Abstract
Purpose
Thinning of the RPE and the underlying vascular layer, the choroid, is observed with age in many human eye disorders. The reasons for this thinning are ill-defined. Here, we highlight the possible role of T lymphocyte recruitment in choroidoretinal thinning in aged and light-challenged mice.
Methods
In age and light challenge models, we measured chemokine concentrations using enzyme-linked immunosorbent assay and used flow cytometry to characterize lymphocyte populations. We quantified thinning in eye immunosections and RPE65 expression using quantitative PCR.
Results
Age and light challenge led to increased levels of the lymphotactic protein CXCL10 alone (aging) or in conjunction with CXCL9 (light challenge). Increased numbers of CD3+ T lymphocytes, most of them CD8+ cytotoxic T lymphocytes, were also observed in the choroid and retina of old mice and following light challenge. Influx of T lymphocytes was associated with RPE and choroidal thinning and diminished expression of RPE65 mRNA, an essential enzyme of the visual cycle.
Conclusions
The observations from this study suggest that cytotoxic CD8+ T lymphocytes might participate in choroidal and RPE degeneration and that modulation of T lymphocyte recruitment might be a novel strategy to reduce choroidoretinal dysfunctions observed with age and following photo-oxidative stress.
Introduction
The choroidal vasculature is essential when it comes to bringing oxygen and nutrients to the functioning retina and evacuating debris resulting from the normal visual cycle. Choroidal thinning is a common feature in many human eye diseases, including high myopia [1,2] and retinitis pigmentosa [3,4], and has been reproducibly observed with age [5-7]. However, the association between choroidal thinning and age-related macular degeneration (AMD) remains controversial. Some authors have reported the loss of choriocapillaries in eyes with exudative AMD [8], and choroidal thinning has been detected in some studies [9-11]. Choroidal thinning has also been associated with geographic atrophy (GA), the dry form of late AMD [12-15]. A morphometric analysis by Ramrattan et al. more than two decades ago showed a decrease in choriocapillary density and diameter with age and in GA, but choroidal thinning was only significant with age [6]. Moreover, it has been reported that the choriocapillaries and choroid are thinner in areas where the RPE has degenerated [8]. However, all studies agree that aging is associated with significant choroidal thinning [16-18]. The exact mechanisms behind choroidal thinning with age or disease are not clear.
The RPE is a monolayer of pigmented cells situated between photoreceptors and Bruch’s membrane; its plays an essential role in the visual cycle. RPE65, which is also called 11-cis retinol isomerase and is strongly expressed in the RPE, participates in the production of 11-cis retinal [19], which is essential for photoreceptor function [20]. Mutations in the RPE65 gene cause progressive photoreceptor degeneration [21,22] and adult RPE65−/− mice develop degenerative RPE changes that are also observed in aged wild-type mice to a lesser degree [23]. The RPE also plays an important role in the maintenance of the choroid, secreting angiogenic factors such as vascular endothelial growth factor and cyclooxygenase-2 [24,25]. The primary insult in GA appears to be at the level of the RPE and the choriocapillaris [8,26], with the most obvious change being the patchy loss of RPE visible in fundoscopy.
No treatment for GA exists at the moment [27]. GA is a complex multifactorial event influenced by aging [28], smoking history [29], oxidative stress [30], and genetic polymorphisms [31-33]. Moreover, macrophage recruitment plays a role in the physiopathology of GA [34]. Recently, animal and human studies have also suggested that T lymphocytes directed toward the retina or the RPE/choroid interface could be involved in the pathogenesis of AMD [35,36]. In mice, a microarray study of gene expression has shown that several mRNAs specific to T cells (CD3, CD8, T-cell receptor) and T cell–chemoattractants (CXCL9, CXCL10) are overexpressed in the RPE/choroid complex and retina during aging [37]. In addition, it has been reported that moderate light challenge induces mild T-cell infiltration in albino rats [38]. Recently, Cruz-Guilloty et al. have observed carboxyethylpyrrole (CEP)-specific T cells in the eyes of CEP-immunized mice in vivo [39]. In humans, lymphocytes, including CD8+ cells, have been observed in the choroid of eyes from AMD patients [40,41].
In the present study, we investigated the potential association between T-cell recruitment and RPE and choroidal thinning and dysfunction in aged mice and a model of photo-oxidative stress. Using flow cytometry, we demonstrated that T cells are indeed recruited in the choroid of mice following aging or light exposure. Increased numbers of T lymphocytes were correlated with enhanced levels of the lymphocyte-chemoattractant cytokine CXCL10 alone or in association with CXCL9. Moreover, the influx of T lymphocytes was associated with choroidal thinning and a reduction of RPE65 mRNA expression or RPE thinning. Our results suggest that T lymphocytes could participate in choroid/RPE alterations and consequent degeneration associated with age or following photo-oxidative stress.
