Abstract
There is cross-sectional evidence that neurocognitive intra-individual variability (IIV), or dispersion, is elevated in HIV disease and is associated with declines in activities of daily living, including medication adherence. This longitudinal study extends this literature by examining whether increased neurocognitive IIV in HIV+ persons over time predicts declines in medication adherence above and beyond changes in mean level of performance over a six-month observation. After controlling for drug use, declines in mean performance, and changes in depressive symptoms, results confirmed that increases in IIV were associated with overall poorer antiretroviral medication adherence. HIV+ individuals with the greatest increases in dispersion demonstrated marked reductions in adherence by the third month that exceed that observed in less variable individuals. Our results indicate that increases in dispersion are associated with poorer declines in medication adherence in HIV disease, which may have implications for the early detection and remediation of suboptimal antiretroviral adherence.
Neurocognitive impairment remains a significant concern in HIV/AIDS. Despite the development of potent antiviral drugs that target the virus, HIV-associated neurocognitive disorders (HAND) remain common, with prevalence rates of approximately 40–60% (Heaton et al., 2010; Sacktor, 2002). Such impairments can interfere with health-maintenance behaviors including medication adherence (Hinkin et al., 2002; 2004; Woods, Moore, Weber, & Grant, 2009). In turn, poor adherence can lead to additional neurocognitive impairment, precipitating a deteriorating cycle (Ettenhofer, Foley, Castellon, & Hinkin, 2010a).
Neurocognitive impairment is most typically measured by mean scores on validated testing instruments. However, recent attention in the literature has turned to the phenomenon of intra-individual variability (IIV) within neurocognitive scores (Ettenhofer et al., 2010b; Levine et al., 2008; Morgan, Woods, Delano-Wood, Bondi, & Grant, 2011; Morgan, Woods, & Grant, 2012). IIV addresses within-person differences in test performance and is demarcated by differences across scores during a single session (dispersion) as well as on a single task across multiple time points (inconsistency) (Hilborn, Strauss, Hultch, & Hunter, 2009; Ettenhofer et al., 2010; Stuss, Murphy, Binns, & Alexander, 2003; Stuss, Pogue, Buckle, & Bondar, 1994). There is growing evidence that increased IIV may be a sensitive measure of decline in functioning (Hilborn et al., 2009; Rapp, Schnaider-Beeri, Sano, Silverman, & Haroutunian, 2005). Cross-sectional studies report that increased IIV has been observed in individuals with traumatic brain injury (Stuss et al., 1994) and multiple sclerosis (Bruce, Bruce, & Arnett, 2010), and has been linked to focal brain lesions in the frontal lobe as well as white-matter hyperintensities (Stuss et al., 2003; Nilsson et al., 2014). IIV is also more pronounced in older patients and may signal a neurodegenerative condition (Morgan et al., 2011).
Within HIV infected populations, our group has identified a cross-sectional link between reaction time variability and medication adherence (Ettenhofer et al., 2010b; Levine et al., 2008); individuals with more variability (i.e. inconsistency) on a test of sustained attention and reaction time (i.e., Continuous Performance Test) evidenced poorer medication adherence as well as poorer global neurocognition. This phenomenon may be related to relationships between white matter degradation, slowed processing speed, and increased reaction time variability observed in neurologically compromised individuals (Nilsson et al., 2014), in that HIV positive individuals exhibit incipient cognitive changes related to neuronal degeneration associated with the disease.
It has been proposed that IIV is also related to changes in executive control (Bellgrove et al., 2004). Declines in executive control are common sequelae of brain pathology and may affect response consistency on neurocognitive measures (Stuss et al., 1999). This framework lends support to existing findings on neurocognitive dispersion and adherence in HIV. It is well-established that individuals with HIV exhibit a subcortical pattern of deficits that impact executive functioning (Woods et al., 2009). In turn, executive functioning is a predictor of poorer medication adherence in HIV/AIDS (Hinkin et al., 2002). It may be that as individuals experience cognitive decline, they lose the ability to maintain focus and top-down attentional control on neuropsychological tasks, which results in increased variability in performance. At the same time, these executive control deficits may lead to attentional lapses in medication-cue monitoring and poorer adherence behaviors. Neuropsychological variability also reflects vagaries in attention, concentration, and memory that may occur in the earlier stages of cognitive impairment. Patients who have yet to exhibit mean level impairment may still incur lapses in their IADLS, such as medication management, as they experience these fluctuations in their cognitive abilities. In line with this, dispersion has been found to predict self-reported activities of daily living and medication adherence in patients with HIV even before they developed an HIV-associated neurocognitive disorder (HAND; Morgan et al., 2012). Dispersion may therefore be a proxy measure for cognitive changes in HIV and is therefore a promising method of predicting poorer medication adherence.
