Abstract
Objective
To summarize and critically review the existing literature on the prevalence of posttraumatic stress disorder (PTSD) following organ transplantation, risk factors for post-transplantation PTSD and the relationship of post-transplant PTSD to other clinical outcomes including health-related quality of life (HRQOL) and mortality.
Methods
We conducted a systematic literature review using PubMed, CINAHL Plus, the Cochrane Library, PsycInfo and a search of the online contents of 18 journals.
Results
Twenty-three studies were included. Post-transplant, the point prevalence of clinician-ascertained PTSD ranged from 1% to 16% (n = 738), the point prevalence of questionnaire-assessed substantial PTSD symptoms ranged from 0% to 46% (n = 1,024), and the cumulative incidence of clinician-ascertained transplant-specific PTSD ranged from 10% to 17% (n = 482). Consistent predictors of post-transplant PTSD included history of psychiatric illness prior to transplantation and poor social support post-transplantation. Post-transplant PTSD was consistently associated with worse mental HRQOL and potentially associated with worse physical HRQOL.
Conclusions
PTSD may impact a substantial proportion of organ transplant recipients. Future studies should focus on transplant-specific PTSD, and clarify potential risk factors for, and adverse outcomes related to, post-transplant PTSD.
Keywords: posttraumatic stress disorder, organ transplantation, quality of life, outcome assessment (health care)
Introduction
Over 100,000 organ transplantations are performed annually worldwide [1]. While graft survival has generally improved across organ types [2], there is interest in identifying modifiable risk factors for adverse outcomes following organ transplantation. Though a growing number of studies have identified that depression is substantially more common in organ transplant recipients than the general population and is associated with increased risk of post-transplant treatment non-adherence and mortality [3-6], relatively little is known about the prevalence and potential health impacts of other psychiatric disorders in this population.
Organ transplant recipients are exposed to extreme physiological and psychological stressors, including life-threatening illness, transplant surgery, pain, and intensive care unit (ICU) stays with mechanical ventilation and possible delirium, all of which make posttraumatic stress disorder (PTSD) a reasonable concern for this population [7, 8]. PTSD has been estimated to affect 3.5% of the U.S. population [9], and has been found to be especially prevalent in other medically ill populations and associated with both worse health-related quality of life (HRQOL) and increased healthcare utilization [7, 8, 10, 11]. Therefore, understanding the epidemiology of PTSD in organ transplant recipients is important, especially since PTSD is amenable to treatment and could represent a modifiable risk factor for adverse outcomes post-transplantation.
The current report details the results of a systematic review of studies examining PTSD in adult organ transplant recipients. The authors' objectives were to: (1) determine the prevalence of PTSD post-transplant; (2) identify potential risk factors for post-transplantation PTSD; and (3) examine the relationship of PTSD symptoms following organ transplantation to other post-transplantation outcomes such as HRQOL and mortality.
Materials and Methods
Approach and Search Strategy
We conducted a systematic review of the literature utilizing electronic databases and online journal content. We searched PubMed (1966–2014), CINAHL Plus (1969–2014), the Cochrane Library (2014, Issue 12) and PsycInfo (1967–2014) as of January 21, 2015. Our search strategy utilized the following terms mapped to the appropriate MeSH subject headings and “exploded”: “mental disorders” AND (“transplants” OR “transplantation”). The following terms were also included as text words: (“depress*” OR “stress” OR “anxi*”) (See Supplementary Appendix A). In addition, we searched the online contents of 18 transplantation and psychiatry journals (see Supplementary Appendix B) using the search terms (“posttraumatic” OR “PTSD”) AND “transplantation.” The search was limited to English language articles.
Study Selection
We sequentially reviewed citations, abstracts and full text articles in order to select eligible studies. Articles were selected for review if they met the following a priori eligibility criteria: (1) the population was composed of solid organ transplantation survivors ≥ 18 years of age; (2) PTSD assessments were conducted using validated measures (e.g., self-report questionnaires or structured/semi-structured diagnostic interviews) ≥ 1 month following transplantation; and (3) the study size was ≥ 15 participants. Abstracts, case reports/case series and review articles were excluded.
Data Abstraction and Assessment of Study Quality
For each eligible study, we abstracted information on study cohorts, PTSD measures, potential risk factors for post-transplant PTSD and associations between PTSD following organ transplantation and post-transplant health outcomes (e.g., HRQOL, mortality, treatment non-adherence, graft rejection) using a data abstraction tool (see Supplementary Appendix B). Authors of eligible studies were contacted for additional information when necessary.
We assessed study quality regarding assessment of potential risk factors for post-transplant PTSD using the following five criteria adapted from the US Preventive Services Task Force and prior systematic reviews of heterogeneous outcome data [12-14]: (1) enrollment of consecutive participants; (2) no loss to follow-up of > 10% prior to first PTSD symptom assessment; (3) description of participants lost to follow-up; (4) at least one statistical comparison of participants lost to follow-up versus those completing the study; and (5) adjustment for confounding by stratification, statistical adjustment, randomization or comparison with a matched population. Study quality criteria were not used in decisions on study inclusion or exclusion.
Results
Search Results, Study Characteristics and Quality
We reviewed 10,219 citations, 988 abstracts and 591 full-text articles (Figure). Twenty-three articles describing 21 cohorts of organ transplant recipients were eligible for data abstraction (Table 1) [15-37].
Figure. Flow diagram of literature search results.
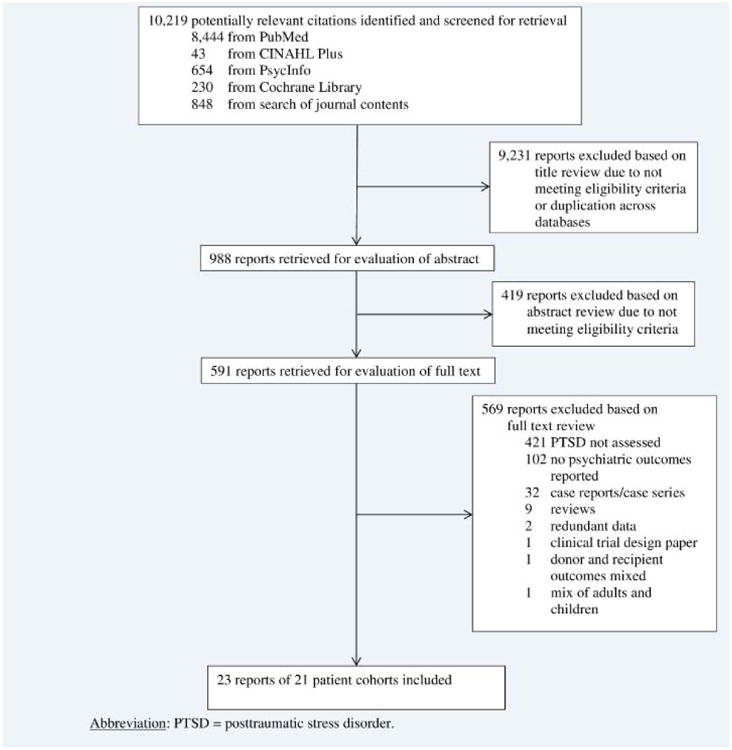
Table 1. Study cohort characteristics, ordered by follow-up time.
