Abstract
Rationale
There is a current need for development of new therapies for patients with heart failure.
Objective
To test the effects of members of the Corticotropin-Releasing Factor (CRF) family of peptides on myocyte contractility to validate them as potential heart failure therapeutics.
Methods and Results
Adult feline left ventricular myocytes (AFMs) were isolated and contractility was assessed in the presence and absence of CRF peptides Urocortin 2 (UCN2), Urocortin 3 (UCN3), Stresscopin (SCP), and the β-adrenergic agonist isoproterenol (Iso). An increase in fractional shortening and peak Ca2+ transient amplitude was seen in the presence of all CRF peptides. A decrease in Ca2+ decay rate (Tau) was also observed at all concentrations tested. cAMP generation was measured by ELISA in isolated AFMs in response to the CRF peptides and Iso and significant production was seen at all concentrations and time points tested.
Conclusions
The CRF family of peptides effectively increases cardiac contractility and should be evaluated as potential novel therapeutics for heart failure patients.
Keywords: heart failure, contractility, urocortin, corticotropin-releasing factor, calcium
1. INTRODUCTION
The Corticotropin-Releasing Factor (CRF) family of peptides is widely expressed throughout the central nervous system and a variety of peripheral tissues in all vertebrate species and consists of the structurally related peptides CRF, Urocortin (UCN)1, UCN2, UCN3, and stresscopin (SCP) [1–5]. These peptides have been implicated in the control of stress responses by mediating the release of adrenocorticotropin (ACTH) from the anterior pituitary[1, 5] and have been linked to the regulation of the cardiovascular[6, 7] and gastrointestinal systems[8, 9] and inflammatory processes[10].
The biological actions of CRF and related peptides are mediated via binding to two G-protein coupled receptors (GPCRs), CRF receptors type 1 and 2 (CRFR1 and CRFR2), found throughout the central nervous system and periphery [11–13]. CRFR2 is further classified by its three known splice variants: CRFR2α, CRFR2β, and CRFR2γ[14]. The receptors have different tissue distribution, pharmacology, and affinities for the various CRF peptides. Ligand binding to the CRFRs leads to the activation of adenylyl cyclase and 3’, 5’-cyclic adenosine monophosphate (cAMP) production in target cells[15]. Comparisons of the binding affinities and efficacies of the known UCN peptides to the CRFRs show that UCN2, UCN3, and SCP exclusively bind to type 2 receptors whereas UCN1 appears to bind to both types[2, 3, 16]. The UCNs, SCP, and CRFR2 are expressed throughout the cardiovascular system [16–18]. Interestingly, activation of CRFR2 can also stimulate the inositol phosphate pathway through Gq, leading to beneficial cardiac remodeling[19]. Mechanistically, in failing hearts UCN2 facilitates nuclear export of NFATc1 and NFATc3, potentially preventing maladaptive hypertrophy[20]. Therefore, CRFR2 signaling represents an opportunity to impact multiple beneficial signaling processes in heart failure, with a diversity of cardiovascular effects including protection from cell death after exposure to hypoxia/reoxygenation injury[21, 22] and acute vasodilation[18].
UCN has also been reported to have positive inotropic and lusitropic effects via CRFR2-mediated stimulation of PKA, resulting in increased L-type Ca2+ current (ICa), sarcoplasmic reticulum (SR) Ca2+ load, SR Ca2+ release, and cytosolic Ca2+ transients[23]. Intravenous infusion of UCN2 induced beneficial hemodynamic, neurohormonal, and renal effects in experimental models of severe heart failure[24, 25]. In a small clinical study of eight human patients with stable HF, UCN2 treatment improved cardiac output (CO) and left ventricular ejection fraction (LVEF) while increasing heart rate (HR) and decreasing systemic vascular resistance (SVR) and systolic and diastolic blood pressure (SBP, DBP)[26]. The UNICORN (Urocortin2 in the treatment of acute heart failure as an adjunct over conventional therapy) clinical trial performed in patients with acute decompensated heart failure (ADHF) showed that UCN2 administration resulted in increased CO without significant reflex tachycardia and vasodilation as measured by renal indices[27]. A clinical trial examining the therapeutic efficacy of SCP (JNJ-39588146) on patients with HF and reduced LVEF demonstrated that SCP increased cardiac index (CI) and decreased SVR without significantly affecting HR or SBP, which highlights its potential advantage over standard inotropic agents[28]. However, the direct effects of CRF family members on cardiac myocyte contraction and Ca2+ transients have not been clearly defined.
In the current study, we set out to analyze the effects of UCN2, UCN3, and SCP on myocyte contractions, Ca2+ transients and cAMP generation in an in vitro system of isolated adult feline left ventricular myocytes (AFMs). AFMs were chosen as a model system for this study because their fundamental electrophysiological and contractile properties more closely mimic those of human myocytes than rodent myocytes, making them an ideal for in vitro studies of electrical and mechanical properties [29–31]. Experiments were performed to test the effects of UCN2, UCN3, and SCP on Ca2+ transients, contractions, and cAMP production in comparison to the well-described β-adrenergic agonist isoproterenol (Iso).
2. MATERIALS AND METHODS
The Temple University School of Medicine Institutional Animal Care and Use Committee approved all animal procedures.
