Abstract
Background
We used a simple experimental design to test for the effects of microcosm scaling on the growth and survival of the mosquito, Culex pipiens. Microcosm and mesocosm studies are commonly used in ecology, and there is often an assumption that scaling doesn't affect experimental outcomes. The assumption is implicit in the design; choice of mesocosms may be arbitrary or based on convenience or cost. We tested the hypothesis that scale would influence larvae due to depth and surface area effects. Larvae were predicted to perform poorly in microcosms that were both deep and had small openings, due to buildup of waste products, less exchange with the environment, and increased competition. To determine if the choice of scale affected responses to other factors, we independently varied leaf litter quantity, whose effects on mosquitoes are well known.
Results
We found adverse effects of both a lower wall surface area and lower horizontal surface area, but microcosm scale interacted with resources such that C. pipiens is affected by habitat size only when food resources are scarce. At low resource levels mosquitoes were fewer, but larger, in microcosms with smaller horizontal surface area and greater depth than in microcosms with greater horizontal surface area and shallower depth. Microcosms with more vertical surface area/volume often produced larger mosquitoes; more food may have been available since mosquitoes browse on walls and other substrates for food.
Conclusions
The interaction between habitat size and food abundance is consequential to aquatic animals, and choice of scale in experiments may affect results. Varying surface area and depth causes the scale effect, with small horizontal surface area and large depth decreasing matter exchange with the surrounding environment. In addition, fewer resources leads to less leaf surface area, and the effects of varying surface area will be greater under conditions of limiting resources. This leads to smaller size, which limits fecundity and survival. Choice of container size, either by ovipositing females or researchers, interacts with a major aspect of the ecology of animals; obtaining resources in a resource-limited environment.
Background
Microcosm and mesocosm studies are commonly used in ecological studies of aquatic and terrestrial habitats [1-3]. Microcosms and mesocosms are generally subsets of communities and abiotic factors of particular ecosystems, with restricted exchange of matter and energy [3]. These containers are by definition smaller than the organism's actual habitat, and controversy exists about the relevance of these studies because of the scaling of simulated habitats and the lack of replication and complexity [1,3-5]. There is often an implicit, and untested, assumption that scaling doesn't affect experimental outcomes in microcosm studies [3].
Aquatic insects such as mosquitoes are often studied in micro- and mesocosms; in fact, much of what we know about mosquitoes has come from microcosm and laboratory studies [e.g., 6-14]. These types of studies may be more relevant for those mosquitoes that breed in phytotelmata (i.e., plant-held waters) or artificial containers, as these experimental units may hold as much volume as many natural treeholes, pitcher plants, or other containers [15,16]. However, there may still be fundamental ecological effects of changing habitat size for aquatic insects living in a variety of habitats, such as phytotelmata and ponds.
One species of mosquito that breeds in natural and artificial habitats of various sizes is Culex pipiens L., more commonly known as the house mosquito [17,18]. This culicid has a more or less global distribution, and is found in North America, Africa, Europe, Asia, and Australia. Although C. pipiens primarily utilizes birds as bloodmeal hosts [19,20], it inhabits human-made containers, and is a known pest of humans [21].
Culex pipiens females oviposit, and larvae survive, in a wide array of aquatic habitats, including gutters, birdbaths, pools, rain barrels, treeholes, and stagnant pools of water [18,21]. In many of these aquatic habitats, leaf litter forms the basis of the food web. Microbes grow on the leaf litter and other detritus, and mosquitoes such as C. pipiens browse substrate or filter water for microbes and detritus [23]. Because the size of C. pipiens habitat varies, and because there is often an implicit assumption in experimental designs that microcosm scale does not affect experimental outcomes [3], it is a logical species to test for scale effects.
Varying habitat size affects species and communities living in small aquatic habitats, and many of these species appear to be flexible in their choice of habitat [16,23-25]. However, we need to be able to distinguish between fundamental effects of scale, that is, how habitats of different size affect growth and survival of individual C. pipiens, and artifacts caused by scaling, which may be determined as much by experimental design as by actual differences in habitat scale [3]. Vertical, or wall, surface area is thought to be the source of artifacts in micro- and mesocosm experiments that are scaled down from actual habitat size and artificially enclose the system in question [3]. Wall area is an artifact, because as microcosms are scaled down wall area per unit volume increases, which may affect composition of the microbial community and increase dominance of periphyton [3]. Determining the source of effects is critical for understanding the ecology of scaling and for how microcosm design affects experimental outcomes. If scaling of habitat affects C. pipiens, which inhabits aquatic systems of varying size, it might likely affect many other organisms that either live in small aquatic habitats, or have been tested in small-scale microcosms.