| Study | Study Type | Organ Type | N enrolled | Inclusion (I) and Exclusion (E) criteria | Sex (%) | % or Mean (SD) or median (interquartile range) [absolute range] | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age (years) | Time on transplant wait list (months) | Post-transplant follow-up period (months) | ||||||
| Grandi et al. 2001 [15] | Cross-sectional | Heart | 129 | I: Outpatients post-heart transplantation E: - |
♂: 81% ♀: 19% |
51 (5) | - | 1 |
| Dew et al. 1996 [16] | Prospective cohort | Heart | 154 | I: ≥ 18 years old, survived ≥ 6 weeks post-transplant E: Not fluent in English |
♂: 84% ♀: 16% |
< 50: 48% ≥ 50: 52% |
≥ 6 months: 56% | 2, 7, 12 |
| Dew et al. 1999 [17] | Prospective cohort | Heart | 174 | I: ≥ 18 years old, survived ≥ 6 weeks post-transplant E: Not fluent in English |
♂: 83% ♀: 17% |
< 50: 47% ≥ 50: 53% |
- | 2, 7, 12 |
| Stukas et al. 1999 [18] | Prospective cohort | Heart | 182 | I: ≥ 18 years old, survived ≥ 6 weeks post-transplant E: Not fluent in English, did not have a primary caregiver |
♂: 83% ♀: 17% |
< 50: 43% ≥ 50: 57% |
≥ 6 months: 54% | 2, 7, 12 |
| Dew et al. 2001a [19], 2001b [20] | Prospective cohort | Heart | 191 | I: ≥ 18 years old, survived ≥ 6 weeks post-Transplant E: Not fluent in English |
♂: 82% ♀: 18% |
< 50: 44% ≥ 50: 56% |
≥ 6 months: 54% | 2, 7, 12, 36 |
| Dew et al. 2012 [21] | Prospective cohort | Heart or Lung | 304 (126 heart and 178 lung) | I: ≥ 18 years old, receiving first lung or hearttransplant, survival ≥ 6 weeks post-transplant E: - |
♂: 59% ♀: 41% |
Heart: 52 (12) Lung: 51 (12) |
- | 2, 7, 12, 18, 24 |
| Fukunishi et al. 2002 [22] | Prospective cohort | Kidney or Liver | 96 (65 kidney and 31 liver) | I: Age > 20 E: Pre-transplant psychiatric disorders (e.g., delirium) |
♂: 49% ♀: 51% |
Kidney: 47 [20, 62] Liver: 43 [20, 65] |
- | 3, 12 |
| Fukunishi et al. 2001 [23] | Prospective cohort | Kidney or Liver | 275 adults (234 kidney and 41 liver) | I: Adults and children surviving living-related kidney or living-related liver transplantation E: - |
- | - | - | 3, 12, 36 |
| Possemato et al. 2009 [24] | Cross-sectional | Kidney | 63 | I: Received a kidney transplant at their transplant center within the previous 3 to 6 months, English-speaking E: Gross cognitive impairment |
♂: 73% ♀: 27% |
57 (13) | - | 3 to 6 |
| Cohen et al. 2014 [25] | Retrospective cohort | Lung | 42 | I: ≥ 18 years old; listed for lung transplantation or received single or bilateral lung transplantation E: - |
♂: 48% ♀: 52% |
60(57, 64) | - | 8 (2, 16) |
| Jin et al. 2012 [26] | Cross-sectional | Liver | 296 | I: age ≥ 18, understanding of Chinese, > 6 months post-transplant E: Severe medical complication s preventing completing questionnaire s, limited ability of expression |
♂: 81% ♀: 19% |
47 (10) | - | ≤ 12: 22% > 12 to ≤36: 45% > 36 to ≤60: 24% > 60: 9% |
| Guimaro et al. 2011 [27] | Cross-sectional | Liver | 24 | I: Acute liver failure E: - |
♂: 17% ♀: 83% |
41 (17) | - | 13 (14) |
| Possemato et al. 2010 [28] | Randomized controlledtrial | Kidney | 52 | I: ≥ 18 years old, able to read/write in English E: - |
♂: 46% ♀: 54% |
46 (12) [20, 70] | 24 (24) [0, 96]a | 24 (24) [3, 48] |
| Baranyi et al. 2013 [29] | Retrospective cohort | Heart or Liver or Heart/ Lung or Lung | 126 (62 Heart and 43 Liver and 21 Lung or Heart/Lung) | I: Survived solid organ transplantation E: - |
♂: 69% ♀: 31% |
52 (12) | - | 25 (12) |
| Gries et al. 2013 [30] | Cross-sectional | Lung | 210 | I: ≥ 18 years old, ≥ 6 months post-transplant, lung transplantation or post-lung transplant f/u care at their transplant center, English speaking E: - |
♂: 50% ♀: 50% |
59 (48, 63) | 3 (1, 6) | 29 (8, 60) |
| Einsle et al. 2012 [31] | Cross-sectional | Heart or lung | 82 | I: - E: Psychiatric disorder (e.g., psychosis); neurologic disease (e.g., dementia); lack of language comprehension |
♂: 81% ♀: 19% |
54 (12) | - | 32 (19) [4, 86] |
| Köllner et al. 2002 [32] | Cross-sectional | Heart or Lung | 82 (72 Heart and 8 Lung and 2 Heart/Lung) | I: > 4 months post-transplant, German-speaking E: - |
♂: 81% ♀: 19% |
54 | - | 32 [4, 86] |
| Favaro et al. 2011 [33] | Prospective cohort | Heart | 107 | I: ≥ 18 years old, > 1 year and < 5 years post-transplant E: Severe brain injury, mental retardation, schizophreni a-spectrum disorders |
♂: 79% ♀: 21% |
58 (12) [18, 75] | - | 36 (12) [12, 60] |
| Rothenhäusler et al. 2002 [34] | Retrospective cohort | Liver | 75 | I: Surviving > 5 months post-liver transplant, ≥ 16 years old, had measurable cyclosporine and/or tacrolimus levels E: Received corticosteroids |
♂: 57% ♀: 43% |
52 (11), 54 [16, 71] | 0.7 (0.4) [0.03, 7] | 46 (28) [5, 129] |
| Bunzel et al. 2007 [35], 2008 [36] | Cross-sectional | Heart | 38 | I: Survived heart transplantation following bridging with a mechanical circulatory assist device ≥ 6 months before study start, able to read/write in German, ≥ 17 years old E: Cognitive impairment |
♂: 95% ♀: 5% |
48 (12) [17, 67] | 5 (4) [1, 24]b | 53 (36) [6, 135] |
| Bunzel et al. 2005 [37] | Cross-sectional | Heart | 41 | I: Adults who survived heart transplantation after bridging with a mechanical circulatory assist device E: Cognitive impairment, inability to read/write German |
♂: 93% ♀: 7% |
46 (12) | 6 (5) [1, 24]b | 55 (34) [7, 122] |
Abbreviations (in alphabetical order): f/u = follow-up; SD = standard deviation.
This data refers to the mean time on hemodialysis, not the time on the transplant wait list, which was not reported.
This data refers to the mean time of having a mechanical circulatory assist device, not the time on the transplant wait list, which was not reported.
Table 1 presents the study designs and baseline descriptive data for the 23 studies, ordered by follow-up timing. Follow-up ranged from one month post-transplant [15] to 10.2 years post-transplant [37]. The studies enrolled 2,833 participants.
Only slightly over one-quarter of studies enrolled consecutive transplant patients (see Supplementary Table) [18, 19, 21, 29, 34]. Among cohort studies, more than half had over 10% of participants lost to follow-up [18, 19, 21, 28]. All of these studies provided descriptions of participants lost to follow-up and conducted analyses to compare those lost to follow-up to study completers. Only six of the 16 studies that examined potential risk factors for post-transplant PTSD adjusted for confounding in their analyses of these risk associations [18, 21, 26, 28-30].
Method of Assessment and Prevalence of Post-Transplant PTSD Symptoms
Fourteen studies utilized in-person assessments of post-transplant PTSD [15-23, 25, 30, 32-34]; five, mailed questionnaires [24, 29, 35-37]; two, a combination of in-person assessment and mailed questionnaire [26, 31]; one, internet questionnaire [28]; and one study did not report how follow-up assessments were conducted [27]. Six studies employed clinicians to make post-transplant PTSD diagnoses using versions of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID) [15, 21, 31-34]. In three of these studies, the investigators also used questionnaires to assess post-transplant PTSD symptoms [31, 32, 34]. Five studies employed clinicians to make post-transplant PTSD diagnoses using the Composite International Diagnostic Instrument (CIDI), a structured diagnostic interview [16-20]. In two studies, psychiatrists conducted diagnostic interviews using DSM-IV criteria [22, 23]. The remaining ten studies used only questionnaires to ascertain post-transplant PTSD symptoms. Of these studies, four used the Impact of Events Scale-Revised (IES-R) [27, 35-37]; three, the PTSD Checklist-Civilian version (PCL-C) [24, 28, 30]; two, the Posttraumatic Symptom Scale-10 (PTSS-10) [25, 29]; and one, the PTSD Self-Report Scale (PTSD-SS) [26].
