Abstract
Background
Neural activity within the prefrontal cortex (PFC) is altered by alcohol and alcohol-associated stimuli, and is mediated by genetic susceptibility to alcoholism. However, very little is known about how genetic risk for excessive drinking might mediate neural firing in the PFC during alcohol consumption.
Methods
To determine how genetic risk influences alcohol seeking, intake, and neural activity, a Pavlovian alcohol consumption task was used - the 2-Way Cued Access Paradigm (2CAP). Alcohol preferring ‘P’ rats and relatives of their (heterogeneous) founding Wistar population were used for these studies. After acquisition of 2CAP, extinction of responding for alcohol was evaluated by substituting water for alcohol. Following these experiments, in vivo electrophysiological recordings were obtained during 2CAP from the PFC in a separate cohort of Wistar and P rats implanted with moveable tetrode microdrives.
Results
P and Wistar rats increased daily alcohol seeking and intake with P rats consuming roughly twice as much alcohol as Wistar. Both rat populations decreased seeking behavior during extinction. However, P rats displayed persistent increases in seeking after controlling for intake vs Wistar. Higher firing rates (FR) were observed in P rats prior to 2CAP, and throughout alcohol and water consumption compared with Wistars that were matched for alcohol drinking history. Differences in FR were driven, in part, by a larger percentage of neurons in P rats versus Wistars that increased FR compared with those that decreased, or didn’t change.
Conclusions
These data provide additional evidence of increased alcohol consumption and persistent alcohol seeking in alcohol preferring P vs. Wistar rats. Differences in PFC neural firing observed in P rats prior to drinking could be heritable and/or related to an enhanced response to alcohol-associated contextual cues. FR differences observed during alcohol drinking might be related to an augmented sensitivity of PFC neurons to orally consumed alcohol.
Keywords: Pre-Frontal Cortex (PFC), Alcohol Preferring (‘P’)rats, neural firing, electrophysiology, voluntary alcohol intake/consumption
Introduction
The prefrontal cortex (PFC) plays an important role in processing the subjective properties of addictive substances such as alcohol (Goldstein and Volkow, 2011). Alcohol has been shown to increase blood flow to the PFC (Volkow et al., 1988, Sano et al., 1993, Tiihonen et al., 1994), and this effect is influenced by risk for developing alcoholism (Tolentino et al., 2011). Imaging studies in alcoholics have shown that baseline hemodynamic activity in the PFC is substantially lower than in non-alcoholic controls (Catafau et al., 1999). However, exposure to cues associated with alcohol experience elicits robust increases in the hemodynamic activation of this brain region (George et al., 2001, Kareken et al., 2010). Furthermore, genetic susceptibility to the development of (i.e. family history of) alcoholism influences hemodynamic activation of the PFC following acutely administered alcohol (Kareken et al., 2010). These results suggest that genetic vulnerability for developing alcoholism interacts with a history of drinking to modify, and possibly enhance, the representation of alcohol-related information. While these data highlight the PFC as a key brain region that mediates excessive drinking, especially in those genetically vulnerable, it is not clear if, or how, alcohol changes neural firing in these populations.
Studies in animal models also suggest that the PFC plays a central role in mediating the subjective effects of alcohol. Acute alcohol exposure increases immediate early gene expression in several PFC sub-regions (Segovia et al., 2013), as does exposure to alcohol-associated cues (Dayas et al., 2007, Groblewski et al., 2012). Furthermore, alcohol has been shown to decrease the firing rate of neurons in the PFC of anesthetized rats (Tu et al., 2007), and lesions of the PFC decrease voluntary unsweetened alcohol intake (Deckel et al., 1996). While these observations suggest that alcohol influences neural activity in the frontal cortex, which may modulate its reinforcing properties; it is not known if neural firing in the PFC is differentially influenced by voluntary self-administered alcohol. Furthermore, it is not clear how a genetic predisposition for excessive drinking might mediate neural firing following alcohol consumption. Rodent models of a genetic predisposition for excessive drinking can provide an answer to these questions, thus yielding insight into the neural processes that guide alcohol seeking/consumption behaviors and how they are altered in genetically vulnerable populations.
Indiana alcohol preferring ‘P’ rats serve as a validated genetic rodent model of alcohol use disorder (AUD; (McBride and Li, 1998, McBride et al., 2014) as they demonstrate a number of phenotypic alterations observed in excessive drinking populations (Bell et al., 2014, Lumeng and Li, 1986, Gatto et al., 1987, Stewart et al., 1991, Kampov-Polevoy et al., 2000, Waller et al., 1982). Wistar rats are a genetically heterogeneous population that served as the founder population for P rats and are not considered vulnerable to excessive drinking. While they vary considerably in the amount of alcohol they consume as individuals, they drink less compared to P rats overall (Lumeng et al., 1977). For these reasons, Wistar rats provide a heterogeneous “control” phenotype well suited to compare with P rats.
The first goal of the current study was to determine if alcohol seeking and intake behaviors are acquired and extinguished differently in rodent populations with low and high genetic vulnerability for excessive drinking. The second goal was to characterize if neural activity in the PFC might be mediated by differential risk for excessive alcohol intake.
Materials
Animals
Twenty-two male Indiana University Alcohol Preferring (P) rats and 21 non-genetically predisposed (heterogeneous) Wistar rats were bred in-house or purchased from Harlan (Indianapolis, IN), respectively. Eighteen P and 18 Wistar rats were used for behavioral experiments only, whereas 4 P and 3 Wistar rats were used for both behavioral and electrophysiological experiments. Animals were acclimated to single housing and a 12 hour reverse light/dark cycle. Animals were ≈70 days of age prior to testing and had ad lib access to food and water. All procedures were approved by the Purdue School of Science Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academic Press, 2003).
Apparatus
Two compartment rectangular boxes (20.3cm*15.9cm*21.3cm; Med Associates, St Albans, VT) were used for ‘2-way Cued Access Protocol’ (2CAP) procedures. Each end of the box contained a retractable sipper with the drinking orifice 2.5 cm above the grid floor. Approximately 16.5 cm above each sipper orifice was a single white stimulus light.
Methods
Intermittent Alcohol Procedure (IAP)
The procedural timeline and methods for all experiments are outlined in Table 1. All animals first underwent an IAP using previously published procedures (Simms et al., 2008). Rats were given access to 2 bottles, one containing 20% alcohol and the other tap water, for 24 hours every other day (Mon/Wed/Fri) in the home cage. These procedures were continued for 4 weeks; animals had 12 total 24-hour alcohol/water access sessions.
Table 1.
Procedures
| IAP [12 Days] |
(N) | 2CAP [15 Days] |
(N) | |||||
|---|---|---|---|---|---|---|---|---|
| Behavior (N=36) |
P | (18) | 10% Alcohol 20% Alcohol |
(9) (9) |
2CAP Water Extinction [5 Days] |
Home Cage No Manipulation [30 Days] |
2CAP Alcohol [5 Days]* |
|
| Wistar | (18) | 10% Alcohol 20% Alcohol |
(9) (9) |
|||||
| E-phys (N=7) |
P | (4) | 10% Alcohol | (4) | Surgery/ Recovery [7 Days] |
Habituation Acclimation [7 Days] |
2CAP Alcohol [1–3 Days] |
2CAP Water [1–3 Days]† |
| Wistar | (3) | 10% Alcohol | (3) | |||||
Intermittent Alcohol Procedures (IAP); 2-Way Cued Alcohol Procedures (2CAP)
Blood samples were taken on the final (5th) day.
Brains were removed for histology after the final day.
