Abstract
Background
Successful eradication of Helicobacter pylori is becoming more difficult, mainly due to emerging antibiotic resistance. Treatment regimens containing bismuth have increased efficacy, but the mechanism is unknown. H. pylori is a neutralophile adapted to survive the acidic gastric environment via acid acclimation, but demonstrates more robust growth at neutral pH. Many antibiotics used to treat H. pylori rely on bacterial growth.
Aim
To investigate the mechanism of increased efficacy of bismuth-containing H. pylori treatment regimens.
Methods
RNAseq and qPCR, urease activity in permeabilized and intact bacteria, internal pH, and membrane potential were measured with and without colloidal bismuth subcitrate (CBS). Bacterial survival was assessed with CBS and/or ampicillin.
Results
Genes involved with metabolism and growth were upregulated in the presence of CBS at acidic pH. Urease activity of permeabilized H. pylori at pH 7.4 and 4.5 decreased in the presence of CBS, but intact urease activity only decreased at acidic pH. The fall in cytoplasmic pH with external acidification was diminished by CBS. The increase in membrane potential in response to urea addition at acidic medium pH was unaffected by CBS. The impact of CBS and ampicillin on H. pylori survival was greater than either agent alone.
Conclusions
Bismuth is not acting directly on urease or the urea channel. CBS impedes proton entry into the bacteria, leading to a decrease in the expected fall in cytoplasmic pH. With cytoplasmic pH remaining within range for increased metabolic activity of a neutralophile, the efficacy of growth dependent antibiotics is augmented.
Keywords: Helicobacter pylori, bismuth, cytoplasmic pH
Background
Helicobacter pylori is a highly prevalent gram negative bacterium that infects the gastric mucosa of about 50% of the world’s population. Infection at a minimum causes gastritis and is a prominent etiologic agent of gastric and duodenal ulcer disease and gastric cancer 1. Treatment success using standard regimens has become more difficult in recent years. Standard first line triple therapy, including a proton pump inhibitor (PPI) and two antibiotics, typically clarithromycin and amoxicillin or metronidazole, has seen a drop in efficacy to close to 70%, far below the original goal of 80% 2. Increased antibiotic resistance is considered the most important reason for decreased treatment efficacy, 3. Quadruple therapy regimens containing bismuth are considered to have increased efficacy, and are recommended for first line treatment in areas of high clarithromycin resistance 4. Three recent clinical trials using colloidal bismuth subcitrate (CBS) combined with tetracycline and metronidazole in a single pill formulation, given with a PPI, achieved eradication rates ranging from 88–93% 6–8. Resistance of H. pylori to bismuth has not been reported 9–11. Knowledge of the mechanism of action of bismuth compounds against H. pylori would be beneficial in the development of improved treatment regimens in this era of declining eradication success rates.
Bismuth is a trivalent heavy metal element used in many medical applications 12. This is based on the ability of bismuth to replace other metals such as iron, nickel, and zinc, which have catalytic or structural roles 13. CBS and bismuth subsalicylate (BSS) are commonly used in H. pylori treatment regimens. CBS is water soluble while BSS is water insoluble, and both have low systemic absorption 10. Bismuth absorption is not required for efficacy in H. pylori treatment regimens 14, suggesting a local mechanism of action. Acid suppression further decreases bismuth absorption 10, augmenting the local effect in the gastric lumen as part of H. pylori treatment regimens. An effective bismuth concentration approximating 1 mg/mL has been suggested at standard therapeutic dosing 15. Bismuth compounds also protect the gastric mucosa and aid in ulcer healing 16, 17. Bismuth alone can sometimes clear H. pylori infection, and eradication has been reported on rare occasions with CBS 18–20.
H. pylori is a neutralophile, yet it colonizes an acidic niche in the stomach 21–23. The bacterium is able to survive in acid via a mechanism of acid acclimation, centered around the neutral pH optimum, cytoplasmic urease and the inner membrane localized, proton gated urea channel, UreI 24, 25. At acidic medium pH, the pH of the periplasmic space falls, leading to opening of UreI and movement of urea into the cytoplasm 24, 25. Urease, which is activated at the inner membrane, facilitated by a trafficking response at acidic pH, catalyzes the breakdown of urea into carbonic acid, then carbon dioxide and ammonia 26–28. The two gases diffuse back through the urea channel and through the membrane into the periplasm 27. Carbon dioxide is converted to bicarbonate with the help of α-carbonic anhydrase, an inner membrane protein with a periplasmic catalytic domain, and bicarbonate and ammonia buffer the periplasm into the range of survival for a neutralophile 29. With periplasmic buffering, the cytoplasmic pH is maintained within acceptable levels as well 30.
Neutralophiles survive between pH 4–8 and divide between pH 6–8, 31 and many of the antibiotics used to treat H. pylori are growth dependent. There is increased transcription of growth-dependent genes and higher in vitro efficacy of ampicillin against H. pylori at higher medium pH 32. This suggests a higher rate of bacterial growth and division, allowing for increased antibiotic efficacy. This has been demonstrated in clinical models as well. Dual therapy with amoxicillin and omeprazole is successful in the setting of supratherapeutic omeprazole dosing, with gastric pH maintained at a mean of ≥5.5 and >4 for ≥80% of the time over the course of therapy 33. A more recent study showed a 95% eradication rate in treatment naïve patients and 89% in non-naïve patients using a four times daily high dose PPI and amoxicillin regimen 34. If more of the bacteria are exposed to a higher pH for a greater percentage of time, the resultant increase in bacterial division will lead to increased treatment efficacy, and this degree of acid suppression is not typically seen with recommended dosing of currently available PPIs 32, 35.
The mechanism of action of bismuth compounds against H. pylori is not definitively known. It has been suggested, among other possible mechanisms, that bismuth inhibits H. pylori urease by blocking the active site of the enzyme 36, 37. The results of these in vitro experiments have not been confirmed in vivo, where very minor direct inhibition has been noted 38, 39. This discrepancy is most likely related to the degree of access of bismuth to cytoplasmic urease in the intact bacteria. Bismuth requires transport into the bacterial cytoplasm. Bismuth uptake into H. pylori is limited by the presence of ferric ion, suggesting a competitive uptake process using transporters designed for other metal ions 40. Uptake of bismuth ions from CBS is linear up to 20 µg/mL and does not further increase beyond a concentration of 40 µg/mL, and growth inhibition was also maximized at this concentration 40.
Taking into account that both profound acid inhibition and the addition of bismuth independently increase the efficacy of H. pylori treatment regimens, the aim of the current study was to determine the mechanism of action of bismuth in the treatment of H. pylori infection, with particular attention to the impact of pH on this system. Gene expression using RNAseq and confirmation by qPCR were completed after incubation of H. pylori at acidic pH with and without CBS. Urease activity in permeabilized and intact bacteria, internal pH, membrane potential, and survival were determined at acidic and neutral pH. The results reported here demonstrate that, in the intact bacteria, CBS does not act directly on urease or the urea channel, but rather impedes proton entry into the bacterial cytoplasm. The consequence is that the degree of cytoplasmic acidification in response to medium acidification is diminished, remaining within the range of bacterial growth of a neutralophile. This reduces the dependence on profound acid inhibition for optimal efficacy of growth dependent antibiotics, leading to higher eradication rates.
Materials and Methods
Bacterial strains and culture conditions
H. pylori strain ATCC 43504 was used. Strain 43504 was isolated from the antrum of an Australian patient in 1985 and is CagA and VacA positive 41. Bacteria were grown under microaerobic conditions (5% O2, 10% CO2, 85% N2) either on Trypticase Soy Agar (TSA) plates supplemented with 5% sheep blood (Gibco-BRL, Life Technologies, Carlsbad, CA) or in brain heart infusion (BHI) medium (Difco laboratories, Becton Dickenson, and Co, Franklin Lakes, NJ) supplemented with 7% horse serum (Gibco) and 0.25% yeast extract (Difco). Bacteria in media were grown in the presence of Dent selective supplement (Oxoid Limited, Hampshire, UK).