In examining the prevalence of substantial PTSD symptoms or PTSD diagnosis following organ transplantation, we addressed several challenges as follows. First, since five studies reported the prevalence of post-transplant PTSD from overlapping cohorts presented in larger studies [16-18, 20, 36], we excluded these five studies from our descriptive analyses. Second, four studies ascertained the cumulative incidence of PTSD specifically related to the transplant experience [19, 21, 28, 33]. Twelve of the remaining 14 studies did not specify whether ascertainment of PTSD was limited to only symptoms related to the transplant (two studies noted that they examined both transplant-specific PTSD and PTSD symptoms related to other traumatic exposures [32, 34]). Therefore, we report the ranges in point prevalence of PTSD diagnosis or substantial PTSD symptoms ascertained post-transplant and cumulative incidence of transplant-specific PTSD. For the lone interventional trial included, we used the baseline prevalence for both the intervention and control group under the reasoning that the control group was not necessarily more representative than the intervention group. Notably, four of the five studies that used the IES-R to assess post-transplant PTSD symptoms used a regression formula-based threshold to determine substantial PTSD symptoms [31, 32, 35, 37]; the remaining study used a cut-off score of ≥ 20 [27]. Since five studies reported the prevalence of both the full PTSD syndrome and sub-threshold PTSD symptoms [24, 26, 28, 33, 34], we also report the prevalence range of sub-threshold PTSD symptoms.
The point prevalence of clinician-ascertained PTSD across all organ types ranged from was 1% at 3 months post-transplant [23] to 16% at a mean of 2.7 years post-transplant [32] (6 studies, n = 738), while the point prevalence of questionnaire-assessed substantial PTSD symptoms ranged from 0% at 8 months post-transplant [25] to 46% at a mean of 1.1 years post-transplant [27] (11 studies, n = 1,024). The cumulative incidence of clinician-ascertained transplant-specific PTSD ranged from 10% at 7 months post-transplant [19] to 17% at 3 years post-transplant [19] (3 studies, n = 482), while the cumulative incidence of questionnaire-assessed, transplant-specific substantial PTSD symptoms was 15% at 2 years post-transplant (1 study, n = 48) [28]. The prevalence of sub-threshold PTSD symptoms ranged from 5% out to over 5 years post-transplant [26] to 20% at a mean of 3 years post-transplant [33] (5 studies, n = 534).
Of note, three studies used both the SCID and the PTSS-10 [31, 32, 34]. In one study only three of the participants diagnosed with PTSD post-transplant with the SCID also had substantial PTSD symptoms on the PTSS-10 (score > 35) [32], while in another study all of the participants with SCID-diagnosed PTSD had substantial PTSD symptoms on the PTSS-10 [34] (the third study did not report concordance between the SCID and PTSS-10 among the transplant recipient cohort [31]).
Potential Risk Factors for PTSD Symptoms Post-Transplant
Four demographic factors were significantly associated with increased PTSD symptoms in some studies: younger age in two of eight studies [18, 21, 24, 26, 29, 30, 33, 34]; female sex in one of eight studies [18, 21, 24, 26, 29, 30, 33, 34]; lower educational status in two of four studies [24, 26, 29, 33]; and lower income in one of three studies (Table 3) [18, 26, 30]. Having private insurance was associated with fewer PTSD symptoms following lung transplantation in one study [30].
Table 3. Potential risk factors for posttraumatic stress disorder symptoms following solid organ transplantation.
| Study (N) | Measure of association | Potential risk factor | Outcome: posttraumatic stress symptoms/disorder |
|---|---|---|---|
| Stukas et al. 1999 [18] (N = 300) a | Hierarchical logistic regressionb |
|
|
| Hierarchical linear regressionb |
|
|
|
| Dew et al. 2001b [20] (N = 63) | χ2 test | VAD as a bridge to heart transplantation | n.s. |
| Dew et al. 2012 [21] (N = 239) | Cox proportional hazards regression |
|
|
| Fukunishi et al. 2002 [22] (N = 95) | χ2 test | Pre-transplant alexithymia | n.s. |
| Fukunishi et al. 2001 [23] (N = 275) | χ2 test | Hospital LOS, liver or kidney function, allograft rejection, major complications | n.s. |
| Possemato et al. 2009 [24] (N = 63) | Spearman's correlation |
|
|
| Cohen et al. 2014 [27] (N = 25) | Spearman's correlation | Higher resilience | R = -0.45, P = 0.02 |
| Jin et al. 2012 [26] (N = 241) | Stepwise multiple linear regression |
|
|
| Guimaro et al. 2011 [27] (N = 24) | Mann-Whitney U | Time from liver transplant (< 5 months vs. > 5 months) | 24 vs. 15 IES-R points, P = 0.09 |
| Possemato et al. 2010 [28] (N = 48) | Repeated measures analysis of covariance | Medical fact writing vs. Expressive writing | 36 vs. 32 PCL-C points, P = 0.14 10 vs. 8 PCL-C intrusive symptom points, n.s. 13 vs. 12 PCL-C avoidant symptom points, n.s. 12 vs. 10 PCL-C arousal symptom points, P = 0.09 |
| Baranyi et al. 2013 [29] (N = 126) | Mann-Whitney U, χ 2, or Fisher's exact test |
|
Comparisons: PTSD vs. No PTSD
|
| Multiple linear regression |
|
|
|
| Gries et al. 2013 [30] (N = 210) | Bivariate linear regression |
|
|
| Multiple linear regressionc |
|
||
| Favaro et al. 2011 [33] (N = 107) | χ2 test |
|
|
| Rothenhäusler et al. 2002 [34] (N = 75) | Kruskal-Wallis one way analysis or χ2 test |
|
Comparisons: Full PTSD vs. Sub-threshold PTSD vs. No PTSD
|
| Bunzel et al. 2007 [35] (N = 38) | Mann-Whitney U or Wilcoxon or Fisher's exact test |
|
|
| Bunzel et al. 2005 [37] (N = 41) | Wilcoxon test | Pulsatile vs. non-pulsatile mechanical circulatory assist device | n.s. |
Abbreviations (in alphabetical order): ARDS = acute respiratory distress syndrome; β = linear regression coefficient in multivariable model; CI = confidence interval; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; HAM-D = Hamilton Rating Scale for Depression; HR = hazard ratio; HBV = hepatitis B virus; HCV = hepatitis C virus; ICU = intensive care unit; IES-R = Impact of Events Scale-Revised; LOS = length of stay; MELD = Model for End-Stage Liver Disease; n.s. = not significant; OR = odds ratio; PCL-C = PTSD Checklist-Civilian version; PTSD = posttraumatic stress disorder; PBC = primary biliary sclerosis; PSC = primary sclerosing cholangitis; SF-36 MCS = Short-Form 36 Mental Component Summary; SF-36 PCS = Short Form-36 Physical Component Summary; SF-36 PF = Short-Form-36 Physical Functioning score; SKT = Short Cognitive Performance Test; VAD = ventricular assist device.
The analyses examining potential risk factors for post-transplant PTSD included both transplant recipients and their caregivers.
Regression models were adjusted for recipient vs. caregiver status.
In the multivariable linear regression models, the PCL-C outcome underwent inverse transformation. Therefore, a negative β coefficient corresponds to greater PTSD symptoms, while a positive β coefficient corresponds to fewer PTSD symptoms.
