Abstract
Purpose
To study retinal extracellular ATP levels and to assess the changes in the vesicular nucleotide transporter (VNUT) expression in a murine model of glaucoma during the development of the disease.
Methods
Retinas were obtained from glaucomatous DBA/2J mice at 3, 9, 15, and 22 months together with C57BL/6J mice used as age-matched controls. To study retinal nucleotide release, the retinas were dissected and prepared as flattened whole mounts and stimulated in Ringer buffer with or without 59 mM KCl. To investigate VNUT expression, sections of the mouse retinas were evaluated with immunohistochemistry and western blot analysis using newly developed antibodies against VNUT. All images were examined and photographed under confocal microscopy. Electroretinogram (ERG) recordings were performed on the C57BL/6J and DBA/2J mice to analyze the changes in the electrophysiological response; a decrease in the scotopic threshold response was observed in the 15-month-old DBA/2J mice.
Results
In the 15-month-old control and glaucomatous mice, electrophysiological changes of 42% were observed. In addition, 50% increases in the intraocular pressure (IOP) were observed when the pathology was fully established. The responses in the retinal ATP net release as the pathology progressed varied from 0.32±0.04 pmol/retina (3 months) to 1.10±0.06 pmol/retina (15 months; threefold increase). Concomitantly, VNUT expression was significantly increased during glaucoma progression in the DBA/2J mice (58%) according to the immunohistochemical and western blot analysis.
Conclusions
These results may indicate a possible correlation between retinal dysfunction and increased levels of extracellular ATP and nucleotide transporter. These data support an excitotoxicity role for ATP via P2X7R in glaucoma. This modified cellular environment could contribute to explaining the functional and biochemical alterations observed during the development of the pathology.
Introduction
The role of extracellular ATP in almost all biologic systems has been shown since the idea was first proposed in the mid-1950s. The role of this nucleotide and other nucleotides became even more attractive when this molecule was identified as a component of the secretory granules and synaptic vesicles in neurosecretory systems [1]. Costorage with acetylcholine, monoamines, and even the acidic neurotransmitters glutamate and gamma-aminobutyric acid (GABA) makes ATP a relevant modulator of the activity of most synapses in the central nervous system (CNS) and in the autonomic nervous system [2].
In the eye, where the autonomic and central nervous systems control this organ, ATP, as a neurotransmitter or neuromodulator together with other nucleotides, plays an important function in the regulation of relevant neurophysiological processes. Examples of the participation of nucleotides in eye functions are involvement in tear secretion and ocular surface mucin release, a role in wound repair, the modulation of intraocular pressure (IOP), and the modulation of the retinal visual output [3]. In this sense, nucleotide and dinucleotide synthetic analogs may act as therapeutic agents for pathologies such as dry eye, glaucoma, and retinal detachment [4, 5]. In the retina, ATP receptors, ionotropic P2X, and metabotropic P2Y receptors are present and probably modulate the synaptic transmission in this intricate ocular structure [6, 7].
Despite knowledge of the existence of receptors for ATP in the retina, little is known about the levels of ATP and its release in this area. This is a relevant point since ATP levels may change with aging and pathology, as was recently shown in the retinas of a murine model with Alzheimer disease [8].
One prevalent retinal disease that leads to blindness is glaucoma. This neuropathy affects retinal cells, mainly retinal ganglion cells [9-11]. Nucleotides such as ATP and diadenosine tetraphosphate are present in higher concentrations in the human aqueous humor in cases of acute and high-pressure glaucoma, which suggests that these nucleotides participate in the etiology of the disease [12, 13]. Therefore, nucleotides elevated several fold may contribute to the ocular hypertension often associated with glaucoma probably by the activation of P2Y2 receptors present in the ciliary body as previously demonstrated [14]. This increase in nucleotide levels occurs in the anterior pole of the eye and may not reflect similar changes at the retinal level. Nevertheless, P2Y2 receptor activation in the ciliary body increases IOP, and this elevation in pressure could be, at least in part, responsible for concomitant retinal damage. These alterations have been related to ATP receptors, P2X7 receptors in particular, present in retinal cells such as ganglion cells [15-19]. Interestingly, the expression of P2X7 receptors changes with the development of glaucoma. Nevertheless, information regarding the levels of ATP during the development of the disorder, another critical point to fully establish the contribution of the P2X7 receptor to the glaucomatous pathology, is lacking. ATP is normally stored in synaptic vesicles with the vesicular nucleotide transporter (VNUT) [20-22]. This transporter is also useful to know what cells have ATP as a neurotransmitter, which is relevant in a complicated structure such as the retina.
In the present study, variation in the expression of the VNUT in the retina of normal and glaucomatous animals was investigated. In addition, and using the same models, the basal and stimulated release of ATP from whole mount retinas of normal and pathological animals was measured.
Methods
Animal handling
Female C57BL/6J (n=16, for each stage) and DBA/2J (n=16, for each stage) mice obtained from the European distributor of Jackson Laboratory mice (Charles River Laboratories, Barcerlona, Spain) were used for this study. Mice were housed one to four mice per cage in temperature- and light-controlled rooms maintained on a 12 h:12 h light-dark cycle (light from 8 AM to 8 PM) and with food and water ad libitum. The DBA/2J and C57BL/6J mice were divided into groups corresponding to different ages: 3, 6, 9, 15, and 22 months. All animal maintenance and experimental procedures were performed following the Spanish and European regulations of animal care in the laboratory and animal research (Guide for the Care and Use of Laboratory Animals) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. This study has been approved by the UCM-CEA with reference 45-2014 and by the Madrid Enviromental Department with reference 05/312824.9/14.
For IOP and electroretinogram (ERG) measurements, the mice were weighed and anesthetized with an intraperitoneal (i.p.) injection of a mixture of ketamine (95 mg/kg, Imalgene 1000®, Merial, Barcelona, Spain) and xylazine (5 mg/kg, Rompún®, Bayer, S.A., Barcelona, Spain).
IOP measurements
Control (n=8, for each stage) and glaucomatous mice (n=8, for each stage) were anesthetized and maintained on a warmed pad. The intraocular pressure was recorded by using a non-invasive rebound tonometer (Tono-lab®; iCare Finland Oy, Helsinki, Finland) [23-26]. Both eyes of each animal were measured immediately after sedation [27] at the age of 3, 6, 9, 15, and 22 months, and IOP was always measured at the same time to avoid fluctuations due to the circadian rhythm [28, 29]. For each animal, IOP was presented as the average of six measurements taken for each eye.