2.1. Isolation of Adult Feline Left Ventricular Myocytes
Adult feline left ventricular myocytes (AFMs) were isolated as extensively described [29–32]. Adult felines (2–3 kg) were anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/kg). The heart was rapidly excised and placed in a beaker of ice cold Krebs-Henseleit buffer (KHB) containing, in mmol/L: glucose 12.5, KCl 5.4, lactic acid 1, MgSO4 1.2, NaCl 130, NaH2PO4 1.2, NaHCO3 25, and Na-pyruvate 2, and rinsed to remove blood from the chambers. A cannula was placed in the aorta and the heart was mounted on a constant-flow Langendorff apparatus where the coronaries were perfused antegradely with KHB supplemented with 10 mmol/L Taurine and warmed to 37°C. Hearts were perfused with KHB until the initial perfusate was clear of blood (approximately 100 mL) and then switched to a recirculating KHB-based, collagenase-containing digestion solution (type II Collagenase, 180 U/mL, Worthington Biochemical) supplemented with 50 µmol/L CaCl2. The pH of all buffers was maintained at 7.4 by aeration with 95% O2 and 5% CO2 and temperature were held stable at 37°C by incubation in a water bath. Perfusion was terminated when the heart became flaccid (20–35 minutes), at which time the atria were trimmed off and the left ventricle was isolated from the right ventricular free wall and septum and gently minced with scissors, filtered through 210 µm nylon mesh, and equilibrated in KHB with 200 µmol/L CaCl2 and 1% bovine serum albumin (BSA) at room temperature. Routinely, our initial yield is > 80% rod-shaped myocytes, and > 70% calcium-tolerant, rod-shaped myocytes survive by the end of the isolation.
2.2. Fluo-4 AM Loading of Myocytes
Myocytes were loaded with 1 µmol/L Fluo-4 by incubation with the acetoxymethyl ester form of the dye (Fluo-4 AM, Molecular Probes) for 15 minutes at room temperature. After loading, the cells were switched to normal Tyrode’s solution containing (in mmol/L): CaCl2 1, glucose 10, HEPES 5, KCl 5.4, MgCl2 1.2, NaCl 150, sodium pyruvate 2, pH 7.4, for 30 minutes to allow for the washout of extracellular dye and de-esterification of the intracellular Fluo-4.
2.3. Ca2+ Transients and Fractional Shortening
Myocytes were placed in a heated chamber (37°C) on the stage of an inverted Nikon microscope adapted for epifluorescence measurements and perfused with a normal physiological Tyrode’s solution with 1 mmol/L CaCl2 and paced by field-stimulation at 0.5 Hz (100 ms pulse duration) through a pair of platinum electrodes embedded at the bottom of the chamber. Fluo-4 was excited by the 488 nm line of an argon-ion laser and emitted fluorescence collected at > 505 nm. Changes in [Ca2+]i are expressed as changes of normalized fluorescence, F/F0, where F denotes the maximal fluorescence (peak) and F0 (or F unstimulated), is the resting fluorescence at the beginning of each recording (average fluorescence of the cell 50 msec prior to stimulation). Background fluorescence was subtracted from each parameter before calculating the peak Ca2+ transient[33, 34]. Tau was measured as the decay rate of the average Ca2+ transient trace. Simultaneous fractional shortening measurements were taken from both ends of each cell using a video edge detection system (Crescent Electronics).
Each data point represents a single cell measured at baseline and with the addition of drug/peptide at a single concentration. AFMs were paced for 1–2 minutes to establish stable baseline recordings. Once steady-state baseline conditions were reached, myocytes were superfused with a drug-containing solution and recordings were maintained for a period of 1–15 minutes until maximal drug effects were observed and a new steady state was reached. All data were analyzed using pClamp software. To account for variation in baseline fractional shortening and Ca2+ transient recordings, baseline values were set at 1 and the changes seen after the addition of drug/compound were expressed relative to their corresponding baseline value.
2.4. In vitro cAMP Assay
AFMs were buffer-exchanged to normal Tyrode’s solution supplemented with 1 mmol/L CaCl2 and 1 mmol/L ATP (SIGMA). 5×104 myocytes were incubated with the appropriate concentration of drug/peptide for the indicated period of time at 37°C and the reactions were terminated by immediate transfer of the samples to ice. Reactions were centrifuged for 10 minutes at 600×g at 4°C to pellet myocytes and cells were lysed in 400 µl of 0.1 M HCl solution containing 0.5% Triton X-100. Samples were centrifuged again at 600×g for 10 minutes at room temperature and the cleared cAMP containing lysates were transferred to fresh tubes for subsequent immediate analysis by cAMP ELISA. Where indicated, myocytes were pre-treated for 10 minutes with the β1-adrenergic receptor (AR) antagonist CGP 20712A (1 µmol/L CGP) for assay validation.
2.5. cAMP ELISA
cAMP ELISAs were performed using the Monoclonal Anti-cAMP Antibody Based Direct cAMP ELISA Kit (non-acetylated version) from NewEast Biosciences (#80204) as per the manufacturer’s protocol. The ELISA kit is a competitive immunoassay used to measure cAMP levels from cell extracts. Multi-well plates are provided in the kit and are pre-coated in goat-anti-mouse serum. cAMP in the samples competitively binds to a monoclonal anti-cAMP antibody in the presence of fixed amounts of cAMP-conjugated horseradish peroxidase. Known amounts of cAMP were diluted in 0.1 M HCl solution containing 0.5% Triton-X100 and used as standards to generate the calculation curve. After a short incubation, the excess reagents are washed away and substrate is added. The multiwell plates are then read on a microplate reader at 450 nm. The intensity of the yellow color developed is inversely proportional to the concentration of cAMP in the samples. The measured optical density (OD) is used to calculate the concentration of cAMP in samples based on the curve from the cAMP standards as plotted as the percent bound. Percent Bound was calculated as the Net OD of each pair of standards as a percentage of the maximum binding wells.
2.6. Pharmacology
All drugs and peptides were used at 1 µmol/L, 100 nmol/L, and 10 nmol/L concentrations. Isoproterenol and the β1-adrenergic receptor (AR) antagonist, CGP 20712A (CGP, 1 µmol/L), were purchased from SIGMA. Urocortin 2, Urocortin 3, and Stresscopin peptides were provided by Johnson & Johnson.
2.7. Mouse Myocardial Infarction (MI) and Trans-aortic Constriction (TAC)
All mice were C57BL/6 background. For our myocardial infarction (MI) model, mice were subjected to permanent ligation of the left main descending coronary artery (LCA) or a sham surgery as we have described [35]. For our trans-aortic constriction (TAC) model, aortic constriction was performed by tying a 7.0 nylon suture ligature against a blunted 27-gauge needle that was promptly removed to yield a 0.4mm constriction as previously described[36]. Pressure gradients were determined by in vivo echocardiography of the transverse aorta and mice with gradients greater than 45 mmHg were used.