Methods
Animals
C57BL/6J mice (without the retinal degeneration 8 [Rd8] mutation) were obtained from Janvier Labs (Le Genest St Isle, France). All animals were housed in cyclic 12h:12h normal animal facility conditions (50–250 lux) with food and water available ad libitum. Animal experiments were approved by the local Institutional Animal Care and Use Committee and all studies were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Animals used for aging experiments were between 12 and 18 months old.
The light-challenge model
Three-month-old C57BL/6J mice were adapted to complete darkness for 6 h and pupils were fully dilated with 1% atropine (Novartis, Nanterre, France). Mice were then exposed to green light-emitting diode (LED) light (4500 Lux, JP Vezon Équipements, Chamalières, France) for 4 days and subsequently kept in cyclic 12h:12h normal animal facility conditions. The typical dominant LED wavelength is 505 nm (min = 500 nm; max = 510 nm). RPE and choroidal layer thicknesses were assessed histologically at 21 days after light exposure.
Flow cytometry
Animals, anesthetized using intraperitoneal (IP) pentobarbital (100 mg/kg), were perfused with a Dulbecco's PBS 1X (NaCl 137 mmol/l, KCl 2.7 mmol/l, Na2HPO4 10 mmol/l, KH2PO4 1.8 mmol/l) intracardiac injection to eliminate the blood before enucleation. The isolated fresh RPE/choroid complex or inner retina was digested in a 3 ml digestion cocktail (0.05% Liberase [Sigma-Aldrich], 0.025 U/ml DNase I [Sigma-Aldrich, St Quentin Fallavier, France]) at 37 °C for 30 min before being passed through a cell strainer to obtain a single cell suspension. After washing and centrifugation, cells were incubated in ice-cold PBS medium containing 5 mM EDTA (Sigma), 1% fetal calf serum (FCS), 3% normal rat and mouse serum, and 10% mouse Seroblock FcR (anti-CD16/CD32, Abd Serotec, Kidlington, UK). Cells were then stained with anti-CD3-PerCPCy5.5, CD4-PE, and CD8-allophycocyanin (APC; BD Biosciences, Le Pont de Claix, France) to discriminate the lymphocyte main subpopulations (CD4+ “helper” and CD8+ “cytotoxic” cells); they were analyzed using an LSRII Flow Cytometer (BD Biosciences). First, cells were analyzed using the forward-scattered light (FSC) parameter, which is proportional to cell-surface size, and the side-scattered light (SSC) parameter, which is proportional to cell granularity. Doublet cell exclusion gates were applied so that each dot on flow cytometry corresponded to a unique cell. Next, cells with the morphometric characteristics of lymphocytes were analyzed for expression of the T-lymphocyte marker CD3+ and for their CD8 and CD4 expressions using Flow Jo V7.9 software. The number of cells per run was between 500,000 and 1,000,000 for pooled retinas and about 500,000 for pooled choroids.
Histology
Eyes were fixed in 0.5% glutaraldehyde and 4% paraformaldehyde for 2 h, dehydrated, and mounted in Historesin (Leica, Nanterre, France). Five-micrometer oriented sections crossing the inferior pole, optic nerve, and superior pole were cut and stained with toluidine blue. Photomicrographs were taken along the whole section and all thicknesses were measured every 400 µm between -3,000 µm and +3,000 µm (distance from the optic nerve, 0 µm). Images were captured with a DM5500 microscope (Leica) and analyzed using Metamorph software (Molecular Devices). Choroidal and RPE thicknesses were measured and evaluated as the area under the curve (AUC).
Enzyme-linked immunosorbent assay
Murine CXCL9 and CXCL10 concentrations were measured in retina and choroid lysates using multiplex assay following the manufacturer’s protocol (Luminex, Biosource, Saint Aubin, France). For the aging protocol, we used eight eyes from eight different mice per group in two independent experiments. For the illumination protocol, we used four eyes from four mice per group in two independent experiments.