RNA extraction
H. pylori were incubated in dialysis cassettes (Slide-A-lyzer, MWCO 10 kD, 3 mL capacity, Thermo, Waltham, MA) suspended in a large volume of media at pH 4.5 with urea to maintain constant pH and urea concentrations over an extended period as described previously 32. Bacteria were incubated with or without CBS for 4 hours. The presence of CBS did not alter the pH of the media. RNA was then extracted using a combination of Trizol® reagent (Life Technologies) and the RNAeasy mini kit (Qiagen, Valencia, CA) as described previously 30. Quality of RNA was confirmed using an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA) and was only used if the RNA integrity number was >8.
RNAseq and qPCR
RNAseq analysis was done at the UCLA Clinical Microarray Core within the Jonsson Comprehensive Cancer Center (http://pathology.ucla.edu/body.cfm?id=126). Sequencing was generated using the Illumina HiSeq 2000 machine (Illumina Inc., San Diego, CA) with a read length of 1×50 and depth of coverage of 15 million reads. Statistical analysis was done with the assistance of the core director.
For qPCR experiments, 3 distinct RNA preparations from H. pylori incubated at pH 4.5 with or without CBS for 4 hours were completed. Immediately following incubation and RNA extraction as above, cDNA was created from 6 µg RNA using Omniscript® reverse transcriptase (Qiagen). qPCR was then completed in triplicate for each sample and each primer pair, using a Bio-Rad iCycler. Primer design was aided by the Primer3 software available at biotools.umassmed.edu 42, 43. Unique primers were designed for 100–300 base pair regions of genes of interest that changed in the RNAseq analysis. Primer sequences are shown in table 1. Standard PCR performed with genomic DNA from H. pylori strain ATCC 43504 as the template was used to assure that all primer pairs resulted in amplification of a single product (data not shown). H. pylori genomic DNA was diluted serially by 10 fold to produce the standard curve for the qPCR reaction. qPCR was completed using 20 µL reactions in a 96 well plate with SYBR Green (SsoFast EvaGreen® Supermix, Bio-Rad, Irvine, CA), conditions were 92° for three minutes followed by 40 cycles of 92° for 30 seconds, 55° for 30 seconds, 72° for 40 seconds. Data were collected during the extension step and mean starting quantity was calculated using the Bio-Rad software. A melting curve was used at the end of the run to confirm that there was only one peak and only one product for each primer.
Table 1.
Primers used for qPCR*
| atpF (HP0623)-5’ | atatcccgcatgatccaaaa |
| atpF (HP0623)-3’ | gccgtgatactgctctttcc |
| murC (HP0748)-5’ | ggcgtggttttccaagatta |
| murC (HP0748)-3’ | ggccttatggcgtaaatcaa |
| exbD (HP1129)-5’ | ccttctggctcaaaaactgc |
| exbD (HP1129)-3’ | ccgcacggatactaacccta |
| ftsE (HP1136)-5’ | taagccctttgtgcgctact |
| ftsE (HP1136)-3’ | tcactttgagttgggcttga |
Gene numbers refer to H. pylori strain 26695
Urease activity measurement
Bacteria were added to 100 mM phosphate buffer containing 5 mM KCl, 138 mM NaCl, 0.5 mM MgCl2, 1 mM CaCl2, 10 mM glucose, 1 mM glutamine, and 5 mM [14C]-urea (specific activity 10 mCi/mmol), with or without CBS at 500 µg/mL. For experiments measuring total urease activity, 0.01% C12E8 (Sigma) was added to permeabilize the bacteria and bypass the urea channel, UreI. Buffer pHs were 4.5 or 7.4. The pH of the buffer during the course of the experiment did not change by more than 0.1 pH units. Urease activity was measured radiometrically 44, 45. 10 µL bacteria was added to the buffer mixture containing 5mM [14C]-urea. Plastic wells containing 500 mM KOH-soaked filter paper hung from rubber stoppers were used to collect the total 14CO2 from the hydrolysis of urea. Urease activity was measured for 30 minutes at 37°C with constant agitation. The reaction was terminated with 5N H2SO4 to release all the generated CO2 and incubated for 30 minutes at 37°C. The wells were placed in scintillation cocktail (HiIonicFluor; Packard Instruments, Meriden, CT), and the radioactivity was measured by scintillation counting (1216 RackBeta; LKB Instruments, Victoria, Australia). Protein concentration was determined by the BCA method (Pierce). Results were expressed in µmol/min/mg protein.
Measurement of internal pH (pHi)
Internal (cytoplasmic) pH was measured using the membrane-permeant, pH-sensitive fluorescent probe 2,7-bis-(2-carboxyethyl)-5-carboxyfluorescein acetoxymethyl ester (BCECF-AM, Life Technologies) as described previously 30. Experiments were done with or without CBS at 500 µg/mL. Experiments were also completed at 40 µg/mL and 80 µg/mL CBS with the same results (data not shown). Experiments were initiated at pH 7.4, then after about 175 seconds, HCl was added to drop the pH to 4.5.
Each experiment was calibrated independently. Once the fluorescence of the internal BCECF had been measured, 150 nM of the protonophore 3,3,4,5-tetrachlorosalicyanilide (TCS) was added to equilibrate the internal pH with that of the medium. HCl was then added to obtain minimum fluorescence of the dye, followed by the addition of NaOH to obtain maximum fluorescence of the dye. The internal pH was calculated using the equation:
where pKa = pKa of BCECF = 6.98, R = value at 502 nm/value at 436 nm for each data point, RA = ratio at minimum fluorescence, RB = ratio at maximum fluorescence, FA(λ2) = minimum fluorescence at 436 nm, and FB(λ2) = maximum fluorescence at 436 nm.
Membrane potential measurement
Membrane potential was determined using the fluorescent membrane potential sensitive dye, diSC3(5) as previously described 46.
The calibration of the membrane potential was carried out as previously described by the addition of valinomycin (Sigma, St. Louis, MI) followed by the addition of K+ until no further change in fluorescence was observed 46. This enables calculation of the K+ equilibrium potential found with the addition of the K+ selective ionophore, valinomycin, using the Nernst equation:
where [K+]in is equal to the external K+ concentration at which the potential difference becomes zero in the presence of valinomycin, i.e. where [K+]out = [K+]in, and 5 mM is medium concentration when valinomycin is added. The membrane potential in the absence of valinomycin can then be calculated. Membrane potential was measured at medium pH 4.5 and 7.4 with or without 500 µg/mL CBS. Experiments were also done at 40 µg/mL and 80 µg/mL CBS with similar results. 5 mM urea was added to each experiment after addition of bacteria.
Survival assays
Bacteria were harvested from plates after overnight incubation and resuspended in BHI. For 4 conditions, 2 plates were resuspended in 13 mL BHI and 3 mL was used for each condition. Bacteria were placed in dialysis cassettes (Slide-A-lyzer, MWCO 10 kD, 3 mL capacity, Thermo) suspended in a large volume of media (750 mL) adjusted to pH 4.5 with HCl or 7.4 and 5 mM urea as described previously 32. Either bismuth (40 µg/mL), ampicillin (250 µg/mL), or ampicillin and bismuth were added to one beaker at pH 4.5 and 7.4 for each experiment, and each was completed in direct comparison to controls without additives. Bacteria were incubated for 8 hours to incorporate one doubling time. At the end of the incubation period, the pH was confirmed to be unchanged in the bulk media. Bacteria were removed from dialysis cassettes, serially diluted, and plated in duplicate. Colonies were counted after 3–5 days. Data is expressed as percent of each pH control. Each condition was completed in triplicate.