Electroretinogram recordings
After the IOP measurements, the same animals were dark-adapted overnight before the ERG recordings. All manipulation procedures were performed under dim red light (λ>600 nm) in absolute darkness. Mice were anaesthetized with an i.p. injection of a solution of ketamine (95 mg/kg) and xylazine (5 mg/kg) and maintained on a heating pad to preserve the normal body temperature at 37 °C. Pupil mydriasis was induced in each mouse by applying a drop of topical 1% tropicamide (Colircusí Tropicamida 1% ®; Alcon-Cusí, S.A., El Masnou, Barcelona, Spain) in the right eye of each mouse. Scotopic threshold responses were recorded in response to light stimuli produced by a Ganzfeld stimulator using a Burian-Allen bipolar lens electrode (Briot Weko España, Barcelona, Spain) located on the cornea. Previously, the corneal surface was protected with a non-allergenic ionic conductive drop of methylcellulose (Methocel 2%®; Novartis Laboratories CIBA Vision, Annonay, France). A copper electrode was placed in the mouth and served as the reference electrode, and the ground electrode was a needle placed subcutaneously at the base of the tail. Anesthetized mice were placed on the warming table in a Ganzfeld stimulator. The scotopic threshold responses (STRs) were recorded from the right eye in response to light stimuli (intensities ranged from 10−5 to 10−2 cd·s·m−2), provided by a Ganzfeld dome light source, which ensures the uniform illumination of the retina. For each light intensity, a series of ERG responses were averaged (approximately 20 ERG responses for each trace), and the interval between light flashes was adjusted to ensure timing that allowed response recovery (10 s for the dimmest stimulus intensities). Electrical signals generated in the retina were amplified (1,000X) and band filtered between 0.3 and 1000 Hz with a Grass amplifier (CP511 AC amplifier, Grass Instruments, Quincy, MA). Electrical signals were digitized at 20 kHz using a Power Lab data acquisition board (AD Instruments, Chalgrove, UK) and displayed on a PC computer. Light stimuli were calibrated periodically with a photometer (Mavo Monitor USB, Gossen, Nürenberg, Germany). The recordings were analyzed with the normalization criteria established by the International Society for Clinical Electrophysiology of Vision (ISCEV) for measurement of the amplitude and implicit time of the different waves studied.
The STR was analyzed for each stimulus; positive STR (pSTR) was measured from the baseline to the peak of the positive deflection, approximately 110–120 ms from the flash onset, and the negative STR (nSRT) was measured from the baseline to the peak of the negative deflection after the pSTR, approximately 220 ms from the onset of the flash. ERG wave amplitudes were calculated for each animal group, and the percentage difference between the DBA/6J eyes and the control eyes was obtained for each stimulus and was further averaged (mean ± standard error of the mean, SEM).
Statistical analysis
The results were analyzed with GraphPad Instat® 3 for Windows® (GraphPad Software, San Diego, CA). Descriptive statistics were calculated, and the normality of the distribution of the data was examined. The Student t test was applied for the comparison between the mean ERG response of right eyes at −3.69 cd·sm−2 in the two animal models at different ages.
Retinal isolation and ATP samples analysis
The C57BL/6J and glaucomatous mice were euthanized at 3, 9, 15, and 23 months (n=8 for each age) through decapitation on a sterile gauze pad. Both eyes were enucleated using sterile curved scissors to cut the optic nerve. Extra caution was needed to avoid damaging the retina. Eyecups were placed in Ringer’s solution and dissected under a stereomicroscope (SteREO Discovery.V8, Zeiss, Madrid, Spain). With 0.8 mm tip curved forceps and sterile fine-angled dissecting scissors, the cornea was severed. The forceps were then gently moved at a slight backward angle to pull up the cornea. Then the lens was removed with slight pressure on the eyecup. Gently, the retina was separated from the sclera, and the retina was cleaned of choroid pigment and vitreous.
Tissue samples were homogenized with dissecting scissors and mechanic lysis in 10 volumes of ice-cold 5% trichloroacetic acid (TCA) and transferred to 1.5 ml Eppendorf tubes. They were then centrifuged at 22,000 ×g for 10 min at 4 °C. Supernatants were collected in a fresh tube and were neutralized with 2.5% hydroxide potassium (KOH) adjusted to an approximate pH value of 7.5. The samples were frozen until they were analyzed with high-pressure liquid chromatography (HPLC).
After thawing, the samples were strongly vortexed for 2 min. The ATP levels were analyzed in 20 μl of the TCA tissue extracts with HPLC using an isocratic pump (model 1515; Waters Madrid, Spain). The chromatographic conditions consisted of a C18 reverse-phase column, 250 mm long and 4.6 mm in diameter (Hyperchrome, Scharlab, Madrid, Spain). It was equilibrated with a mobile phase containing 10 mM KH2PO4, 2 mM tetrabutylammonium bisulfate (TBA), and 20% acetonitrile adjusted to pH 7.5 with 5 M KOH. The flow rate throughout the chromatographic runs was 2 ml/min. ATP was measured at the 260 nm wavelength.
The control retinas were maintained in Ringer solution, and the stimulated retinas were treated with 59 mM potassium chloride (KCl) in Ringer solution for 1 min. The supernatants (1,000 μl) were collected in 1.5 ml tubes and maintained in ice for 5 min. The tubes were heated in a 98 °C bath for 2 min and then transferred to ice for 10 min, to precipitate the proteins. To pellet the proteins, the tubes were centrifuged at 22,000 ×g for 10 min at 4 °C. The concentrations of released ATP were measured using reverse-phase HPLC and ultraviolet (UV) detection as described above.