RNA isolation and quantitative PCR
Total RNA was isolated with Nucleospin RNAII (Macherey Nagel, Hoerdt, France). Single-stranded cDNA was synthesized from total RNA (pretreated with DNaseI amplification grade) using oligo-dT as a primer and SuperScript® reverse transcriptase (RT) (Life Technologies, Saint Aubin, France). Subsequent RT–PCR was performed using cDNA, SYBR® green Gene Expression Master Mix (Life Technologies) and primers (0.5 pmol/ml). Results were normalized via the expression of β-actin. PCR reactions were performed in 45 cycles of 15 s at 95 °C and 45 s at 60 °C. The primers used for PCR were as follows: Actin forward primer, 5′-AAG GCC AAC CGT GAA AAG AT-3′; Actin reverse primer, 5′-GTG GTA CGA CCA GAG GCA TAC-3′; RPE65 forward primer, 5′-TGT GGC AAG AGC CAG ATT C-3′; and RPE65 reverse primer, 5′-GCT CAC CAC CAC ACT CAG AA-3′.
Statistics
Graph Pad Prism 6 (GraphPad Software, La Jolla, CA) was used for data analysis and graphic representation. All values are reported as means ± standard error of the mean (SEM). Statistical comparisons used unpaired t tests, non-parametric Mann–Whitney, or one-way analysis of variance (ANOVA) followed by Tukey multiple comparison post hoc tests, as indicated in the figure legends, and p values less than 0.05 were considered statistically significant. RPE, choroidal, and outer nuclear layer (ONL) thicknesses were calculated as AUCs and tested as described above.
Results
T cells are recruited in the choroid/RPE complex in aged C57BL/6J mice
First, flow cytometry analysis (representative dot plots in Figure 1A) was performed on retinal and choroid/RPE suspensions of young (3 months old) and aged (12–18 months old) C57BL/6J mice (Figure 1B-C). Choroidal CD3+ T cells were predominantly CD8+, wShereas the number of choroidal CD4+ cells did not change significantly (Figure 1B, n= 4-6 pooled sample; ANOVA then Tukey test). As observed in the choroid, CD8+ cells represented the main population of T cells recruited in retinas of aging C57BL/6 mice (Figure 1C, n= 4-6 pooled sample; ANOVA then Tukey test), but the amount of lymphocytes was lower by far than in the RPE/choroid complex. Protein expression of the lymphocyte-chemoattractant CXCL10, but not CXCL9, measured by enzyme-linked immunosorbent assay (ELISA) in RPE choroidal extracts, was significantly increased in the eyes of aged mice (Figure 1D n= 8/group; Mann-Whitney test). These results show a significant, preferential recruitment of CD8+ T cells in the choroid/RPE complex during aging in C57BL/6 mice (the experiments were repeated three times).
Figure 1.
Recruitment of T cells in aged C57BL/6 mice and effects in the chorioretinal layer. A: Flow cytometry analysis. Representative dot plots of CD4 and CD8 expression in CD3+-gated cells from cell suspensions of pooled choroid/RPE complexes from two eyes of 18-month-old C57BL/6 mice. SSC-A: Side-scattered light–area; FSC-H: Forward-scattered light–height; APC: Allophycocyanin; PE: Phycoerythrin. B-C: Absolute quantification of CD4 and CD8+ T cells in pooled choroid/RPE layers from young or aged C57BL/6 mice (B). Absolute quantification of CD4 and CD8+ T cells in pooled inner retina from young or aged C57BL/6 mice (C; n = 4–6 pooled sample, analysis of variance (ANOVA) and Tukey test). D: CXCL9 and CXCL10 protein expression in eyes from young and aged mice (n = 8/group, Mann–Whitney test) 4 days after the onset of illumination. E-F: Representative photomicrograph of 3-month-old C57BL/6 retina (E). Representative photomicrograph of a retina taken at the same distance from the optic nerve of an 18-month-old C57BL/6 mouse (F). G: RPE thickness in µm2 (-3,000 μm: inferior pole, +3,000 μm: superior pole, 0 μm: optic nerve) in young (n = 4) and old C57BL/6J mice (n = 10). Area under the curve (AUC) in young and old C57BL/6 mice (Student t test) in the whole retina and restricted to the central retina. H: Quantitative PCR of RPE65 mRNA of young and aged C57BL/6 mice (n = 5–6 per group, Student t test). I: Choroidal thickness in µm2 (-3,000 μm: inferior pole, +3, 000 μm: superior pole, 0 μm: optic nerve) in young (n = 4) and old C57BL/6J mice (n = 10). AUC in young and old C57BL/6 mice (Student t test) in the whole retina and restricted to the central retina. ONL: Outer nuclear layer; ret; Retina; cho: Choroid, rpe; Retinal pigment epithelium. All scale bars represent 20 µm. All values are represented as mean ± standard error of the mean (SEM), *p≤0.05, **p≤0.01, ***p≤0.001.