Results
RNAseq analysis of H. pylori incubated with CBS shows upregulation of genes involved with metabolism and growth
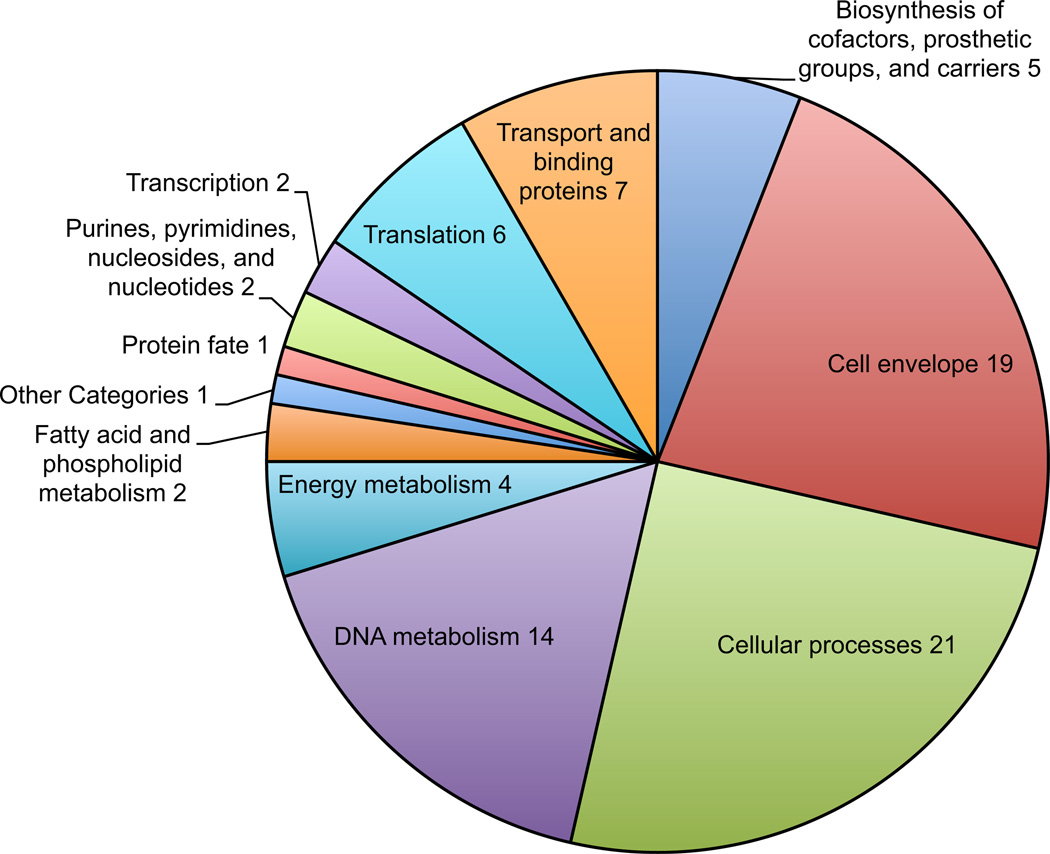
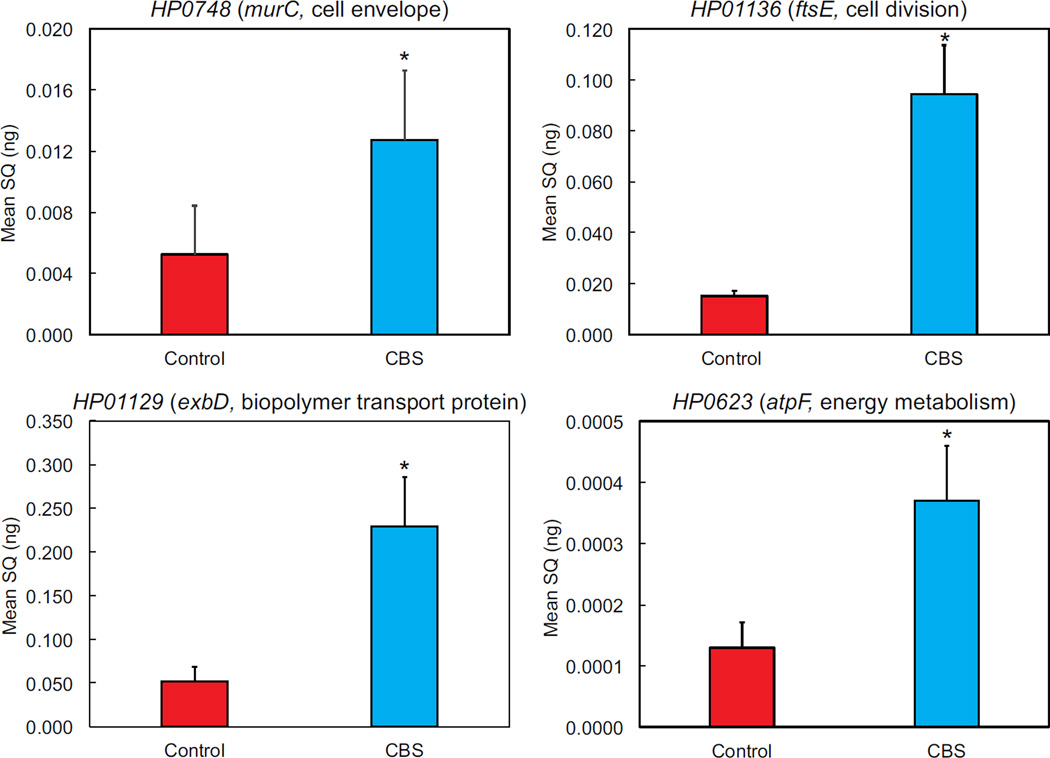
A total of 187 genes were upregulated at pH 4.5 in the presence of CBS. Of these, 84 were of known function (figure 1, table 2). The most common gene categories were cell envelope, cellular processes, DNA and energy metabolism. Increased transcription of the cell envelope synthesis genes, murC and murD, cell division genes ftsE and ftsN and genes involved energy metabolism, atpF and atpB are indicative of replication and increased metabolism. Upregulation of select genes from each category was confirmed by qPCR (figure 2).
Figure 1.
Genes involved with metabolism and growth are upregulated in the presence of CBS. H. pylori were incubated in dialysis cassettes suspended in 750 mL media at pH 4.5 with urea and with or without CBS for 4 hours. Media pH did not change over the course of the experiment. RNA extraction and RNAseq were performed, showing 187 upregulated genes. Genes of known function are represented in the pie chart by category.
Table 2.
Genes up-regulated in the presence of CBS
| Accession number* | CBS/Control | Description/gene name |
|---|---|---|
| Cell envelope | ||
| HP0623 | 4.85 | UDP-N-acetylmuramate-alanine ligase (murC) |
| HP0805 | 4.59 | lipooligosaccharide 5G8 epitope biosynthesis-associated protein (lex2B) |
| HP0494 | 3.52 | UDP-N-acetylmuramoylalanine-D-glutamate ligase (murD) |
| HP0230 | 3.30 | CMP-3-deoxy-D-manno-octulosonate-cytidylyl-transferase (kdsB) |
| HP1581 | 3.19 | undecaprenyl phosphate N-acetylglucosaminyltransferase (llm) |
| HP1416 | 3.06 | lipopolysaccharide 1,2-glucosyltransferase (rfaJ) |
| HP0159 | 2.87 | lipopolysaccharide 1,2-glucosyltransferase (rfaJ) |
| Cell division | ||
| HP0647 | 6.89 | cell division protein FTSN |
| HP0316 | 2.87 | Predicted GTPases (dynamin-related) |
| HP0748 | 2.74 | cell division protein (ftsE) |
| Motility | ||
| HP0384 | 7.19 | motility accessory factor |
| HP1462 | 3.13 | secreted protein involved in flagellar motility |
| HP0496 | 2.68 | motility accessory factor |
| HP1031 | 2.68 | flagellar motor switch protein (fliM) |
| HP1420 | 2.59 | flagellar export protein ATP synthase (fliI) |
| DNA metabolism | ||
| HP0260 | 5.36 | adenine specific DNA methyltransferase (mod) |
| HP0051 | 3.83 | cytosine specific DNA methyltransferase |
| HP0142 | 3.73 | A/G-specific adenine glycosylase (mutY) |
| HP0675 | 3.70 | integrase/recombinase (xerC) |
| HP0425 | 3.67 | recombinase (recJ) |
| HP0478 | 3.56 | adenine specific DNA methyltransferase |
| Energy metabolism | ||
| HP1136 | 3.37 | ATP synthase F0, subunit b |
| HP1133 | 2.14 | ATP synthase F1, subunit gamma (atpF) |
| HP0277 | 2.40 | ferredoxin (atpG) |
| HP0388 | 2.75 | ubiquinone menaquinone biosynthesis methlytransferase |
Accession number from H. pylori strain 26695
Figure 2.