Given the documented degree of normal variability seen on neuropsychological testing (Schretlin et al., 2003), dispersion measured at a single time-point may be difficult to interpret. There may be clinical utility in assessing changes in dispersion over time. Just as significant decreases in test scores over time are hallmark indicators of poorer medication adherence, increases in within-subject variability may similarly signal dysfunction. However, while several cross-sectional studies on dispersion have emerged, no study has yet established whether longitudinal changes in dispersion across multiple time points might prove a useful marker of medication decline. To this end, our study examined whether changes in dispersion predicted overall medication adherence in an HIV+ cohort over a 6-month period. Based on prior research, we hypothesized that increased IIV over time (i.e., between baseline and 6-month follow-up) would be associated with poorer overall adherence, signaling that increasing IIV over time may be associated with functional decline in IADLs. Such a finding would indicate that longitudinal changes in within-test neurocognitive dispersion have unique value in predicting health outcomes within HIV.
Methods
Participants
This sample included 150 HIV+ participants who were involved in a larger study examining risk factors for poor antiretroviral medication adherence. All participants were recruited from HIV treatment facilities throughout the greater Los Angeles area. Participants included in the parent study were currently taking at least one antiretroviral medication, and were responsible for administering their own medication(s). Prior to analyses, individuals were excluded if they reported a history of neurological disorder including stroke (n = 10), seizure disorder (n = 15), anoxic event (n = 2), or a head injury involving loss of consciousness exceeding 60 minutes (n = 19). Individuals were also excluded if they met diagnostic criteria for past or current psychotic disorder (n = 13) or bipolar disorder (n = 2), as determined by a modified Structured Clinical Interview for the DSM-IV (First, Spitzer, Gibbon, & Williams, 1995). An additional four participants were excluded for using the MEMS cap incorrectly (i.e., reflected by medication adherence rates less than 5% at the first time point) and two more were excluded for having more than two missing MEMS time points, resulting in our final sample of 150.
Participants completed a baseline assessment that included eligibility screening, psychiatric questionnaires, and neuropsychological testing. Participants returned for monthly follow-up over the course of six months, during which their medication adherence rates for the preceding month were recorded. Urinalysis screening for recent drug use was also conducted at each appointment. All participants received repeat neuropsychological testing during the final (i.e., six month) evaluation, with the battery identical to that given at baseline. On average, participants were 41.9 years of age (SD = 7.4) with 13.1 years of education (SD = 2.1). The majority were African American (66.0%) and male (82.0%). Table 1 provides full demographic and clinical information.
Table 1.
Demographic and Clinical Characteristics of the Sample
| Full Sample (n = 150) | Reduced DispersionΔ ≤ −0.5 (n = 49) |
Stable DispersionΔ −0.5 < z-score < 0.5 (n=62) |
Increased DispersionΔ ≥ 0.5 (n = 39) |
Partial η2/τ | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Demographics | |||||
| Age (years) | 41.9 (7.4) | 42.5 (6.1) | 41.0 (7.4) | 42.6 (8.7) | 0.01 |
| Education (years) | 13.1 (2.1) | 13.4 (2.0) | 13.1 (2.1) | 12.8 (2.1) | 0.01 |
| Sex (% male) | 82.0 | 69.2 | 83.9 | 89.8 | 0.21* |
| Ethnicity | 0.08 | ||||
| % Caucasian | 14.7 | 12.2 | 16.1 | 15.4 | |
| % African American | 66.0 | 61.2 | 64.5 | 74.4 | |
| % Latino | 13.3 | 18.4 | 12.9 | 7.7 | |
| % Other | 6.0 | 8.2 | 6.4 | 2.5 | |
| Estimated IQ | 105.4 (9.5) | 106.4 (9.9) | 104.9 (9.0) | 104.8 (10.0) | 0.01 |
| HIV Status | |||||
| AIDS diagnosis (% yes) | 64.4 | 71.7 | 62.9 | 57.9 | 0.11 |
