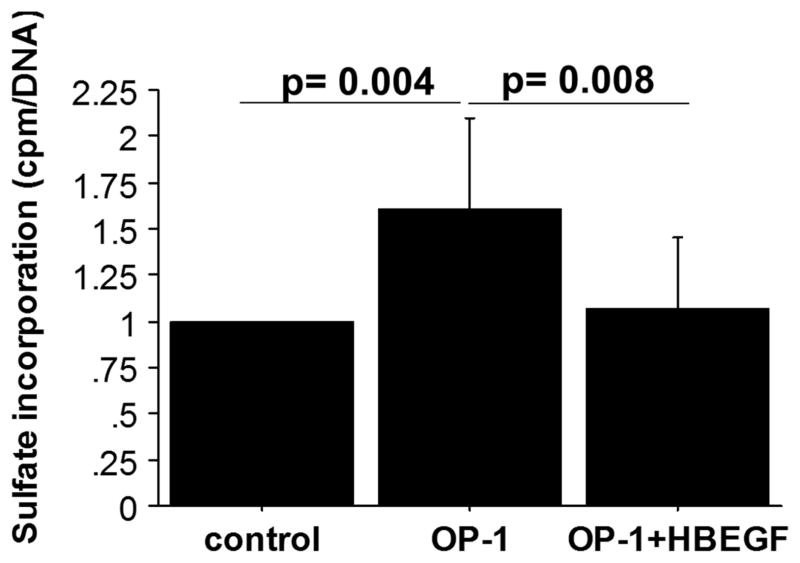
Having shown that HB-EGF can phosphorylate inhibitory residues on Smad1, we determined the effect of HB-EGF treatment on BMP mediated anabolic effects in chondrocytes. We stimulated chondrocytes with 100ng/mL OP-1 (BMP-7) overnight, either with or without co-treatment with 10ng/mL HB-EGF, and measured the synthesis of proteoglycans using sulfate incorporation. OP-1 stimulated proteoglycan synthesis was inhibited in the presence of HB-EGF (Fig. 6). This suggests that HB-EGF can antagonize chondrocyte anabolic signaling mediated by OP-1. Proteoglycan synthesis in cultures treated with HB-EGF alone did not differ from untreated controls, indicating HB-EGF does not inhibit basal proteoglycan synthesis (data not shown).
Fig. 6.
HB-EGF inhibits OP-1 mediated proteoglycan synthesis. Chondrocytes were stimulated overnight with 100ng/mL OP-1 or the combination of OP-1 and 10ng/mL HB-EGF. Proteoglycan synthesis was measured using [35S]sulfate incorporation and DNA was analyzed with a PicoGreen assay. Results were normalized to unstimulated controls set as 1 and are mean±sem of four independent donors.
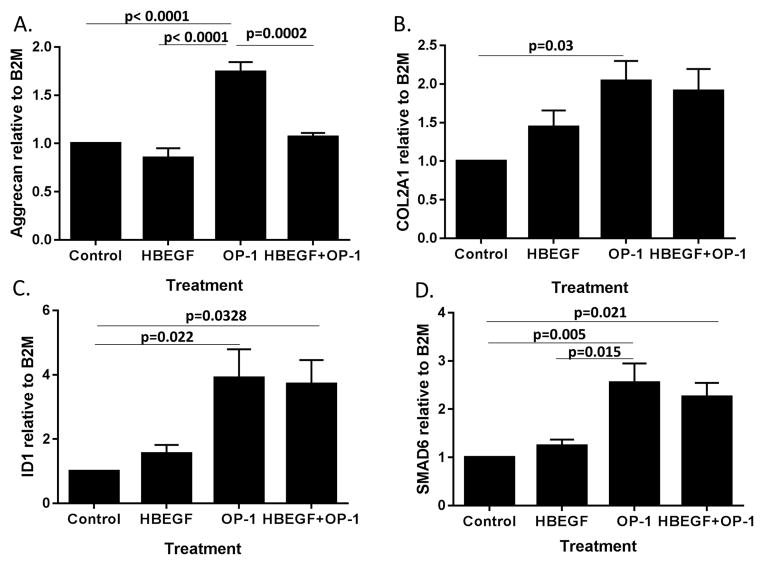
Finally, we examined the effect of HB-EGF on OP-1 mediated gene expression. HB-EGF inhibited OP-1 stimulation of aggrecan expression but did not alter expression of COL2A1 (Fig. 7A,B). We also examined expression of ID1 and SMAD6 which are two BMP target genes but did not detect an effect of HB-EGF on the ability of OP-1 to upregulate these genes (Fig. 7C,D). Therefore, the inhibitory effects of HB-EGF, at the 10ng/ml dose tested, appeared to be limited to OP-1 stimulation of aggrecan expression and proteoglycan synthesis suggesting a different mechanism of regulation from COL2A1, ID1, and SMAD6 or that higher amounts of HB-EGF would be needed to inhibit these genes.