Resource quantity, in the form of leaf litter, also varies in these habitats, and we sought to test for an interaction between the effects of food abundance on mosquitoes, which are well known [22], and the effects of habitat size. This would give us a better understanding of how scaling impacts the biology of the mosquitoes, especially as higher quantities of leaf litter increase growth of bacteria, some of which might grow over the vertical surfaces of microcosms, and consequently provide more food to mosquito larvae. We hypothesized that there would be detrimental effects of increasing depth and decreasing horizontal surface area on survival and growth of C. pipiens. Specifically, microcosms with a lower horizontal surface area to depth ratio will have an adverse effect on developing mosquitoes because of lowered exchange of gases, which may increase growth of anaerobic bacteria that produce methane or hydrogen sulfide (CJP, unpublished data), or allow accumulation of mosquito wastes. Hydrogen sulfide, for instance, is a known toxin to animals [26], and affects growth of the eastern treehole mosquito Aedes triseriatus (Paradise, unpublished data). Microcosms with less vertical (wall) surface area per unit volume than others will be detrimental to mosquitoes, as there will be less surface area from which to browse for microbes. Finally, we hypothesized that effects of leaf litter quantity could interact with effects of container size, as increased leaf litter may compensate for lower wall area per unit volume, since microbes grow, and mosquitoes browse, on leaf litter [22]. High food abundance appears to decrease stress and compensate for detrimental abiotic factors [11,27,29]. Our experimental design allowed us to test for both vertical and horizontal surface area effects, which may be both natural effects of changing habitat size or artifacts of experimental microcosm design [3].
Results
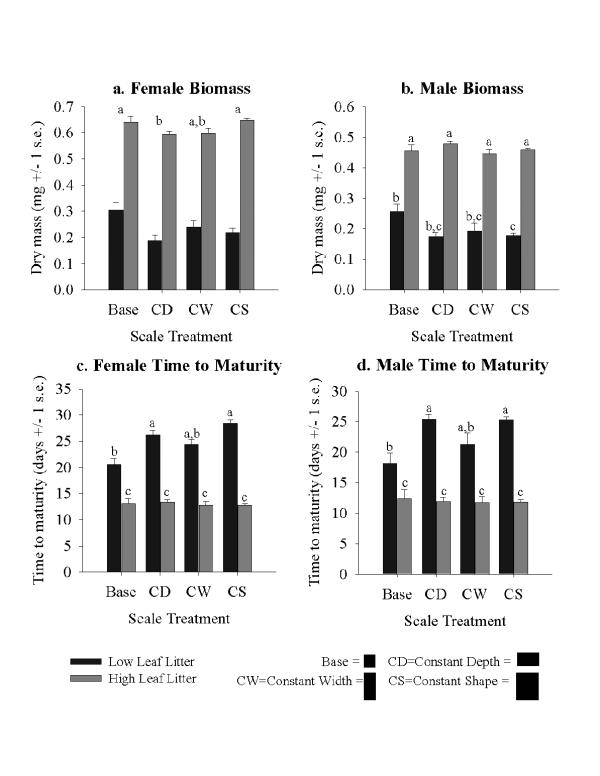
The interaction between food availability and container size was significant for all response variables except female biomass, which was affected only by main effects (Table 1; Fig. 1a). Not surprisingly, high leaf litter quantity led to larger mass, shorter larval development time and higher survival (Figs. 1 and 2a). When the interaction was present, there was no effect of scale at high food abundance, but when food abundance was low, container size affected male and female development time and mass of males (Figs. 1b,c,d). There were significant differences between B (Base size – used in other experiments [16]; see Materials & Methods and Table 2) and CD (Constant Depth) microcosms for female biomass and male and female time to maturity, such that B microcosms, with more wall area per unit volume, had larger, earlier emerging mosquitoes (Figs. 1a, c, and 1d; Table 1). This is evidence for experimental artifacts caused by decreasing wall surface area per unit volume. However, differences between CW (Constant Width) and CS (Constant Shape – Table 2) were not evident.