| Most recent CD4+ count (median) | 382.5 (320.8) | 378.0 (284.4) | 392.0 (267.3) | 511.0 (429.5) | 0.02 |
| Nadir absolute CD4+ count (median) | 189.0 (188.6) | 164.0 (193.2) | 194.0 (188.7) | 190.5 (186.2) | <0.01 |
| Most recent viral load (log) | 2.2 (1.9) | 2.2 (1.8) | 2.3 (1.8) | 2.1 (2.0) | <0.01 |
| Drug Use (% overall positive) | 67.3 | 59.0 | 71.0 | 69.4 | 0.07 |
| % Methamphetamine | 13.3 | 10.2 | 17.7 | 10.3 | 0.01 |
| % Barbiturates | 2.7 | 2.0 | 3.2 | 2.6 | 0.01 |
| % Benzodiazepines | 12.7 | 14.3 | 11.3 | 12.8 | 0.02 |
| % Cocaine | 45.3 | 42.9 | 51.6 | 38.5 | 0.02 |
| % Opiates | 15.1 | 14.3 | 21.0 | 4.8 | 0.08 |
| Psychiatric Status | |||||
| Major Depressive Disorder (% current) | 13.5 | 8.8 | 14.9 | 16.7 | 0.08 |
| BDI-II Baseline | 12.1 (8.6) | 10.7 (7.4) | 13.5 (9.6) | 11.8 (9.0) | 0.02 |
| BDI-II Follow-up | 10.9 (10.4) | 10.4 (9.0) | 11.0 (11.0) | 11.4 (11.1) | <0.01 |
| BDI-II Change | −.05 (1.07) | .14 (1.33) | −.15 (.96) | −.14 (.83) | 0.02 |
| Neurocognition | |||||
| Mean Baseline | 42.9 (5.6) | 43.1 (5.4) | 42.9 (6.2) | 42.5 (5.1) | <0.01 |
| Mean Follow-up | 43.8 (5.7) | 44.3 (5.4) | 43.5 (6.3) | 43.5 (5.1) | 0.01 |
| Mean Change | .02 (.08) | .03 (.07) | .01 (.06) | .03 (.09) | 0.01 |
| Dispersion Baseline1 | 9.2 (1.9) | 10.0 (1.8) | 9.3 (1.9) | 7.8 (1.5) | 0.18* |
| Dispersion Follow-up2 | 8.9 (2.0) | 7.5 (1.7) | 9.4 (2.0) | 10.0 (1.5) | 0.24* |
| Dispersion Change3 | −.01 (.23) | −.25 (.09) | .03 (.08) | .28 (.18) | 0.75* |
Note. MEMS = Medication Event Monitoring System; BDI-II = Beck Depression Inventory, 2nd Edition;; IQ estimated from the North American Adult Reading Test. Drug use was counted if participants tested positive at least once over the 6-month interval.
Note. Increased DispersionΔ < Stable DispersionΔ, Reduced DispersionΔ.
Note. Stable DispersionΔ Reduced DispersionΔ < Increased DispersionΔ.
Note. Reduced DispersionΔ < Stable DispersionΔ < Increased DispersionΔ.
Note. p < 0.05
Measures
Participants completed a neuropsychological battery consisting of several standardized neuropsychological tests. See Table 2 for a list of subtests and neurocognitive domains.
Table 2.
Neuropsychological subtests and domains
| Domain | Test Name | Variable | Normative Data |
|---|---|---|---|
| Information Processing Speed | WAIS-III | Digit Symbol | Taylor & Heaton, 2001 |
| WAIS-III | Symbol Search | Taylor & Heaton, 2001 | |
| TMT | Part A | Heaton et al., 2004 | |
| Learning and Memory | CVLT/CVLT-II | Trials 1–5 Total | Test Manual |
| CVLT/CVLT-II | LDFR Trial | Test Manual | |
| Attention | WAIS-III | Letter-Number | Taylor & Heaton, 2001 |
| WAIS-III | Digit Span | Taylor & Heaton, 2001 | |
| PASAT | Series 1 | Diehr et al., 2003 | |
| Verbal Fluency | COWAT | FAS | Heaton et al., 2004 |
| Executive Functioning | TMT | Part B | Heaton et al., 2004 |
| Stroop | Interference Trial | Test Manual | |
| WCST-64 | Perseverative Errors | Test Manual | |
| Motor Functioning | GPT | Dominant Hand | Heaton et al., 2004 |
| GPT | Nondominant Hand | Heaton et al., 2004 |
Note. WAIS-III = Wechsler Adult Intelligence Scale – Third Edition; TMT = Trail Making Test; CVLT-II = California Verbal Learning Test Second Edition; LDFR = Long Delay Free Recall; PASAT = Paced Auditory Serial Addition Test; COWAT = Controlled Oral Word Association Test; WCST = Wisconsin Card Sorting Test; GPT = Grooved Pegboard Test
Medication adherence was assessed using the Medication Event Monitoring System (MEMS cap) system. The MEMS pill bottle includes a programmed microchip embedded in its cap that records the date and time of each bottle opening. One prescribed medication was assigned for each participant with priority based on medication type in the following order: protease inhibitors, nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and nucleotide reverse transcriptase inhibitors. Data from the microchip were downloaded at monthly follow-up visit. Adherence rates for each interval were determined by percent of doses recorded by the MEMS cap relative to the total number of doses prescribed. Monthly adherence rates (i.e., at each of the six 1-month intervals) as well as an overall 6-month mean adherence rate were calculated.