Immunohistochemical studies
The C57BL/6J and DBA/2J mice at 3, 9, 15, and 22 months of age (n=4 animals for each group) were anesthetized (see above) and perfused pericardially with 4% PBS (20 mM NaCl, 20 mM KCl, 10 mM NaPO4, 5 mM KPO4, pH 7.4) paraformaldehyde in PBS 0.1 M at pH 7.4. The eyes were enucleated, fixed in 4% paraformaldehyde (PFA) in PBS for 2 h at 4 °C, rinsed in 33% sucrose, and embedded in tissue freezing medium until frozen with liquid N2. Vertical sections of the retinas (10 μm thick) were collected from the central retinal regions. Frozen sections were rinsed in PBS 1X and permeated with PBS-0.25% Tx-100 solution for 30 min. To avoid non-specific staining, sections were blocked in 10% normal donkey serum (NDS; Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. Then, the primary antibody rabbit anti-solute carrier family 17 member 9 (anti-VNUT, ABN110; Millipore, Billerica, MA) was incubated at 1:300 dilution at 4 °C overnight. The primary antibody used in this study has been used in the mouse retina [30]. Then sections were washed in PBS-0.1% Tx-100 and incubated with donkey anti-immunoglobulin (IgG) rabbit antibody conjugated with fluorescein isothiocyanate (FITC; green; 1:200; Jackson ImmunoResearch), diluted in PBS-0.1% Tx-100 for 1 h in darkness at room temperature. The nuclei were stained with propidium iodide (red, Sigma) diluted 1:500 in PBS for 10 min. The sections were finally mounted in Vectashield (Vector Laboratories, Palex Medical, Barcelona, Spain) and coverslipped. For the analysis, the images were acquired using a laser-scanning microscope (Zeiss LSM 5, Jena, Germany).
Western blot
Thirty-two mice were used for the western blot analysis. Twelve C57BL/6J mice (n=4 for each stage) were used as the control, and 12 DBA/2J mice (n=4 for each stage) were used to evaluate the possible expression changes in the VNUT transporter at 3, 9, 15, and 22 months of age. Retinas were removed and homogenized in ice with radioimmunoprecipitation assay (RIPA) buffer (1:5 v/v) containing 50 mM HEPES, pH 8, 150 mM NaCl, 1% NP-40 (w/v), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and inhibitor proteases (1 mM phenyl methanesulfonyl fluoride (PMSF), 10 µg/ml leupeptin, 5 µg/ml pepstatin, 10 µg/ml aprotinin, 1 mM sodium fluoride, and 2 mM sodium orthovanadate). The lysates were centrifuged at 15,000 ×g for 15 min at 4 °C. The supernatant was stored at −80 °C until use.
The protein concentration was determined with Bradford assay. Retinal samples (40 µg protein) were diluted in Laemmli’s sample buffer, loaded on 10% SDS–polyacrylamide gel electrophoresis (PAGE) gels, and transferred to a nitrocellulose membrane. Blots were blocked with 5% non-fat dry milk for 1 h at room temperature, and then they were incubated overnight at 4 °C in TBS 1X 0.1% Tween-20 containing 5% non-fat milk (blocking buffer) and VNUT primary antibody (Millipore). Mouse monoclonal glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH 1:500; Santa Cruz, Dallas, CA) served as a loading control. Membranes were incubated with goat anti-IgG rabbit antibody or goat anti-IgG mouse antibody conjugated with horseradish peroxidase secondary antibody (Jackson ImmunoResearch) for 1 h at room temperature. Then the proteins were revealed with chemiluminescence using enhanced chemiluminescence (ECL) detection (Amersham Phamacia Biotech, Barcelona, Spain). Films were scanned with the Gel Logic 200 Imaging System (Kodak, Rochester, NY). The densitometric analysis was performed by using Kodak Molecular Imaging software (Kodak). The densitometry values of each sample were normalized to the respective densitometric GAPDH values.
Statistical analysis
Data were plotted as the mean ± standard error of the mean (SEM). Where indicated, analyses of significance were performed using the Student t test followed by Welch’s test or one-way analysis of variance (ANOVA) followed by the Bonferroni correction to determine statistical significance (GraphPad InStat software, Prism, La Jolla, CA). For correlations, Pearson bivariate regression was used. p<0.05 was considered statistically significant.
Results
Changes in intraocular pressure in control and glaucomatous mice
To evaluate whether IOP changes occurred during the progression of the pathology, measurements were performed in the right and left eyes of female DBA/2J mice between 3 and 15 months. IOP experiments were not performed at 22 months of age since the pathology also produces corneal thickness increase and calcification, which makes it difficult to accurately measure IOP at that age [31].
IOP was measured for the C57BL/6J mice aged 3 and 15 months as the control. The mean IOP before the development of glaucomatous damage was 13±3 mmHg (3 months).
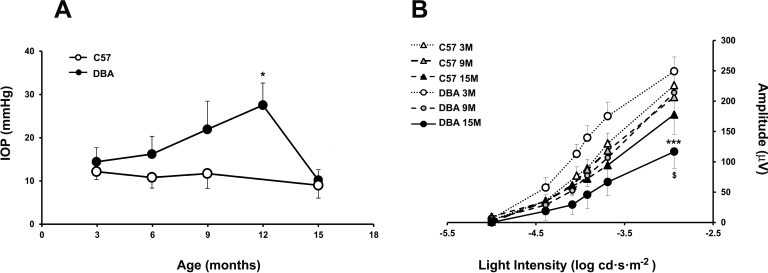
At 9 months of age, an increase of 50% in the IOP was observed in the DBA/2J mice compared with the same strain at 3 months, which was the maximum at 12 months (27±5 mmHg). The evolution of IOP is shown in Figure 1A at 3, 6, 9, 12, and 15 months. Between 3 and 9 months, the IOP remained constant in the eyes with glaucoma. No differences were found in IOP in the control mice. Measurements from a total of eight mice per age group were included.
Figure 1.
Changes in the intraocular pressure and electroretinogram recordings in glaucomatous and control mice. A: Assessment of intraocular pressure (IOP) measurements in the glaucomatous (DBA/2J) and control (C57) mice as a function of age. Animals were measured at 3, 9, and 15 months of age. A significant increase was observed in DBA/2J mice at 12 months when compared with the control mice (*p<0.05). B: Scotopic threshold responses from control C57BL/6J or DBA/2J glaucomatous mice as a function of age. Superposed averaged positive scotopic threshold response (pSTR) amplitudes were measured from the electroretinogram (ERG) flash response as a function of stimulus light intensity recorded from C57BL/6J (triangles) and DBA/2J (circles) mice at 3 (white symbols), 9 (gray symbols), and 15 months (black symbols). Plot data correspond to mean values ± standard deviation (SD) (n=8). A significant reduction in the STR amplitudes in the DBA/2J mice between 3 and 15 months is observed for pSTR amplitudes (***p<0.001) and when compared with the control mice at 15 months ($p<0.001).