A history of pre-transplant psychiatric illness was associated with increased risk of PTSD symptoms post-transplant in four of four studies [18, 21, 29, 33]. Prior exposure to traumatic events was associated with increased risk of PTSD symptoms post-transplant in two of three studies [24, 30, 33]. One study found that pre-transplant benzodiazepine use, though not alcohol abuse, was associated with increased risk of PTSD symptoms post-transplant, though this association did not retain significance in multivariable regression [29].
One of two studies found that a shorter duration on the transplant wait list was associated with increased risk of PTSD post-transplant [33, 34]. One study of liver transplant recipients found that higher pre-operative Model for End-Stage Liver Disease score was associated with increased PTSD symptoms post-transplant [26]. While four studies enrolled transplant recipients of different organ types and assessed potential risk factors for post-transplant PTSD [21-23, 29], only one explicitly examined organ type transplanted as a potential risk factor [29], finding that liver transplantation was associated with increased prevalence of post-transplant PTSD vs. heart or lung transplantation [29].
Three transplant-related clinical characteristics were significantly associated with risk of post-transplant PTSD in some studies: re-transplantation in one of two studies [29, 34]; postoperative complications in two of four studies [23, 26, 29, 34]; and acute rejection in one of four studies [23, 30, 33, 34]. In a study of lung transplant recipients, lower post-transplant forced expiratory volume in 1 second (FEV1) and/or forced vital capacity (FVC) as well as development of bronchiolitis obliterans were associated with increased post-transplant PTSD symptoms [30]. One study of liver transplant recipients found that a longer ICU length of stay (LOS) was associated with increased risk of post-transplant PTSD [34].
Among post-transplantation factors, poor social support was associated with increased risk of PTSD or increased PTSD symptoms in three of four studies [18, 21, 33, 34]. One study of heart transplant recipients found that a low sense of personal control over events (i.e., mastery) was associated with increased PTSD symptoms [18]. One study of liver transplant recipients found that post-transplant depressive symptoms as well as worse cognition post-transplant, both assessed cross-sectionally (i.e., at the same time as PTSD symptoms) were associated with greater risk of post-transplant PTSD [34]. In a mixed cohort of organ transplant recipients, better post-transplant physical and mental HRQOL was correlated with fewer PTSD symptoms post-transplant [29]. One study identified that post-transplant benzodiazepine use was associated with increased risk of post-transplant PTSD, though this association did not persist in multivariable regression [29]. One study of heart transplant recipients found that having a spouse with PTSD was associated with increased PTSD symptoms in the recipient [35]. One study of lung transplant recipients identified a correlation between higher resilience and lower post-transplant PTSD symptoms [25]. Notably, time from transplant to follow-up assessment was not associated with either PTSD diagnosis or symptom severity in any study that examined this potential risk factor [26, 27, 29, 30, 34].
Associations of PTSD Symptoms and Post-Transplant Outcomes
Five studies examined associations between PTSD following organ transplantation and post-transplantation HRQOL (Table 4) [24, 26, 29, 32, 34]. All five found that post-transplant PTSD symptoms were associated with worse mental HRQOL, while three of four identified that post-transplant PTSD symptoms were associated with worse social functioning [24, 26, 29, 34], and three of three found that post-transplant PTSD symptoms were associated with worse general health [26, 29, 34]. Also, three [26, 29, 34] of the five studies ascertaining associations between post-transplant PTSD and post-transplant HRQOL found that post-transplant PTSD or substantial PTSD symptoms were associated with significantly worse physical functioning HRQOL domain scores.
Table 4. Associations of posttraumatic stress disorder symptoms and post-transplant outcomes.
| Post-transplant outcome: HRQOL | |||||
|---|---|---|---|---|---|
|
| |||||
| Study | Measure of association | Follow-up (months) | PTSD symptom instrument | HRQOL instrument | Test value (s) |
| Possemato et al. 2009 [24] | Spearman's correlation | 3 to 6 | PCL-C | NHP Total | R = 0.41, P ≤ 0.01 |
| NHP ER | R = 0.54, P ≤ 0.01 | ||||
| NHP SI | R = 0.48, P ≤ 0.01 | ||||
| NHP M | R = 0.18, n.s. | ||||
| NHP P | R = 0.14, n.s. | ||||
| NHP E | R = 0.23, n.s. | ||||
| Hierarchical linear regressiona | 3 to 6 | PCL-C | NHP Total | β = 0.43, P = 0.001 | |
| NHP ER | β = 0.63, P < 0.001 | ||||
| NHP SI | β = 0.58, P < 0.001 | ||||
| Jin et al. 2012 [26] | ANOVA | ≤ 12: 22% > 12 to ≤ 36: 45% > 36 to ≤ 60: 24% > 60: 9% |
PTSD-SS | SF-36 Components | Comparisons: Mean SF-36 Component Scores for PTSD vs. Sub-threshold PTSD vs. No PTSD |
| SF-36 PF | 71 vs. 76 vs. 80, P = 0.47 | ||||
| SF-36 RP | 31 vs. 36 vs. 60, P = 0.04 | ||||
| SF-36 BP | 61 vs. 63 vs. 76, P = 0.01 | ||||
| SF-36 GH | 51 vs. 40 vs. 60, P = 0.003 | ||||
| SF-36 V | 55 vs. 50 vs. 62, P = 0.04 | ||||
| SF-36 SF | 54 vs. 58 vs. 72, P = 0.003 | ||||
| SF-36 RE | 21 vs. 38 vs. 65, P = 0.002 | ||||
| SF-36 MH | 54 vs. 56 vs. 78, P < 0.001 | ||||
| Baranyi et al. 2013 [29] | Mann-Whitney U | 25b | PTSS-10 | SF-36 Components | Comparisons: Mean SF-36 Component Scores for PTSD vs. No PTSD |
| SF-36 PF | 40 vs. 69, P < 0.001 | ||||
| SF-36 RP | 25 vs. 58, P < 0.001 | ||||
| SF-36 BP | 35 vs. 69, P < 0.001 | ||||
| SF-36 GH | 31 vs. 61, P < 0.001 | ||||
| SF-36 V | 31 vs. 58, P < 0.001 | ||||
| SF-36 SF | 53 vs. 85, P < 0.001 | ||||
| SF-36 RE | 35 vs. 82, P < 0.001 | ||||
| SF-36 MH | 42 vs. 79, P < 0.001 | ||||
| Köllner at al. 2002 [32] | χ2 test | 32b | SCID-IV | SF-36 Component Summaries | Comparisons: Mean SF-36 Component Summary Scores for PTSD vs. No PTSD |
| SF-36 PCS | 33 vs. 43, P = 0.06 | ||||
| SF-36 MCS | 47 vs. 54, P < 0.05 | ||||
| Rothenhäusler et al. 2002 [34] | Kruskal-Wallis analysis of variance | 46b | SCID-III-R | SF-36 Components | Comparison: Mean SF-36 Component Scores for PTSD vs. Sub-threshold PTSD vs. No PTSD |
| SF-36 PF | 40 vs. 54 vs. 71, P < 0.05 | ||||
| SF-36 RP | 21 vs. 50 vs. 71, P < 0.05 | ||||
| SF-36 BP | 56 vs. 61 vs. 77, P = 0.13 | ||||
| SF-36 GH | 33 vs. 53 vs. 63, P < 0.05 | ||||
| SF-36 V | 20 vs. 42 vs. 62, P < 0.001 | ||||
| SF-36 SF | 56 vs. 79 vs. 83, P = 0.18 | ||||
| SF-36 RE | 19 vs. 77 vs. 83, P < 0.01 | ||||
| SF-36 MH | 30 vs. 67 vs. 79, P < 0.001 | ||||
|
| |||||
| Post-transplant outcome: mortality | |||||
|
| |||||
| Study | Measure of association | Follow-up in months | PTSD symptom instrument | Test value (s) | |
|
| |||||
| Dew et al. 1999 [17] | Hierarchical logistic regression | 36 | CIDI | OR: 13.74,c P < 0.01 | |
| Favaro et al. 2011 [33] | Cox proportional hazards regression | 136b | SCID-IV | HR: 1.4 (95%CI: 0.5, 3.6) | |
|
| |||||
| Post-transplant outcome: graft rejection | |||||
|
| |||||
| Study | Measure of association | Follow-up in months | PTSD symptom instrument | Test value (s) | |
|
| |||||
| Dew et al. 1999 [17] | χ2 test | 36 | CIDI | n.s. | |
|
| |||||
| Post-transplant outcome: treatment adherence | |||||
|
| |||||
| Study | Measure of association | Follow-up in months | PTSD symptom instrument | Test value (s) | |
|
| |||||
| Favaro et al. 2011 [33] | χ2 test Logistic regression | 136b | SCID-IV | PTSD intrusive symptoms | |
| χ2 = 4.5, P < 0.04 | |||||
| OR:d 4.9 (95%CI: 1.0, 23.8) | |||||
| OR:e 7.2 (95%CI: 1.2, 43.6) | |||||