2-Way Cued Access Protocol (2CAP)
Acquisition/maintenance
Twenty-four hours following the final (12th) IAP access session animals received access to an unsweetened 10% or 20% alcohol solution or tap water for daily 2CAP sessions as previously described (McCane et al., 2014). 2CAP sessions occurred during the dark phase of the light dark cycle, starting no earlier than 1 hour after lights off. During 2CAP, a stimulus light was illuminated for 2 seconds on one side of the rectangular box at random. One second after this light was turned off, a sipper tube containing the alcohol solution was extended into the box on the same side as the light cue. Thus, the light was a CS+ that predicted the location that the alcohol was made available. To ensure the sipper motor sound did not serve as a directional cue, both tube motors were turned on for the same duration but only the appropriate sipper entered the chamber. The tube remained available for 10 seconds. Each trial was separated by a 20–180 second inter-trial interval (ITI; 90 seconds on average). Thus, each daily 2CAP session was approximately 72 minutes in duration (71.67 minutes). A total of 40 trials were conducted for 5 out of 7 days a week for 3 weeks (15 total sessions over days 1–19). The time spent at the alcohol extended sipper (TAS), the latency to approach this sipper (LTS), and the amount of total alcohol consumed were the dependent variables of interest. All animals went through these procedures in an identical fashion.
Extinction
All animals, except the subset of 7 animals used for electrophysiology experiments, were given 5 daily consecutive extinction sessions beginning 3 days after the final 2CAP alcohol session (5 total sessions over days 22–26). Animals slated for electrophysiology experiments underwent surgery/recovery (see below) and had extinction sessions at a later date following reacquisition of 2CAP. Extinction sessions were identical to alcohol session except the sippers contained water. During extinction sessions, a tube containing 10% or 20% alcohol was present outside the fluid delivery port to ensure that the presence or absence of the alcohol odor did not predict alcohol availability/unavailability. Chambers were cleaned thoroughly between subjects.
Stereotaxic Surgery and Behavioral Electrophysiology
All animals in the electrophysiology studies were in the 10% alcohol groups. Following the acquisition/maintenance of 2CAP, electrophysiology animals were unilaterally implanted with multi-tetrode arrays in the medial PFC using previously described methods and coordinates (Lapish et al., 2008).
After a full recovery from surgery, animals were given a period of about a week of habituation/acclimation prior to electrophysiological recordings. Animals were habituated to the handling required for incremental lowering of tetrodes prior to the task, and also to the tether. Following habituation and acquisition of ensemble recordings, 1–3 days of 2CAP reinforced with 10% alcohol were conducted while electrophysiology was recorded. Animals were then given 1–3 extinction sessions where the sippers contained water. Following the completion of behavioral testing and electrophysiological recordings, placements were verified via histology (see suppl. Figure 3).
Blood Sampling
Blood samples were not collected at any point during conditioning, but rather as a final separate experiment with 34 of the total 43 animals. One month following the final 2CAP session, animals were given 5 consecutive days of 2CAP alcohol access at the concentration they were trained with. Immediately following the final (5th) session, 50µl blood samples were drawn from the tip of the tail. Samples were centrifuged and plasma was withdrawn and stored at −20°C. Blood alcohol concentrations (BACs) were then determined using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Statistics
Mean total fluid intake (IAP/2CAP) and TAS, and LTS (2CAP only) were analyzed using a mixed 3-way analysis of variance (ANOVA) with day as the within subject’s factor and 2CAP alcohol concentration (10%/20%) and rat population (P/Wistar) as the between groups factor. BACs and alcohol intake on BAC day were analyzed by 2-way ANOVA with alcohol concentration and rat populations as factors. Residual scores were calculated as the difference between the actual Y-axis data value and the Y-axis value predicted by the linear model (i.e. prediction error). TAS and LTS residuals were analyzed separately via ANOVA with rat population and day as factors. Subsequent ANOVAs broken down on one or more factors were conducted to facilitate the interpretation of interactions, and to evaluate within-rat population changes in behavior over days. Correlation and regression analyses were conducted to determine the strength and significance of particular variables of interest. Behavioral data were all tested for normality using Kolmogorov-Smirnov Tests (K–S), and in the few instances where not normally distributed, were evaluated using Mann-Whitney U test. Tukey, Sidak, and Dunnett’s post-hoc tests were performed when appropriate. Post hoc significance indications in the results are all Sidak unless otherwise noted.
Firing Rate (FR)
Baseline FR from each neuron was assessed as the mean firing frequency during the 3 minutes immediately preceding the onset of the first trial. Changes in FR over the course of the behavioral task were evaluated by normalizing the FR of each neuron to its baseline FR. For this and subsequent FR analyses, neurons with fewer than 10 spikes during the 3 minute baseline were also excluded (20/134=114 P-Alcohol, 13/138=125 P-Water, 22/123=101 Wistar-Alcohol, 16/122=106 Wistar-Water). The cumulative FR frequency was also determined from each group over the course of the behavioral task. In some instances FR data tested for normality using K-S test were further evaluated using Mann-Whitney U test. The change in FR throughout 2CAP (i.e. slope) was determined using linear and polynomial regression on the firing rate from all neurons in a treatment group. The fit of each group’s data to one of two polynomial (1st order vs. 2nd order) equations were compared using an F-statistic to determine the equation that best described the change in FR throughout 2CAP (i.e. explained the highest proportion of variance), with the null hypothesis that a first order polynomial was a better fit than a second order polynomial. Regression analyses were also performed on each neuron individually to determine the proportion of neurons within a group that increased, decreased, or didn’t change FR. The significance of group differences in number of neurons with increases, decreases, or no change was determined using chi-squared statistics. All data were considered significant at p<0.05.
Results
Fluid Intake: Behavior only cohort
Alcohol Intake (Acquisition/Maintenance)
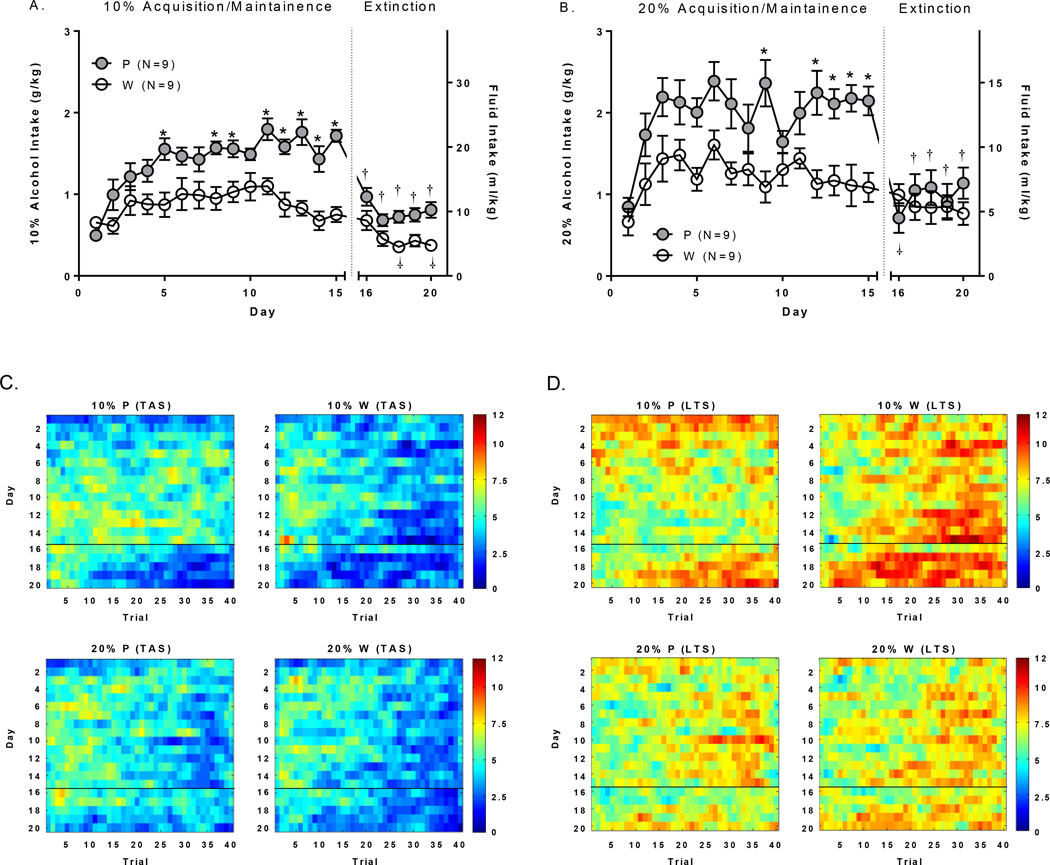
The 36 animals used for behavior only were evaluated separate from those used for electrophysiology and are reported here. IAP results can be found in supporting information (suppl. Figure 1). 10% and 20% alcohol intake during the 15 day acquisition and maintenance of 2CAP drinking procedures can be seen in Figure 1A and B respectively. It was necessary for direct comparisons of alcohol intake between 10% and 20% alcohol concentration groups to evaluate g/kg (not ml/kg). Analyses of the first 15 alcohol days revealed significant main effects of rat population [F(1,32)=26.16;p<0.0001], alcohol concentration [F(1,32)=12.75;p<0.01], and day [F(14,448)=12.89;p<0.0001]. P rats consumed more alcohol than Wistars, the 20% alcohol concentration group had higher total alcohol intake, and total alcohol intake increased over days.