Several measures of dispersion were computed. First, neurocognitive dispersion was calculated both as variability at baseline and as change in variability over time. Baseline neurocognitive dispersion was defined as the standard deviation of the 18 neuropsychological test T-scores, following the method described prior work on dispersion studies (Hilborn et al., 2009; Morgan et al., 2011). Higher dispersion scores indicate greater variability, which suggests that individuals exhibited less consistency across subtests. Conversely, lower dispersion scores indicate relatively consistent and stable performance across tests. Change in neurocognitive dispersion was conceived as a change score to account for baseline performance. To calculate this variable, we subtracted the dispersion score at 6-month follow-up from the baseline dispersion score and divided the difference by the baseline score (DispersionΔ). We also examined the change score for global neuropsychological test performance. First, global neuropsychological T-scores were computed for both baseline and 6-month follow-up by averaging performance across all neuropsychological tests at each time point. Change in global neurocognition was defined as the difference between the two baseline and 6-month follow-up neurocognitive score that was then divided by the baseline score (MeanΔ).
Data Analysis
Baseline and 6-month mean and dispersion scores as well as change in mean and dispersion scores were all normally distributed. Simple correlations were run to confirm that mean performance and dispersion both correlated with adherence. Hierarchical linear regression models were used for the main analysis with overall MEMS adherence serving as the outcome variable. Given that a large proportion of the sample tested positive for use of at least one drug throughout the entire evaluation, positive drug use as determined by a urinalysis test in any of the six evaluations was entered as a covariate. Change in the BDI-II score (BDI-2Δ) was also entered as a covariate given the known association between depression and medication adherence (Safren et al., 2009). We entered the MeanΔ variable into the second block, and the DispersionΔ variable into the third block to test their incremental value to the regression model.
Secondary analyses were then conducted to further characterize the DispersionΔ variable. A cutoff score of a 0.5 standard deviation above the average DispersionΔ score within the HIV sample was set to characterize individuals with higher levels of dispersion change (Increased DispersionΔ) while a score of a 0.5 standard deviation below the average DispersionΔ score represented individuals, and whose dispersion dropped between the intervals (Reduced DispersionΔ). Individuals between these two values were labeled the Stable DispersionΔ group. We used a series of univariate analyses to compare the Increased DispersionΔ, Stable DispersionΔ, and Reduced DispersionΔ groups on demographic and clinical variables. Following this, a mixed-model ANOVA examined medication adherence with each month of the 6 intervals serving as the within-subjects factor and DispersionΔ group serving as the between-subjects factor.
Results
Pearson correlations confirmed that increases in mean neurocognition positively correlated with adherence, r(148) = .19, p = .019, while increases in dispersion negatively correlated with adherence, r(148) = −.18, p = .024. When drug use, BDI-2Δ, MeanΔ and DispersionΔ variables were entered as predictors of overall adherence, the overall model was significant, adjusted R2 = .152, p < .001. As seen in Table 3, both increase in depressive symptoms, β = −.17, p = .039 and active drug use, β = −.26, p = .002 predicted poorer overall medication adherence across all three blocks. In addition, there was a negative association between change in dispersion of neurocognitive scores and adherence, β = −.22, p = .005 that significantly contributed to the model after accounting for drug use, change in depression, and change in mean neurocognitive performance, R2 change = .049, p = .005. The results confirmed that participants who exhibited an increase in their neurocognitive test score dispersion had poorer mean 6-month adherence rates. The association between change in mean neurocognition and adherence was non-significant although a trend remained, (β = .14, p = .087).
Table 3.