The RPE and choroid are thinner in aged than in young C57BL/6 mice
Next, we studied the effects of aging on C57BL/6J mouse eyes. We compared the number of photoreceptor nucleic rows in the ONL, choroidal thickness, and RPE thickness in young and old mice (Figure 1E-F). Photomicrographs were taken at an equal distance from the optic nerve on histological sections (Figure 1E-F). There was no statistical difference in the number of nuclei of photoreceptors between old and young mice (data not shown). In contrast, the RPE cell layer was statistically thinner in old C57BL/6 mice compared to young mice (Figure 1G, young n= 4, aged= 10; Student t test). When restricted to the “central” retina, RPE thickness calculated as the AUC was still significantly reduced with age (Figure 1G). Moreover, analysis of RPE65 mRNA levels (Figure 1H, n= 5-6/group; Student t test) showed a drastic reduction (10-fold) in RPE65 mRNA expression in the RPE/choroid complex of old C57BL/6 mice. The RPE showed patchy depigmentation in 18-month-old mice (Figure 1F), but not in young mice (Figure 1E). Furthermore, we observed a significant reduction of choroidal thickness in old mice compared to young mice (Figure 1I, young n= 4, aged= 10; Student t test). When restricted to the central retina, choroidal thickness calculated as the AUC was no longer significantly reduced with age (Figure 1I); only an age-related tendency was found (p = 0.07). Taken together, our observations show that the observed T-cell accumulation was associated with RPE and choroidal thinning and likely impaired RPE function, as the RPE65 mRNA expression was severely reduced.
T-cell recruitment in the choroid/RPE complex in light-challenged mice
Oxidative stress is one of the recognized risk factors of various ocular diseases [30,42-45]. To determine whether oxidative stress per se could induce T-cell recruitment, we induced oxidative stress through light exposure of 3-month-old C57BL/6 mice (continuous 4,500 lux of green light for 4 days), which does not in itself induce photoreceptor degeneration in pigmented mice, as previously described [34]. Illumination accelerates retinal disorders [46] and induces mild T-cell recruitment in the retina of albino rats [38]. Flow cytometry analysis performed on choroid/RPE and retinal cell suspensions from two eyes confirmed T-cell recruitment (these experiments were repeated three times). As observed in aged mice, recruited T cells in choroids following illumination were predominantly CD8+ cells (Figure 2A, n= 4-6 pooled sample; ANOVA then Tukey test), whereas the number of CD4+ T cells did not significantly increase. In retinal cell suspensions, significantly more T cells (CD4+ and CD8+) were detected in the retinas of illuminated mice than in non-illuminated control mice (Figure 2B, n= 4-6 pooled sample; ANOVA then Tukey test). Similar to our observation in aged mice, the number of T cells recruited in the retina was much lower than that detected in the choroid. Importantly, the expressions of the lymphotactic CXCL9 and CXCL10 chemokines increased significantly after 4 days of illumination (Figure 2C, n= 4/group; Mann-Whitney test).
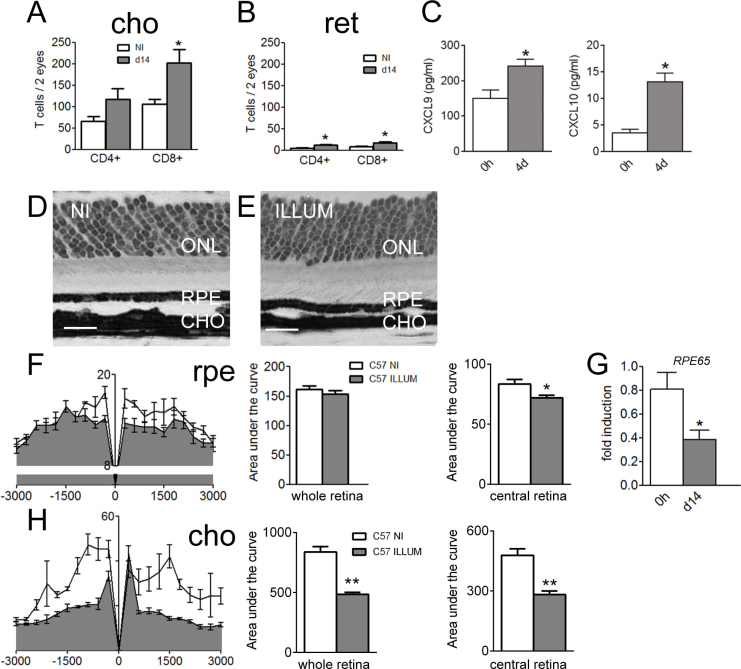
Figure 2.