Figure 1.
Fluid intake and TAS/LTS. A+B) Alcohol intake was greater overall and increased to a larger degree over days in P vs. Wistar at both alcohol concentrations. Water consumption (ml/kg) decreased on extinction days to levels below those observed on the final day alcohol consumption (day 15) in both P and Wistar at the 10% alcohol concentration, and in P rats only at the 20% alcohol concentration. Time course (over trials; x-axis) of mean C) time at the sipper (TAS) and D) latency to the sipper (LTS) for alcohol acquisition (Days 1–15; y-axis) and water extinction (Days 16–20; y-axis) days of all animals. Color intensity reflects time in seconds according to the color bar scales to the right of the figures. *’s indicate between rat population group differences; p’s<.05). †’s indicate within-rat population differences from day 15 (p’s<.05).
Water Intake (Extinction)
Analyses of water intake (ml/kg) revealed main effects of rat population [F(1,32)=5.26;p<0.05], and alcohol concentration [F(1,32)=6.98;p<0.05]. P rats consumed more water, and more water was consumed during extinction in groups assigned to the 10% concentration compared to groups assigned to the 20% concentration.
Alcohol vs. Water Intake (Acquisition/Maintenance/Extinction)
Analysis of intake for all 20 days was then conducted and followed by post hoc testing. Main effects of day were as follows: P/10% [F(19,152)=16.71;p<0.0001]; Wistar/10% [F(19,152)=6.34;p<0.0001]; P/20% [F(19,152)=9.66;p<0.0001], Wistar/20% [F(19,152)=2.96;p<0.0001]. Between group differences (*’s) and differences in water consumption compared to the final alcohol day (†’s; Day 15; Dunnets) are indicated in Figure 1A+B.
TAS, LTS, and Intake Relationships (Alcohol): Behavior only cohort
Mean TAS and LTS data are presented in Table 2. Mean TAS and LTS were also examined for each trial per day in Figure 1C+D. For alcohol days, there were significant trial*rat population*alcohol concentration interactions for both TAS [F(39,2145)=2.24;p<.0001] and LTS [F(39,2145)=2.34;p<.0001], which were driven by relatively sustained TAS/LTS over trials in the P rats at the 10% alcohol concentration, and was the motivating factor for choosing this concentration in the electrophysiology experiments. The relationship between time spent at the alcohol cued sipper, latency to the alcohol cued sipper, and alcohol intake can be found in Table 3. Time spent at the alcohol cued sipper was positively correlated with alcohol intake at both concentrations in both rat populations (p’s<0.01). Latency to the alcohol cued sipper was significantly negatively correlated with alcohol intake at both concentrations in P rats only (p’s<0.05). Time spent at the alcohol cued sipper was significantly negatively correlated with latency to the alcohol cued sipper at both concentrations in both rat populations (p’s<0.001).
TABLE 2.
Mean TAS/LTS
| TAS (10%) | LTS (10%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | WISTAR | P | WISTAR | |||||||||
| Day | ||||||||||||
| Mean | ±SEM | N | Mean | ±SEM | N | Mean | ±SEM | N | Mean | ±SEM | N | |
| 1 | 2.72 | 0.41 | 9 | 3.53 | 0.21 | 9 | 8.79 | 0.44 | 9 | 7.30 | 0.25 | 9 |
| 2 | 3.57 | 0.49 | 9 | 3.42 | 0.30 | 9 | 8.34 | 0.46 | 9 | 7.87 | 0.42 | 9 |
| 3 | 4.39 | 0.44 | 9 | 4.35 | 0.48 | 9 | 7.56 | 0.43 | 9 | 7.19 | 0.46 | 9 |
| 4 | 4.37 | 0.37 | 9 | 3.36 | 0.45 | 9 | 7.72 | 0.32 | 9 | 8.18 | 0.48 | 9 |
| 5 | 5.34 | 0.27 | 9 | 3.68 | 0.35 | 9 | 6.75 | 0.25 | 9 | 7.96 | 0.35 | 9 |
| 6 | 5.08 | 0.33 | 9 | 4.61 | 0.50 | 9 | 7.13 | 0.32 | 9 | 6.94 | 0.43 | 9 |
| 7 | 4.90 | 0.35 | 9 | 4.22 | 0.43 | 9 | 7.24 | 0.32 | 9 | 7.02 | 0.40 | 9 |
| 8 | 5.48 | 0.25 | 9 | 4.12 | 0.50 | 9 | 6.64 | 0.25 | 9 | 7.58 | 0.45 | 9 |
| 9 | 5.28 | 0.26 | 9 | 4.30 | 0.36 | 9 | 6.69 | 0.29 | 9 | 7.63 | 0.32 | 9 |
| 10 | 5.23 | 0.21 | 9 | 4.67 | 0.49 | 9 | 6.68 | 0.27 | 9 | 7.23 | 0.47 | 9 |
| 11 | 5.65 | 0.42 | 9 | 4.69 | 0.39 | 9 | 6.42 | 0.34 | 9 | 7.09 | 0.38 | 9 |