2.8. Human Tissue
Samples were acquired from the Temple University human heart tissue bank (IRB #21319). All samples were core biopsies of post-MI heart failure patients at the time of transplant.
2.9. RNA Isolation and Semi-quantitative PCR
RNA isolation and analysis was performed as previously described[37]. Semi-quantitative PCR was carried out on cDNA using SYBR Green (Bio-Rad) and 100nM of gene-specific oligonucleotides for18S and ANF on a CFX96 Real Time System with BioRad CFX Manager 2.1 software. (Bio-Rad). Quantitation was established by comparing 18s rRNA, which was similar between groups, for normalization and compared using the ΔΔCt method.
2.10. Statistics
Data are reported as mean ± SEM. Unpaired t-tests, paired t-tests, and one-way ANOVAs were performed to detect significance using GraphPad Prism6 software. P≤0.05 was considered significant (ns p > 0.05; * p≤0.05; ** p≤0.01; *** p≤0.001; **** p≤0.0001).
3. RESULTS
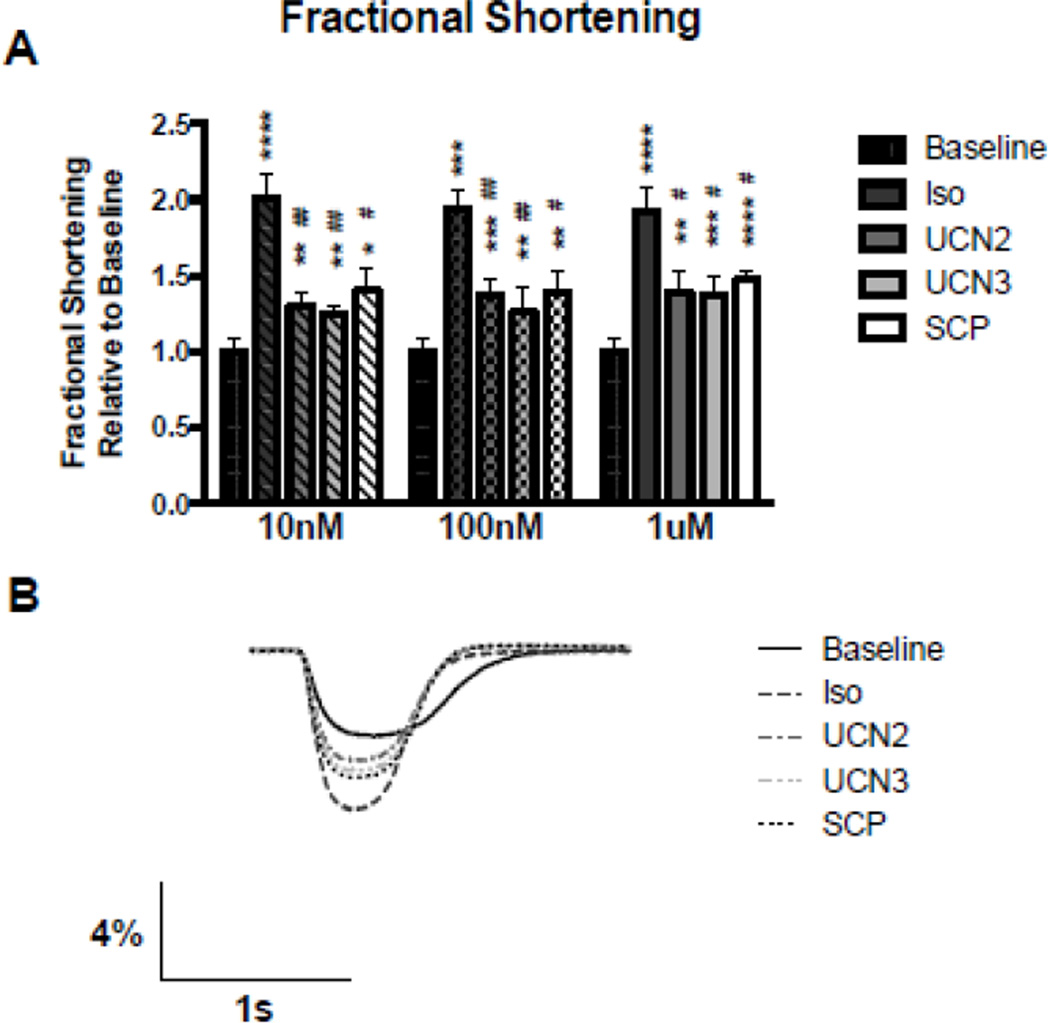
To analyze the effects of the CRF family of peptides on cardiomyocyte contractility to validate them as potential heart failure therapeutics, we used a system of isolated adult feline left ventricular myocytes (AFMs). Freshly isolated AFMs were paced by field stimulation to establish stable baseline contractile function. The bath was rapidly switched to drug containing solutions (10 nmol/L, 100 nmol/L or 1 µmol/L) for a period of 5–15 minutes until stable inotropic effects were observed and a new steady state was reached. Maximal inotropic effects were observed at the lowest concentration of Iso (10 nmol/L) while UCN2, UCN3, and SCP all showed incremental changes in contractility relative to baseline (Figure 1). All concentrations tested resulted in statistically significant increases in fractional shortening relative to baseline. However, even at the highest concentration tested (1 µmol/L), all of the CRF peptides had significantly smaller increases in contraction compared to Iso. Given the extremely high efficacy of catecholamines (Iso), this result was not entirely unexpected. Representative fractional shortening traces illustrate the increased magnitude of contraction and faster relaxation rate seen in drug-treated cells (Figure 1B). No treatments caused significant changes in resting cell length.
Figure 1. Fractional Shortening.