Recruitment of T cells in illuminated C57BL/6 mice and effects in the chorioretinal layer. A-B: Number of CD4+ and CD8+ in pooled choroidal (A) and pooled retinal (B) cell suspensions from non-illuminated (NI) versus illuminated (d14) C57BL/6 mice (n = 4–6/group; ANOVA and Tukey test). C: CXCL9 and CXCL10 protein expression in eyes from non-illuminated and light-challenged mice (n = 4/group, Mann–Whitney test) 4 days after the onset of illumination. D-E: Representative photomicrographs taken 1,000 μm from the optic nerve of a non-illuminated mouse (E) and from a C57BL/6 mouse 21 days following illumination (D). Scale bar: 20 µm. F: RPE thickness in µm2 in non-illuminated (n = 4) versus illuminated C57BL/6 mice (n = 7). Area under the curve (AUC) in illuminated and non-illuminated C57BL/6 mice (Student t test) in the whole retina and restricted to the central retina. G: Quantitative–PCR of RPE65 mRNA of non-illuminated versus illuminated C57BL/6 mice (n = 5–6 per group, Student t test). H: Choroidal thickness in µm2 (-3,000 μm: inferior pole, +3,000 μm: superior pole, 0 μm; optic nerve) in young (n = 4) and old C57BL/6J mice (n = 7) in the whole retina and restricted to the central retina. The AUC in illuminated and non-illuminated C57BL/6 mice (Student t test). ONL: Outer nuclear layer; ret: Retina; cho: Choroid; rpe; Retinal pigment epithelium. All scale bars represent 20 µm. All values are represented as mean ± standard error of the mean (SEM), *p≤0.05, **p≤0.01, ***p≤0.001.
Light challenge reduces RPE65 mRNA expression and choroidal thicknesses
The influence of light challenge on photoreceptor degeneration and RPE/choroid homeostasis was evaluated. Photomicrographs were taken at equal distance from the optic nerve on histological sections of non-illuminated (Figure 2D) and illuminated C57BL/6 mice (Figure 2E). As previously reported [47], photoreceptor nucleic rows did not change following light exposure (data not shown). The RPE was not significantly thinner in illuminated mice compared to control mice (Figure 2F, non-illuminated n= 4, illuminated n= 7; Student t test) if we analyzed the AUC between -3,000 µm and +3,000 µm (distance from the optic nerve). When restricted to the central retina, RPE thickness calculated as the AUC was significantly reduced in light-challenged compared to non-illuminated mice (Figure 2F). Illuminated C57BL/6 mice displayed a twofold-reduced expression of RPE65 mRNA compared to non-illuminated controls (Figure 2G, n= 5-6/group; Student t test). Choroids were significantly thinner in illuminated compared to non-illuminated mice (Figure 2H, non-illuminated n= 4, illuminated n= 7; Student t test). When restricted to the central retina, choroidal thickness calculated as the AUC was still significantly reduced with light challenge (Figure 2H). In conclusion, recruitment of T cells in the choroid/RPE complexes and retinas of light-challenged mice was associated with choroidal thinning and reduction of RPE65 mRNA expression.
Discussion
Using flow cytometry, we demonstrated that normal aging (Figure 1), as well as light challenge (Figure 2), induces T-cell recruitment in the choroid and retina of pigmented C57BL/6 mice. These observations are in agreement with previous reports from gene expressions studies in aged, pigmented mice [37], as well as with observations from immunohistochemistry of the retina in albino rats following moderate light challenge [38]. During both aging and oxidative stress, we observed that T-cell recruitment was predominantly located in the choroid rather than in the retina. Our flow cytometric analysis also demonstrated that most T cells expressed CD8 rather than CD4. Moreover, we report here that T-cell recruitment correlates with increased expression of the lymphotactic protein CXCL10 alone (aging) or in conjunction with CXCL9 (light challenge). This increase of lymphotactic chemokines might participate in the observed T-cell accumulation.
More importantly, we also report that aging and light-induced oxidative stress induce alterations of the choroid/RPE complexes in mice. Indeed, both aging and light challenge induce choroidal thinning. Interestingly, thinning of the choroid has been reported in humans with age [5-7]; it has also been observed in the dry form of AMD [12,13,15].