| 12 | 5.66 * | 0.36 | 9 | 3.68 | 0.53 | 9 | 6.45 | 0.38 | 9 | 8.06 | 0.55 | 9 |
| 13 | 6.06 * | 0.39 | 9 | 3.89 | 0.36 | 9 | 5.96 * | 0.38 | 9 | 7.98 | 0.39 | 9 |
| 14 | 5.18 * | 0.41 | 9 | 3.35 | 0.60 | 9 | 6.66 * | 0.37 | 9 | 8.36 | 0.58 | 9 |
| 15 | 5.59 * | 0.21 | 9 | 3.36 | 0.31 | 9 | 6.45 * | 0.20 | 9 | 8.41 | 0.29 | 9 |
| 16 | 4.82 | 0.25 | 9 | 4.42 | 0.46 | 9 | 6.18 | 0.29 | 9 | 6.82 † | 0.38 | 9 |
| 17 | 3.76 † | 0.37 | 9 | 2.69 | 0.53 | 9 | 7.27 | 0.38 | 9 | 8.78 | 0.49 | 9 |
| 18 | 3.42 † | 0.37 | 9 | 2.55 | 0.54 | 9 | 7.56 | 0.39 | 9 | 9.21 | 0.57 | 9 |
| 19 | 3.15 † | 0.32 | 9 | 2.61 | 0.38 | 9 | 8.13 † | 0.35 | 9 | 9.09 | 0.37 | 9 |
| 20 | 3.10 † | 0.38 | 9 | 2.82 | 0.31 | 9 | 8.33 † | 0.45 | 9 | 8.73 | 0.30 | 9 |
| TAS(20%) | LTS (20%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | WISTAR | P | WISTAR | |||||||||
| Day | ||||||||||||
| Mean | ±SEM | N | Mean | ±SEM | N | Mean | ±SEM | N | Mean | ±SEM | N | |
| 1 | 3.75 | 0.45 | 9 | 3.11 | 0.27 | 9 | 7.10 | 0.48 | 9 | 7.34 | 0.30 | 9 |
| 2 | 4.20 | 0.44 | 9 | 4.02 | 0.36 | 9 | 7.21 | 0.40 | 9 | 6.71 | 0.34 | 9 |
| 3 | 4.90 | 0.28 | 9 | 4.97 | 0.54 | 9 | 6.80 | 0.26 | 9 | 6.24 | 0.37 | 9 |
| 4 | 5.20 | 0.25 | 9 | 4.67 | 0.37 | 9 | 6.39 | 0.23 | 9 | 6.66 | 0.33 | 9 |
| 5 | 4.30 | 0.18 | 9 | 4.15 | 0.26 | 9 | 7.35 | 0.20 | 9 | 7.39 | 0.23 | 9 |
| 6 | 5.13 | 0.43 | 9 | 4.62 | 0.30 | 9 | 6.34 | 0.51 | 9 | 6.78 | 0.18 | 9 |
| 7 | 4.57 | 0.31 | 9 | 3.97 | 0.47 | 9 | 6.94 | 0.35 | 9 | 7.72 | 0.45 | 9 |
| 8 | 4.38 | 0.34 | 9 | 4.24 | 0.41 | 9 | 6.97 | 0.38 | 9 | 6.94 | 0.39 | 9 |
| 9 | 5.13 | 0.30 | 9 | 4.05 | 0.39 | 9 | 6.37 | 0.29 | 9 | 7.10 | 0.45 | 9 |
| 10 | 3.91 | 0.29 | 9 | 3.94 | 0.36 | 9 | 7.61 | 0.35 | 9 | 7.61 | 0.29 | 9 |
| 11 | 4.51 | 0.49 | 9 | 4.52 | 0.29 | 9 | 6.97 | 0.44 | 9 | 6.45 | 0.21 | 9 |
| 12 | 4.27 | 0.49 | 9 | 4.32 | 0.37 | 9 | 7.00 | 0.48 | 9 | 6.92 | 0.27 | 9 |
| 13 | 4.59 | 0.37 | 9 | 3.62 | 0.38 | 9 | 6.87 | 0.45 | 9 | 7.60 | 0.25 | 9 |
| 14 | 4.48 | 0.29 | 9 | 3.91 | 0.48 | 9 | 6.93 | 0.28 | 9 | 7.07 | 0.38 | 9 |
| 15 | 4.27 | 0.45 | 9 | 3.73 | 0.38 | 9 | 7.08 | 0.48 | 9 | 7.50 | 0.28 | 9 |
| 16 | 4.80 | 0.35 | 9 | 3.85 | 0.27 | 9 | 6.03 | 0.35 | 9 | 6.43 | 0.19 | 9 |
| 17 | 5.15 | 0.47 | 9 | 3.73 | 0.39 | 9 | 5.86 | 0.35 | 9 | 7.06 | 0.29 | 9 |
| 18 | 4.22 | 0.44 | 9 | 3.33 | 0.39 | 9 | 6.77 | 0.38 | 9 | 7.07 | 0.28 | 9 |
| 19 | 3.67 | 0.30 | 9 | 3.05 | 0.33 | 9 | 6.80 | 0.36 | 9 | 7.98 | 0.29 | 9 |
| 20 | 3.25 | 0.27 | 9 | 2.98 | 0.30 | 9 | 7.48 | 0.25 | 9 | 7.89 | 0.36 | 9 |
’s indicate significant between-group differences (p<.05; Sidak)
’s indicate significant within-group differences from day 15 (p<.05; Dunnets)
TABLE 3.
Relationship between Seeking Behaviors (TAS/LTS) and Alcohol Intake
| Ethanol % | Behavior | Line | r | p | df | |
|---|---|---|---|---|---|---|
| 10% | TAS vs. Intake | P | 0.98 | <.0001 | 13 | |
| Wistar | 0. 87 | <. 0001 | 13 | |||
| LTS vs. Intake | P | −0. 95 | <. 0001 | 13 | ||
| Wistar | −0.59 | <.05 | 13 | |||
| TAS vs. LTS | P | −0.99 | <.0001 | 13 | ||
| Wistar | −0.83 | <.0001 | 13 | |||
| 20% | TAS vs. Intake | P | 0.75 | <.01 | 13 | |
| Wistar | 0. 87 | <. 0001 | 13 | |||
| LTS vs. Intake | P | −0.54 | <.05 | 13 | ||
| Wistar | −0.48 | >05 | 13 | |||
| TAS vs. LTS | P | −0.89 | <.0001 | 13 | ||
| Wistar | −0.74 | <.001 | 13 | |||
Blood Alcohol Concentrations (BACs): Behavior only cohort
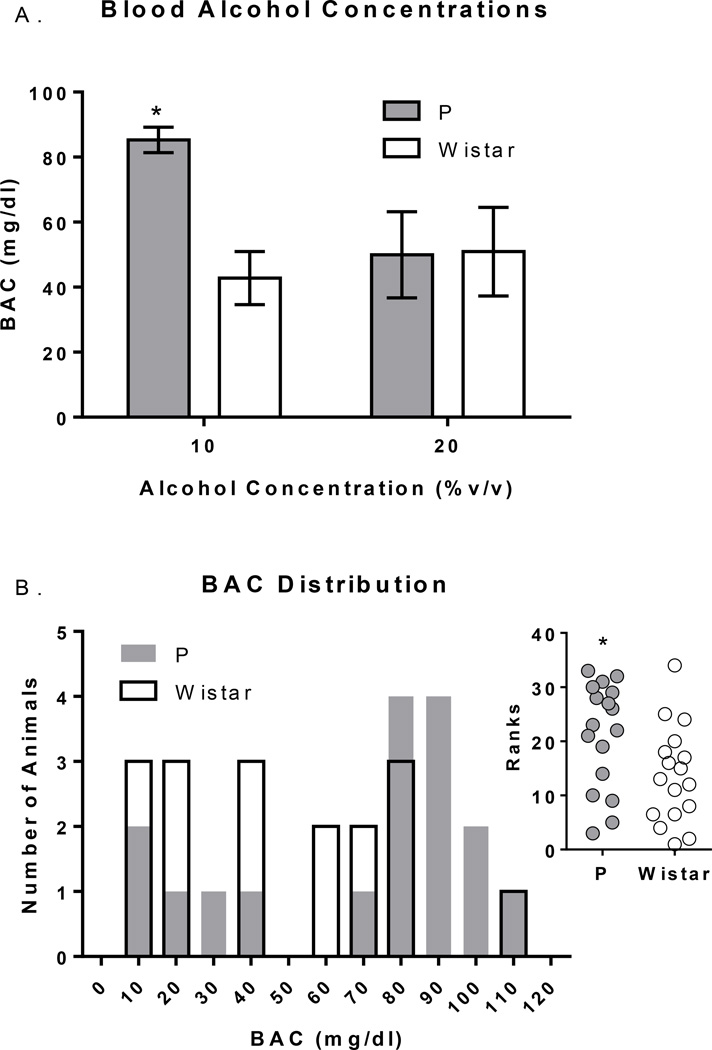
Mean BACs immediately following 2CAP were higher in P vs. Wistar rats at the 10% alcohol concentration (p<.05), while no differences in BACs were observed between rat populations at the 20% alcohol concentration (Figure 2). Notably, 4/17 Wistar and 11/17 P rats had ‘binge-like’ BACs between 80–120 mg/dl (Figure 2B), and P rats had significantly greater BACs than Wistar rats overall [U(32)=80;p<0.05;Figure 2B inset]. Additional/detailed results of these analyses can be found the supplementary text.