Visual function is reduced in glaucomatous mice
To study the affectation of glaucomatous changes in the ganglion cells of the DBA/2J mice, the STR of the ERG was evaluated during the progression of the pathology. Glaucomatous mice and age-matched control mice were measured at 3, 9, and 15 months of age.
Figure 1B shows the STRs. ERG recordings were elicited with light stimuli of intensity ranging −5.00 to −2.94 log cd·s·m−2. The pSTR amplitudes for the DBA/2J mice were studied as a function of age. The average amplitude of the pSTR waves was measured from the ERG flash response as a function of stimulus light intensity. Significantly decreases (39.2% and 75%) were observed between 3, 9, and 15 months of age in the glaucomatous mice.
A significant decrease of 42% in the ERG wave amplitude was also observed in the DBA/2J mice compared to the C57BL/6J animals tested at 15 months of age. The STR traces of the control mice showed no significant differences when the different time intervals were compared. A slight non-significant age-dependent decrease in the amplitudes of the pSTR obtained from 3- and 15-month-old C57BL6/J mice was observed.
Presence of extracellular ATP in stimulated and non-stimulated retinas
The experiment with the nucleotide ATP in the retinas of the normal and glaucomatous animals showed interesting results. The evolution of the ATP concentrations in the wild-type mouse (C57BL6/J) showed a gradual decrease in the basal extracellular and stimulated ATP release (Figure 2A).
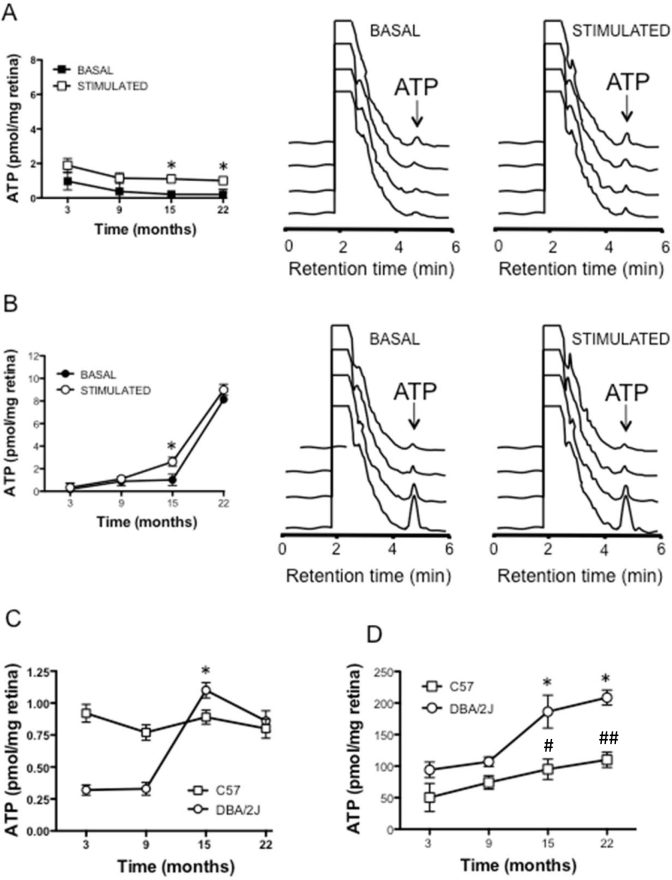
Figure 2.
Study of the ATP release in control (C57BL/6J) and glaucomatous (DBA/2J) mice. A: Basal and KCl stimulated ATP release from whole mount retinas obtained from the C57BL/6J mice. The left panel represents the mean of the ATP amounts at the given mice ages either under basal (solid symbol) or stimulated conditions (open symbol). The middle and right panels are representative high-pressure liquid chromatography (HPLC) elution profiles of basal (middle panel) and stimulated conditions (right panel). B: Basal and KCl stimulated ATP release from whole mount retinas obtained from glaucomatous DBA/2J mice. The left panel shows the mean of the ATP amounts at the given mice ages either under basal (solid symbol) or stimulated conditions (open symbol). The middle and right panels are representative HPLC elution profiles of the basal (middle panel) and stimulated conditions (right panel). C: Net ATP release in the glaucomatous DBA/2J mice (open circles) and the control C57BL/6J mice (open squares) followed from 3 to 22 months. D: Total ATP content in the retinas of the control and glaucomatous mice at the different ages under study (n=8). *p<0.05 between 9 and 15 months of age in the DBA/2J animals. #p<0.05 C57BL/6J mice versus DBA/2J mice at 15 months and ##p<0.01 C57BL/6J versus DBA/2J mice at 22 months.
In contrast, when the same experiments were performed in the glaucomatous DBA/2J mice, there were no significant changes in the basal ATP levels until the 22nd month (at 15th months, the ATP value was 1.02±0.50 pmol/mg per retina) while there was a huge increase to 8.13±0.33 pmol/mg per retina at 22 months of age (Figure 2B).
The release of ATP from the retinas of the glaucomatous mice when they were challenged with 59 mM KCL demonstrated that changes were measurable at 15 months (ATP: 2.62±0.45 pmol/mg per retina) compared to 9 months (ATP: 1.13±0.31 pmol/mg per retina), and increased enormously at 22 months; the ATP concentration was 8.99±0.57 pmol/mg per retina (Figure 2B).
Interestingly, when the net release was studied in the DBA/2J mouse, a clear increase in the ATP-stimulated release at 15 months was observed (ATP: 1.10±0.06 pmol/mg per retina) while at 22 months, the ATP concentration was not as high (0.86±0.08 pmol/mg per retina; Figure 2C).
When the total amount of ATP was measured in both mice strains at the indicated ages, an increase was observed in the nucleotide in the control and glaucomatous animals. The changes were more marked in the case of the DBA/2J mice, from 106.96±15.15 pmol/mg per retina at 9 months to 186.33±45.23 pmol/mg per retina (Figure 2D, n=8).