We noted a reduction in the thickness of the RPE layer in aging mice. This observation is in agreement with findings of flattened RPE cells, displaying variable sizes and shapes of their nuclei, in OXYS rats (a model of accelerated male senescence) compared to age-matched Wistar rats [48]. We also observed a drastic reduction (10 times) of the RPE65 mRNA expression in aging compared to young mice. Interestingly, adult RPE65−/− mice have been shown to develop degenerative RPE changes that are also observed in aged wild-type mice to a lesser degree [23]. It is therefore tempting to speculate that the observed age-dependent decrease in RPE65 expression in wild-type mice participates in the degenerative RPE changes. These findings confirm that aging itself induces general alterations of the entire choroid/RPE complex. In our photo-oxidative stress model, we noted a tendency toward diminution in the RPE thickness, but this did not reach significance. However, there was a twofold reduction of the RPE65 mRNA expression following light challenge. Possibly, increasing the time or intensity of light exposure could reduce the RPE layer thickness significantly. Here, we used RPE65 mRNA level as a marker of RPE function. Its decrease reflects age- and light-induced RPE dysfunction, but we do not show or propose that RPE65 mRNA levels are directly responsible for RPE thinning or eventual electroretinogram (ERG) changes.
In aging and following light challenge, the RPE/choroid complex alterations were associated with cytotoxic T-cell influx, as discussed above. It is tempting to speculate that the chronic presence of cytotoxic T cells in the choroid participates in the observed choroidal thinning and RPE65 mRNA expression differences. Further studies are needed to determine the role and precise mechanisms of action of T lymphocytes. One hypothesis could involve interleukin (IL)-17 production by T cells, which could participate in RPE atrophy [49], but other studies have shown that cytotoxic T cells could also kill RPE cells via Fas/Fas Ligand (Fas/FasL or CD95/CD95L) interaction [50]. Interestingly, Gregerson et al. [51] showed that cytotoxic CD8+ lymphocytes could kill murine RPE cells in vitro via the induction of apoptosis. Furthermore, CD8+ cells have been shown in the choroid of frozen sections of donor eyes with drusen and fibrovascular scarring [52]. The recent proteomic comparison between thick and thin RPE/choroids from donor eyes by Sohn et al. [13] demonstrated lower levels of serine protease inhibitors (SERPINs) in thin RPE/choroids. Low protease inhibitor levels could allow recruited cytotoxic T cells to exert their deleterious effects by allowing, for example, granzyme-dependent fibronectin cleavage of choroidal endothelial cell membranes [53].
The association of cytotoxic T lymphocytes with choroidal thinning needs further development to decipher the precise mechanisms of lymphocyte-dependent choroid/RPE complex thinning and alterations with age and during photo-oxidative stress. However, from these observations and data from the literature, we propose that the modulation of T lymphocyte recruitment or activation might be a novel strategy to reduce the choroid/RPE dysfunctions observed in several eye diseases.
Acknowledgments
The authors wish to thank the Plateforme « Phénotypage du petit animal et Microdosages » of Hôpital Saint-Antoine, Paris. This work was supported by grants from INSERM, ANR Maladies Neurologiques et Psychiatriques (ANR-08-MNPS-003), ANR Geno 2009 (R09099DS), and ERC starting Grant (ERC-2007 St.G. 210345). Author contributions: S.C. and W.R. designed the study, performed experiments, analyzed data and wrote the paper; B.C., S.L.L., E.Do, J.H., E.De. and X.G. performed experiments and/or analyzed data; F.S. designed the study, analyzed data and wrote the paper. Dr. Raoul (william.raoul@univ-tours.fr) and Dr. Camelo (sergecamelo2@hotmail.com) are co-corresponding authors for this paper.