Upregulation of genes involved with metabolism and growth in the presence of CBS was confirmed by qPCR. Bacteria were incubated under the same conditions used for RNAseq, in dialysis cassettes suspended in 750 mL media at pH 4.5 with urea and with or without CBS for 4 hours, followed by RNA isolation, RT reaction, and qPCR using primers from selected genes in each relevant category (gene numbers are from strain 26695). SQ=starting quantity of RNA, N=9 (3 preparations each run in triplicate), error bars represent SEM, p values calculated by t-test, *p<0.05.
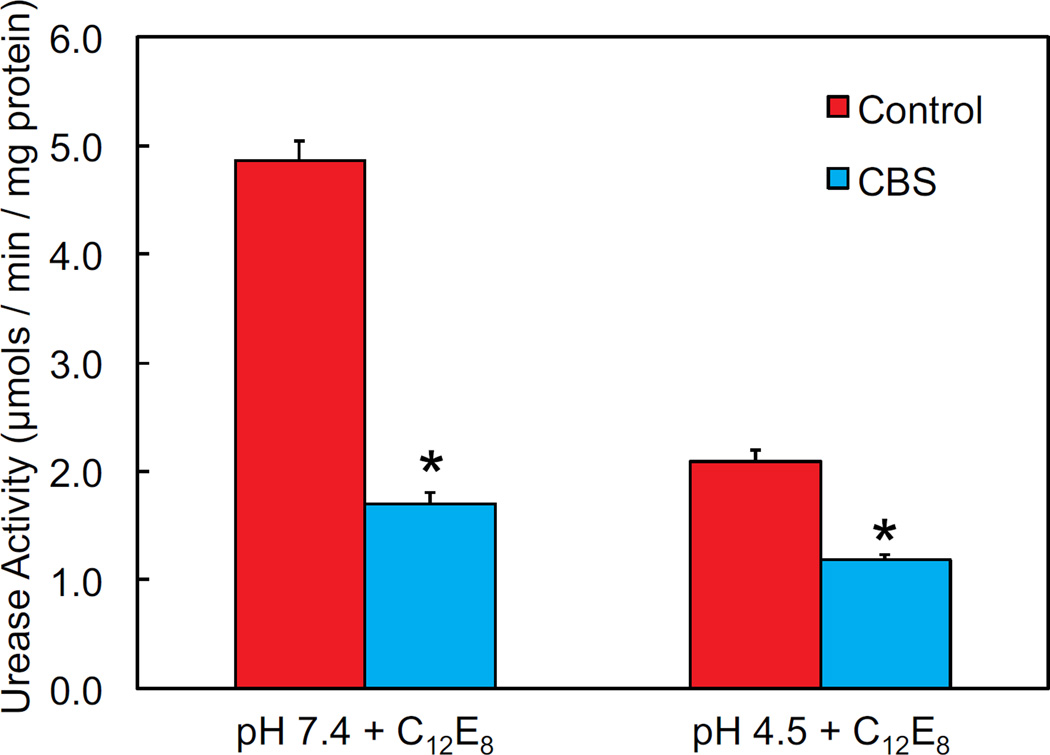
Urease activity in permeabilized bacteria is decreased in the presence of CBS
Total urease activity was measured radiometrically in the presence of C12E8, which permeabilizes the membrane and allows free access of urea to cytoplasmic urease enzyme, bypassing the proton-gated urea channel, UreI, 24, 25. As expected in control experiments, the urease activity was high (close to 5 µmol/min/mg protein) at pH 7.4, which is near the pH optimum of the urease enzyme, and lower at pH 4.5 (~2 µmol/min/mg protein). In the presence of CBS, there was a significant decrease in urease activity at both pHs, with a >50% decrease at pH 7.4 (figure 3). In permeabilizing the membrane, there is likely greater access of bismuth to the bacterial cytoplasm, and there appears to be a direct inhibitory effect of supraphysiologic internal concentrations of bismuth on the urease enzyme.
Figure 3.
Urease activity of permeabilized H. pylori is decreased at both pH 4.5 and pH 7.4 in the presence of CBS. Urease activity was measured quantitatively by using [14]C-labeled urea and detecting output of 14C-labeled carbon dioxide. Results are normalized to total protein in each sample. Bacteria were permeabilized with C12E8, allowing free access of urea and bismuth to the bacterial cytoplasm. N=6, error bars represent SEM, p values calculated by t-test, *p<0.05.
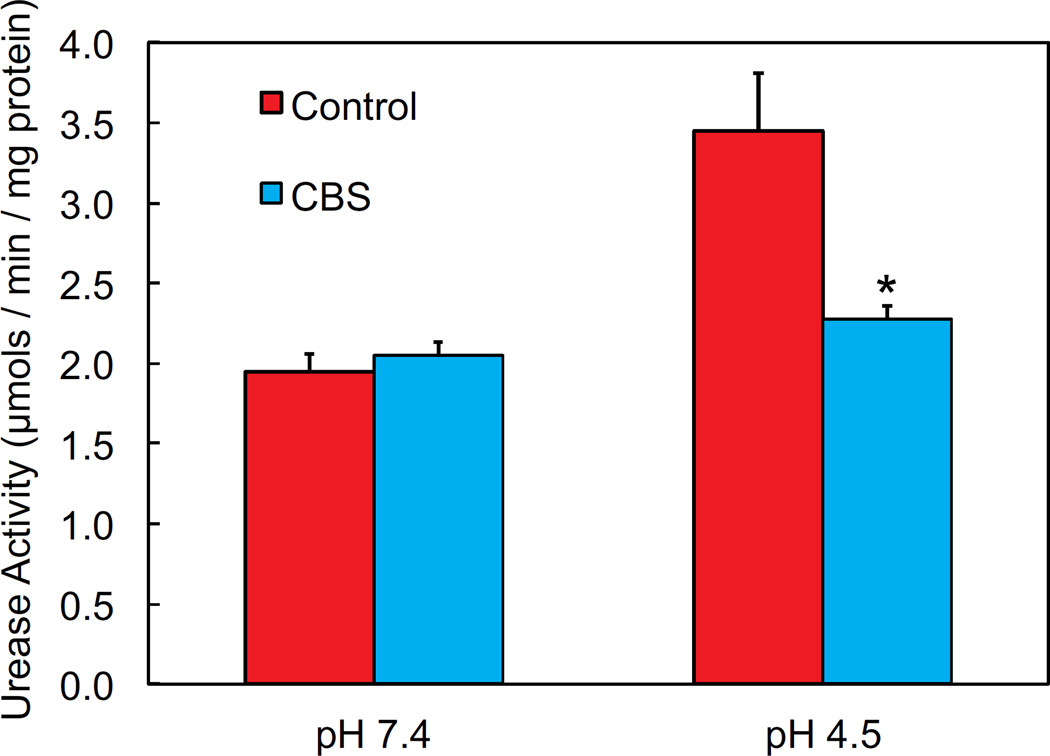
Urease activity of intact bacteria in acid is decreased in the presence of CBS
Urease activity was measured in intact H. pylori in the presence and absence of CBS. In the intact bacteria, urea enters the cytoplasm via UreI, which has a higher open probability at acidic pH 25. In intact H. pylori, there was an increase in urease activity at acidic pH, when the urea channel is more likely to be open 24, 25. In the presence of CBS, there was no significant difference in the already low level of urease activity at pH 7.4 in the intact bacteria (figure 4). At pH 4.5 in the presence of CBS, there was a significant decrease in the intact urease activity, by approximately 30%, suggesting an impediment of urea access to urease. This explanation is favored over direct inhibition of the enzyme since an equivalent decrease was not seen at pH 7.4.