| Logistic regression | PTSD avoidance symptoms | ||||
| OR:d 0.5 (95%CI: 0.1, 3.9) | |||||
| Logistic regression | PTSD hyper-arousal symptoms | ||||
| OR:d 1.3 (95%CI: 0.4, 4.5) | |||||
| Logistic regression | Full PTSD | ||||
| OR:d 0.6 (95%CI: 0.1, 5.4) | |||||
|
| |||||
| Post-transplant outcome: malignancy | |||||
|
| |||||
| Study | Measure of association | Follow-up in months | PTSD symptom instrument | Test value (s) | |
|
| |||||
| Favaro et al. 2011 [33] | Logistic regression | 136b | SCID-IV | PTSD intrusive symptoms | |
| OR:d 1.4 (95%CI: 0.6, 3.2) | |||||
| Logistic regression | PTSD avoidance symptoms | ||||
| OR:d 1.0 (95%CI: 0.3, 2.9) | |||||
| Logistic regression | PTSD hyper-arousal symptoms | ||||
| OR:d 1.9 (95%CI: 0.9, 4.3) | |||||
| Logistic regression | Full PTSD | ||||
| OR:d 1.4 (95%CI: 0.5, 4.1) | |||||
|
| |||||
| Post-transplant outcome: incident coronary artery disease | |||||
|
| |||||
| Study | Measure of association | Follow-up in months | PTSD symptom instrument | Test value (s) | |
|
| |||||
| Dew et al. 1999 [17] | χ2 test | 36 | CIDI | n.s. | |
Abbreviations (in alphabetical order): ANOVA = analysis of variance; β = linear regression coefficient in multivariable model; CI = confidence interval; CIDI = Composite International Diagnostic Instrument; HR = hazard ratio; NHP E = Nottingham Health Profile Energy score; NHP ER = Nottingham Health Profile Emotional Reaction score; NHP M = Nottingham Health Profile Mobility score; NHP P = Nottingham Health Profile Pain score; NHP SI = Nottingham Health Profile Social Isolation score; NHP Total = Nottingham Health Profile total score; n.s. = not significant; OR = odds ratio; PCL-C = PTSD Checklist-Civilian version; PTSD = posttraumatic stress disorder; PTSD-SS = Posttraumatic Stress Disorder Self-Report Scale; PTSS-10 = Posttraumatic Symptom Scale-10; SCID = Structured Clinical Interview for DSM; SF-36 BP = Short Form-36 Bodily Pain score; SF-36 GH = Short Form-36 General Health score; SF-36 MCS = Short Form-36 Mental Component Summary; SF-36 MH = Short Form-36 Mental Health score; SF-36 PCS = Short Form-36 Physical Component Summary; SF-36 PF = Short Form-36 Physical Functioning score; SF-36 RE = Short-Form 36 Role Emotional score; SF-36 RP = Short Form-36 Role Physical score; SF-36 SF = Short Form-36 Social Functioning score; SF-36 V = Short Form-36 Vitality score.
This hierarchical linear regression model was adjusted for years of education and number of prior traumatic events.
Mean
This hierarchical logistic regression model was adjusted for donor age, donor race, donor sex, requirement for IABP or LVAD, recipient age, cold ischemic time, diabetes, CMV serostatus, and grade 3A acute rejection or greater during the first 12 months post-transplant.
The odds ratios are unadjusted.
This logistic regression model was adjusted for age and receipt of prednisolone.
Only two studies, both of heart transplant recipients, ascertained potential associations between post-transplant PTSD and post-transplant mortality [17, 33]. While one of these studies did not find an association between post-transplant PTSD and mortality [33], the other found that post-transplant PTSD was associated with nearly 14-times greater odds of mortality [17]. Only one study examined potential associations between post-transplant PTSD and treatment non-adherence, finding that PTSD intrusive symptoms, though not the full PTSD syndrome, were associated with greater odds of treatment non-adherence [33]. The single study that assessed a potential association between post-transplant PTSD and graft rejection in heart transplant recipients did not find a significant association [17]. No associations were found between post-transplant PTSD and incident coronary artery disease (determined by coronary arteriography) or post-transplant malignancies [17, 33].
Discussion
To our knowledge, this paper is the first systematic review of the existing literature on PTSD in adult organ transplant recipients. This review highlights several key issues. First, we identified that PTSD, either as ascertained by diagnostic interview or defined as questionnaire-ascertained substantial PTSD symptoms, may be two to five-times higher than the US general population estimated one year prevalence [9]. The prevalence of PTSD in organ transplant recipients is similar to studies of PTSD in acute coronary syndrome survivors [38], though not quite as high as that found in general ICU or acute respiratory distress syndrome (ARDS) survivors [7, 8]. Importantly, if sub-threshold PTSD symptoms are also considered, then over one-quarter of organ transplant recipients may experience at least some degree of PTSD following transplantation.
Second, the two factors most consistently associated with increased risk of post-transplant PTSD include pre-transplant psychiatric illness and poor social support post-transplant. These findings are not unexpected since a pre-trauma history of psychiatric illness is known to increase risk for developing PTSD [8, 39], while strong social support has been shown to potentially moderate PTSD risk following trauma exposure [40]. We also found that demographic factors and transplant-related clinical characteristics appear to be less consistent predictors of PTSD post-transplant, findings similar to those in systematic reviews of PTSD in critical illness survivors [8].
Third, PTSD in organ transplant recipients may have a substantial negative impact on HRQOL that goes beyond mental HRQOL. While these results are consistent with the literature in critical illness survivors as well as the general PTSD literature [7, 8, 41], the impact of post-transplant PTSD on self-reported general health appears to be more pronounced than in other populations. Although only two studies examined the association of post-transplant PTSD with other post-transplant clinical outcomes, there is evidence that PTSD symptoms in transplant recipients could increase the risk of both treatment non-adherence and mortality. These findings warrant replication in additional organ transplant recipient cohorts, particularly since similar results have been reported in other studies of patients with chronic diseases [42, 43].
The existing literature has several important limitations. Many studies were cross-sectional in design, therefore limiting the ability to determine the direction of the association between potential risk factors and PTSD symptoms. Also, the questionnaires used to ascertain PTSD symptoms displayed sub-optimal discriminatory characteristics compared to structured and semi-structured diagnostic interviews. In addition, several studies had substantial losses to follow-up, potentially introducing selection bias. Furthermore, the majority of included studies did not ascertain only PTSD symptoms that were specifically related to the transplant experience. While it is conceivable that the extreme stress of the transplant experience could lead to recurrences of PTSD symptoms related to prior traumatic exposures, understanding the exact nature of PTSD in organ transplant recipients is essential for designing effective interventions. Moreover, while PTSD is highly comorbid with major depression [44], and comorbidity between depression and anxiety disorders such as PTSD has been associated with adverse health outcomes [45], none of the included studies examined the relationship of comorbidity between post-transplant PTSD and depression with other longer-term post-transplant outcomes. Therefore, additional prospective studies of PTSD specific to the organ transplantation experience are needed that also identify self-report PTSD symptom measures with improved case-finding properties and that examine the impact of comorbid post-transplant psychiatric conditions on post-transplant HRQOL, mortality and graft rejection.