Table 1.
Results of two-way analyses of variance. a. two-way analyses of variance. b. Logistic regression.
| a. Analysis of variance | Litter | Scale | Litter* Scale | ||||||
| F | df | P | F | df | P | F | df | P | |
| Female mass | 351.9 | 1,15 | 0.0001 | 5.34 | 3, 15 | 0.011 | 1.21 | 3,15 | 0.34 |
| Log female development | 245.2 | 1,15 | 0.0001 | 4.55 | 3, 15 | 0.018 | 5.27 | 3,15 | 0.011 |
| Male mass | 516.1 | 1,15 | 0.0001 | 3.93 | 3, 15 | 0.03 | 5.12 | 3,15 | 0.012 |
| Log male development | 240.6 | 1,15 | 0.0001 | 4.56 | 3, 15 | 0.018 | 6.21 | 3,15 | 0.006 |
Figure 1.
Average (+/- 1 s.e.) effects of scale and leaf litter on growth and development of Culex pipiens. a. Female biomass. b. Male biomass. c. Time for female larvae to become adults. d. Time for male larvae to become adults. Dark bars are low leaf litter, and light bars are high leaf litter treatments. For each graph, bars with the same letter above them are not significantly different. For female biomass, the letters refer to comparisons of the scale main effect, since there was no statistical interaction, and for all others, the letters refer to the leaf litter by scale interaction. The relative sizes of the scale treatments are shown pictorially at the lower right.
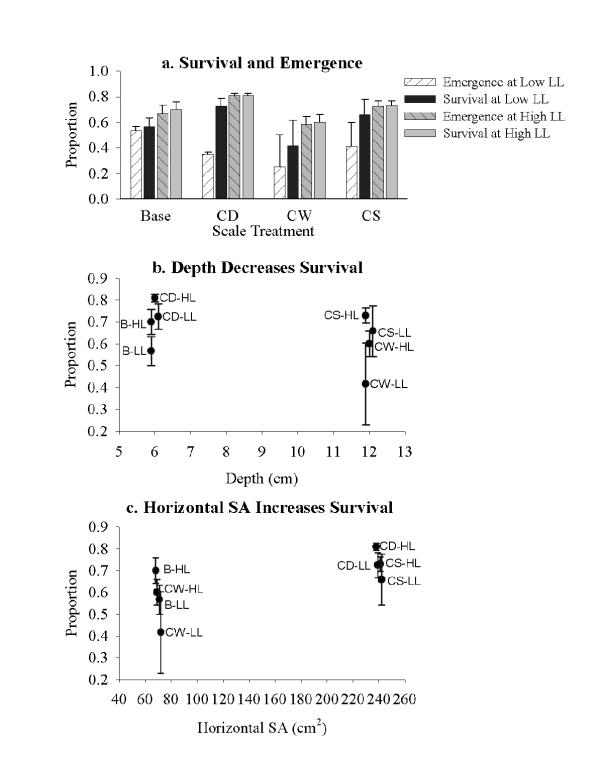
Figure 2.
Survival effects. a. Average survival (+/- 1 s.e.) for all eight treatment combinations. White and grey, hatched bars (first and third in each set) are emergence at low and high leaf litter, respectively, and dark and light blue bars (second and fourth in each set) are survival at low and high leaf litter, respectively. b. Mean proportion survival for four treatments at each of two depths. c. Mean proportion survival for four treatments at each of two horizontal surface areas. For b and c, points are offset slightly on the x-axis to better view error bars (which = 1 s.e.). HL = high leaf litter treatments, and LL = low leaf litter treatments, and codes for scale are as in text.
Table 2.