A. Peak fractional shortening values expressed as maximal drug treated response relative to baseline shortening. B. Sample representative fractional shortening traces at 100nM. ns p > 0.05; * or # p≤0.05; ** or ## p≤0.01; *** p≤0.001; **** p≤0.0001
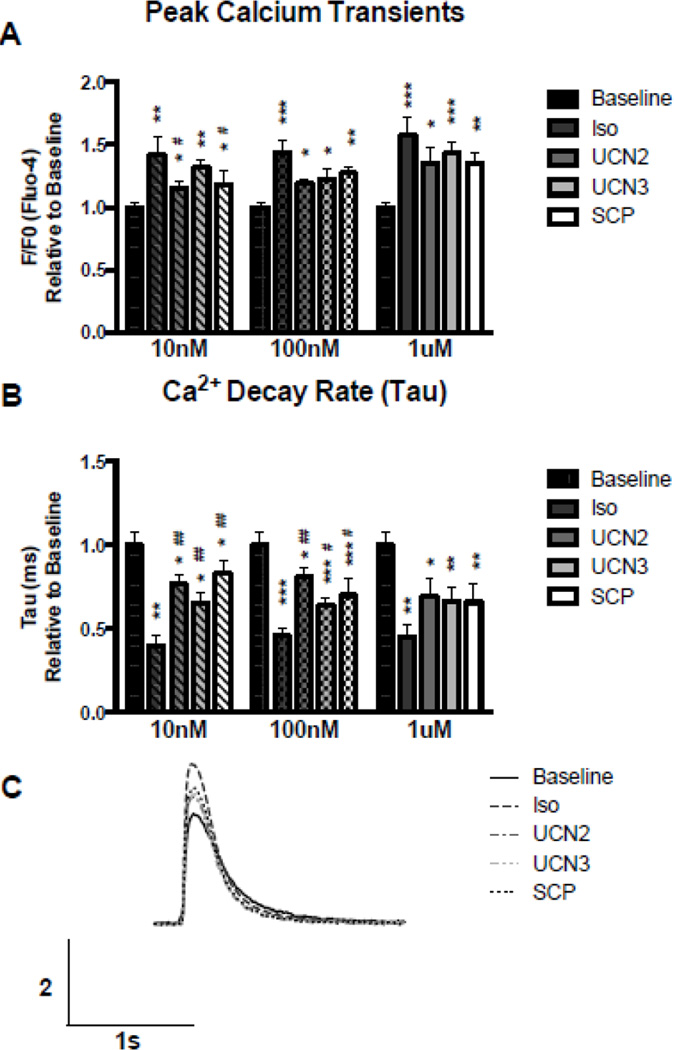
Maximal effects on Ca2+ transient amplitude were reached at the lowest concentration of Iso (10 nmol/L) tested. Similar to the effects on contraction, UCN2, UCN3, and SCP all had smaller effects on peak Ca2+ transient amplitude relative to baseline at the lowest concentrations tested (Figure 2A, 10nmol/L), but the effects of UCN2, UCN3, and SCP on peak Ca2+ transients were not significantly less than those produced by Iso at either 100nmol/L or 1 µmol/L.
Figure 2. Ca2+ Transients.
A. Peak Ca2+ transient values expressed as maximal drug treated response relative to baseline. B. Exponential decay curves were fitted to the downward slope of Ca2+ transients and average values expressed as maximal drug treated response relative to baseline. C. Sample representative Ca2+ transient traces at 100nM. ns p > 0.05; * or # p≤0.05; ** p≤0.01; *** p≤0.001; **** p≤0.0001
Analysis of individual Ca2+ transients (Figure 2B,C) showed that Iso and CRF peptides reduced cardiomyocyte relaxation time, indicative of increased SR Ca2+-ATPase (SERCA2a) reuptake of Ca2+. The time constant of cytosolic Ca2+ decline (Tau) was determined from the Ca2+ transient by fitting an exponential decay curve to the downward slope of the transient. The Tau value reflects SERCA2a-mediated Ca2+ reuptake. A lower Tau value reflects a faster uptake of cytosolic Ca2+ into the SR (and a more active SERCA2a) and can be visualized by a steeper slope in the decay of the Ca2+ transient which is evident in myocytes treated with Iso or any of the CRF peptides (Figure 2C). Each concentration tested resulted in statistically significant reductions in Tau relative to baseline (Figure 2B). None of the CRF peptides had as strong of an effect on Tau as Iso, although at the highest concentration examined, 1 µmol/L, the effects of UCN2, UCN3, and SCP on Tau values were not significantly different from that seen with Iso.