References
- 1.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Nishida Y, Fujiwara T, Imamura Y, Lima LH, Kurosaka D, Spaide RF. Choroidal thickness and visual acuity in highly myopic eyes. Retina. 2012;32:1229–36. doi: 10.1097/IAE.0b013e318242b990. [DOI] [PubMed] [Google Scholar]
- 3.Ayton LN, Guymer RH, Luu CD. Choroidal thickness profiles in retinitis pigmentosa. Clin Experiment Ophthalmol. 2013;41:396–403. doi: 10.1111/j.1442-9071.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- 4.Dhoot DS, Huo S, Yuan A, Xu D, Srivistava S, Ehlers JP, Traboulsi E, Kaiser PK. Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol. 2013;97:66–9. doi: 10.1136/bjophthalmol-2012-301917. [DOI] [PubMed] [Google Scholar]
- 5.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–9. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–64. [PubMed] [Google Scholar]
- 7.Ruiz-Medrano J, Flores-Moreno I, Pena-Garcia P, Montero JA, Duker JS, Ruiz-Moreno JM. Macular choroidal thickness profile in a healthy population measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:3532–42. doi: 10.1167/iovs.14-13868. [DOI] [PubMed] [Google Scholar]
- 8.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–91. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118:840–5. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Coscas F, Puche N, Coscas G, Srour M, Francais C, Glacet-Bernard A, Querques G, Souied EH. Comparison of macular choroidal thickness in adult onset foveomacular vitelliform dystrophy and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:64–9. doi: 10.1167/iovs.13-12931. [DOI] [PubMed] [Google Scholar]
- 11.Fein JG, Branchini LA, Manjunath V, Regatieri CV, Fujimoto JG, Duker JS. Analysis of short-term change in subfoveal choroidal thickness in eyes with age-related macular degeneration using optical coherence tomography. Ophthalmic Surg Lasers Imaging Retina. 2014;45:32–7. doi: 10.3928/23258160-20131220-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adhi M, Lau M, Liang MC, Waheed NK, Duker JS. Analysis of the thickness and vascular layers of the choroid in eyes with geographic atrophy using spectral-domain optical coherence tomography. Retina. 2014;34:306–12. doi: 10.1097/IAE.0b013e3182993e09. [DOI] [PubMed] [Google Scholar]
- 13.Sohn EH, Khanna A, Tucker BA, Abramoff MD, Stone EM, Mullins RF. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014;55:1352–60. doi: 10.1167/iovs.13-13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Lee DH, Lee JY, Yoon YH. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7812–8. doi: 10.1167/iovs.13-12284. [DOI] [PubMed] [Google Scholar]
- 15.Lindner M, Bezatis A, Czauderna J, Becker E, Brinkmann CK, Schmitz-Valckenberg S, Fimmers R, Holz FG, Fleckenstein M. Choroidal thickness in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:875–82. doi: 10.1167/iovs.14-14933. [DOI] [PubMed] [Google Scholar]
- 16.Yiu G. Advances in choroidal imaging with EDI-OCT. Retina Today. 2014 [Google Scholar]
- 17.Jonas JB, Forster TM, Steinmetz P, Schlichtenbrede FC, Harder BC. Choroidal thickness in age-related macular degeneration. Retina. 2014;34:1149–55. doi: 10.1097/IAE.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 18.Yiu G, Chiu SJ, Petrou PA, Stinnett S, Sarin N, Farsiu S, Chew EY, Wong WT, Toth CA. Relationship of central choroidal thickness with age-related macular degeneration status. Am J Ophthalmol. 2015;159:617–26. doi: 10.1016/j.ajo.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–51. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 20.Wolf G. Function of the protein RPE65 in the visual cycle. Nutr Rev. 2005;63:97–100. doi: 10.1111/j.1753-4887.2005.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 21.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 22.Redmond TM. Focus on Molecules: RPE65, the visual cycle retinol isomerase. Exp Eye Res. 2009;88:846–7. doi: 10.1016/j.exer.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivert L, Keldbye H, Gouras P. Age-related changes in the basement membrane of the retinal pigment epithelium of Rpe65 -/- and wild-type mice. Graefe's archive for clinical and experimental ophthalmology = Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2005;243:250–6. doi: 10.1007/s00417-004-0967-y. [DOI] [PubMed] [Google Scholar]
- 24.Houssier M, Raoul W, Lavalette S, Keller N, Guillonneau X, Baragatti B, Jonet L, Jeanny JC, Behar-Cohen F, Coceani F, Scherman D, Lachapelle P, Ong H, Chemtob S, Sennlaub F. CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents. PLoS Med. 2008;5:e39. doi: 10.1371/journal.pmed.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA. 2009;106:18751–6. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35:2562–73. doi: 10.1016/j.neurobiolaging.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:1079–91. doi: 10.1016/j.ophtha.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Bonilha VL. Age and disease-related structural changes in the retinal pigment epithelium. Clin Ophthalmol. 2008;2:413–24. doi: 10.2147/opth.s2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuner B, Komm A, Wellmann J, Dietzel M, Pauleikhoff D, Walter J, Busch M, Hense HW. Smoking history and the incidence of age-related macular degeneration--results from the Muenster Aging and Retina Study (MARS) cohort and systematic review and meta-analysis of observational longitudinal studies. Addict Behav. 2009;34:938–47. doi: 10.1016/j.addbeh.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the Proctor lecture. Invest Ophthalmol Vis Sci. 2010;51:1275–81. doi: 10.1167/iovs.09-4478. [DOI] [PubMed] [Google Scholar]