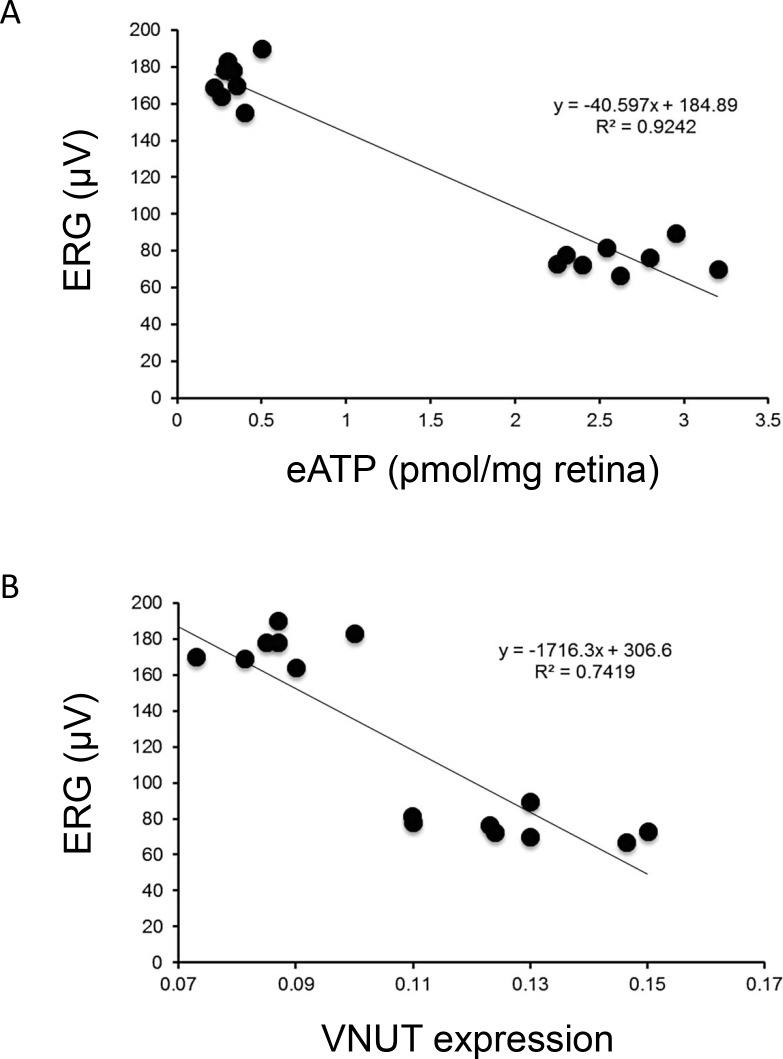
The ATP concentration and VNUT expression showed a strong correlation in the DBA/2J model; the Pearson’s correlation coefficient was −0.961 (p<0.0001; Figure 3).
Figure 3.
Correlation between electroretinogram recordings, ATP and vesicular nucleotide transporter expression. Relationship between ERG amplitude and ATP levels (A) and ERG amplitude and VNUT expression (B). Correlation was performed in glaucomatous animals comparing 3 versus 15 months of age.
VNUT expression of DBA/2J mice increases with the development of the pathology
Immunohistochemistry was performed to evaluate the expression pattern of the VNUT in the retinas of the glaucomatous and control mice. Figure 4A-H shows a fixed mouse retina labeled for the vesicular nucleotide transporter. VNUT immunoreactivity was observed in the outer segments and in photoreceptors in the glaucomatous retinas (Figure 4G), in the outer plexiform layer (OPL), inner plexiform layer (IPL), inner nuclear layer (INL), and ganglion cell layer (GCL).
Figure 4.
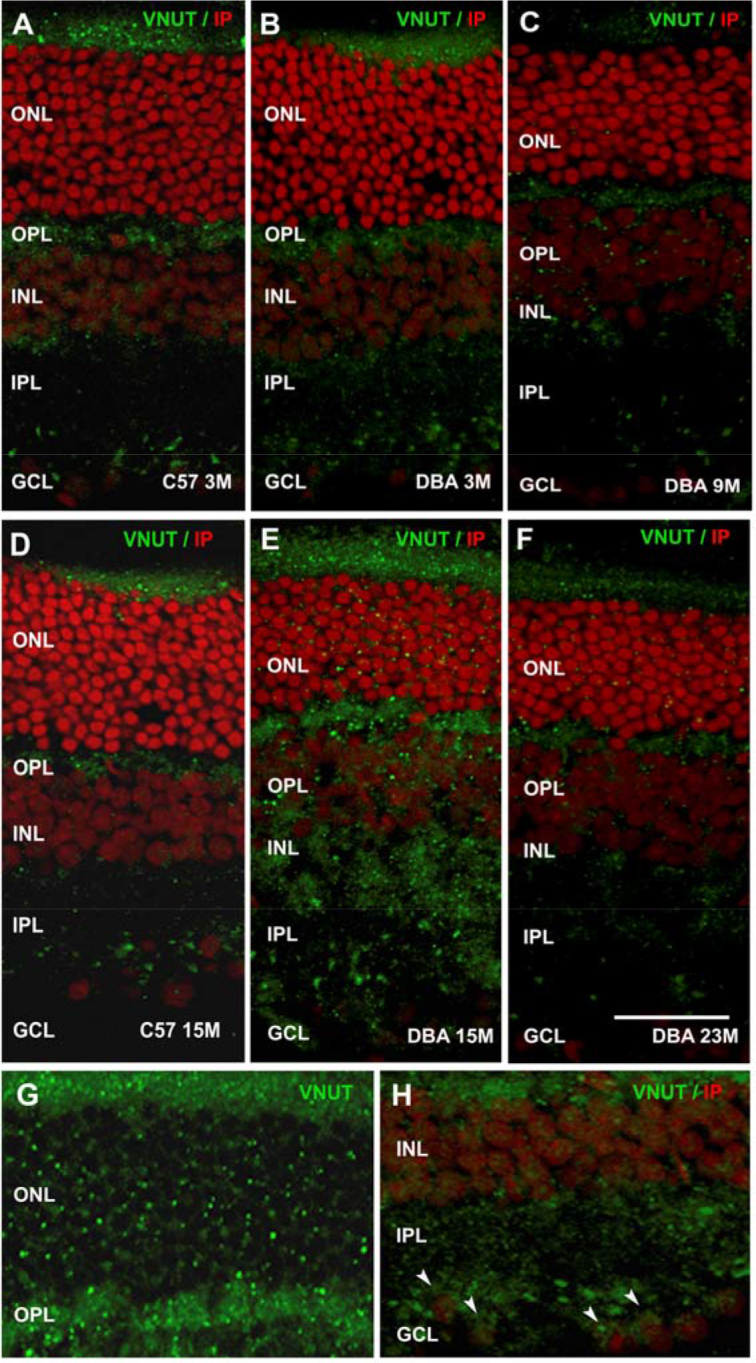
Immunohistochemistry for VNUT in C57BL/6J and DBA/2J retinas. Vertical sections at 3, 9, 15, and 22 months of retinas from glaucomatous mice (B, C, E, F) and control mice at 3 and 15 months (A, D). Vesicular nucleotide transporter (VNUT)-stained retinas (green) showed a significant increase at 15 months in the DBA/2J mice (E) compared to that observed in the DBA/2J and C57BL/6J mice at 3 months (A, B) and 23 months (F). G–H: High magnification of the photoreceptor layer, showing an increase in VNUT labeling in the DBA/2J mice at 15 months (G) and VNUT staining found in ganglion cells (arrows; H). Nuclei were stained with propidium iodide (red). All images were collected from the central area of the retina. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar: 20 μm.