Further research is needed to increase understanding of potential risk factors for PTSD following organ transplantation. One study of lung transplant recipients and one study of liver transplant recipients identified clinical characteristics (e.g., post-transplant bronchiolitis obliterans, ICU LOS) significantly associated with risk of post-transplant PTSD [30, 34]. These results warrant replication since bronchiolitis obliterans in lung transplant recipients was previously shown to be associated with increased risk of depression [46], and longer ICU LOS has been found to be associated with increased risk of PTSD in ARDS survivors [7]. Since one study found that liver transplant recipients may be at greater risk of post-transplant PTSD compared to heart or lung transplant recipients [29], more studies are needed to clarify whether liver transplant recipients are at especially heightened risk of developing PTSD.
Notably, none of the studies assessed early post-transplant psychiatric symptoms as potential predictors for later PTSD; psychiatric symptoms (e.g., acute stress, anxiety and/or depressive symptoms) prior to, or shortly following, hospital discharge, have been found to be potent predictors for the development of later PTSD in critical illness survivors [8, 47]. Also, while one study examined both pre-transplant and post-transplant benzodiazepine use as potential risk factors for post-transplant PTSD [29], no studies assessed in-hospital benzodiazepine exposure. Studies of critical illness survivors have found that in-ICU exposure to benzodiazepines, particularly to high doses of benzodiazepines for sedation, may be a risk factor for subsequent PTSD [8, 48]. In addition, further research is needed on the potential role of immunosuppressant regimens, particularly corticosteroid exposure, in the development of post-transplant PTSD. While corticosteroids have been found to produce anxiety symptoms [49], studies in the critical care literature have found that in-ICU corticosteroid exposure may reduce the risk of subsequent PTSD [48, 50]. Given these results in critical illness survivors, future studies of potential risk factors for PTSD in organ transplant recipients should examine in-hospital benzodiazepine and corticosteroid exposure as well as early post-transplant psychiatric symptoms as part of a comprehensive model that also incorporates pre-transplant factors (e.g., prior psychiatric illness), transplant patient group (e.g., liver transplant recipients vs. other transplant recipients) and post-transplant factors (e.g., social support).
A pathophysiological mechanism underlying how organ transplantation may lead to subsequent PTSD in recipients could be through transplantation-related systemic inflammation. Studies have found that genetic polymorphisms involved in regulating the inflammatory cascade are associated with increased risk of PTSD [51, 52], including in surgical populations [53], potentially through increasing systemic inflammation [52]. Furthermore, following organ transplantation the complement cascade is activated by human leukocyte antigen (HLA) antibodies [54], and studies have found that the complement cascade is hyperactive in individuals who have undergone acute psychological stress and potentially those with PTSD [55, 56]. Additional research is needed to determine if increased systemic inflammation could be a common pathway linking organ transplantation and subsequent PTSD risk.
Our systematic review has several potential limitations. Due to the methodological issues of the original studies described above, confidence regarding the precision and validity of our findings should be tempered. In particular, due to substantial heterogeneity in PTSD assessment methods and timing as well as relatively few studies adjusting for confounding in their analyses of potential risk factors for post-transplant PTSD, we are unable to conduct a meta-analysis of the results presented here with sufficient precision that minimizes the possibility of spurious conclusions being made. Also, we were unable to review potential risk factors for post-transplant PTSD for each organ transplant recipient group due to an insufficient number of studies examining these associations among different transplant populations. Furthermore, we did not assess for potential publication bias. Finally, despite a comprehensive search of 10,219 citations, potentially eligible studies may have been omitted due to inconsistent indexing in electronic databases.
In conclusion, a substantial proportion of solid organ transplant recipients may have some degree of PTSD symptoms which negatively impact HRQOL and possibly increase the risk of mortality. Risk factors for PTSD post-transplant appear to include a prior history of psychiatric illness and poor social support after transplant, although additional research into modifiable clinical risk factors is necessary. Since PTSD takes a substantial toll on transplant patients, their families, and society, collaboration between transplant specialists, primary care physicians, and mental health clinicians is necessary in order to facilitate prompt, comprehensive evaluation and treatment.
Supplementary Material
Table 2. Measurements of posttraumatic stress disorder symptoms, ordered by follow-up time.
| Study | Follow-up (months) | N at follow-up | Instrument | Mean (SD) or Median (IQR) [range] | Cut-off score | Point prevalence (%) |
|---|---|---|---|---|---|---|
| Grandi et al. 2001 [15] | 1 | 129 | SCID-IV | N/A | N/A | 7 |
| Dew et al. 2012 [21] | 2 | 304 | SCID-IV | N/A | N/A | Lung: 12 Heart: 13 |
| 7 | - | Lung: 12 Heart: 13 |
||||
| 12 | - | Lung: 15 Heart: 14 |
||||
| 24 | 239 | Lung: 15 Heart: 14 |
||||
| Fukunishi et al. 2001 [23] | 3 | 275 | DSM-IV Criteria | N/A | N/A | Total: 1 Kidney: 0.4 Liver: 2 |
| Possemato et al. 2009 [24] | 3 to 6 | 63 | PCL-C | - | Algorithmb | Full: 21 Sub-thresholdc: 19 |
| Dew et al. 2001a [19] | 7 | - | CIDI | N/A | N/A | 10 |
| 12 | - | 16 | ||||
| 36 | 136 | 17 | ||||
| Cohen et al. 2014 [25] | 8d | 42 | PTSS-10 | 16 (13, 22) | 35 | 0 |
| Fukunishi et al. 2002 [22] | 12 | 95 | DSM-IV Criteria | N/A | N/A | Total: 4 Kidney: 3 Liver: 6 |
| Jin et al. 2012 [26] | ≤ 12: 22% > 12 to≤ 36: 45% > 36 to≤ 60: 24% > 60: 9% |
241 | PTSD-SS | - | Algorithmg | Full: 4 Sub-thresholdh: 5 |
| Guimaro et al. 2011 [27] | 13i | 24 | IES-R | 20 (13) | 20 | 46 |
| Possemato et al. 2010 [28] | 24i | 48 | PCL-C | 37 (11) | Algorithmb | Full: 15 Sub-thresholdc: 10 |
| Baranyi et al. 2013 [29] | 25i | Total: 126 Heart: 62 Liver: 43 Lung or Heart/Lung: 21 |
PTSS-10 | 32 (7) | 35 | Total: 15 Heart: 6 Liver: 30 Lung or Heart/Lung: 9 |
| Gries et al. 2013 [30] | 29d | 210 | PCL-C | 25 (21, 34) | Algorithmb | 13 (95% CI: 8, 18) |
| Einsle et al. 2012 [31] | 32i | 82 | PTSS-10 IES-R SCID-IV-TR |
26 (9) Intrusion: 7 (6) Avoidance: 9 (8) Arousal: 8 (7) N/A |
35 Maercker & Schützwohl formula N/A |
15 5 10 |
| Köllner et al. 2002 [32] | 32i | 82 | PTSS-10 IES-R SCID-IV |
- N/A |
35 Maercker & Schützwohl formulaN/A | 15 9 16 |
| Favaro et al. 2011 [33] | 36i | 107 | SCID-IV | N/A | N/A | Full: 13 Sub-thresholdj: 20 |
| Rothenhäusler et al. 2002 [34] | 46i | 75 | PTSS-10 SCID-III-R |
18 [10, 52] N/A |
35 N/A |
5 Full: 5 Sub-thresholdk: 17 |
| Bunzel et al. 2007 [35] | 53i | 38 | IES-R | 18 (13) | Maercker & Schützwohl formula | 0 |
| Bunzel et al. 2005 [37] | 55i | 41 | IES-R | 21 (15) | Maercker & Schützwohl formula | 0 |
Abbreviations (in alphabetical order): CIDI = Composite International Diagnostic Instrument; DSM = Diagnostic and Statistical Manual of Mental Disorders; GAD = Generalized Anxiety Disorder; IES-R = Impact of Events Scale-Revised; IQR = interquartile range; MDD = Major Depressive Disorder; N/A = not applicable; NOS = not otherwise specified; PCL-C = PTSD Checklist-Civilian version; PTSD-SS = Posttraumatic Stress Disorder Self-Report Scale; PTSS-10 = Posttraumatic Symptom Scale-10; SCID = Structured Clinical Interview for DSM; SD = standard deviation.