Fig. 7.
HB-EGF inhibits OP-1 stimulation of aggrecan but not COL2A1, ID1, or SMAD6 expression. Chondrocytes were treated overnight with control media, 10ng/ml HB-EGF, 100ng/ml OP-1, or the same amounts of HB-EGF + OP-1. RNA was isolated and used for quantitative PCR with primers for aggrecan (A), COL2A1 (B), ID1 (C), and SMAD6 (D). Results shown are the mean±sem of samples from four independent donors.
Discussion
Using results from a prior gene array study to identify potential contributors to the development of OA, we found that the EGF receptor ligand HB-EGF is present in OA cartilage and is expressed by OA chondrocytes. Production of HB-EGF by normal chondrocytes was stimulated by FN-f, a catabolic stimulus, and promoted FN-f stimulation of MMP-13 production. HB-EGF was also found to have anti-anabolic properties through stimulation of Smad1 phosphorylation at an inhibitory site and this was associated with inhibition of OP-1 mediated aggrecan expression and proteoglycan synthesis. Thus, increased HB-EGF in OA could contribute to the well characterized imbalance in catabolic and anabolic activity that promotes joint tissue destruction.
Like the other EGFR ligands, HB-EGF is synthesized in a pro-form as a transmembrane protein that is cleaved by proteolytic enzymes including ADAM-10 and -12 and MMP-2, -3, and - 923. Increased MMP production by OA chondrocytes could promote HB-EGF release where it would act in an autocrine and paracrine fashion to stimulate EGFR signaling. We found that treatment of chondrocytes with FN-f increased HB-EGF expression and resulted in increased release of HB-EGF into the conditioned media. We have previously shown that FN-f stimulates production of multiple MMPs by chondrocytes including MMP-310 and more recently found that they also stimulate meniscal cells to produce MMPs that would be capable of cleaving and releasing HB-EGF15.
Although HB-EGF was not required for FN-f stimulation of MMP-13 expression, addition of HB-EGF with FN-f resulted in greater MMP-13 production than FN-f alone. Inhibition of the EGF receptor blocked this effect, consistent with a mechanism involving HB-EGF stimulation of EGF receptor signaling. Because FN-f requires activation of the ERK and p38 MAP kinases to stimulate MMP-13 expression7,13, it is possible that HB-EGF, which also activates these pathways through the EGFR, promotes a higher level of signaling resulting in increased MMP-13. The ability of HB-EGF to increase α5 integrin expression could also promote the response to FN-f by increasing the number of receptors for FN-f. A previous study in Vero, A431, and MG63 cells found that HB-EGF could form a complex with the diphtheria toxin receptor-associated protein (DRAP27)/CD9 and the α3β1 integrin but not with α5β124 making it unlikely that HB-EGF is directly interacting with the chondrocyte α5β1 integrin.
A role for EGFR-mediated signaling in OA has been previously proposed and the findings to date support a role for EGFR ligands in promoting cartilage catabolic gene expression while inhibiting chondrocyte anabolic activity. Treatment of rabbit articular chondrocytes with EGF was found to increase COX-2 expression and PGE2 levels and to inhibit collagen expression and proteoglycan synthesis25. The EGFR ligand TGFα was shown to be present in OA cartilage and to promote cartilage degradation through increased MMP expression as well as to inhibit expression of Sox-9 and matrix genes5, 6, 26. We found that HB-EGF at 10ng/ml inhibited aggrecan expression but not COL2A1. This finding is similar to a previous study from our lab9 where we found that activation of the ERK MAP kinase with constitutively active MEK inhibited aggrecan but not COL2A1 expression. Because HB-EGF activates ERK it may represent a similar mechanism for differential regulation of aggrecan and collagen expression. Similar to our findings with HB-EGF, both EGF and TGFα signaling in chondrocytes included activation of the MAP kinases ERK as well as p386, 25. It is possible that the level of activation of ERK and p38 differs among the EGFR ligands resulting in differential effects on gene expression or that there is a dose effect, such that higher doses of HB-EGF would be needed to inhibit COL2A1, ID1, and SMAD6 expression than what are needed to inhibit aggrecan expression.
Further evidence that EGFR signaling can promote OA is provided by studies of mitogen-inducible gene 6 (Mig-6) mice. Mig-6 is a negative regulator of EGFR signaling and deletion of Mig-6 in mice has been used to study the effects of excessive EGFR activity on joint tissues. Mice with systemic deletion of Mig-6 were found to have fibrocartilagenous hyperplasia, excessive osteophyte formation and early onset OA that was evident by 3 months of age27. Cartilage specific deletion of Mig-6 in floxed mice using Col2a1Cre also resulted in early-onset OA in knee joints after an initial thickening of the cartilage due to excessive chondrocyte proliferation28. Of potential relevance to our finding of HB-EGF in the meniscus, Mig-6 was also found to be expressed in the meniscus and deletion in the meniscus appeared to contribute to the development of knee OA28.