Predictors of Medication Adherence
| Block | Predictor |
Adjusted R2 |
ΔR2 | F | Significant F change |
Beta Weight |
p |
|---|---|---|---|---|---|---|---|
| 1 | .092 | 8.18 | <.001 | ||||
| BDI-2Δ | −.17 | .039 | |||||
| Positive Drug Test (any) | −.26 | .002 | |||||
| 2 | .109 | .017 | 6.74 | .06 | <.001 | ||
| BDI-2Δ | −.16 | .044 | |||||
| Positive Drug Test (any) | −.25 | .003 | |||||
| MeanΔ | .15 | .062 | |||||
| 3 | .152 | .043 | 7.33 | <.01 | <.001 | ||
| BDI-2Δ | −.17 | .033 | |||||
| Positive Drug Test (any) | −.26 | .001 | |||||
| MeanΔ | .14 | .087 | |||||
| DispersionΔ | −.22 | .005 |
Note. BDI-2Δ = change of the Beck Depression Inventory – Second Edition; MeanΔ = change of mean neuropsychological performance; DispersionΔ = change of the dispersion among subtest scores.
The DispersionΔ variable was next split into groups comprising an Increased DispersionΔ group (z-score ≥ 0.5; mean change = .28; n = 39), a Stable DispersionΔ group (−0.5 < z-score < 0.5; mean change = .03; n = 62), and a Reduced DispersionΔ group (z-score ≤ −0.5; mean change = −.25; n = 49). See Table 2 for group differences. There were no significant differences between the groups on most demographic and clinical variables although there were more women in the Increased DispersionΔ group.
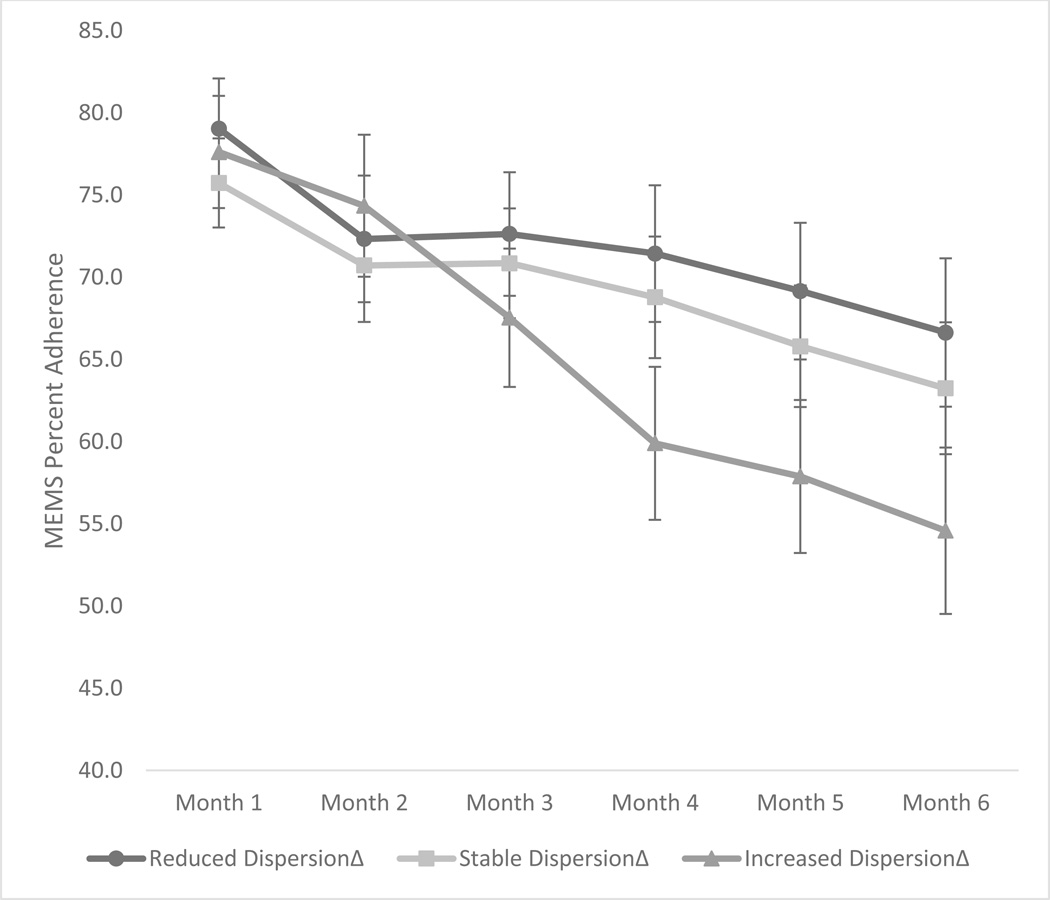
Mixed-model ANCOVA found that gender was not significantly associated with adherence and so it was not retained as a covariate. Mixed-model ANOVA with group (Increased, Stable, Reduced DispersionΔ) as the between subjects factor and time (monthly adherence rates) as the within subjects factor found a significant main effect for time, F(5, 735) = 18.85, p < .001, partial η2 = .11 though the main effect for group was not significant, F(2, 147) = 0.86, p = .425. However, there was a significant group by time interaction effect, F(10, 483) = 1.86, p = .047, partial η2 = .03. As seen in Figure 1, which illustrates the plot of the interaction effect, groups exhibited similar adherence rates during the first two months. However, by the third month, there was a dramatic drop in adherence for the Increased DispersionΔ group that continued to decrease each subsequent month at a sharper rate than the Stable or Reduced DispersionΔ groups. By the sixth month, there was over a 12-point discrepancy in adherence rates between the Increased DispersionΔ (M = 54.6, SD = 33.8) and Reduced DispersionΔ (M = 66.6, SD = 30.3) groups.