Scale dimensions, leaf litter quantities, and mosquito abundance and density for each scale treatment.
| Scale Treatment: | ||||
| Parameter | Base | Constant Depth | Constant Width | Constant Scale |
| Depth (cm) | 6.0 | 6.0 | 12.0 | 12.0 |
| Width (cm) | 8–9.5 | 17.0 | 8–9.5 | 17.0 |
| Width:depth ratio | 1.25 | 2.50 | 0.625 | 1.25 |
| Horizontal SA (cm2) | 70 | 240 | 70 | 240 |
| Vertical SA (cm2) | 205 | 350 | 410 | 700 |
| Total Volume (ml) | 425 | 1550 | 900 | 3050 |
| High LL (g) | 5 | 10 | 10 | 40 |
| Low LL (g) | 1 | 4 | 2 | 8 |
| # of larvae | 10 | 40 | 20 | 80 |
| Larval density (#/i) | 23.5 | 25.8 | 22.2 | 26.2 |
SA = surface area; LL = leaf litter
Fundamental scale effects caused by increased volume were also present, but only for the main effect of scale on female biomass (Fig. 1a). B microcosms compared to CW microcosms were never significantly different, and CD vs. CS comparisons were only different for female biomass, where mosquitoes from CS, the largest and deepest containers, were significantly larger. In addition, mosquitoes reached maturity quicker and males were larger in B/low litter microcosms than in CS/low litter microcosms (Figs. 1b,c,d). These latter comparisons include both fundamental effects and wall area effects, but also indicate adverse effects on mosquitoes as container size increases.
The logistic regression analysis revealed that survival was dependent on litter quantity, horizontal surface area, and depth (Fig. 2; Table 3). More mosquitoes survived in high leaf litter treatments than in low leaf litter treatments (Fig. 2a). Emergence was markedly lower than survival in most low litter treatments, while almost all mosquitoes that survived also emerged from high litter treatments (Fig. 2a). This is partly an artifact caused by experimental duration. Microcosms with low horizontal surface area (B and CW) had lower survival than those with high horizontal surface area (Fig. 2c), and deeper microcosms (CW and CS) had lower survival than shallower microcosms (Fig. 2b). CD microcosms had higher survival than B and CS, and yet the latter produced larger females than CD (Figs. 1a and 2a).
Table 3.
Logistic regression analysis for survival. SA = surface area, G is the G-test.
| Constant | Coefficient | Log Likelihood | G | P | |
| Leaf Mass | 0.47 | 0.106 | -556.0 | 8.69 | 0.003 |
| Volume | 0.51 | 0.0001 | -558.9 | 2.81 | 0.09 |
| Vertical SA | 0.73 | 0.0001 | -560.3 | 0.81 | 0.81 |
| Horizontal SA | -0.07 | 0.0042 | -551.9 | 16.9 | 0.0001 |
| Depth | 1.44 | -0.065 | -557.2 | 6.30 | 0.012 |
Discussion
Petersen et al. [3] make a number of recommendations about using artificial habitats to test ecological questions, among them the suggestion to perform scale-sensitive experiments that allow an independent understanding of volume and wall effects. In this experiment, we independently varied different dimensions of small aquatic habitats to look at how microcosm design choices may affect experimental outcomes, and how natural changes in habitat size and shape may affect aquatic insect larvae in a potentially resource-limited environment.
Our two habitat size hypotheses predicted: 1) an adverse effect of a lower wall area per unit volume, a possible artifact of microcosm design (compare B to CD and CW to CS – see Materials & Methods and Table 2), and 2) an adverse effect of a lower horizontal surface area to depth ratio (compare B to CW and CD to CS), which may be a factor in experimental design or in choice of oviposition site by females. We found more support for the former hypothesis than the latter, but regardless, we found that container size interacted with resources such that C. pipiens is affected by habitat size only when resources are scarce. Mosquitoes in the CW microcosms, with the smallest surface area and the greatest depth, had the lowest survival. Both small horizontal surface area and large depth decrease matter exchange between the container and the surrounding environment, and increase waste accumulation from microbes and mosquitoes [8]. Small horizontal surface area and small volume may also increase direct interactions among individual mosquitoes, thus increasing interference competition. However, mosquitoes from CW microcosms were not significantly different from other mosquitoes in size or time to maturity. Differences did appear in comparisons of B to CD microcosms, indicating possible wall effects. Our prediction was that B microcosms, with more wall area per unit volume would allow for greater growth and shorter development time of mosquitoes, which it did at low resource levels, possibly by providing more substrate on which periphyton could grow [3]. In addition, at low litter abundance there is less leaf surface area, and the effects of varying wall surface area will be greater under conditions of limiting resources. Shorter development time is important in ephemeral aquatic habitats such as temporary ponds and phytotelmata, allowing adults to escape prior to dry down in late spring or early summer [29,30]. Short time to maturity allows the insects to escape their larval habitat before it dries up, and males that mature before females are ready to mate when females emerge.