Prevalence of any psychiatric disorder prior to transplant.
The algorithm to ascertain probable PTSD using the PCL-C considers a score of ≥ 3 on at least one intrusive symptom, at least three avoidant symptoms, and at least two arousal symptoms as consistent with DSM diagnostic criteria.
The authors defined sub-threshold PTSD using the PCL-C as a score of ≥ 3 on at least one intrusive symptom and either at least 3 avoidant symptoms, or at least two arousal symptoms.
Median
Prevalence of lifetime history of depression or an anxiety disorder.
The authors defined sub-threshold PTSD using the CIDI as either: 1) meeting the one month duration criterion but lacking one symptom in one of the three symptom domains; or 2) meeting the symptom thresholds but having a duration of symptoms between 2 weeks and 1 month.
The algorithm to ascertain probable PTSD using the PTSD-SS considers a score of ≥ 3 on at least one intrusive symptom and either at least 3 avoidant symptoms, or at least two arousal symptoms as consistent with likely clinical diagnosis.
The authors defined sub-threshold PTSD using the PTSD-SS as meeting symptom thresholds on any two of three PTSD symptom domains.
Mean
The authors defined sub-threshold PTSD using the SCID-IV as the presence of at least one intrusive, one avoidance and one arousal symptom.
The authors defined sub-threshold PTSD using the SCID-III-R as the presence of symptoms in at least two of the three PTSD symptom domains.
Acknowledgments
The authors thank Brigitta Bunzel, PhD, Mark Mikkelsen, MD and Kyle Possemato, PhD for clarification of their study designs.
Funding: This work was supported by grant KL2 TR000421 from the National Institutes of Health. The sponsor had no role in the study design, collection, analysis or interpretation of data, or in the decision to submit the article for publication.
Footnotes
Disclosures: The authors have no relevant conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. GKT1 activity and practices. [Accessed December 18, 2014]; http://www.who.int/transplantation/gkt/statistics/en/
- 2.OPTN/SRTR 2012 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration; 2014. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) [Google Scholar]
- 3.Rosenberger EM, Dew MA, Crone C, DiMartini AF. Psychiatric disorders as risk factors for adverse medical outcomes after solid organ transplantation. Curr Opin Organ Transplant. 2012;17:188–192. doi: 10.1097/MOT.0b013e3283510928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jindal RM, Neff RT, Abbott KC, Hurst FP, Elster EA, Falta EM, et al. Association between depression and nonadherence in recipients of kidney transplants: analysis of the United States Renal Data System. Transplant Proc. 2009;41:3662–3666. doi: 10.1016/j.transproceed.2009.06.187. [DOI] [PubMed] [Google Scholar]
- 5.Novak M, Molnar MZ, Szeifert L, Kovacs AZ, Vamos EP, Zoller R, et al. Depressive symptoms and mortality in patients after renal transplantation: a prospective prevalent cohort study. Psychosom Med. 2010;72:527–534. doi: 10.1097/PSY.0b013e3181dbbb7d. [DOI] [PubMed] [Google Scholar]
- 6.DiMartini AF, Dew MA, Chaiffetz D, Fitzgerald MG, Devera ME, Fontes P. Early trajectories of depressive symptoms after liver transplantation for alcoholic liver disease predicts long-term survival. Am J Transplant. 2011;11:1287–1295. doi: 10.1111/j.1600-6143.2011.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker EA, Katon W, Russo J, Ciechanowski P, Newman E, Wagner AW. Healthcare costs associated with posttraumatic stress disorder symptoms in women. Arch Gen Psychiatry. 2003;60:369–374. doi: 10.1001/archpsyc.60.4.369. [DOI] [PubMed] [Google Scholar]
- 11.Davydow DS, Hough CL, Zatzick D, Katon WJ. Psychiatric symptoms and acute care service utilization over the course of the year following medical-surgical intensive care unit admission: a longitudinal investigation. Crit Care Med. 2014;42:2473–2481. doi: 10.1097/CCM.0000000000000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 13.Dowdy DW, Eid MP, Sedrakyan A, Mendez-Tellez PA, Pronovost PJ, Herridge MS, et al. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med. 2005;31:611–620. doi: 10.1007/s00134-005-2592-6. [DOI] [PubMed] [Google Scholar]
- 14.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandi S, Fabbri S, Tossani E, Mangelli L, Branzi A, Magelli C. Psychological evaluation after cardiac transplantation: the integration of different criteria. Psychother Psychosom. 2001;70:176–183. doi: 10.1159/000056250. [DOI] [PubMed] [Google Scholar]
- 16.Dew MA, Roth LH, Schulberg HC, Simmons RG, Kormos RL, Trzepacz PT, et al. Prevalence and predictors of depression and anxiety-related disorders during the year after heart transplantation. Gen Hosp Psychiatry. 1996;18:48S–61S. doi: 10.1016/s0163-8343(96)00077-1. [DOI] [PubMed] [Google Scholar]
- 17.Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J Heart Lung Transplant. 1999;18:549–562. doi: 10.1016/s1053-2498(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 18.Stukas AA, Jr, Dew MA, Switzer GE, DiMartini A, Kormos RL, Griffith BP. PTSD in heart transplant recipients and their primary family caregivers. Psychosomatics. 1999;40:212–221. doi: 10.1016/s0033-3182(99)71237-5. [DOI] [PubMed] [Google Scholar]
- 19.Dew MA, Kormos RL, DiMartini AF, Switzer GE, Schulberg HC, Roth LH, et al. Prevalence and risk of depression and anxiety-related disorders during the first three years after heart transplantation. Psychosomatics. 2001;42:300–313. doi: 10.1176/appi.psy.42.4.300. [DOI] [PubMed] [Google Scholar]
- 20.Dew MA, Kormos RL, Winowich S, Harris RC, Stanford EA, Carozza L, Griffith BP, et al. Quality of life outcomes after heart transplantation in individuals bridged to transplant with ventricular assist devices. J Heart Lung Transplant. 2001;20:1199–1212. doi: 10.1016/s1053-2498(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 21.Dew MA, DiMartini AF, DeVito Dabbs AJ, Fox KR, Myaskovsky L, Posluszny DM, et al. Onset and risk factors for anxiety and depression during the first two years after lung transplantation. Gen Hosp Psychiatry. 2012;34:127–138. doi: 10.1016/j.genhosppsych.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukunishi I, Sugawara Y, Takayama T, Makuuchi M, Kawarasaki H, Surman O. Association between pretransplant psychological assessments and post-transplant psychiatric disorders in living-related transplantation. Psychosomatics. 2002;43:49–54. doi: 10.1176/appi.psy.43.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Fukunishi I, Sugawara Y, Takayama T, Makuuchi M, Kawarasaki H, Surman O. Psychiatric disorders before and after living-related transplantation. Psychosomatics. 2001;42:337–343. doi: 10.1176/appi.psy.42.4.337. [DOI] [PubMed] [Google Scholar]
- 24.Possemato K, Geller PA, Ouimette P. Posttraumatic stress and quality of life in kidney transplantation recipients. Traumatology. 2009;15:34–39. [Google Scholar]
- 25.Cohen DG, Christie JD, Anderson BJ, Diamond JM, Judy RP, Shah RJ, et al. Cognitive function, mental health, and health-related quality of life after lung transplantation. Ann Am Thorac Soc. 2014;11:522–530. doi: 10.1513/AnnalsATS.201311-388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin SG, Yan LN, Xiang B, Li B, Wen TF, Zhao JC, et al. Posttraumatic stress disorder after liver transplantation. Hepatobiliary Pancreat Dis Int. 2012;11:28–33. doi: 10.1016/s1499-3872(11)60122-7. [DOI] [PubMed] [Google Scholar]