Figure 1.
Medication adherence across six months stratified by increased, stable, and reduced % dispersion groups.
Note. MEMS = Medication Event Monitoring System
Note. The y-axis has been truncated for effect.
We next compared the percentage of participants in each group who declined form adequate adherence (defined at 90% or better) to sub-optimal adherence (<90%). Results revealed that 10.0% of the Stable and 8.1% of the Reduced DispersionΔ participants who initially demonstrated adequate adherence became sub-optimal adherers at six-month follow-up. In contrast, 20.5% of the Increased DispersionΔ participants, or over twice that of the other two groups, converted to sub-optimal adherence levels over the same time frame.
Discussion
Intra-individual variability (IIV) is an emerging topic of interest in the neuropsychological literature. The current study examined if longitudinal changes in neurocognitive dispersion may signal declines in medication adherence. While cross-sectional research has reported that IIV during a single assessment predicted outcome (Ettenhofer et al., 2010; Morgan et al., 2012), to our knowledge no study has focused specifically on the potential role of longitudinal changes in IIV. As expected, declines in mean neurocognitive performance predicted worse medication adherence (Hinkin et al., 2002; Woods et al., 2009). Increases in dispersion also predicted adherence, indicating that this variable contributed unique variance to the model. Therefore, increases in neurocognitive dispersion may important to assess when medication adherence is a concern in HIV populations.
Consistent with prior work, adherence rates were found to drop over the six-month time period for all participants, which in part may be related to demotivation in study participation and other factors (Becker et al, 2011). However, the mixed-model group analyses indicated that individuals with increases in dispersion demonstrated a pronounced drop in adherence by the third month that was not observed in participants whose dispersion levels either did not substantially change or diminished over the same time period. Further descriptive analyses revealed that over the six months, more than twice as many participants with increasing IIV became poor adherers than participants with stable or decreasing IIV. This further illustrates the deleterious impact of increasing dispersion on longitudinal medication adherence. Of some significance, neither mean level performance nor changes in mean level performance differentiated the three groups, suggesting that increasing dispersion may be a significant clinical factor even when mean scores have yet to be affected. It is also noteworthy that differences over the six time points in adherence rates between the Increased and Stable/Reduced DispersionΔ groups did not appear during the first two months. This finding is in line with the notion that cognitive changes relating to the acceleration of dispersion scores over time may not have been present at baseline or in the earlier months of follow-up for the Increased DispersionΔ group.
Results suggest that increasing neurocognitive IIV over time is linked to adherence behaviors in HIV infection. The mechanisms underlying increased IIV likely reflect the heterogeneity of cognitive decline in HIV (Morgan et al., 2012). Individuals with higher variability may be experiencing mild or temporary cognitive perturbations that do not yet translate to generalized impairment. However, these perturbations nonetheless impact day-to-day functioning. For example, patients with more cognitive variability may have difficulty with adherence because they sometimes but not always have the ability to maintain focus, remember adherence schedules, organize pill boxes, and similar strategies. In other words, variability across neuropsychological tests may translate into variability with medication management. Other measures of IIV, such as inconsistency in reaction time tasks, have been tied to neuroanatomical changes in both white and gray matter (Nilsson et al., 2014; Stuss et al., 2003). It remains to be seen if within-subtest IIV also has existing neurobiological correlates, though it is possible that white matter disruptions tied to frontal-subcortical dysfunction also provoke erratic subtest performance in HIV/AIDS.