- 31.Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–92. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 32.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 33.McKay GJ, Patterson CC, Chakravarthy U, Dasari S, Klaver CC, Vingerling JR, Ho L, de Jong PT, Fletcher AE, Young IS, Seland JH, Rahu M, Soubrane G, Tomazzoli L, Topouzis F, Vioque J, Hingorani AD, Sofat R, Dean M, Sawitzke J, Seddon JM, Peter I, Webster AR, Moore AT, Yates JR, Cipriani V, Fritsche LG, Weber BH, Keilhauer CN, Lotery AJ, Ennis S, Klein ML, Francis PJ, Stambolian D, Orlin A, Gorin MB, Weeks DE, Kuo CL, Swaroop A, Othman M, Kanda A, Chen W, Abecasis GR, Wright AF, Hayward C, Baird PN, Guymer RH, Attia J, Thakkinstian A, Silvestri G. Evidence of association of APOE with age-related macular degeneration: a pooled analysis of 15 studies. Hum Mutat. 2011;32:1407–16. doi: 10.1002/humu.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sennlaub F, Auvynet C, Calippe B, Lavalette S, Poupel L, Hu SJ, Dominguez E, Camelo S, Levy O, Guyon E, Saederup N, Charo IF, Rooijen NV, Nandrot E, Bourges JL, Behar-Cohen F, Sahel JA, Guillonneau X, Raoul W, Combadiere C. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med. 2013;5:1775–93. doi: 10.1002/emmm.201302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;144:618–26. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitcup SM, Sodhi A, Atkinson JP, Holers VM, Sinha D, Rohrer B, Dick AD. The role of the immune response in age-related macular degeneration. Int J Inflamm. 2013;2013:348092. doi: 10.1155/2013/348092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PLoS ONE. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collier RJ, Wang Y, Smith SS, Martin E, Ornberg R, Rhoades K, Romano C. Complement deposition and microglial activation in the outer retina in light-induced retinopathy: inhibition by a 5-HT1A agonist. Invest Ophthalmol Vis Sci. 2011;52:8108–16. doi: 10.1167/iovs.10-6418. [DOI] [PubMed] [Google Scholar]
- 39.Cruz-Guilloty F, Saeed AM, Duffort S, Cano M, Ebrahimi KB, Ballmick A, Tan Y, Wang H, Laird JM, Salomon RG, Handa JT, Perez VL. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS ONE. 2014;9:e88201. doi: 10.1371/journal.pone.0088201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penfold P, Killingsworth M, Sarks S. An ultrastructural study of the role of leucocytes and fibroblasts in the breakdown of Bruch's membrane. Aust J Ophthalmol. 1984;12:23–31. [PubMed] [Google Scholar]
- 41.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefe's archive for clinical and experimental ophthalmology = Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 42.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 43.Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–27. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 45.Payne AJ, Kaja S, Naumchuk Y, Kunjukunju N, Koulen P. Antioxidant drug therapy approaches for neuroprotection in chronic diseases of the retina. Int J Mol Sci. 2014;15:1865–86. doi: 10.3390/ijms15021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29:113–34. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Combadière C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am J Pathol. 2012;181:472–7. doi: 10.1016/j.ajpath.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Camelo S. Potential sources and roles of adaptive immunity in Age-related Macular Degeneration: Shall we rename AMD into Autoimmune Macular Disease? Autoimmune Dis. 2014;2014:532487. doi: 10.1155/2014/532487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrington DA, Tran TN, Lew KL, Van Remmen H, Gregerson DS. Different death stimuli evoke apoptosis via multiple pathways in retinal pigment epithelial cells. Exp Eye Res. 2006;83:638–50. doi: 10.1016/j.exer.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Gregerson DS, Lew KL, McPherson SW, Heuss ND, Ferrington DA. RPE cells resist bystander killing by CTLs, but are highly susceptible to antigen-dependent CTL killing. Invest Ophthalmol Vis Sci. 2006;47:5385–94. doi: 10.1167/iovs.06-0636. [DOI] [PubMed] [Google Scholar]
- 52.Ezzat MK, Hann CR, Vuk-Pavlovic S, Pulido JS. Immune cells in the human choroid. Br J Ophthalmol. 2008;92:976–80. doi: 10.1136/bjo.2007.129742. [DOI] [PubMed] [Google Scholar]
- 53.Hendel A, Granville DJ. Granzyme B cleavage of fibronectin disrupts endothelial cell adhesion, migration and capillary tube formation. Matrix Biol. 2013;32:14–22. doi: 10.1016/j.matbio.2012.11.013. [DOI] [PubMed] [Google Scholar]