HB-EGF is expressed in vascular tissues and mice with a deletion of HB-EGF were noted to die soon after birth due to defects in heart and lung development29. Of interest to our results showing HB-EGF inhibition of chondrocyte OP-1 signaling, lack of HB-EGF in mice resulted in excessive Smad1/5/8 activation in heart valves, suggesting that HB-EGF serves as a negative regulator of BMP signaling. We noted prominent HB-EGF staining in blood vessel walls in the vascular region of the meniscus consistent with its known expression by endothelial cells which can be promoted by VEGF30. A recent study also found HB-EGF to be expressed at higher levels in inflamed areas of synovium compared to normal/reactive areas from patients undergoing knee replacement for OA31.
Further studies will be needed to measure the amounts of HB-EGF present in normal and OA cartilage and to clarify the role of HB-EGF in joint tissues in vivo. Because mice with a general deletion of HB-EGF die at a very young age, tissue specific deletion will be necessary to examine the role of HB-EGF in mice. Based on the present findings and on studies to date with other EGFR ligands, as well as with overactivation of EGFR signaling in mice with the Mig-6 deletion, we expect that excessive production of HB-EGF in joint tissues will contribute to OA through altering the balance of chondrocyte anabolic and catabolic activity.
Supplementary Material
Fig. S1. Immunohistochemical staining for HB-EGF in meniscus. Sections taken from normal (A,B) or OA (C,D) meniscus were immunostained with an antibody to HB-EGF as detailed in the methods. The outer third of the meniscus is the vascularized region which is more fibrous while the inner third is avascular and is more similar to articular cartilage. Strong staining for HB-EGF was noted in blood vessels in the outer zone but also in the matrix of both zones. Staining was similar in normal and OA meniscus.
Fig. S2. Effects of fibronectin fragments on ITGA5 (α5 integrin subunit) and TGFα expression. Cultured chondrocytes were stimulated with FN-f in a time course experiment followed by RNA isolation and measurement of gene expression by quantitative PCR. Results shown are mean±sem from experiments using cells from four independent donors.
Table S1. Primer sequences TBP, B2M and ACAN
Acknowledgments
We thank Kathryn Kelley, Mary Zhou and Kadie Vanderman for technical assistance, the Gift of Hope Organ and Tissue Donor Network and the donor families for providing normal donor tissue and Dr. Martin Lotz (supported by NIH grant AG007996) for providing cartilage and meniscus sections for immunohistochemistry.
Role of the funding source
This project was funded by NIH grants AR049003 and AG044034. The study sponsors did not have a role in the study design, collection, analysis or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Authors’ contributions
DL and VU designed and carried out the experiments and helped to draft the manuscript. SC designed experiments, interpreted data, and assisted with manuscript writing. RL designed and supervised experiments, analyzed and interpreted the data, and completed the writing of the manuscript. All authors approved the content of the manuscript.
Competing interests
There are no competing interests to disclose for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
D.L. Long, Email: dllong@wakehealth.edu.