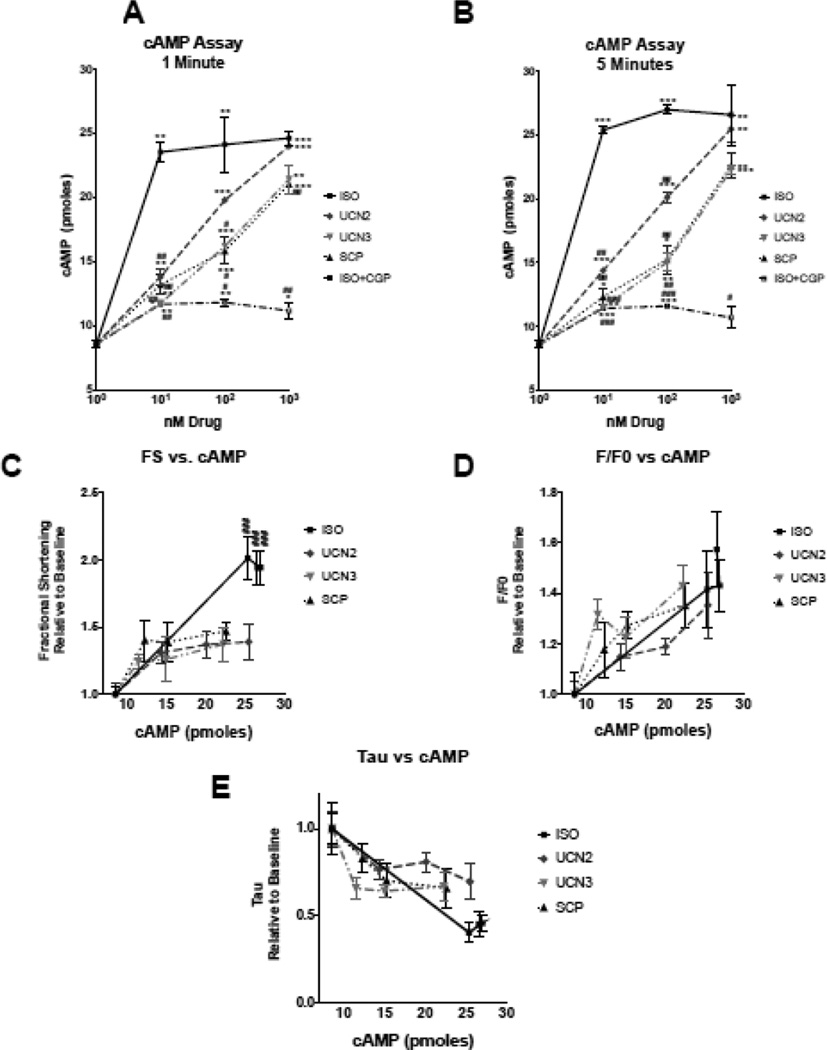
CRFR2 couples to Gs-cAMP-PKA signaling[6]. We next determined if increased contractility seen in AFMs treated with UCN2, UCN3, SCP, and Iso was related to increased cAMP levels. We performed a standard assay to quantify cAMP generation (Figure 3). In accordance with our contractility studies, we found maximal cAMP generation in Iso-treated cells at the lowest concentration tested (10 nmol/L) (Figure 3). To validate our assay, we pretreated cells with the β1-adrenergic receptor (AR) antagonist CGP 20712A (1 µmol/L CGP) before the addition of Iso. In these samples, we saw significant inhibition of cAMP production at all concentrations and time points assayed. The three CRF-related peptides elicited a concentration-dependent, statistically significant increase in cAMP production relative to baseline. All compounds showed a slight trend towards higher cAMP generation at the five-minute versus one-minute time points, but this was not shown to be significant. UCN2 was slightly more effective than UCN3 or SCP at any given concentration tested; however, the cAMP generation in UCN3 and SCP was significantly greater than baseline at each concentration and time point tested. At the highest concentration tested, 1 µmol/L, cAMP generated by UCN2, UCN3, and SCP at the five-minute time point did not significantly differ from that of Iso. At the lower concentrations assayed, 10 nmol/L and 100 nmol/L, the CRF peptides all produced significantly less cAMP than Iso. More detailed mechanistic studies will need to be performed to tease out the differences in signaling that lead to the pronounced cAMP effect seen in UCN2-treated cells versus UCN3- or SCP-treated cells. These studies show that UCNs and SCP are less effective activators of cAMP signaling than Iso at lower concentrations but are comparable at the highest concentrations examined.
Figure 3. cAMP ELISA.
cAMP production was measured in isolated myocytes after drug treatment for 1 minute (A) or 5 minutes (B) and plotted as a function of drug concentration. C-E. Fractional shortening (FS, C) of isolated myocytes, peak Ca2+ transients (F/F0, D) or Ca2+ decay rates (Tau, E) expressed as a function of cAMP production. ns p > 0.05; * or # p≤0.05; ** or ## p≤0.01; *** or ### p≤0.001; **** p≤0.0001
Interestingly, the inotropic efficacy of CRF peptides was less than that of Iso, even after accounting for cAMP-generating capacity. When myocyte contraction was plotted as a function of cAMP generation, the CRF peptides all had significantly smaller effects than Iso (Figure 3C). Therefore, Iso and CRF peptides elicited different contractile responses at comparable cAMP levels, indicating potential divergent inotropic mechanisms. These differences appear to be independent of intracellular Ca2+ levels as seen when maximal peak Ca2+ transients (Figure 3D) or intracellular Ca2+ decay rates (Figure 3E) were examined as a function of cAMP generation. These results suggest that the contractile differences seen between Iso and the CRF peptides are likely at the level of the myofilaments and point toward different signaling than produced by Iso.
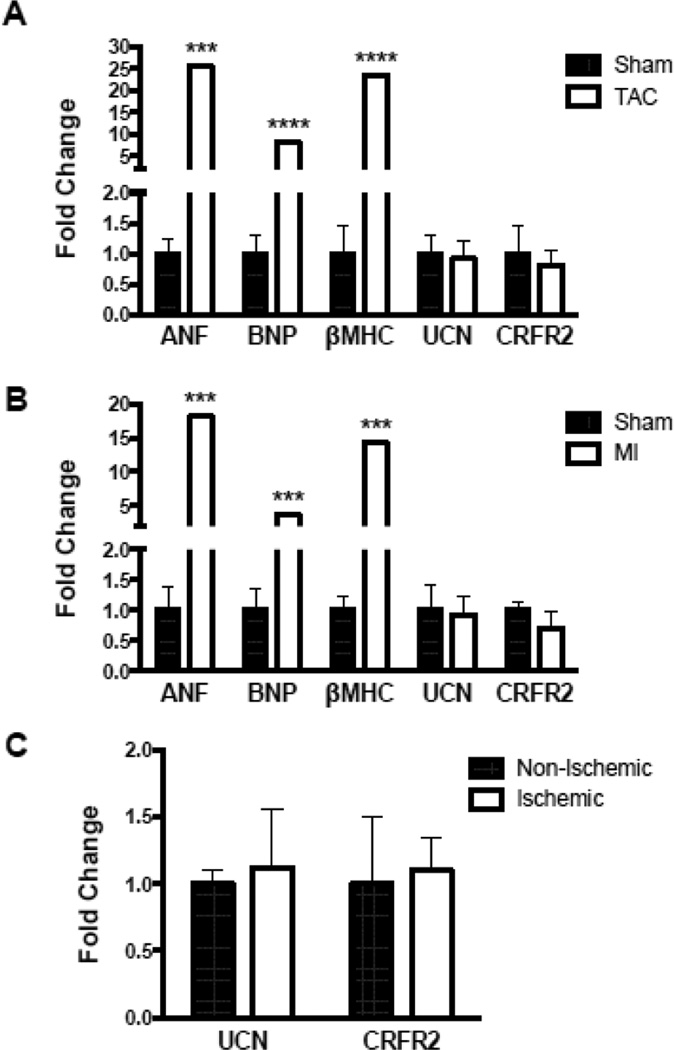
We next determined if the CRF family member receptors were altered in disease, since this could influence the responses to their agonists. To address this issue, we first used semi-quantitative PCR on heart tissue from mice 14-weeks after trans-aortic constriction (TAC). These experiments revealed no significant changes in the levels of UCN or CRFR2 in TAC animals as compared to control (sham) animals. We validated our TAC model by examining classical markers of the cardiac stress response and saw significant upregulation of ANF, BNP, and β-MHC (Figure 4A) as expected. UCN and CRFR2 levels were further assessed in a mouse model of heart failure where animals underwent myocardial infarction (MI) or sham procedures. Heart tissue was collected 6-weeks post-MI and again analyzed by qPCR. Similar to our TAC studies, mice that underwent MI had significantly elevated levels of ANF, BNP and β-MHC as compared to sham animals but showed no changes in UCN or CRFR2 levels (Figure 4B). We extended our findings to human heart failure patients by performing the same qPCR analysis on tissues from heart biopsies from ischemic and non-ischemic HF patients and again saw no significant alterations in UCN or CRFR2 levels (Figure 4C). Taken together, these data indicate that CRFR2 expression levels are not altered in human or mouse models of heart failure and therefore serve as attractive targets for novel heart failure therapeutics. These results help explain why HF patients showed favorable cardiovascular responses to UCN2[26, 27] and SCP[28] in early phase clinical trials.