Figure 4.
Urease activity of intact H. pylori is decreased at pH 4.5 in the presence of CBS, but unchanged at pH 7.4. Urease activity was measured quantitatively by using 14C-labeled urea and detecting output of 14C-labeled carbon dioxide. Results are normalized to total protein in each sample. N=6, error bars represent SEM, p values calculated by t-test, *p<0.05.
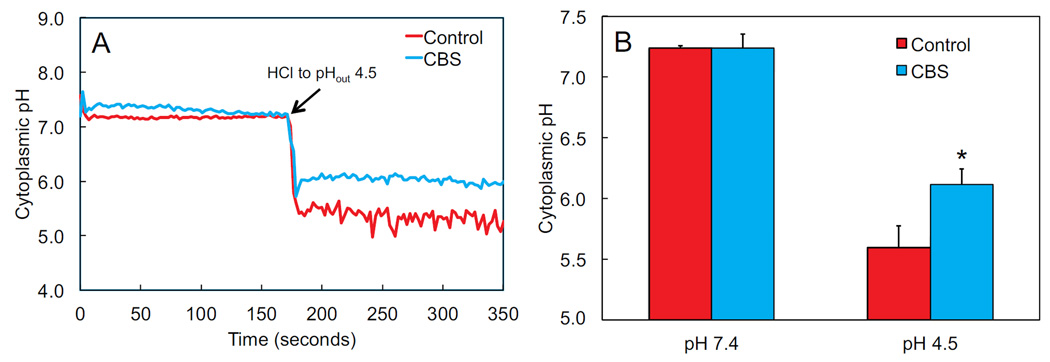
Fall in H. pylori cytoplasmic pH in response to acid challenge is diminished in the presence of CBS
Internal pH of H. pylori was measured using BCECF-AM. As expected, at media pH 7.4, the cytoplasmic pH is about 7.2. There was no change at external pH 7.4 in the presence of CBS. At media pH 4.5, the cytoplasmic pH fell to about 5.6, which is in the range for survival but below the level for bacterial division in a neutralophile 31. In the presence of CBS at pH 4.5, the cytoplasmic pH fell to about 6.2, which is now within the range for bacterial division and growth (figure 5). With internal pH increasing in acid in the presence of CBS, there will be increased bacterial growth and therefore increased susceptibility to growth dependent antibiotics.
Figure 5.
The drop in cytoplasmic pH of H. pylori in response to acid challenge is diminished in the presence of CBS. Internal pH was measured using BCECF-AM, and each experiment was calibrated independently. A representative tracing with and without CBS is shown in (A). Combined data are shown in (B). Experiments were started at external pH 7.4, and then HCl was added to lower the pH to 4.5. N=3, error bars represent SEM, p values calculated by t-test, *p<0.05.
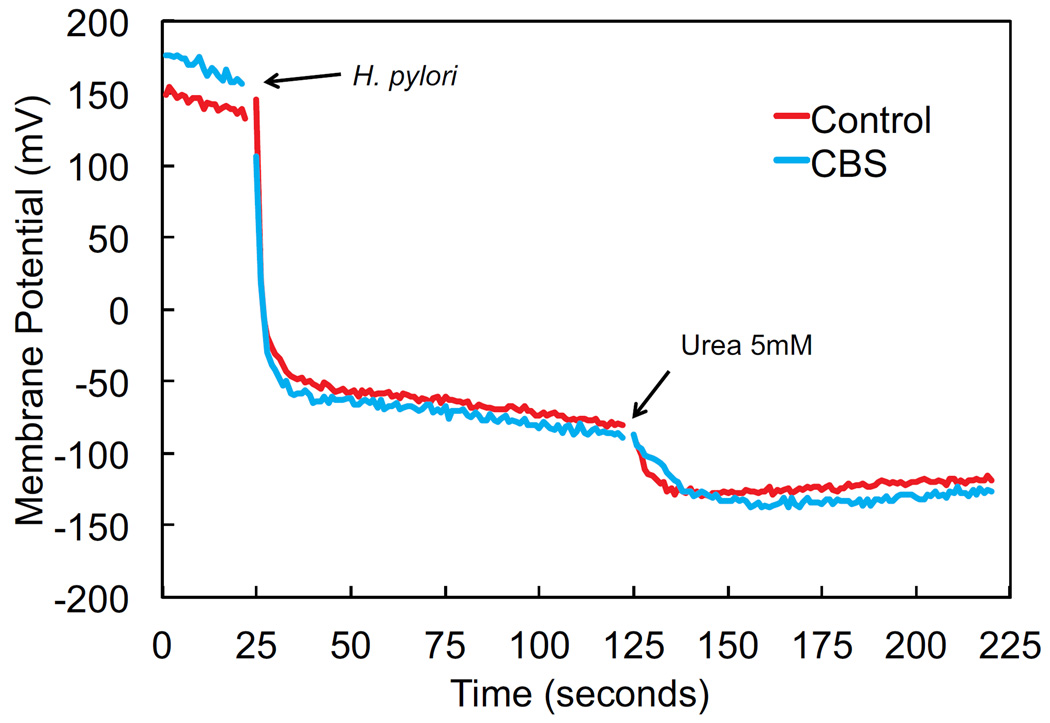
CBS has no effect on membrane potential of H. pylori
H. pylori, as a neutralophile, is unable to maintain sufficient membrane potential for survival at low pH in the absence of urea 24, 46. In the presence of urea, membrane potential with acidic media pH is restored to levels seen at neutral pH, due to opening of the proton gated urea channel, UreI, and urea access to cytoplasmic urease, allowing for periplasmic and cytoplasmic buffering and survival in acid 24. CBS does not alter this response, the addition of urea at media pH 4.5 leads to an equivalent increase in membrane potential as is seen in the control experiments, measured using the potential sensitive dye DiSC3(5) (figure 6). Since CBS does not alter the response of membrane potential to the addition of urea, UreI remains unhindered. This suggests that the mechanism of action of CBS on H. pylori does not involve direct blockage or inhibition of the urea channel.
Figure 6.
Restoration of H. pylori membrane potential at pH 4.5 with addition of urea is unaffected by CBS, showing that the urea channel is not directly blocked. Membrane potential was measured using diSC3(5), and experiments were calibrated individually using valinomycin and KOH. The tracing shown represents combined data for 3 experiments for each condition.