Figure 2.
Blood alcohol concentrations (BACs) following 2CAP. A) BACs were higher in P vs. Wistar rats at the 10% alcohol concentration, but no different at the 20% alcohol concentration. B) The distribution of BACs in P and Wistar rats. *’s indicate between rat population group differences; p’s<.05).
Alterations in Seeking vs. Intake: Behavior only cohort
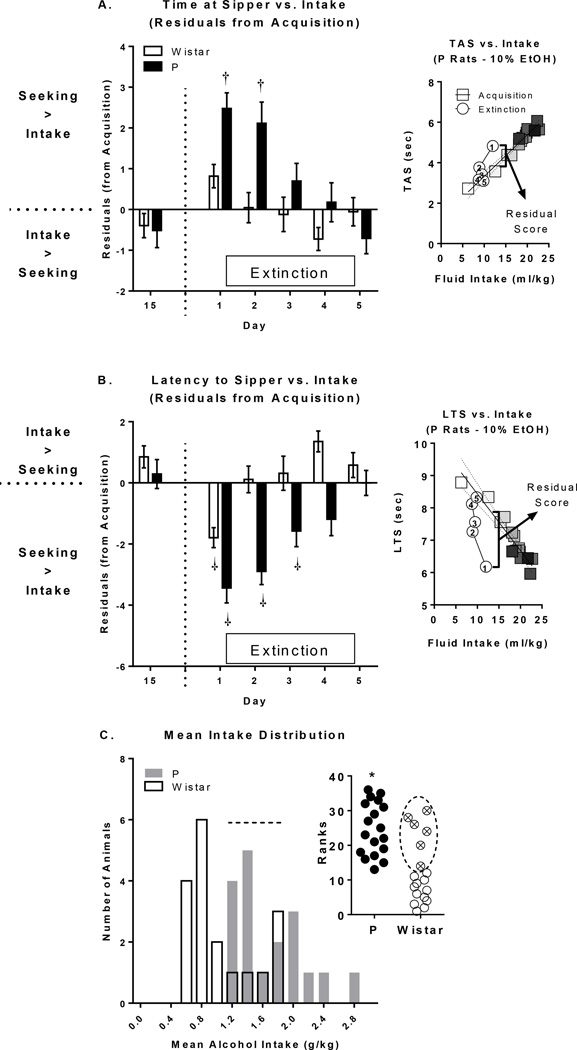
To directly evaluate if the relationship between seeking and drinking behaviors, when compared with acquisition, was altered during extinction, standardized residual scores were computed for each animal on each day. Standardized residuals were computed as the prediction error from the regression of both TAS (Figure 3A) or LTS (Figure 3B) on fluid intake (see Figure 3 right insets for examples). There were significant main effects of day for both TAS [F(19,646)=4.78;p<0.0001] and LTS [F(19,646)=6.93;p<0.0001]. There were also significant day*rat population interactions (TAS [F(19,646)=2.50;p<0.001]; LTS [F(19,646)=3.77;p<0.0001]), driven by greater differences on extinction days in P rats vs. Wistar rats. Dunnett’s post-hoc tests confirmed these rat population effects (see Figure 3). The direction of these effects suggest a dissociation of seeking behavior vs. intake behavior, with greater seeking vs. intake on extinction days, which were larger in magnitude and duration (days) in P rats. Mean alcohol intake (g/kg) was not normally distributed in Wistar rats (K-S;D(17)=0.2263;p<0.01; Figure 3C), so rat population differences in mean alcohol intake were evaluated using a Mann-Whitney test. P rats had significantly greater mean alcohol intake than Wistar rats [U(34)=49;p<0.001;Figure 3C inset], although there was a subset of Wistar rats whose mean alcohol intake fell within the distribution of P rats. Importantly, residual score differences could not be solely explained by alcohol exposure/experience (See Suppl. Text).
Figure 3.
Alterations in the relationship between fluid intake and time at sipper (TAS) and latency to sipper (LTS). A-B) The relationship between TAS/LTS and fluid consumption that was established during acquisition broke down during extinction in P rats for both TAS/LTS measures and for LTS only in Wistar rats. Furthermore, this effect was larger in magnitude and duration in P rats compared to Wistar rats. Insets to right of Panels A and B serve as representative examples of how residuals scores were determined. Square symbols reflect alcohol days 1–15 and are increasingly shaded from white (Day 1) to black (Day 15). Circles reflect each of the 5 days of extinction and are numbered accordingly. Regression lines and associated confidence intervals for alcohol days are shown. C) The distribution of mean 2CAP alcohol intake was significantly different between P and Wistar rats (inset). However, there were 6 Wistar rats that had mean alcohol intake that fit within the P rat mean alcohol intake distribution; these Wistar animals are highlighted by dotted lines. †’s indicate within-rat population differences from day 15 (p’s <.05). *’s indicate between rat population group differences; p’s<.05).