Figure 4. RT-PCR Analysis.
Transcript levels for ANF, BNP, β-MHC, UCN and CRFR2 were quantified by real-time RT-PCR in heart tissue. Fold change in abundance is presented after standardization to 18S. ANF, BNP, and β-MHC mRNA transcript levels are significantly increased in mouse models of TAC (A) and MI (B), but no changes in UCN or CRFR2 levels were detected in either of these mouse models or in ischemic heart tissue from humans (C). ns p > 0.05; *** p≤0.001; **** p≤0.0001
4. DISCUSSION AND CONCLUSIONS
Heart failure is a complex syndrome that is a significant cause of premature morbidity and mortality in the United States and a huge financial burden on our healthcare system[38, 39]. Heart failure patients have poor cardiac function with chronic, excessive activation of adrenergic and renin-angiotensin signaling cascades, increased systemic vascular resistance, and fluid accumulation[40]. Current treatments for heart failure include β-adrenergic antagonists, angiotensin signaling inhibitors, diuretics, vasodilators, and inotropic agents[40], but the need for new therapies is well documented. CRF peptides have biological actions in the cardiovascular system that makes them appealing as heart failure therapeutics. Attractive features include vasodilation[18], positive inotropy[23], cardioprotection against ischemia/reperfusion injury[21, 22], and suppressive actions against the renin-angiotensin and sympathetic nervous systems[24, 41, 42]. In the present study, we show that CRFR2 expression levels are not altered in human or mouse models of heart failure, hence they serve as attractive targets for novel heart failure therapeutics.
The present study explored the direct effects of CRF peptides on myocyte contractility and Ca2+ dynamics. It is now well established that abnormalities of intracellular Ca2+ handling play a crucial role in the pathogenesis of heart failure[43]. Studies of Ca2+ handling in isolated cardiac myocytes have established the involvement of SR calcium ATPase (SERCA2a) in alterations of cardiac signaling and excitation-contraction coupling[44] in heart failure. Decreased peak systolic Ca2+ with prolonged Ca2+ transient duration produces reduced force-generating capacity and slower rates of force decay that characterize the failing heart[43, 45, 46]. Slower rates of SR Ca2+ uptake and changes in Ca2+ efflux (via the Na+-Ca2+ exchanger, NCX) produce rate-dependent elevations in diastolic Ca2+ and thus explain certain diastolic defects in the failing heart[43]. A prominent feature of the failing myocyte is reduced contractile effects of β-adrenergic agonists due to disruption of signaling cascades. In the present study, we sought to validate UCN2, UCN3, and SCP as regulators of myocyte contractility and to determine if these peptides modulate contractility via cAMP production. Adult feline ventricular myocytes were used because they have similar Ca2+ handling properties to those of humans [29, 47].
CRFR2 agonist-induced signaling has been shown to exert positive inotropic and lusitropic effects in isolated cardiac myocytes via stimulation of PKA, which augments ICa and SR Ca2+ load to increase SR Ca2+ release and intracellular Ca2+ transients[23]. In our experiments, UCN2, UCN3, and SCP significantly increased myocyte contractility in a concentration-dependent manner as characterized by increased fractional shortening and peak systolic Ca2+ transients and reduced Ca2+ transient decay rates relative to baseline. At the highest concentrations tested, the CRF peptides produced increases in peak Ca2+ transients and increased rates of Ca2+ reuptake that were statistically similar to those of Iso. UCNs or SCP also increased myocyte fractional shortening, but these effects were not as great as those produced by Iso. Concentration dependent increases in contractility correlated with increased cAMP generation in cells treated with UCN2, UCN3, and SCP, with UCN2 having a slightly greater effect. More detailed mechanistic studies are required to define the differences in signaling that lead to the pronounced cAMP effect seen in UCN2- versus UCN3- or SCP-treated cells. Also of interest is the observation that, at the highest concentration tested (1 µM), the CRF peptides produce similar levels of cAMP and Ca2+ signaling as isoproterenol, yet their effect on contraction was significantly less robust (Figure 3). The simplest explanation is that UCNs and SCP reduce myofilament Ca2+ binding affinity more so than Iso to produce a more modest inotropic effect.