To test horizontal surface area effects, we can compare CD to CS and B to CW (Table 2). Females from CS microcosms were larger than females from CD microcosms, regardless of resource levels. Both have the same wall area per unit volume, but CD microcosms had more horizontal surface area per unit volume than CS microcosms, and we predicted that CD microcosms would yield larger and faster growing larvae. The extremely large volume in CS containers may have supported high densities of microbes in the water column and prevented leaf litter from packing together, thus allowing more surface area for microbial growth and less interference among mosquitoes browsing for food than in smaller volume containers where leaves were more tightly packed. We observed more tightly packed leaf litter in smaller containers with high leaf litter quantities, but we did not measure microbial growth, so this idea remains to be tested. Although wall area per unit volume was equivalent in those two treatments, the high volume of CS indirectly produced more "wall" surface area. The other comparison between B and CW microcosms yielded mosquitoes that were similar in size and development rate. However, mosquitoes in B microcosms had higher survival than in CW, which had a lower horizontal surface area to depth ratio than B, in accordance with our second hypothesis.
A final comparison of B to CS microcosms (Table 2) is of interest because it represents a comparison of constant scaling, which encapsulates both horizontal and vertical scaling effects [3]. Here the major effect is in time to maturity and male biomass, with the smaller volume container producing larger males with shorter development times (Fig. 1). The large CS microcosms had both less horizontal and vertical surface area per unit volume, but a larger horizontal surface area to depth ratio. Even though B microcosms had a lower horizontal surface area to depth ratio, the actual depth was only 6 cm, and positive effects of more wall area and more horizontal surface area per unit volume may have outweighed adverse effects of depth.
The interaction we found between habitat size and food abundance is consequential for ecology of the organisms within, and there may be a complex relationship between habitat size, habitat drying (which changes volume when it occurs), and resources [30,32]. For instance, leaf litter resources are often limiting to organisms in aquatic detritus-based communities [11,15,22,32,33]. Resources in the form of leaf litter are consumed by microbes, which are then consumed by mosquito larvae, along with small particulate organic matter [22]. When litter abundance is high, microbial growth is also high (CJ Paradise, personal observation), due to both high levels of resources and substrate for microbes to grow on. This may lead to increased detritivore growth and decreased stress due to deficiencies in abiotic factors, as evidenced by the mosquitoes in high leaf litter treatments being unaffected by habitat size and shape. Higher growth by larvae leads to larger size, and larger female mosquitoes have higher fecundity and both males and females have higher adult survival [7,34,35].
Growth and survival may thus be affected by habitat size, which varies greatly among small aquatic habitats [23,24,31,36]. There is a positive relationship between size, or volume, of the habitat and species richness, and between volume and growth responses of individual species [16,23-25,31,37]. Levels of water and resources may be important cues to ovipositing females, with resulting impacts on individuals and communities [9,16,38,39]. Oviposition site selection is an important variable affecting survival and development of non-dispersing larvae, and large volumes may be preferred by females laying eggs in aquatic environments due to increased stability and decreased risk of drought [29,39]. However, allochthonous plant material from surrounding terrestrial areas is the major input of energy resources to many freshwater detritus-based food webs, and resource quantity in these habitats is highly heterogeneous and dependent on location of the habitat relative to resource inputs [14,40]. Clearly, the choice of habitat by ovipositing C pipiens females will have growth and survival consequences for offspring.
Conclusions
Choice of scale in experimental design is important, even when studying mosquitoes adapted to a wide variety of habitats. The scale effect is likely to be caused by a combination of horizontal surface area, wall area, and depth. When food resources are low, high horizontal and vertical surface area per unit volume allow for greater growth than in conditions of low horizontal and vertical surface area per unit volume. Larval densities used in this experiment were fairly low, but competition for resources may still have occurred when leaf litter quantities were low. The effects of container dimensions thus constrain larval growth, possibly by decreasing exchange of materials or reducing inputs of resources, an area of future investigation. Choice of container size, either by ovipositing females or researchers, interacts with a major aspect of the ecology of these organisms; obtaining resources in a potentially resource-limited environment.