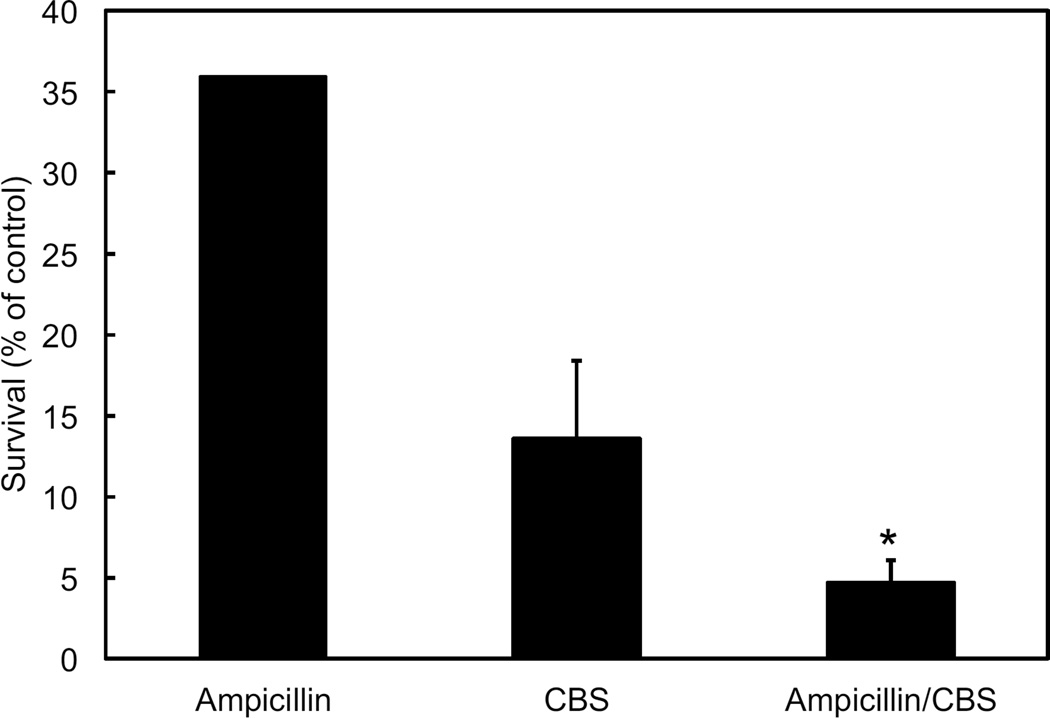
CBS in combination with the growth dependent antibiotic ampicillin leads to decreased survival of H. pylori
Survival of H. pylori after an 8-hour incubation is decreased by both CBS and ampicillin. Bacteria were incubated in dialysis cassettes suspended in 750 mL media with urea and with or without CBS, ampicillin, or both. Susceptibility to growth dependent antibiotics was greater at pH 7.4 than 4.5 (data not shown), but this difference was not significant (significant difference is instead seen between pH 3 and 7.4 32). Although CBS alone results in a significant loss of survival, the decrease in survival at pH 4.5 with CBS and ampicillin is greater than with either agent alone, demonstrating that CBS augments the effect of the growth dependent antibiotics in this model system (figure 7).
Figure 7.
H. pylori survival with ampicillin and CBS is less than with either agent alone. Bacteria were incubated for 8 hours in dialysis cassettes suspended in 750mL media at 4.5 with urea, then diluted and colonies counted. Ampicillin and/or bismuth were added as indicated. Data are expressed as percent of control. N=3 experiments for each condition, error bars represent the standard deviation, p values calculated by t-test, *p<0.05.
Discussion
H. pylori has a significant worldwide disease burden, and the efficacy of standard treatment regimens is declining. Although there are many possible reasons for decreased treatment efficacy, antibiotic resistance, particularly to clarithromycin, has been identified as the most important 4. As no specific new medications have been introduced in recent years, novel treatment regimens have been created using different combinations, durations, and sequences of available medications 47. The addition of bismuth has shown increased efficacy of a variety of treatment regimens. Metronidazole resistance can be overcome by addition of bismuth and bismuth containing regimens can be used as first line treatment in areas of high clarithromycin resistance 4, 48. The mechanism of action of bismuth in H. pylori treatment is unknown, and knowledge of this mechanism may aid in the development of new or tailored regimens.
The data presented here suggest that bismuth plays a role in maintenance of cytoplasmic pH in H. pylori in the setting of external acidification. PPI efficacy and gastric pH are important factors in H. pylori eradication 49–51. It is well known that use of twice daily PPI in conjunction with treatment regimens increases efficacy over once daily, and the benefit is further augmented by the use of newer, more potent PPI formulations 52, 53. In addition, patients with a polymorphism in CYP2C19 metabolize omeprazole more slowly, leading to a higher intragastric pH and higher treatment efficacy compared to rapid metabolizers 54. Supra-therapeutic PPI doses allow for treatment with amoxicillin alone regardless of CYP2C19 genotype 34. In in vitro studies, genes involved with metabolism and growth are upregulated at pH 7.4 compared to pH 3, and the efficacy of ampicillin is increased at higher pH, as bacteria are dividing, allowing the antibiotic to work more efficiently 32. Increased transcription of genes involved with metabolism and growth is similarly seen here in acid when CBS is present, suggesting a facilitation of bacterial growth and division. Since PPIs that are commercially available at this time do not provide persistent elevation in gastric pH to the degree required to ensure most bacteria are dividing 55, the presence of bismuth may play a role in augmenting this effect.
It has been reported that the action of bismuth on H. pylori may be related to inhibition of urease activity. Urease depends on nickel for activation, and bismuth is able to replace metals such as nickel13. Much of the data related to urease and bismuth has focused on the free enzyme, and in vivo cytoplasmic bismuth levels, dependent on metal transport systems, are likely much lower 9. Similar results were seen here when bacteria were permeabilized by C12E8, allowing free access of urea and bismuth to urease. The significant drop in urease activity in response to direct interaction with a high concentration of bismuth was seen at both acidic and neutral pH. In contrast, when measuring urease activity of intact bacteria, the drop in urease activity was only seen at acidic pH. Since there was no change in urease activity at neutral pH, this suggests either a barrier to urea diffusion or a barrier to proton diffusion rather than a direct inhibitory effect of bismuth on urease enzyme in the intact bacteria. Internal pH and membrane potential measurements were used to further address this question.
As shown previously, internal pH falls with acidic medium pH, and this is partially rescued by the addition of urea 30. In the presence of bismuth, the fall in cytoplasmic pH in response to medium acidification is not as great. As a result, the cytoplasmic pH remains within the range for metabolism and growth of a neutralophile despite the acidic environment. Measurement of membrane potential at acidic pH confirms that bismuth is not acting directly on the urea channel. At acidic medium pH, H. pylori membrane potential is diminished due to a decreased proton motive force across the inner membrane, and potential is restored by addition of urea 24. The response to urea addition in acid is unaffected by the presence of CBS. There is a consistent but not statistically significant baseline increase in membrane potential in the presence of CBS, which fits the model suggested by the internal pH measurements. By impeding proton entry into the cytoplasm, CBS is able to augment the effect of acid suppression by maintaining cytoplasmic pH in a range that promotes increased transcription of metabolic and cell division genes, thus improving antibiotic efficacy.
Amoxicillin and its parenteral equivalent ampicillin work by inhibiting bacterial cell wall synthesis. H. pylori resistance to these antibiotics is extremely rare 4. Survival studies were done over an 8 hour time period to allow for passage of at least one doubling time, using a system that maintains constant pH and urea concentrations 32. Since the bactericidal effect of CBS and ampicillin is greater than for either agent alone, and there is an overall trend toward an augmented effect at neutral pH, this again supports the idea that CBS augments both the effect of acid suppression and of growth dependent antibiotics.
Other mechanisms of action have been proposed for bismuth, and it should be taken into account that the effect may be multifactorial. Most proposed mechanisms center around enzyme inhibition, metal interference, or bacterial structure and adherence. Bismuth can bind fumarase, part of the tricarboxylic acid cycle, impacting bacterial energetics or motility via decreased flagellar rotation 9, 56, but this again raises the question of the degree of bismuth access to the enzyme in vivo. Hpn, an H. pylori protein that binds nickel and zinc, has been assumed to bind bismuth as well since mutant strains lacking the protein are four times more sensitive to CBS 57, but this also assumes significant bismuth uptake and does not definitively predict mechanism. H. pylori adherence to cultured cells and human mucosal preparations is decreased in the presence of CBS 58–60. Ultrastructural changes in the bacteria have been reported with prolonged incubation with bismuth 15. These and other mechanisms likely contribute to the action of bismuth on H. pylori since bismuth alone can decrease bacterial load and rarely can clear the infection 18, 19. Since bismuth complements regimens that include growth dependent antibiotics and strong acid inhibition enhances bacterial growth, it follows that the concept of reduced proton entry into H. pylori and cytoplasmic pH alteration could represent a dominant mechanism of action. Bismuth appears to partially relieve the dependence on achievement of profound and sustained acid inhibition for augmented efficacy of growth dependent antibiotics, leading to improved H. pylori treatment success.