Electrophysiological Activity within the PFC during 2CAP
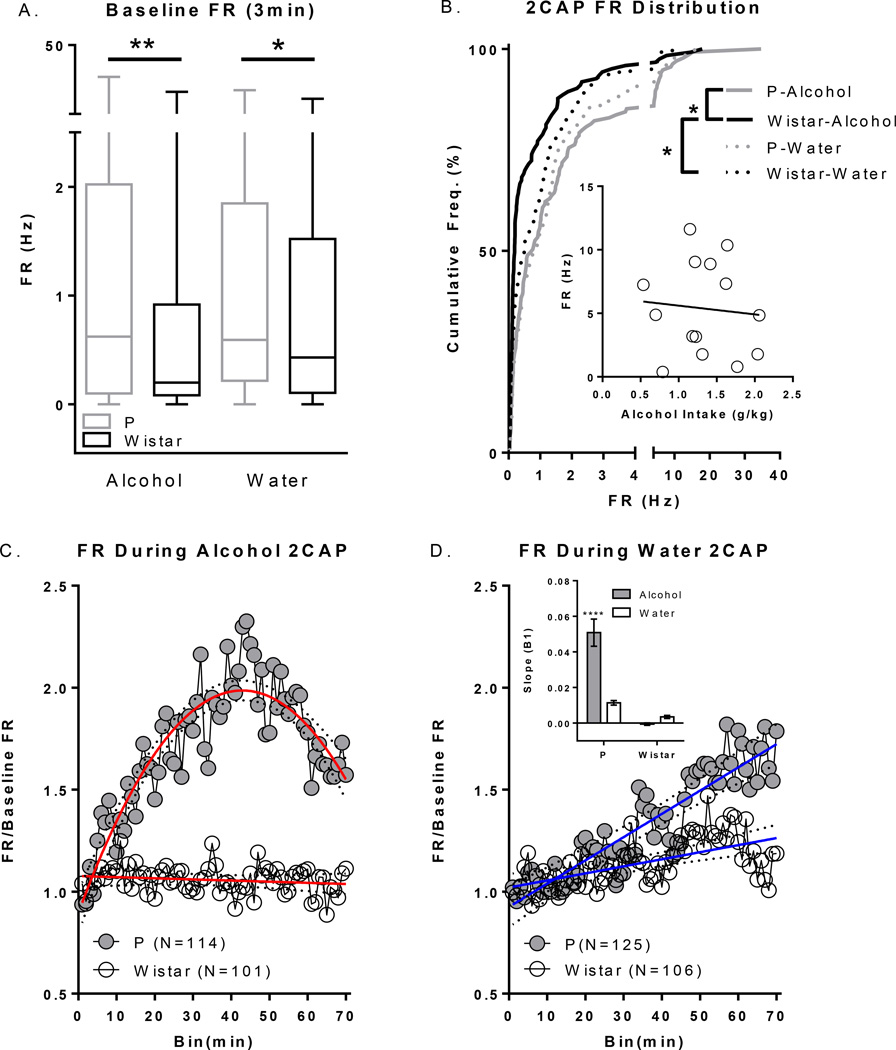
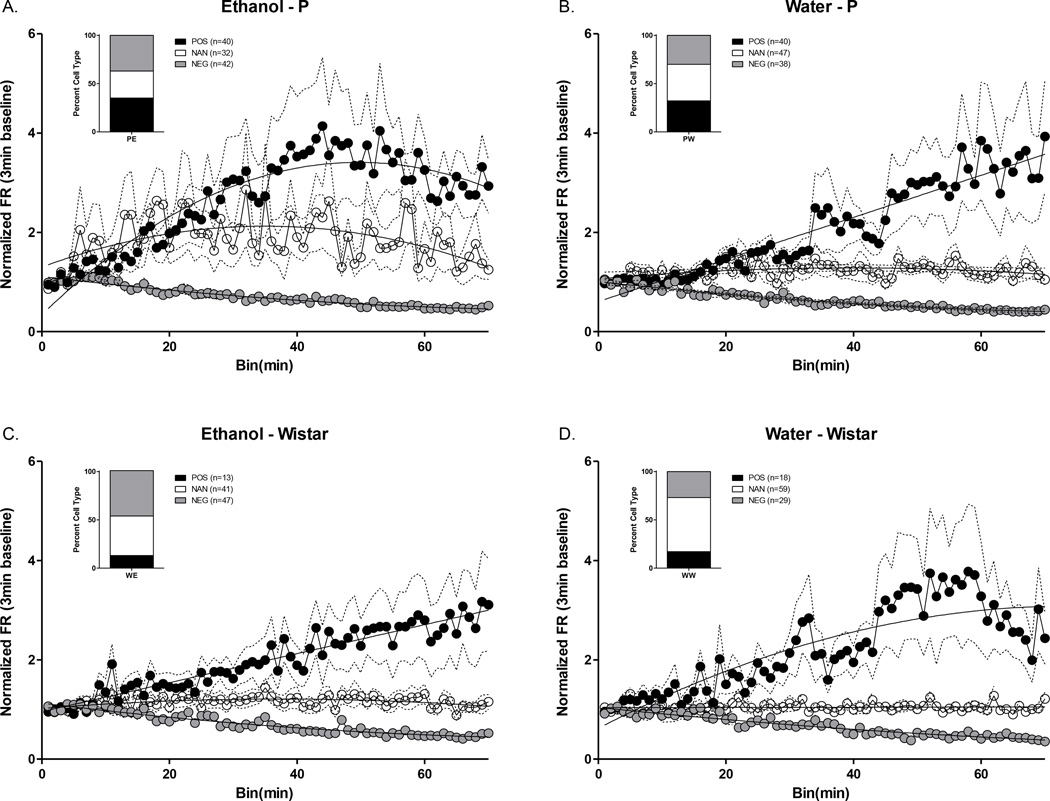
Wistar rats used for these electrophysiology experiments were selected such that they drank alcohol at comparable levels to P rats to minimize the potential confound of differences in alcohol history and/or intake when interpreting electrophysiology data. This allowed us to test the hypothesis that heavy drinkers with a family history of high alcohol consumption would display differences in neural activity compared to heavy drinkers with no family history, and is comparable in this respect to Kareken et al., (2010). There were no differences in intake between P or Wistar groups for IAP (data not shown) or the acquisition of alcohol 2CAP (see suppl Figure 2). Baseline FR and FR over the course of the entire 2CAP session are presented in Figure 4. Baseline FR data were not normally distributed, even after transformation, so non-transformed data were analyzed using a non-parametric K-S test for each fluid type separately. The distribution of baseline P rat FR was significantly higher than that of Wistars for both water (K-S;D(69)=0.1761;p<0.05) and alcohol (K-S;D(69)=0.2250;p<0.01) sessions (Figure 4A). To evaluate changes in FR over the course of the entire 2CAP session(s), FR for each neuron was normalized to its 3 minute baseline FR. There were significant differences between the cumulative distributions constructed from the mean FR of all recorded neurons from alcohol consuming P and alcohol consuming Wistar rats (K-S;D(69)=0.3304;p=9.8107e-7), as well as between alcohol and water consuming days in Wistars (K-S;D(69)=0.2326;p<0.01; Figure 4B). There were no detectable differences in the cumulative distribution of mean firing rate between water and alcohol drinking P rats. In addition, no detectable differences were observed between P’s or Wistars on water days. These findings were additionally confirmed by Mann Whitney tests: P Alcohol vs. Wistar Alcohol [U(256)=5423;p<0.0001]; Wistar Alcohol vs. Wistar Water [U(243)=6093;p=0.01]. No systematic relationship was detected between alcohol intake and mean FR during 2CAP (Figure 4B inset).
Figure 4.
Baseline and 2CAP FR. A) Baseline FR in the PFC was higher in P vs. Wistar rats prior to alcohol and water sessions. B) There were significant differences in cumulative frequency distributions of mean FR in P Alcohol vs. Wistar Alcohol groups and in Wistar Alcohol vs. Wistar Water days during 2CAP. Inset depicts non-significant association between amount of alcohol consumed and mean FR throughout the task across all cells from a given animal. C) FR over the course of 2CAP on alcohol days increased significantly in P rats but did not change in Wistar rats. D) FR over the course of 2CAP water days increased more in P rats vs. Wistar rats.*’s in A and B indicate group differences noted by bars (*p’s<.05;**p<.01). *’s in D inset indicates differences from all other groups (***p<.001). Dotted lines in C+D represent 95% confidence intervals of regression.
Evaluation of the time course of changes in FR during 2CAP indicated that all groups were best fit to 1st order polynomials (linear; p’s >.05) with the exception of the P Alcohol group which was best fit to a 2nd order polynomial [F(1,67)=162.6;p<.0001; Figure 4C+D]. Regression analyses indicated that all groups had significant changes in FR over time with the exception of the Wistar Alcohol group [P-Alcohol: Adj. R2(1,67)=0.80;p<.0001; P Water: R2(1,68)=0.83;p<.0001; Wistar Alcohol: R2(1,68)=0.03;p=0.16; Wistar Water: R2(1,68)=0.35;p<0.0001]. We then evaluated if the rate of change in FR was different between groups by comparing the initial slopes (β1) of each regression equation. There was a significant interaction between fluid type and rat population which was driven by a higher mean slope in alcohol consuming P rats compared to all other groups (p’s<.0001; Figure 4D inset). Thus, alcohol availability/experience during 2CAP was found to produce robust differences in FR in P rats. This was not the case for Wistar rats, which showed no significant alterations in FR during 2CAP sessions in which alcohol was made available.