There was little change in the VNUT labeling in C57BL/6J retinas at 3 months compared to those from DBA/2J animals at the same age (Figure 4A,B). The expression levels of the ATP vesicular transporter remained the same in the control mice at 9 and 22 months (data not shown). The label and distribution of VNUT remained constant in the OPL, the IPL, and the inner cells (the INL, the GCL) at 9 months (Figure 4C). However, in the 15-month-old DBA/2J retinas (Figure 4E), there was a clear increase in VNUT expression in the plexiform layers (OPL and IPL), the INL, and the GCL. In contrast, at 23 months of age, there was a marked reduction in the vesicular nucleotide transporter labeling in the DBA/2J retinas; the expression was similar to that before the development of the pathology (3 months; Figure 4A-H).
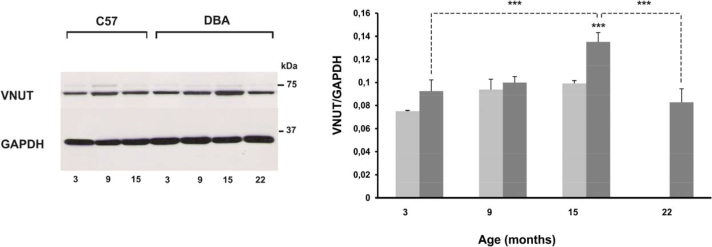
Figure 5A–B shows quantification of VNUT expression with western blot analysis. A significant increase in the VNUT level was found in the DBA/2J mice between 3 and 15 months of age, compared to the age-matched control C57BL/6J mice (47.21%; p<0.001). However, the VNUT levels in the 22-month-old mice decreased compared to 15 months, 38.72% (p<0.001). ERG amplitude and VNUT expression showed a strong correlation in the DBA/2J model; the Pearson’s correlation coefficient was −0.86 (p<0.0001; Figure 3). The VNUT expression of the control mice showed no significant differences among the different age groups studied.
Figure 5.
VNUT expression in the glaucomatous and control retinas at different ages (3, 9, 15, and 22 months). A: Detection of vesicular nucleotide transporter (VNUT) (68 kDa) levels in the C57BL/6J mice at 3, 9, and 15 months and in the DBA/2J mice at 3, 9, 15, and 22 months by western blotting. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 37 kDa) levels are shown as a loading control. B: Western blot analysis of the VNUT at 3, 9, 15, and 22 months of age. Values are the mean ± standard deviation (SD) of six independent animal experiments (***p<0.001).
Discussion
Changes in ATP concentrations and in VNUT in the glaucomatous animals and the control animals were investigated. Animals with glaucoma developed the pathology starting from the 9th month, the moment at which the IOP started to increase and a concomitant deterioration in the retinal electrophysiological pattern occurred [3]. In a parallel way, a clear and gradual increase was observed in the extracellular levels of ATP that was highest in the latest stages of the pathology, particularly at 22 months of age in the DBA/2J mouse. Interestingly, when the total ATP was measured in the animal retinas, a gradual increase with age was observed, which was more evident in the glaucomatous animals. It could be that due to this cytoplasmic increase in the nucleotide in the retinal cells, the elevation in VNUT is a mechanism for reducing the ATP concentrations within the cells. This could be why at 22 months compared to 15 (in the DBA) although the total amount of ATP is almost identical, the release at 22 months is lower. More experiments are necessary to fully demonstrate this point.
Increased levels of retinal ATP have been recently described in a murine model of Alzheimer disease (AD). An increase in the levels of ATP in basal and stimulated conditions was observed in the AD mice (AβPP). Interestingly, the increase in the extracellular concentrations of ATP peaked when this disorder was fully established but decreased during advanced stages of the disease [7]. In contrast, in the present work, in the glaucomatous model, there was an increase in ATP levels that lasted until 22 months of age. Despite this apparent difference between the AD model and glaucoma model, when the net release was studied, a similar behavior was observed. In this sense, the net release of ATP in the glaucomatous mice increased between the 9th and the 12th month decreased at 22 months. In the AD mice model, the net release of ATP peaked at 14 months, and the nucleotide was reduced at 18 months. In both cases, as the disease progressed, the peak in extracellular ATP diminished when the pathology was advanced. The reduction in ATP release when glaucoma is advanced may reflect retinal cell death. Ganglion cell death is a typical feature of this pathology as we have previously described in the DBA/2J model [32].
In parallel, we measured the expression of the vesicular nucleotide transporter in normal and glaucomatous animals. VNUT distribution in the retina suggests an important role for the delivery of ATP as a neurotransmitter. ATP released in the inner retina could contribute to activating the P2 purinergic receptors [6]. Among all receptors and in relation to the possible cell death detected at 22 months of age, the activation of P2X7 receptors may occur. If so, their involvement may activate signaling pathways, which promotes apoptotic mechanisms. Selective apoptosis mediated by P2X7 receptors has been demonstrated in the photoreceptors when intravitreal injections of ATP are performed [33, 34]. This P2X7 receptor–induced apoptosis has also been detected in retinal ganglion cells [35, 36]. Environmental factors such as hypoxia or an acute increase in hydrostatic pressure induced retinal ganglion cell death due to the increase in extracellular ATP, which activates P2X7 receptors [37-39].