The current findings have potential clinical implications, as neuropsychologists may be able to assess for and identify those HIV+ individuals who are most at risk for suboptimal medication adherence by examining changes in dispersion scores over time. These individuals should be targeted for interventions aimed at compensating for cognitive difficulties and improving medication adherence. There is some evidence that cognitive neurorehabilitation of HAND can improve mean level neurocognitive performance (Weber, Blackstone, & Woods, 2013) though less is known about IIV. Research on other clinical populations typified by high levels of cognitive variability may provide some direction. For example, self-monitoring techniques can assist individuals with attention deficit/hyperactivity disorder (Gureasko-Moore et al., 2006) and may similarly be beneficial for patients with HIV who have adequate cognitive reserve. For those with more severe mean neurocognitive impairment, external support systems that provide explicit structure for one’s daily routine, including medication schedules, may be more appropriate. Medication adherence can be improved through the use of pill boxes and electronic reminder systems. Given that dispersion likely represents temporary lapses in cognitive domains, patients who exhibit increased IIV yet do not have noticeable drops in mean levels of cognition may require similar compensatory approaches to treatment. Regardless, it remains to be seen whether rehabilitation efforts might also lower clinically significant levels of IIV as cognitive restoration takes place
This study has some limitations, such as the predominance of men versus women in our sample, which may limit the generalizability of these findings to HIV+ females though it should be noted that the percentage of women in our sample exceeded base rates for the Los Angeles catchment area. However, gender was not a significant covariate in our repeated measures ANOVA analysis and so as a variable does not appear to affect our results. Additionally, while IIV may certainly be a precursor for emerging neurologic illness, it is possible that some of the dispersion on neurocognitive tests may be due to normal IIV and reduced test-retest reliability of certain tests. Furthermore, we had no control group to compare with the HIV sample. Finally, the effect size for differences in adherence among the three groups was modest, and we do not have additional longitudinal data past the six-months. Nonetheless, this study also has several strengths, including the use of an objective measure of medication adherence as opposed to self-report, the longitudinal nature of the data, and inclusion of a large sample of African American individuals who are relatively understudied in the HIV medication adherence literature.
In summary, clinicians should be attuned to and directly assess for IIV in HIV+ patients’ neurocognitive test performance in order to help prevent the cognitive and functional morbidity associated with poor adherence. In addition, our results present evidence that increases in IIV over time may be clinically relevant in evaluating cognitive changes that predict poor adherence. Further research on IIV change is required, including identifying potential “cutoff” points in which IIV increases and then declines, as would be expected in individuals who transition to moderate to severe dementia and fail across multiple neurocognitive measures. Studies of neuroimaging correlates of increased dispersion over time are also warranted to provide a more in-depth understanding of this phenomenon.
Acknowledgments
This work was supported by National Institutes of Health grant R01 DA13799 and the National Institutes of Health Ruth L. Kirschstein National Research Service award T32 MH19535.
Contributor Information
Nicholas S. Thaler, Department of Psychiatry and Biobehavioral Sciences, UCLA Geffen School of Medicine, Los Angeles, CA
Philip Sayegh, Department of Psychiatry and Biobehavioral Sciences, UCLA Geffen School of Medicine, Los Angeles, CA.
Alyssa Arentoft, Department of Psychiatry and Biobehavioral Sciences, UCLA Geffen School of Medicine, Los Angeles, CA.