- 27.Guimaro MS, Lacerda SS, Aguilar MR, Karam CH, Kernkraut AM, Ferraz-Neto BH. Post-traumatic stress disorders, mood disorders and quality of life in transplant recipients with acute liver failure. Transplant Proc. 2011;43:187–188. doi: 10.1016/j.transproceed.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Possemato K, Ouimette P, Geller PA. Internet-based expressive writing for kidney transplant recipients: effects on posttraumatic stress and quality of life. Traumatology. 2010;16:49–54. [Google Scholar]
- 29.Baranyi A, Krauseneck T, Rothenhäusler HB. Posttraumatic stress symptoms after solid-organ transplantation: preoperative risk factors and the impact on health-related quality of life and life satisfaction. Health Qual Life Outcomes. 2013;11:111. doi: 10.1186/1477-7525-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gries CJ, Dew MA, Curtis JR, Edelman JD, DeVito Dabbs A, Pilewski JM, et al. Nature and correlates of post-traumatic symptomatology in lung transplant recipients. J Heart Lung Transplant. 2013;32:525–532. doi: 10.1016/j.healun.2013.01.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Einsle F, Kraft D, Köllner V. Post-traumatic stress disorder (PTSD) in cardiology and oncology—which diagnostic tools should be used? J Psychosom Res. 2012;72:434–438. doi: 10.1016/j.jpsychores.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Köllner V, Schade I, Maulhardt T, Maercker A, Joraschky P, Gulielmos V. Posttraumatic stress disorder and quality of life after heart or lung transplantation. Transplant Proc. 2002;34:2192–2193. doi: 10.1016/s0041-1345(02)03198-6. [DOI] [PubMed] [Google Scholar]
- 33.Favaro A, Gerosa G, Caforio ALP, Volpe B, Rupolo G, Zarneri D, et al. Posttraumatic stress disorder and depression in heart transplantation recipients: the relationship with outcome and adherence to medical treatment. Gen Hosp Psychiatry. 2011;33:1–7. doi: 10.1016/j.genhosppsych.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Rothenhäusler HB, Ehrentraut S, Kapfhammer HP, Lang C, Zachoval R, Bilzer M, et al. Psychiatric and psychosocial outcome of orthotopic liver transplantation. Psychother Psychosom. 2002;71:285–297. doi: 10.1159/000064811. [DOI] [PubMed] [Google Scholar]
- 35.Bunzel B, Laederach-Hofmann K, Wieselthaler G, Roethy W, Wolner E. Mechanical circulatory support as a bridge to heart transplantation: what remains? Long-term emotional sequelae in patients and spouses. J Heart Lung Transplant. 2007;26:384–389. doi: 10.1016/j.healun.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Bunzel B, Roethy W, Znoj H, Laederach-Hofmann K. Psychological consequences of life-saving cardiac surgery in patients and partners: measurement of emotional stress by the Impact of Events Scale. Stress Health. 2008;24:351–363. [Google Scholar]
- 37.Bunzel B, Laederach-Hofmann K, Wieselthaler G, Roethy W, Drees G. Posttraumatic stress disorder after implantation of a mechanical assist device followed by heart transplantation: evaluation of patients and partners. Transplant Proc. 2005;37:1365–1368. doi: 10.1016/j.transproceed.2004.12.248. [DOI] [PubMed] [Google Scholar]
- 38.Edmonson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS One. 2012;7:e38915. doi: 10.1371/journal.pone.0038915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 40.Pietrzak RH, Feder A, Singh R, Schechter CB, Bromet EJ, Katz CL, et al. Trajectories of PTSD risk and resilience in World Trade Center responders: an 8-year prospective cohort study. Psychol Med. 2014;44:205–219. doi: 10.1017/S0033291713000597. [DOI] [PubMed] [Google Scholar]
- 41.Pacella ML, Hruska B, Delahanty DL. The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. J Anxiety Disord. 2013;27:33–46. doi: 10.1016/j.janxdis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Kronish IM, Edmondson D, Li Y, Cohen BE. Post-traumatic stress disorder and medication adherence: results from the Mind Your Health Study. J Psychiatr Res. 2012;46:1595–1599. doi: 10.1016/j.jpsychires.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ladwig KH, Baumert J, Marten-Mittag B, Kolb C, Zrenner B, Schmitt C. Posttraumatic stress symptoms and predicted mortality in patients with implantable cardioverter-defibrillators: results from the prospective living with an implanted cardioverter-defibrillator. Arch Gen Psychiatry. 2008;65:1324–1330. doi: 10.1001/archpsyc.65.11.1324. [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell ML, Alkemade N, Nickerson A, Creamer M, McFarlane AC, Silove D, et al. Impact of the diagnostic changes to post-traumatic stress disorder for DSM-5 and the proposed changes to ICD-11. Br J Psychiatry. 2014;205:230–235. doi: 10.1192/bjp.bp.113.135285. [DOI] [PubMed] [Google Scholar]
- 45.Kemp AH, Quintana DS, Felmingham KL, Matthews S, Jelinek HF. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: implications for cardiovascular risk. PLoS One. 2012;7:e30777. doi: 10.1371/journal.pone.0030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermeulen KM, Groen H, van der Bij W, Erasmus ME, Koëter GH, TenVergert EM. The effect of bronchiolitis obliterans on health related quality of life. Clin Transplant. 2004;18:377–383. doi: 10.1111/j.1399-0012.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 47.Davydow DS, Zatzick D, Hough CL, Katon WJ. A longitudinal investigation of posttraumatic stress and depressive symptoms over the course of the year following medical-surgical intensive care unit admission. Gen Hosp Psychiatry. 2013;35:226–232. doi: 10.1016/j.genhosppsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bienvenu OJ, Gellar J, Althouse BM, Coluantoni E, Sricharoenchai T, Mendez-Tellez PA, et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013;43:2657–2671. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Judd LL, Schettler PJ, Brown ES, Wolkowitz OM, Sternberg EM, Bender BG, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive and behavioral effects. Am J Psychiatry. 2014;171:1045–1051. doi: 10.1176/appi.ajp.2014.13091264. [DOI] [PubMed] [Google Scholar]
- 50.Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhäusler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 51.Binder EB, Bradley RG, Liu LW, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry. 2015;172:353–362. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauer D, Weis F, Papassotiropoulos A, Schmoeckel M, Beiras-Fernandez A, Lieke J, Kaufmann I, et al. Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Crit Care Med. 2011;39:643–650. doi: 10.1097/CCM.0b013e318206bae6. [DOI] [PubMed] [Google Scholar]
- 54.Thomas KA, Valenzuela NM, Reed EF. The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection. Trends Mol Med. 2015;21:319–329. doi: 10.1016/j.molmed.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burns V, Edwards K, Ring C, Drayson M, Carroll D. Complement cascade activation after an acute psychological stress task. Psychosom Med. 2008;70:387–396. doi: 10.1097/PSY.0b013e31816ded22. [DOI] [PubMed] [Google Scholar]
- 56.Hovhannisyan LP, Mkrtchyan GM, Sukiasian SH, Boyajyan AS. Alterations in the complement cascade in post-traumatic stress disorder. Allergy Asthma Clin Immunol. 2010;6:3. doi: 10.1186/1710-1492-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