Results in isolated cardiomyocytes indicate that CRF-peptides increase contractility, which could make this class of peptides potentially useful therapeutic agents for the treatment of heart failure. Clinical studies examining the safety, pharmacokinetics, and hemodynamic effects of SCP in HF patients with reduced LVEF (≤35%) and CI indicated that SCP infusion increased CI and reduced SVR without significant effects on PCWP (pulmonary capillary wedge pressure), HR, or SBP[28]. These observations are further supported by a study in which urocortin-2 infusion in ADHF patients markedly augmented cardiac output without significant reflex tachycardia[27]. An intriguing finding of our study is the disparity of the effect of cAMP on contraction. While this finding is not well understood, one hypothesis for mechanistic differentiation from classic inotropes could include post-receptor signaling through microdomains that house distinct pools of cAMP[48]. In addition, the effects could potentially be at the level of the myofilaments and signaling downstream of cAMP could be different for CRP-peptides versus β1-agonists.
In conclusion, the CRF family of peptides, particularly SCP, has positive inotropic effects on adult ventricular myocytes. In addition to the inotropic effect, activation of the CRFR2 pathway has potential additional benefit to the failing cardiomyocyte, including the prevention of mPTP opening during ischemia reperfusion[49] and impact on myocardial metabolism through AMPK[50]. These peptides should continue to be investigated as a safe and effective therapy to increase cardiac performance, which may prove to be superior to the currently available inotropes and therapeutic options. In this regard, a recent study has shown that intravenous injection of AAV UCN2 resulted in increased heart function, which correlated with increased plasma UCN2 levels[51]. This study not only demonstrates the feasibility and effectiveness of paracrine-based gene transfer via intravenous delivery, but also highlights the therapeutic potential for the CRF family of peptides.
Highlights.
We show that CRF peptides have positive inotropic effects on cardiac myocytes
We show that UCN2, UCN3, and SCP increase cAMP production in cardiomyocytes
We show that CRFR2 expression levels are not altered in models of heart failure
Our finding suggest that CRF peptides are promising therapeutic candidates
Acknowledgments
DISCLOSURES
This project was funded by Janssen, Pharmaceutical Companies of Johnson & Johnson
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- 2.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 5.Lovejoy DA, de Lannoy L. Evolution and phylogeny of the corticotropin-releasing factor (CRF) family of peptides: expansion and specialization in the vertebrates. J Chem Neuroanat. 2013;54:50–56. doi: 10.1016/j.jchemneu.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Nishikimi T, Miyata A, Horio T, Yoshihara F, Nagaya N, Takishita S, et al. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am J Physiol Heart Circ Physiol. 2000;279:H3031–H3039. doi: 10.1152/ajpheart.2000.279.6.H3031. [DOI] [PubMed] [Google Scholar]
- 7.Parkes DG, May CN. Urocortin: A Novel Player in Cardiac Control. News Physiol Sci. 2000;15:264–268. doi: 10.1152/physiologyonline.2000.15.5.264. [DOI] [PubMed] [Google Scholar]
- 8.Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G406–G419. doi: 10.1152/ajpgi.2001.280.3.G406. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto S, Kimura K, Saito M. Anorectic effect of leptin is mediated by hypothalamic corticotropin-releasing hormone, but not by urocortin, in rats. Neurosci Lett. 2001;307:179–182. doi: 10.1016/s0304-3940(01)01959-0. [DOI] [PubMed] [Google Scholar]
- 10.Radulovic M, Spiess J. Immunomodulatory role of the corticotropin-releasing factor. Arch Immunol Ther Exp (Warsz) 2001;49:33–38. [PubMed] [Google Scholar]
- 11.Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- 12.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol. 1998;12:1077–1085. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- 15.Chang CL, Hsu SY. Ancient evolution of stress-regulating peptides in vertebrates. Peptides. 2004;25:1681–1688. doi: 10.1016/j.peptides.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, et al. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab. 2002;87:340–346. doi: 10.1210/jcem.87.1.8160. [DOI] [PubMed] [Google Scholar]
- 18.Wiley KE, Davenport AP. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Br J Pharmacol. 2004;143:508–514. doi: 10.1038/sj.bjp.0705985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossant CJ, Pinnock RD, Hughes J, Hall MD, McNulty S. Corticotropin-releasing factor type 1 and type 2alpha receptors regulate phosphorylation of calcium/cyclic adenosine 3',5'-monophosphate response element-binding protein and activation of p42/p44 mitogen-activated protein kinase. Endocrinology. 1999;140:1525–1536. doi: 10.1210/endo.140.4.6656. [DOI] [PubMed] [Google Scholar]
- 20.Walther S, Awad S, Lonchyna VA, Blatter LA. NFAT transcription factor regulation by urocortin II in cardiac myocytes and heart failure. Am J Physiol Heart Circ Physiol. 2014;306:H856–H866. doi: 10.1152/ajpheart.00353.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 22.Chanalaris A. Protective effects of the urocortin homologues stresscopin (SCP) and stresscopin-related peptide (SRP) against hypoxia/reoxygenation injury in rat neonatal cardiomyocytes. J Mol Cell Cardiol. 2003;35:1295–1305. doi: 10.1016/s0022-2828(03)00244-x. [DOI] [PubMed] [Google Scholar]