Western blot analysis of VNUT demonstrated a significant increase in the expression of this protein at 15 months in the glaucomatous mice in contrast to the decrease observed as the animals were 22 months old. Nonetheless, and as described in [19], the vesicular transporter for ATP is present in the cells, in the outer and inner retina. This indicates that ATP is stored and released by the classical exocytotic way. The reduction in the VNUT when the glaucomatous animals were 22 months old and the reduction in the net release of ATP (although the total extracellular ATP concentrations increased) suggests that most of this ATP is due to cell death and the consequent release to the extracellular milieu.
One important aspect is the sequence of events during the advance of the pathology. The first events that occur in the anterior pole, the increase in IOP, peaked when there were no significant changes in retinal activity, ATP levels, or VNUT expression. Significant changes in ERG, ATP, and VNUT levels did not occur until the mice were 15 months old. Interestingly, when the mice were 22 months old, VNUT expression was clearly reduced. Nevertheless, the extracellular presence of ATP peaked, which suggests massive cell death with the concomitant presence of the nucleotide in the extracellular medium.
Finding in which retinal neurons the VNUT is present is of great interest. ATP is costored with transmitters such as acetylcholine [40], noradrenaline [41], other monoamines [42], and glutamate [43] in the central nervous system. If there is an equivalent cotransmission in the retina, it would be feasible to find ATP as a transmitter in starburst-cholinergic amacrine cells (A18 wide-field and amacrine displaced cells, and others), dopaminergic amacrine and interplexiform cells, and glutamatergic bipolar and ganglion cells. In the present case, we can only suggest the presence of the VNUT in the inner nuclear layer and in ganglion cells. The precise description of which cells contain the VNUT deserves attention, but this is not the aim of the present work, which focused on the presence of the ATP transporter in the plexiform layers.
In summary, we have demonstrated that the levels of ATP and its vesicular transporter change during the development of the pathology. This increase may in part be responsible for the electrophysiological changes in the retinas of the glaucomatous mice. From a pharmacological point of view, considering the connection glaucoma-ATP increase-retinal injury, it might be of interest to abolish or block excess ATP and to see whether this blockade can protect the retina from glaucomatous damage.
Acknowledgments
This work was supported by grants from the Spanish Ministry of Economy and Competitivity (Grants SAF2010–16024 and SAF-2013–44416-R) and the Ministry of Health Social Services and Equality RETICS (Grant RD12/0034/0001). MJPL is a fellowship holder of FPI.
References
- 1.Gualix J, Fideu MD, Pintor J, Rotllan P, Garcia-Carmona F, Miras-Portugal MT. Characterization of diadenosine polyphosphate transport into chromaffin granules from adrenal medulla. FASEB J. 1997;11:981–90. doi: 10.1096/fasebj.11.12.9337151. [DOI] [PubMed] [Google Scholar]
- 2.Trachte GJ, Binder SB, Peach MJ. Indirect evidence for separate vesicular neuronal origins of norepinephrine and ATP in the rabbit vas deferens. Eur J Pharmacol. 1989;164:425–33. doi: 10.1016/0014-2999(89)90250-1. [DOI] [PubMed] [Google Scholar]
- 3.Sanderson J Dartt DA2, Trinkaus-Randall V3, Pintor J4, Civan MM5, Delamere NA6, Fletcher EL7, Salt TE8, Grosche A9, Mitchell CH10. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res. 2014;127:270–9. doi: 10.1016/j.exer.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crooke A, Guzman-Aranguez A, Peral A, Abdurrahman MK, Pintor J. Nucleotides in ocular secretions: their role in ocular physiology. Pharmacol Ther. 2008;119:55–73. doi: 10.1016/j.pharmthera.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Guzman-Aranguez A, Santano C, Martin-Gil A, Fonseca B, Pintor J. Nucleotides in the eye: focus on functional aspects and therapeutic perspectives. J Pharmacol Exp Ther. 2013;345:331–41. doi: 10.1124/jpet.112.202473. [DOI] [PubMed] [Google Scholar]
- 6.Pintor J, Sanchez-Nogueiro J, Irazu M, Mediero A, Pelaez T, Peral A. Immunolocalisation of P2Y receptors in the rat eye. Purinergic Signal. 2004;1:83–90. doi: 10.1007/s11302-004-5072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler-Schilling TH, Marquordt K, Kohler K, Jabs R, Guenther E. Expression of purinergic receptors in bipolar cells of the rat retina. Brain Res Mol Brain Res. 2000;76:415–8. doi: 10.1016/s0169-328x(00)00020-6. [DOI] [PubMed] [Google Scholar]
- 8.Perez de Lara MJ, Pintor J. Presence and release of ATP from the retina in an Alzheimer's disease model. Journal of Alzheimer's disease. JAD. 2015;43:177–81. doi: 10.3233/JAD-141005. [DOI] [PubMed] [Google Scholar]
- 9.Quigley HA. Glaucoma. Lancet. 2011;377:1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 10.Howell GR, Libby RT, Marchant JK, Wilson LA, Cosma IM, Smith RS, Anderson MG, John SW. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 2007;8:45. doi: 10.1186/1471-2156-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castany M, Jordi I, Catala J, Gual A, Morales M, Gasull X, Pintor J. Glaucoma patients present increased levels of diadenosine tetraphosphate, Ap(4)A, in the aqueous humour. Exp Eye Res. 2011;92:221–6. doi: 10.1016/j.exer.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Li A, Zhang X, Zheng D, Ge J, Laties AM, Mitchell CH. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp Eye Res. 2011;93:528–33. doi: 10.1016/j.exer.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Gil A, de Lara MJ, Crooke A, Santano C, Peral A, Pintor J. Silencing of P2Y [(2)] receptors reduces intraocular pressure in New Zealand rabbits. Br J Pharmacol. 2012;165(4b):1163–72. doi: 10.1111/j.1476-5381.2011.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler-Schilling TH, Marquordt K, Kohler K, Guenther E, Jabs R. Identification of purinergic receptors in retinal ganglion cells. Brain Res Mol Brain Res. 2001;92:177–80. doi: 10.1016/s0169-328x(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 16.Ishii K, Kaneda M, Li H, Rockland KS, Hashikawa T. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J Comp Neurol. 2003;459:267–77. doi: 10.1002/cne.10608. [DOI] [PubMed] [Google Scholar]