Approximately 60% of all recorded cells demonstrated significant changes (i.e. increases or decreases) in FR across the entire 2CAP session (Figure 5). A chi-squared test was performed to determine if there was a relationship between rat population and number of neurons with significant changes in FR. A larger proportion of cells changed FR in P rats compared to Wistar rats (χ2(N=446,df=1)=10.74;p=0.001). Chi-squared tests were then performed to see if there was a relationship between rat population and number of neurons with significant positive, non-significant, and significant negative changes in FR. The proportion of cells that displayed increases, decreases, or no significant change in FR over 2CAP sessions were significantly different between rat populations within a fluid type (P Alcohol vs. Wistar Alcohol: χ2(N=215,df=2)=14.41;p<0.001 – P Water vs. Wistar Water : χ2(N=231,df=2)=9.41;p<0.01), and within Wistar rats between fluid types (Wistar Alcohol vs. Wistar Water: χ2(N=207,df=2)=8.194;p<0.01). There were no differences in the proportion of cell FR changes within P rats between fluid types (P Alcohol vs. P Water: χ2 (N=239,df=2)=2.55;p>.05).
Figure 5.
Classification of the percentage of neurons that changed FR. The proportion of cells that displayed increases, decreases, or no change in FR was significantly different between alcohol P and alcohol Wistar (A. vs C. p<.001), water P and water Wistar (B. vs D. p<.01), and water and alcohol Wistar (C. vs D. p<.05). Dotted lines represent standard error of the mean (±SEM).
Discussion
This study finds differences during the acquisition and extinction of cue-evoked alcohol seeking behaviors, whereby acquisition of this behavior is faster in P than Wistar rats. Importantly, the seeking behaviors observed during extinction were dramatically accentuated when controlling for the amount of fluid actually consumed. These data suggest that a genetic vulnerability for increased alcohol preference/consumption leads to a greater and more persistent ‘imbalance’ between seeking and intake when learned cue/alcohol associations are violated. Moreover, these data indicate that while the selection process does not explicitly target seeking behaviors, it seems that they are prevalent and may be critical for the excessive drinking phenotype (Czachowski and Samson, 2002).
This study also finds basal and 2CAP-associated differences in electrophysiological activity within the PFC, whereby P rats exhibited increased FR’s during baseline as well as differences in FR over the course of 2CAP sessions. These data demonstrate that alcohol consumption and, possibly, exposure to alcohol-paired contextual cues affects the firing properties of PFC neurons in these two rat populations differently, despite similar alcohol and alcohol cue exposure.
P rats consumed more alcohol and were slower to extinguish than Wistars
At both the 10% and 20% alcohol concentrations, P rats consumed more alcohol than Wistar rats, and displayed greater increases in intake over 2CAP sessions. However, although alcohol intake was lower in Wistar rats than P rats, Wistars did drink sufficient alcohol in this task to reach pharmacologically relevant BACs; several (4/17) even reached binge-like BACs (≥80 mg/dl).
The observation that TAS and LTS measures were associated with alcohol intake was not surprising given that proximity to the sipper was required to gain access to the alcohol solution (Table 2). While the relationship between intake and TAS/LTS was consistent over the course of the 15 alcohol 2CAP sessions in both P and Wistar rats, this relationship broke down in extinction where water was substituted for alcohol. During extinction, fluid consumption was lower than predicted when the relative amount of seeking (measured by TAS/LTS) was considered. Simply put, this likely reflects the animals continued exploration (i.e. sniffing, licking, and pawing) of the area around the sipper during extinction whereas during acquisition this time would have likely been spent drinking. Interestingly, however, this effect was generally of greater magnitude and duration in P rats, which we interpret as P rats displaying greater and more persistent alcohol seeking behavior.
It is possible that the greater and more pronounced dissociation between seeking and intake in P rats was a direct result of sign-tracking (Brown and Jenkins, 1968) vs. explicit alcohol seeking behavior. If P rats were more likely to engage in sign-tracking of the light cue than Wistars, our conclusions related to alcohol seeking might be confounded by this behavioral difference. In other words, sign-tracking behavior might lead P rats to the area where the fluid is subsequently made available, but the reason for spending time in this area is not directly related to the goal of finding alcohol per se. However, since the cue light and sipper are in slightly different locations, it might be expected that sign-tracking behavior would interfere to some degree with goal-directed intake. The significant and strong relationship between TAS/LTS and alcohol intake we observed argue against this possibility. However, while we cannot rule out sign-tracking as an explanation that P rats continue to approach the CS+, the propensity to engage in sign tracking behaviors is linked with the expression addiction-associated behaviors (Tomie et al., 2008, Tomie and Sharma, 2013). Whether faster acquisition of 2CAP and dissociation between behavior and intake in the P rat is attributable to persistent alcohol seeking or sign tracking (or some combination of both), each of these imply that the excessive drinking phenotype may be attributable to a heritable perturbation in behavioral control.
Firing rate of PFC neurons is higher in P vs. Wistar rats
These studies are the first to evaluate the firing rate of PFC neurons in P and Wistar rats during voluntary alcohol consumption. Robust differences in neural firing were observed between P and Wistar rats, both prior to and during alcohol access, in the absence of significant behavioral differences.
The increases in FR observed during the baseline period prior to fluid consumption may reflect innate differences in the basal firing properties of P rats. Indeed, P rats have been shown to exhibit lower basal levels of dopamine (Engleman et al., 2006), and higher glucose metabolism (Smith et al., 2001) in the PFC compared to Wistar rats and Non-preferring ‘NP’ rats, respectively.
Alternatively, increased firing rate of PFC neurons might be related to differences in the perceived incentive motivational properties associated with the alcohol-paired environment between rat populations. Contextual cues associated with the effects of alcohol have been shown to increase immediate early gene expression in the PFC of rats (Dayas et al., 2007) and mice (Groblewski et al., 2012). Considering the link between neural firing and immediate early gene expression (Sagar et al., 1988) it is tempting to speculate that the increases in neural activity observed in this study might be related to the expression of these genes. While the changes in PFC firing and immediate early gene expression might reflect the expectation of receiving alcohol, we did not detect any differences in the amount of time spent in proximity to the sipper ports between P and Wistar rats prior to the start of the task, nor did we observe any significant relationships between FR during this period and subsequent intake. Nevertheless, the motivational state of the animals may not manifest itself as seeking during the baseline period or amount of alcohol consumed after this time period, particularly in animals that have had previous training sessions in which alcohol access was never available during this period and the duration of access remained constant following this period. While these data argue against the existence of a linear relationship between FR in PFC neurons and the encoding of environmental cues associated with alcohol, it is possible, even likely, that this information is encoded via dynamic interactions across ensembles of neurons in the PFC (Hyman et al., 2012).
P rats displayed a robust increase in FR over time during alcohol access whereas Wistar displayed no overall changes in FR. In addition, a larger proportion of neurons increased their firing rate on alcohol-reinforced days (relative to baseline levels) in P rats compared to Wistars (Figure 5). When comparing water and alcohol reinforced sessions in P rats, a similar proportion of neurons increased their firing rate. However, the increases in FR occurred faster and were more pronounced in P rats on alcohol days, but ultimately returned to the approximate level of FR observed on water days by the end of the sessions (Figure 4C–D; Figure 5A–B). This increase in FR relative to water may reflect the pharmacological effects of alcohol on neural firing as, on water trials, these animals were still engaged in seeking and drinking behaviors. However, there was no evidence that FR systematically changed linearly with the amount of alcohol consumed (Figure 4B, inset), and there were no significant differences in mean alcohol intake between rat populations. These data argue against a linear relationship between alcohol dose and FR, and might suggest that our observed differences in the rate of FR throughout 2CAP were the result of differences between rat populations in alcohol-induced PFC neuroadaptations resulting from many previous experiences with alcohol.