- 23.Yang LZ, Kockskamper J, Heinzel FR, Hauber M, Walther S, Spiess J, et al. Urocortin II enhances contractility in rabbit ventricular myocytes via CRF(2) receptor-mediated stimulation of protein kinase A. Cardiovasc Res. 2006;69:402–411. doi: 10.1016/j.cardiores.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Rademaker MT, Cameron VA, Charles CJ, Richards AM. Integrated hemodynamic, hormonal, and renal actions of urocortin 2 in normal and paced sheep: beneficial effects in heart failure. Circulation. 2005;112:3624–3632. doi: 10.1161/CIRCULATIONAHA.105.561308. [DOI] [PubMed] [Google Scholar]
- 25.Rademaker MT, Charles CJ, Nicholls G, Richards M. Urocortin 2 sustains haemodynamic and renal function during introduction of beta-blockade in experimental heart failure. J Hypertens. 2011;29:1787–1795. doi: 10.1097/HJH.0b013e3283493776. [DOI] [PubMed] [Google Scholar]
- 26.Davis ME, Pemberton CJ, Yandle TG, Fisher SF, Lainchbury JG, Frampton CM, et al. Urocortin 2 infusion in human heart failure. Eur Heart J. 2007;28:2589–2597. doi: 10.1093/eurheartj/ehm340. [DOI] [PubMed] [Google Scholar]
- 27.Chan WYW, Frampton CM, Crozier IG, Troughton RW, Richards AM. Urocortin-2 Infusion in Acute Decompensated Heart Failure. JACC: Heart Failure. 2013;1:433–441. doi: 10.1016/j.jchf.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Gheorghiade M, Greene SJ, Ponikowski P, Maggioni AP, Korewicki J, Macarie C, et al. Haemodynamic effects, safety, and pharmacokinetics of human stresscopin in heart failure with reduced ejection fraction. Eur J Heart Fail. 2013;15:679–689. doi: 10.1093/eurjhf/hft023. [DOI] [PubMed] [Google Scholar]
- 29.Bailey BA, Houser SR. Sarcoplasmic reticulum-related changes in cytosolic calcium in pressure-overload-induced feline LV hypertrophy. Am J Physiol. 1993;265:H2009–H2016. doi: 10.1152/ajpheart.1993.265.6.H2009. [DOI] [PubMed] [Google Scholar]
- 30.Duthinh V, Houser SR. Contractile properties of single isolated feline ventriculocytes. Am J Physiol. 1988;254:H59–H66. doi: 10.1152/ajpheart.1988.254.1.H59. [DOI] [PubMed] [Google Scholar]
- 31.Silver LH, Hemwall EL, Marino TA, Houser SR. Isolation and morphology of calcium-tolerant feline ventricular myocytes. Am J Physiol. 1983;245:H891–H896. doi: 10.1152/ajpheart.1983.245.5.H891. [DOI] [PubMed] [Google Scholar]
- 32.duBell WH, Houser SR. Voltage and beat dependence of Ca2+ transient in feline ventricular myocytes. Am J Physiol. 1989;257:H746–H759. doi: 10.1152/ajpheart.1989.257.3.H746. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Chen X, Gao E, MacDonnell SM, Wang W, Kolpakov M, et al. Increasing cardiac contractility after myocardial infarction exacerbates cardiac injury and pump dysfunction. Circ Res. 2010;107:800–809. doi: 10.1161/CIRCRESAHA.110.219220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Makarewich CA, Kubo H, Wang W, Duran JM, Li Y, et al. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ Res. 2012;110:831–840. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 37.Huang ZM, Gao E, Fonseca FV, Hayashi H, Shang X, Hoffman NE, et al. Convergence of G protein-coupled receptor and S-nitrosylation signaling determines the outcome to cardiac ischemic injury. Science signaling. 2013;6:ra95. doi: 10.1126/scisignal.2004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 40.Joseph SM, Cedars AM, Ewald GA, Geltman EM, Mann DL. Acute decompensated heart failure: contemporary medical management. Tex Heart Inst J. 2009;36:510–520. [PMC free article] [PubMed] [Google Scholar]
- 41.Charles CJ, Jardine DL, Rademaker MT, Richards AM. Urocortin 3 inhibits cardiac sympathetic nerve activity in conscious sheep. J Cardiovasc Pharmacol. 2011;58:418–423. doi: 10.1097/FJC.0b013e31822707a4. [DOI] [PubMed] [Google Scholar]
- 42.Rademaker MT, Charles CJ, Ellmers LJ, Lewis LK, Nicholls MG, Richards AM. Prolonged urocortin 2 administration in experimental heart failure: sustained hemodynamic, endocrine, and renal effects. Hypertension. 2011;57:1136–1144. doi: 10.1161/HYPERTENSIONAHA.111.173203. [DOI] [PubMed] [Google Scholar]
- 43.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92:350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- 44.Prasad AM, Inesi G. Analysis of calcium transients in cardiac myocytes and assessment of the sarcoplasmic reticulum Ca2+-ATPase contribution. Methods Mol Biol. 2012;798:411–421. doi: 10.1007/978-1-61779-343-1_24. [DOI] [PubMed] [Google Scholar]
- 45.Houser SR, Piacentino V, 3rd, Mattiello J, Weisser J, Gaughan JP. Functional properties of failing human ventricular myocytes. Trends Cardiovasc Med. 2000;10:101–107. doi: 10.1016/s1050-1738(00)00057-8. [DOI] [PubMed] [Google Scholar]
- 46.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 47.Bers DM. Cardiac Na/Ca exchange function in rabbit, mouse and man: what's the difference? J Mol Cell Cardiol. 2002;34:369–373. doi: 10.1006/jmcc.2002.1530. [DOI] [PubMed] [Google Scholar]
- 48.Zheng M, Han QD, Xiao RP. Distinct beta-adrenergic receptor subtype signaling in the heart and their pathophysiological relevance. Sheng li xue bao : [Acta physiologica Sinica] 2004;56:1–15. [PubMed] [Google Scholar]
- 49.Townsend PA, Davidson SM, Clarke SJ, Khaliulin I, Carroll CJ, Scarabelli TM, et al. Urocortin prevents mitochondrial permeability transition in response to reperfusion injury indirectly by reducing oxidative stress. Am J Physiol Heart Circ Physiol. 2007;293:H928–H938. doi: 10.1152/ajpheart.01135.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Qi D, Cheng H, Hu X, Miller EJ, Wu X, et al. Urocortin 2 autocrine/paracrine and pharmacologic effects to activate AMP-activated protein kinase in the heart. Proc Natl Acad Sci U S A. 2013;110:16133–16138. doi: 10.1073/pnas.1312775110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao MH, Lai NC, Miyanohara A, Schilling JM, Suarez J, Tang T, et al. Intravenous adeno-associated virus serotype 8 encoding urocortin-2 provides sustained augmentation of left ventricular function in mice. Human gene therapy. 2013;24:777–785. doi: 10.1089/hum.2013.088. [DOI] [PMC free article] [PubMed] [Google Scholar]