- 17.Puthussery T, Fletcher EL. Synaptic localization of P2X7 receptors in the rat retina. J Comp Neurol. 2004;472:13–23. doi: 10.1002/cne.20045. [DOI] [PubMed] [Google Scholar]
- 18.Puthussery T, Yee P, Vingrys AJ, Fletcher EL. Evidence for the involvement of purinergic P2X receptors in outer retinal processing. Eur J Neurosci. 2006;24:7–19. doi: 10.1111/j.1460-9568.2006.04895.x. [DOI] [PubMed] [Google Scholar]
- 19.Vessey KA, Fletcher EL. Rod and cone pathway signalling is altered in the P2X7 receptor knock out mouse. PLoS ONE. 2012;7:e29990. doi: 10.1371/journal.pone.0029990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–6. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sreedharan S, Shaik JH, Olszewski PK, Levine AS, Schioth HB, Fredriksson R. Glutamate, aspartate and nucleotide transporters in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression. BMC Genomics. 2010;11:17. doi: 10.1186/1471-2164-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson M Sawada K, Morland C, Hiasa M, Ormel L, Moriyama Y, Gundersen V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex. 2012;22:1203–14. doi: 10.1093/cercor/bhr203. [DOI] [PubMed] [Google Scholar]
- 23.Danias J, Lee KC, Zamora MF, Chen B, Shen F, Filippopoulos T, Su Y, Goldblum D, Podos SM, Mittag T. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003;44:5151–62. doi: 10.1167/iovs.02-1101. [DOI] [PubMed] [Google Scholar]
- 24.Wang WH, Millar JC, Pang IH, Wax MB, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci. 2005;46:4617–21. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- 25.Cuenca N Pinilla I, Fernández-Sánchez L, Salinas-Navarro M, Alarcón-Martínez L, Avilés-Trigueros M, de la Villa P, Miralles de Imperial J, Villegas-Pérez MP, Vidal-Sanz M. Changes in the inner and outer retinal layers after acute increase of the intraocular pressure in adult albino Swiss mice. Exp Eye Res. 2010;91:273–85. doi: 10.1016/j.exer.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Vidal-Sanz M, Salinas-Navarro M, Nadal-Nicolás FM, Alarcón-Martínez L, Valiente-Soriano FJ, de Imperial JM, Avilés-Trigueros M, Agudo-Barriuso M, Villegas-Pérez MP. Understanding glaucomatous damage: anatomical and functional data from ocular hypertensive rodent retinas. Prog Retin Eye Res. 2012;31:1–27. doi: 10.1016/j.preteyeres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Ding C, Wang P, Tian N. Effect of general anesthetics on IOP in elevated IOP mouse model. Exp Eye Res. 2011;92:512–20. doi: 10.1016/j.exer.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aihara M, Lindsey JD, Weinreb RN. Twenty-four-hour pattern of mouse intraocular pressure. Exp Eye Res. 2003;77:681–6. doi: 10.1016/j.exer.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Drouyer E, Dkhissi-Benyahya O, Chiquet C, WoldeMussie E, Ruiz G, Wheeler LA, Denis P, Cooper HM. Glaucoma alters the circadian timing system. PLoS ONE. 2008;3:e3931. doi: 10.1371/journal.pone.0003931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voas M, Muddasani N, Moriyama Y, Stella S. Characterization of the vesicular nucleotide transporter (VNUT) in mammalian retina. Invest Ophtalmol Vis Sci. 2013; 54 [Google Scholar]
- 31.Inman DM, Sappington RM, Horner PJ, Calkins DJ. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the DBA/2 mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:986–96. doi: 10.1167/iovs.05-0925. [DOI] [PubMed] [Google Scholar]
- 32.Pérez de Lara MJ, Santano C, Guzmán-Aránguez A, Valiente-Soriano FJ, Avilés-Trigueros M, Vidal-Sanz M, de la Villa P, Pintor J. Assessment of inner retina dysfunction and progressive ganglion cell loss in a mouse model of glaucoma. Exp Eye Res. 2014;122:40–9. doi: 10.1016/j.exer.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Puthussery T, Fletcher E. Extracellular ATP induces retinal photoreceptor apoptosis through activation of purinoceptors in rodents. J Comp Neurol. 2009;513:430–40. doi: 10.1002/cne.21964. [DOI] [PubMed] [Google Scholar]
- 34.Notomi S, Hisatomi T, Kanemaru T, Takeda A, Ikeda Y, Enaida H, Kroemer G, Ishibashi T. Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am J Pathol. 2011;179:2798–809. doi: 10.1016/j.ajpath.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, Budak MT, Lu W, Khurana TS, Zhang X, Laties AM, Mitchell CH. Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol Vis. 2006;12:937–48. [PubMed] [Google Scholar]
- 36.Hu H, Lu W, Zhang M, Zhang X, Argall AJ, Patel S, Lee GE, Kim YC, Jacobson KA, Laties AM, Mitchell CH. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp Eye Res. 2010;91:425–32. doi: 10.1016/j.exer.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;25:2741–54. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama T, Oku H, Shibata M, Fukuhara M, Yoshida H, Ikeda T. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Invest Ophthalmol Vis Sci. 2010;51:3236–43. doi: 10.1167/iovs.09-4192. [DOI] [PubMed] [Google Scholar]
- 39.Reigada D, Lu W, Zhang M, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience. 2008;157:396–404. doi: 10.1016/j.neuroscience.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson PJ, Brown SJ. ATP release from affinity-purified rat cholinergic nerve terminals. J Neurochem. 1987;48:622–30. doi: 10.1111/j.1471-4159.1987.tb04138.x. [DOI] [PubMed] [Google Scholar]
- 41.Poelchen W, Sieler D, Wirkner K, Illes P. Co-transmitter function of ATP in central catecholaminergic neurons of the rat. Neuroscience. 2001;102:593–602. doi: 10.1016/s0306-4522(00)00529-7. [DOI] [PubMed] [Google Scholar]
- 42.Krügel U, Kittner H, Franke H, Illes P. Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse. 2003;47:134–42. doi: 10.1002/syn.10162. [DOI] [PubMed] [Google Scholar]
- 43.Mori M, Heuss C, Gahwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol. 2001;535:115–23. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]