April D. Thames, Department of Psychiatry and Biobehavioral Sciences, UCLA Geffen School of Medicine, Los Angeles, CA
Steven A. Castellon, Department of Psychiatry and Biobehavioral Sciences, UCLA Geffen School of Medicine, Los Angeles, CA; Department of Psychology, VA Greater Los Angeles Healthcare System, Los Angeles, CA
Charlie H. Hinkin, Department of Psychiatry and Biobehavioral Sciences, UCLA Geffen School of Medicine, Los Angeles, CA; Department of Psychology, VA Greater Los Angeles Healthcare System, Los Angeles, CA
References
- Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS and Behavior. 2011;15(8):1888–1894. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Dew MA, Aizenstein HJ, Lopez OL, Morrow L, Saxton J, Tárraga L. A pilot study of the effects of internet-based cognitive stimulation on neuropsychological function in HIV disease. Disability and rehabilitation. 2012;34(21):1848–1852. doi: 10.3109/09638288.2012.667188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JM, Bruce AS, Arnett PA. Response variability is associated with self- reported cognitive fatigue in multiple sclerosis. Neuropsychology. 2010;24:77–83. doi: 10.1037/a0015046. [DOI] [PubMed] [Google Scholar]
- Bunce D, Anstey KJ, Christensen H, Dear K, Wen W, Sachdev P. White matter hyperintensities and within-person variability in community-dwelling adults aged 60–64 years. Neuropsychologia. 2007;45:2009–2015. doi: 10.1016/j.neuropsychologia.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology. 2010a;74:1217–1222. doi: 10.1212/WNL.0b013e3181d8c1ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenhofer ML, Foley J, Behdin N, Levine AJ, Castellon SA, Hinkin CH. Reaction time variability in HIV-positive individuals. Archives of Clinical Neuropsychology. 2010b;25:791–798. doi: 10.1093/arclin/acq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis-I DSM-IV Disorders—Patient Edition (SCID-I/P) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Gureasko-Moore S, Dupaul GJ, White GP. The effects of self-management in general education classrooms on the organizational skills of adolescents with ADHD. Behavior modification. 2006;30(2):159–183. doi: 10.1177/0145445503259387. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn J, Strauss E, Hultsch DF, Hunter MA. Intraindividual variability across cognitive domains: Investigation of dispersion levels and performance profiles in older adults. Journal of Clinical and Experimental Neuropsychology. 2009;31:412–424. doi: 10.1080/13803390802232659. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Stefaniak M. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl. 1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Hardy D, Barclay T, Reinhard M, Cole M, Hinkin C. Elements of attention in HIV-infected adults: Evaluation of an existing model. Journal of Clinical and Experimental Neuropsychology. 2008;30:53–62. doi: 10.1080/13803390601186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Li SC, Bäckman L. Neural underpinnings of within-person variability in cognitive functioning. Psychology and aging. 2009;24(4):792–808. doi: 10.1037/a0017798. [DOI] [PubMed] [Google Scholar]
- Manly T, Davison B, Heutink J, Galloway M, Robertson IH. Not enough time or not enough attention? Speed, error and self-maintained control in the Sustained Attention to Response Test (SART) Clinical Neuropsychological Assessment. 2000;3:167–177. [Google Scholar]
- Matarazzo JD, Prifitera A. Subtest scatter and premorbid intelligence: Lessons from the WAIS—R standardization sample. Psychological Assessment. 1989;1:186–191. [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, Bondi MW, Grant I. Intraindividual variability in HIV infection: evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25:645–654. doi: 10.1037/a0023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Grant I. Intra-individual neurocognitive variability confers risk of dependence in activities of daily living among HIV-seropositive individuals without HIV-associated neurocognitive disorders. Archives of Clinical Neuropsychology. 2012;27:293–303. doi: 10.1093/arclin/acs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Thomas AJ, O’Brien JT, Gallagher P. White matter and cognitive decline in aging: a focus on processing speed and variability. Journal of the International Neuropsychological Society. 2014;20:262–267. doi: 10.1017/S1355617713001458. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Sano M, Silverman JM, Haroutunian V. Cross-domain variability of cognitive performance in very old nursing home residents and community dwellers: Relationship to functional status. Gerontology. 2005;51:206–212. doi: 10.1159/000083995. [DOI] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. Journal of neurovirology. 2002;8(2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, Mayer KH. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychology. 2009;28:1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Munro CA, Anthony JC, Pearlson GD. Examining the range of normal intraindividual variability in neuropsychological test performance. Journal of the International Neuropsychological Society. 2003;9:864–870. doi: 10.1017/S1355617703960061. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Pogue J, Buckle L, Bondar J. Characterization of stability of performance in patients with traumatic brain injury: Variability and consistency on reaction time tests. Neuropsychology. 1994;8:316–324. [Google Scholar]
- Stuss DT, Toth JP, Franchi D, Alexander MP, Tipper S, Craik FI. Dissociation of attentional processes in patients with focal frontal and posterior lesions. Neuropsychologia. 1999;37(9):1005–1027. doi: 10.1016/s0028-3932(98)00158-4. [DOI] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Ross LA, Wadley VG, Ball KK. Speed of processing training with middle-age and older adults with HIV: a pilot study. Journal of the Association of Nurses in AIDS Care. 2012;23(6):500–510. doi: 10.1016/j.jana.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Woods SP. Cognitive neurorehabilitation of HIV-associated neurocognitive disorders: a qualitative review and call to action. Neuropsychology review. 2013;23(1):81–98. doi: 10.1007/s11065-013-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]