Acknowledgements
Supported by K08DK100661 (EAM), UCLA CDI (EAM), USVA 2I01BX001006 (GS), R01DK105156-01(GS).
Footnotes
Authorship Statement
Guarantor of article: David R. Scott
Author contributions: EAM and DRS performed the research, collected and analyzed the data, designed the research study and wrote the paper, GS contributed to the design of the study and edited the manuscript.
All authors approved the final version of the article, including the authorship list.
References
- 1.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. The New England journal of medicine. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 3.Megraud F. Detection of Helicobacter pylori, its sensitivity to antibiotics. A step forward in the use of molecular methods. Gastroenterol Clin Biol. 2007;31(10):790–791. doi: 10.1016/s0399-8320(07)73965-9. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 5.Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta-analysis of efficacy and tolerability. The American journal of gastroenterology. 2010;105(1):65–73. doi: 10.1038/ajg.2009.508. [DOI] [PubMed] [Google Scholar]
- 6.Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spenard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. The American journal of gastroenterology. 2003;98(3):562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Bazzoli F, Delchier JC, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377(9769):905–913. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 8.O’Morain C, Borody T, Farley A, et al. Efficacy and safety of single-triple capsules of bismuth biskalcitrate, metronidazole and tetracycline, given with omeprazole, for the eradication of Helicobacter pylori: an international multicentre study. Alimentary pharmacology & therapeutics. 2003;17(3):415–420. doi: 10.1046/j.1365-2036.2003.01434.x. [DOI] [PubMed] [Google Scholar]
- 9.Ge R, Chen Z, Zhou Q. The actions of bismuth in the treatment of Helicobacter pylori infections: an update. Metallomics. 2012;4(3):239–243. doi: 10.1039/c2mt00180b. [DOI] [PubMed] [Google Scholar]
- 10.Lambert JR, Midolo P. The actions of bismuth in the treatment of Helicobacter pylori infection. Alimentary pharmacology & therapeutics. 1997;11(Suppl 1):27–33. doi: 10.1046/j.1365-2036.11.s1.13.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas F, Bialek B, Hensel R. Medical use of bismuth: The two sides of the coin. Clinical Toxicology. 2012:1–5. S3:004. [Google Scholar]
- 12.Mohan R. Green bismuth. Nat Chem. 2010;2(4):336. doi: 10.1038/nchem.609. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Li H, Sadler PJ. Transferrin as a metal ion mediator. Chem Rev. 1999;99(9):2817–2842. doi: 10.1021/cr980430w. [DOI] [PubMed] [Google Scholar]
- 14.Phillips RH, Whitehead MW, Lacey S, Champion M, Thompson RP, Powell JJ. Solubility, absorption, and anti-Helicobacter pylori activity of bismuth subnitrate and colloidal bismuth subcitrate: In vitro data Do not predict In vivo efficacy. Helicobacter. 2000;5(3):176–182. doi: 10.1046/j.1523-5378.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 15.Stratton CW, Warner RR, Coudron PE, Lilly NA. Bismuth-mediated disruption of the glycocalyx-cell wall of Helicobacter pylori: ultrastructural evidence for a mechanism of action for bismuth salts. J Antimicrob Chemother. 1999;43(5):659–666. doi: 10.1093/jac/43.5.659. [DOI] [PubMed] [Google Scholar]
- 16.Konturek SJ, Radecki T, Piastucki I, Drozdowicz D. Advances in the understanding of the mechanism of cytoprotective action by colloidal bismuth subcitrate. Scand J Gastroenterol Suppl. 1986;122:6–10. doi: 10.3109/00365528609102578. [DOI] [PubMed] [Google Scholar]
- 17.Lambert JR. Clinical indications and efficacy of colloidal bismuth subcitrate. Scand J Gastroenterol. 1991;185(Suppl):13–21. doi: 10.3109/00365529109093215. [DOI] [PubMed] [Google Scholar]
- 18.Lambert JR, Borromeo J, Eaves ER, Hansky J, Korman MG. Efficacy of different dosage regimens of bismuth in eradicating Campylobacter pylori . Gastroenterology. 1988;94:A248. [Google Scholar]
- 19.Marshall BJ, Valenzuela JE, McCallum RW, et al. Bismuth subsalicylate suppression of Helicobacter pylori in nonulcer dyspepsia: a double-blind placebo-controlled trial. Digestive diseases and sciences. 1993;38(9):1674–1680. doi: 10.1007/BF01303177. [DOI] [PubMed] [Google Scholar]
- 20.McNulty CA. Bismuth subsalicylate in the treatment of gastritis due to Campylobacter pylori . Rev Infect Dis. 1990;12(Suppl 1):S94–S98. doi: 10.1093/clinids/12.supplement_1.s94. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner HK, Montrose MH. Regulated alkali secretion acts in tandem with unstirred layers to regulate mouse gastric surface pH. Gastroenterology. 2004;126(3):774–783. doi: 10.1053/j.gastro.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 22.Henriksnas J, Phillipson M, Storm M, Engstrand L, Soleimani M, Holm L. Impaired mucus-bicarbonate barrier in Helicobacter pylori-infected mice. American journal of physiology. 2006;291(3):G396–G403. doi: 10.1152/ajpgi.00017.2006. [DOI] [PubMed] [Google Scholar]
- 23.Scott DR, Marcus EA, Wen Y, Oh J, Sachs G. Gene expression in vivo shows that Helicobacter pylori colonizes an acidic niche on the gastric surface. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(17):7235–7240. doi: 10.1073/pnas.0702300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott DR, Marcus EA, Weeks DL, Lee A, Melchers K, Sachs G. Expression of the Helicobacter pylori ureI gene is required for acidic pH activation of cytoplasmic urease. Infection and immunity. 2000;68(2):470–477. doi: 10.1128/iai.68.2.470-477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weeks DL, Eskandari S, Scott DR, Sachs G. Science. 5452. Vol. 287. New York, N.Y: 2000. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization; pp. 482–485. [DOI] [PubMed] [Google Scholar]
- 26.Marcus EA, Sachs G, Wen Y, Feng J, Scott DR. Role of the Helicobacter pylori sensor kinase ArsS in protein trafficking and acid acclimation. Journal of bacteriology. 2012;194(20):5545–5551. doi: 10.1128/JB.01263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott DR, Marcus EA, Wen Y, Singh S, Feng J, Sachs G. Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4(+), is necessary for acid survival of Helicobacter pylori . Journal of bacteriology. 2010;192(1):94–103. doi: 10.1128/JB.00848-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voland P, Weeks DL, Marcus EA, Prinz C, Sachs G, Scott D. Interactions among the seven Helicobacter pylori proteins encoded by the urease gene cluster. American journal of physiology. 2003;284(1):G96–G106. doi: 10.1152/ajpgi.00160.2002. [DOI] [PubMed] [Google Scholar]