V. Ulici, Email: vulici@email.unc.edu.
S. Chubinskaya, Email: Susanna_Chubinskaya@rush.edu.
References
- 1.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olex AL, Turkett WH, Fetrow JS, Loeser RF. Integration of gene expression data with network-based analysis to identify signaling and metabolic pathways regulated during the development of osteoarthritis. Gene. 2014;542:38–45. doi: 10.1016/j.gene.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuritani K, Takeda J, Sakagami J, Ishii A, Eriksson T, Hara T, et al. Cytokine receptor-like factor 1 is highly expressed in damaged human knee osteoarthritic cartilage and involved in osteoarthritis downstream of TGF-beta. Calcif Tissue Int. 2010;86:47–57. doi: 10.1007/s00223-009-9311-1. [DOI] [PubMed] [Google Scholar]
- 5.Appleton CT, Usmani SE, Bernier SM, Aigner T, Beier F. Transforming growth factor alpha suppression of articular chondrocyte phenotype and Sox9 expression in a rat model of osteoarthritis. Arthritis Rheum. 2007;56:3693–3705. doi: 10.1002/art.22968. [DOI] [PubMed] [Google Scholar]
- 6.Appleton CT, Usmani SE, Mort JS, Beier F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab Invest. 2010;90:20–30. doi: 10.1038/labinvest.2009.111. [DOI] [PubMed] [Google Scholar]
- 7.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 8.Loeser RF, Olex A, McNulty MA, Carlson CS, Callahan M, Ferguson C, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64:705–717. doi: 10.1002/art.33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin W, Park JI, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J Biol Chem. 2009;284:31972–31981. doi: 10.1074/jbc.M109.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic Biol Med. 2007;42:1350–1358. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, et al. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6:231–244. doi: 10.1053/joca.1998.0116. [DOI] [PubMed] [Google Scholar]
- 13.Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54:2912–2922. doi: 10.1002/art.22045. [DOI] [PubMed] [Google Scholar]
- 14.Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278:24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone AV, Loeser RF, Vanderman KS, Long DL, Clark SC, Ferguson CM. Pro-inflammatory stimulation of meniscus cells increases production of matrix metalloproteinases and additional catabolic factors involved in osteoarthritis pathogenesis. Osteoarthritis Cartilage. 2014;22:264–274. doi: 10.1016/j.joca.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long DL, Willey JS, Loeser RF. Rac1 is required for matrix metalloproteinase 13 production by chondrocytes in response to fibronectin fragments. Arthritis Rheum. 2013;65:1561–1568. doi: 10.1002/art.37922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y, Yang J, Besner GE. HB-EGF promotes intestinal restitution by affecting integrin-extracellular matrix interactions and intercellular adhesions. Growth Factors. 2013;31:39–55. doi: 10.3109/08977194.2012.755966. [DOI] [PubMed] [Google Scholar]
- 21.Homandberg GA, Costa V, Ummadi V, Pichika R. Antisense oligonucleotides to the integrin receptor subunit alpha(5) decrease fibronectin fragment mediated cartilage chondrolysis. Osteoarthritis Cartilage. 2002;10:381–393. doi: 10.1053/joca.2002.0524. [DOI] [PubMed] [Google Scholar]
- 22.Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 23.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Iwamoto R, Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin alpha 3 beta 1 at cell-cell contact sites. J Cell Biol. 1995;129:1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh YH, Kim SH, Kim SJ, Chun JS. Differentiation status-dependent regulation of cyclooxygenase-2 expression and prostaglandin E2 production by epidermal growth factor via mitogen-activated protein kinase in articular chondrocytes. J Biol Chem. 2003;278:9691–9697. doi: 10.1074/jbc.M211360200. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell PG, Cheung HS. Tumor necrosis factor alpha and epidermal growth factor regulation of collagenase and stromelysin in adult porcine articular chondrocytes. J Cell Physiol. 1991;149:132–140. doi: 10.1002/jcp.1041490117. [DOI] [PubMed] [Google Scholar]
- 27.Zhang YW, Su Y, Lanning N, Swiatek PJ, Bronson RT, Sigler R, et al. Targeted disruption of Mig-6 in the mouse genome leads to early onset degenerative joint disease. Proc Natl Acad Sci U S A. 2005;102:11740–11745. doi: 10.1073/pnas.0505171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staal B, Williams BO, Beier F, Vande Woude GF, Zhang YW. Cartilage-specific deletion of Mig-6 results in osteoarthritis-like disorder with excessive articular chondrocyte proliferation. Proc Natl Acad Sci U S A. 2014;111:2590–2595. doi: 10.1073/pnas.1400744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arkonac BM, Foster LC, Sibinga NE, Patterson C, Lai K, Tsai JC, et al. Vascular endothelial growth factor induces heparin-binding epidermal growth factor-like growth factor in vascular endothelial cells. J Biol Chem. 1998;273:4400–4405. doi: 10.1074/jbc.273.8.4400. [DOI] [PubMed] [Google Scholar]
- 31.Lambert C, Dubuc JE, Montell E, Verges J, Munaut C, Noel A, et al. Gene expression pattern of cells from inflamed and normal areas of osteoarthritis synovial membrane. Arthritis Rheumatol. 2014;66:960–968. doi: 10.1002/art.38315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Immunohistochemical staining for HB-EGF in meniscus. Sections taken from normal (A,B) or OA (C,D) meniscus were immunostained with an antibody to HB-EGF as detailed in the methods. The outer third of the meniscus is the vascularized region which is more fibrous while the inner third is avascular and is more similar to articular cartilage. Strong staining for HB-EGF was noted in blood vessels in the outer zone but also in the matrix of both zones. Staining was similar in normal and OA meniscus.
Fig. S2. Effects of fibronectin fragments on ITGA5 (α5 integrin subunit) and TGFα expression. Cultured chondrocytes were stimulated with FN-f in a time course experiment followed by RNA isolation and measurement of gene expression by quantitative PCR. Results shown are mean±sem from experiments using cells from four independent donors.
Table S1. Primer sequences TBP, B2M and ACAN