There are several studies that have reported dose-dependent decreases in the FR of cortical/hippocampal neurons in awake behaving animals following experimenter administered alcohol (Ludvig et al., 1995, Matthews et al., 1996, White and Best, 2000). However, examination of these data indicate that measures of neuronal FR seem to be heterogeneous and critically influenced by procedural aspects of a given study. For example, does-dependent decreases in FR in hippocampal place cells were found only within place fields; there were no significant alterations in FR outside of place fields in response to alcohol (White and Best, 2000). Additionally, decreases in FR observed after a first infusion of alcohol were absent following a second administration within the same session (Ludvig et al., 1995); an indication of acute functional tolerance to alcohol-induced FR alterations as might be expected in animals receiving many alcohol bouts over the course of the current studies. Furthermore, other studies have found that alcohol has a more heterogeneous effect on FR, such as transient increases in FR within the cortex followed by sustained decreases (Klemm et al., 1976), or complicated interactions between behavior, alcohol, and FR within the PFC (Alexandrov et al., 1991).
The time course of alcohol consumption could also be responsible for the faster increases in FR over time observed in P rats. If this were the case, it would be expected that P rats consume more alcohol in the earlier 2CAP trials than Wistars. While consumption could not be measured on a trial-by-trial basis, time at sipper (which is highly correlated with consumption) was not different across trials between rat populations in the electrophysiology animals. However, since both rat populations of electrophysiology animals drank consistently throughout 2CAP, it is important to consider that brain alcohol concentrations likely continued to increase throughout the recording. This might explain why a non-linear (e.g inverted-U-like) like function was observed in the time course of FR’s in P rats and not the Wistars: If an inverted-U-like relationship exists between brain alcohol concentration and FR, perhaps this function is left-shifted in P rats. Considering this, during the later trials, brain alcohol concentration may reach sufficient levels that the effects of alcohol on FR transition from excitatory to inhibitory (Tu et al., 2007, Klemm et al., 1976) in the P rat. Increased sensitivity of neurons in the PFC of P rats to alcohol may be a heritable abnormality that is important for the expression of the excessive drinking phenotype. In the current study Wistar and P rats were matched for alcohol consumption history, and thus previous alcohol exposure does not provide explanation of the observed changes in neural firing across rat populations. Identifying the heritable factors leading to this physiological difference might provide insight into the genetic or epigenetic basis of excessive seeking and drinking phenotypes.
Supplementary Material
Acknowledgments
This work was supported by NIAAA grant #’s AA007611 (CCL), and AA022268 (DNL), the ABMRF (CCL), and the Indiana Alcohol Research Center P60AA007611 (D. Crabb).
References
- Alexandrov YI, Grinchenko YV, Laukka S, Jarvilehto T, Maz VN. Acute effects of alcohol on unit activity in the motor cortex of freely moving rabbits: comparison with the limbic cortex. Acta Physiol Scand. 1991;142:429–435. doi: 10.1111/j.1748-1716.1991.tb09177.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, Mcbride WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol. 2014;48:225–234. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon's key-peck. J Exp Anal Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catafau AM, Etcheberrigaray A, Perez De Los Cobos J, Estorch M, Guardia J, Flotats A, Berna L, Mari C, Casas M, Carrio I. Regional cerebral blood flow changes in chronic alcoholic patients induced by naltrexone challenge during detoxification. J Nucl Med. 1999;40:19–24. [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcohol Clin Exp Res. 2002;26:1653–1661. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckel AW, Shoemaker WJ, Arky L. Dorsal lesions of the prefrontal cortex: effects on alcohol consumption and subcortical monoaminergic systems. Brain Res. 1996;723:70–76. doi: 10.1016/0006-8993(96)00219-3. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Murphy JM, Waller MB, Mcbride WJ, Lumeng L, Li TK. Chronic ethanol tolerance through free-choice drinking in the P line of alcohol-preferring rats. Pharmacol Biochem Behav. 1987;28:111–115. doi: 10.1016/0091-3057(87)90021-9. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Ryabinin AE, Cunningham CL. Activation and role of the medial prefrontal cortex (mPFC) in extinction of ethanol-induced associative learning in mice. Neurobiol Learn Mem. 2012;97:37–46. doi: 10.1016/j.nlm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2012;109:5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O’;Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm WR, Mallari CG, Dreyfus LR, Fiske JC, Forney E, Mikeska JA. Ethanol-induced regional and dose-response differences in multiple-unit activity in rabbits. Psychopharmacology (Berl) 1976;49:235–244. doi: 10.1007/BF00426822. [DOI] [PubMed] [Google Scholar]
- Ludvig N, Altura BT, Fox SE, Altura BM. The suppressant effect of ethanol, delivered via intrahippocampal microdialysis, on the firing of local pyramidal cells in freely behaving rats. Alcohol. 1995;12:417–421. doi: 10.1016/0741-8329(95)00012-g. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Li TK. The development of metabolic tolerance in the alcohol-preferring P rats: comparison of forced and free-choice drinking of ethanol. Pharmacol Biochem Behav. 1986;25:1013–1020. doi: 10.1016/0091-3057(86)90079-1. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Simson PE, Best PJ. Ethanol alters spatial processing of hippocampal place cells: a mechanism for impaired navigation when intoxicated. Alcohol Clin Exp Res. 1996;20:404–407. doi: 10.1111/j.1530-0277.1996.tb01660.x. [DOI] [PubMed] [Google Scholar]
- Mcbride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Mcbride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats--animal models of alcoholism. Alcohol. 2014;48:209–215. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccane AM, Czachowski CL, Lapish CC. Tolcapone suppresses ethanol intake in alcohol-preferring rats performing a novel cued access protocol. Alcohol Clin Exp Res. 2014;38:2468–2478. doi: 10.1111/acer.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. J Stud Alcohol. 1993;54:369–376. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Segovia KN, Vontell R, Lopez-Cruz L, Salamone JD, Correa M. c-Fos immunoreactivity in prefrontal, basal ganglia and limbic areas of the rat brain after central and peripheral administration of ethanol and its metabolite acetaldehyde. Front Behav Neurosci. 2013;7:48. doi: 10.3389/fnbeh.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, BARTLETT SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Learn JE, Mcbride WJ, Lumeng L, Li TK, Murphy JM. Alcohol-naive alcohol-preferring (P) rats exhibit higher local cerebral glucose utilization than alcohol-nonpreferring (NP) and Wistar rats. Alcohol Clin Exp Res. 2001;25:1309–1316. [PubMed] [Google Scholar]
- Stewart RB, Mcbride WJ, Lumeng L, Li TK, Murphy JM. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology (Berl) 1991;105:530–534. doi: 10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M, Hallikainen T. Acute ethanol-induced changes in cerebral blood flow. Am J Psychiatry. 1994;151:1505–1508. doi: 10.1176/ajp.151.10.1505. [DOI] [PubMed] [Google Scholar]
- Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, Smith TL, Schuckit MA. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res. 2011;35:1034–1040. doi: 10.1111/j.1530-0277.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Sharma N. Pavlovian sign-tracking model of alcohol abuse. Curr Drug Abuse Rev. 2013;6:201–219. doi: 10.2174/18744737113069990023. [DOI] [PubMed] [Google Scholar]
- Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ, Woodward JJ. Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci. 2007;27:4765–4775. doi: 10.1523/JNEUROSCI.5378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Waller MB, Mcbride WJ, Lumeng L, Li TK. Induction of dependence on ethanol by free-choice drinking in alcohol-preferring rats. Pharmacol Biochem Behav. 1982;16:501–507. doi: 10.1016/0091-3057(82)90459-2. [DOI] [PubMed] [Google Scholar]
- White AM, Best PJ. Effects of ethanol on hippocampal place-cell and interneuron activity. Brain Res. 2000;876:154–165. doi: 10.1016/s0006-8993(00)02629-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.