- 29.Marcus EA, Moshfegh AP, Sachs G, Scott DR. The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. Journal of bacteriology. 2005;187(2):729–738. doi: 10.1128/JB.187.2.729-738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen Y, Marcus EA, Matrubutham U, Gleeson MA, Scott DR, Sachs G. Acid-adaptive genes of Helicobacter pylori . Infection and immunity. 2003;71(10):5921–5939. doi: 10.1128/IAI.71.10.5921-5939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashket ER, Wong PT. The intracellular pH of Escherichia coli . Biochimica et biophysica acta. 1969;193(1):212–214. doi: 10.1016/0005-2736(69)90074-1. [DOI] [PubMed] [Google Scholar]
- 32.Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Alimentary pharmacology & therapeutics. 2012;36(10):972–979. doi: 10.1111/apt.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang JC, Wang HL, Chern HD, et al. Role of omeprazole dosage and cytochrome P450 2C19 genotype in patients receiving omeprazole-amoxicillin dual therapy for Helicobacter pylori eradication. Pharmacotherapy. 2011;31(3):227–238. doi: 10.1592/phco.31.3.227. [DOI] [PubMed] [Google Scholar]
- 34.Yang JC, Lin CJ, Wang HL, et al. High-dose Dual Therapy Is Superior to Standard First-line or Rescue Therapy for Helicobacter pylori Infection. Clin Gastroenterol Hepatol. 2015;13(5):895–905. doi: 10.1016/j.cgh.2014.10.036. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugimoto M, Shirai N, Nishino M, et al. Rabeprazole 10 mg q.d.s. decreases 24-h intragastric acidity significantly more than rabeprazole 20 mg b.d. or 40 mg o.m., overcoming CYP2C19 genotype. Alimentary pharmacology & therapeutics. 2012;36(7):627–634. doi: 10.1111/apt.12014. [DOI] [PubMed] [Google Scholar]
- 36.Benini S, Rypniewski WR, Wilson KS, Ciurli S, Mangani S. The complex of Bacillus pasteurii urease with β-mercaptoethanol from X-ray data at 1.65-Å resolution. JBIC Journal of Biological Inorganic Chemistry. 1998;3(3):268–273. doi: 10.1007/s007750050014. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Mulrooney SB, Leung AF, et al. Inhibition of urease by bismuth(III): implications for the mechanism of action of bismuth drugs. Biometals. 2006;19(5):503–511. doi: 10.1007/s10534-005-5449-0. [DOI] [PubMed] [Google Scholar]
- 38.Butcher GP, Ryder SD, Hughes SJ, et al. Use of an ammonia electrode for rapid quantification of Helicobacter pylori urease: its use in the endoscopy room and in the assessment of urease inhibition by bismuth subsalicylate. Digestion. 1992;53(3–4):142–148. doi: 10.1159/000200989. [DOI] [PubMed] [Google Scholar]
- 39.Ge R, Sun X, Gu Q, et al. A proteomic approach for the identification of bismuth-binding proteins in Helicobacter pylori . J Biol Inorg Chem. 2007;12(6):831–842. doi: 10.1007/s00775-007-0237-7. [DOI] [PubMed] [Google Scholar]
- 40.Tsang CN, Ho KS, Sun H, Chan WT. Tracking bismuth antiulcer drug uptake in single Helicobacter pylori cells. J Am Chem Soc. 2011;133(19):7355–7357. doi: 10.1021/ja2013278. [DOI] [PubMed] [Google Scholar]
- 41.Akopyants NS, Jiang Q, Taylor DE, Berg DE. Corrected identity of isolates of Helicobacter pylori reference strain NCTC11637. Helicobacter. 1997;2(1):48–52. doi: 10.1111/j.1523-5378.1997.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 42.Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23(10):1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 43.Untergasser A, Cutcutache I, Koressaar T, et al. Primer3--new capabilities and interfaces. Nucleic acids research. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald JA, Speeg KVJ, Campbell JW. Urease: a sensitive and specific radiometric assay. Enzymologia. 1972;42(1):1–9. [PubMed] [Google Scholar]
- 45.Scott DR, Weeks D, Hong C, Postius S, Melchers K, Sachs G. The role of internal urease in acid resistance of Helicobacter pylori . Gastroenterology. 1998;114(1):58–70. doi: 10.1016/s0016-5085(98)70633-x. [DOI] [PubMed] [Google Scholar]
- 46.Meyer-Rosberg K, Scott DR, Rex D, Melchers K, Sachs G. The effect of environmental pH on the proton motive force of Helicobacter pylori . Gastroenterology. 1996;111(4):886–900. doi: 10.1016/s0016-5085(96)70056-2. [DOI] [PubMed] [Google Scholar]
- 47.Malfertheiner P, Selgrad M. Helicobacter pylori. Curr Opin Gastroenterol. 2014;30(6):589–595. doi: 10.1097/MOG.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 48.Liang X, Xu X, Zheng Q, et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol. 2013;11(7):802–7. doi: 10.1016/j.cgh.2013.01.008. e1. [DOI] [PubMed] [Google Scholar]
- 49.Hunt RH. Hp and pH--the relevance of gastric acid to the treatment of Helicobacter pylori infection. Journal of gastroenterology. 1994;29(Suppl 7):128–133. [PubMed] [Google Scholar]
- 50.Labenz J, Stolte M, Blum AL, et al. Intragastric acidity as a predictor of the success of Helicobacter pylori eradication: a study in peptic ulcer patients with omeprazole and amoxicillin. Gut. 1995;37(1):39–43. doi: 10.1136/gut.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugimoto M, Furuta T, Shirai N, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12(4):317–323. doi: 10.1111/j.1523-5378.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 52.Vallve M, Vergara M, Gisbert JP, Calvet XSingle vs. double dose of a proton pump inhibitor in triple therapy for Helicobacter pylori eradication: a meta-analysis. Alimentary pharmacology & therapeutics. 2002;16(6):1149–1156. doi: 10.1046/j.1365-2036.2002.01270.x. [DOI] [PubMed] [Google Scholar]
- 53.Villoria A. [Acid-related diseases: are higher doses of proton pump inhibitors more effective in the treatment of Helicobacter pylori infection?] Gastroenterol Hepatol. 2008;31(8):546–547. doi: 10.1157/13127103. [DOI] [PubMed] [Google Scholar]
- 54.Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Digestive diseases and sciences. 2014;59(6):1235–1243. doi: 10.1007/s10620-014-3093-7. [DOI] [PubMed] [Google Scholar]
- 55.Hunt RH. Importance of pH control in the management of GERD. Archives of internal medicine. 1999;159(7):649–657. doi: 10.1001/archinte.159.7.649. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Zhou Q, Ge R. Inhibition of fumarase by bismuth(III): implications for the tricarboxylic acid cycle as a potential target of bismuth drugs in Helicobacter pylori . Biometals. 2012;25(1):95–102. doi: 10.1007/s10534-011-9485-7. [DOI] [PubMed] [Google Scholar]
- 57.Mobley HL, Garner RM, Chippendale GR, Gilbert JV, Kane AV, Plaut AG. Role of Hpn and NixA of Helicobacter pylori in susceptibility and resistance to bismuth and other metal ions. Helicobacter. 1999;4(3):162–169. doi: 10.1046/j.1523-5378.1999.99286.x. [DOI] [PubMed] [Google Scholar]
- 58.Goodwin CS, Armstrong JA, Cooper M. Colloidal bismuth subcitrate inhibits the adherance of H. pylori to epithelial cells. Italian Journal of Gastroenterology. 1991;23(Supplement 2):40. [Google Scholar]
- 59.Nilius M, Schieffer S, Malfertheiner P. Inhibition of H. pylori adhesion to human SMC by CBS and different proteases. Ir J Med Sci. 1992;161(Supplement 10):10. [Google Scholar]
- 60.Wagner S, Beil W, Mai UE, Bokemeyer C, Meyer HJ, Manns MP. Interaction between Helicobacter pylori and human gastric epithelial cells in culture: effect of antiulcer drugs. Pharmacology. 1994;49(4):226–237. doi: 10.1159/000139238. [DOI] [PubMed] [Google Scholar]