Materials and Methods
The experimental design consisted of twenty-four microcosms, each of which fell into four different scale types: 1) a "base" size (B), which has been used in microcosm studies of treehole mosquitoes [16],[31],[33], 2) "constant width" (CW), using the same size container and doubling the depth, 3) "constant depth" (CD), using the same depth as the base, but doubling the width, and 4) "constant shape" (CS), which doubles both the width and depth of the base treatment (Table 2).
By comparing B to CD and CW to CS we can assess whether there were experimental artifacts caused by decreasing wall surface area per unit volume. Both pairs have containers with the same depth, but the second container in each pair has more total wall surface area and less wall area per unit volume. By comparing B to CW and CD to CS we could test for fundamental scale effects caused by increased volume. Both pairs here have containers with the same width and have the same wall area per unit volume. However, the second container in each pair is deeper and has less horizontal surface area per unit volume.
Microcosms used for B and CW treatments were 1.0 liter polyethylene containers. Dimensions were 8 cm × 8 cm at the microcosm bottom, widening to 9.5 cm × 9.5 cm at the top, with a total depth of 13.8 cm. Microcosms used for CD and CS treatments were 5.0 liter polyethylene containers, with full width of 17 cm and a total depth of 20.5 cm. These containers were not completely square, as the vertical comers were rounded. Actual dimensions are shown in Table 2. The overall habitat sizes are within the range of natural habitats used by C. pipiens. Netting (0.2 mm mesh) covered holes cut in the lids.
Within each scale type, half of the microcosms had high leaf litter (about 10 g · l-1 of dried red oak (Quercus rubra L.) leaves) and half had low leaf litter (approximately 2 g · I-1 of dried red oak leaves; Table 2), with three replicates of each treatment combination. These resource levels have been shown to affect growth of treehole mosquitoes and colonization of artificial treeholes in the field [16,28,31,33]. Leaves were collected in January 2000, dried for one week at 80°C, weighed to within 0.05 g of the desired mass, and added to the appropriate microcosms. The drying of leaves may mimic natural conditions, as water in container habitats is ephemeral, with containers drying and filling with precipitation inputs [10,29,30].
After addition of leaf litter, distilled water was added to a line that marked the appropriate depth. We allowed the microcosms to incubate for one week while leaves were wetted and microbes grew. We then added first instar C. pipiens larvae obtained from Carolina Biological Supply to each microcosm in such a way that the density of each microcosm would be relatively constant. However, because of the slight differences in shape noted above, the exact initial densities ranged from 22 to 26 larvae · I-1 (Table 2).
The mosquito larvae were added on 17 February 2000, and by early March adults had begun to emerge from high leaf litter treatments. As the adults emerged each day, they were captured, counted, and sexed. Adults were frozen and later dried at 100°C for 3–5 days for determination of biomass. The adults were collected daily until 25 March 2000, at which point emergence was less than five adults per day for one week, and we ended the experiment. At that point, the microcosms were searched for remaining larvae and pupae, which were counted for determination of total survival to that day.
Percentage survival, length of larval period, and total female biomass were calculated. Statistical analysis consisted of two-way analyses of variance (ANOVA) on the mean male and female biomass and time to maturity from each microcosm, and logistic regression on proportion survival. For the ANOVAs, we used the means because individual mosquito responses were not independent, and an ANOVA model with microcosm nested within the fixed effects of leaf litter and container size did not work because some microcosms had no mosquito emergence. The Tukey procedure for comparisons of treatment means was used when tests were significant for interactions at or below the α of 0.0125 (=0.05/# of ANOVAs performed = 0.05/4; [41]). Time to maturity was log-transformed to approximate normality and reduce heteroscedasticity. For logistic regression, we tested for relationships between survival and horizontal surface area, volume, vertical surface area, depth, and leaf litter quantity. We did not test proportion emergence because more adults might have emerged from low leaf litter treatments had the experiment been run longer, possibly biasing those results.
Acknowledgments
Acknowledgements
We thank Dr. David Glick for assistance and support, the King's College Department of Biology for funds and Petersen, Cornwell, and Kemp (1999) for inspiration.
Contributor Information
Gregory Wynn, Email: glw1178@hotmail.com.
Christopher J Paradise, Email: chparadise@davidson.edu.
References
- Drake JA, Huxel GH, Hewitt CL. Microcosms as models for generating and testing community theory. Ecology. 1996;77:670–677. [Google Scholar]
- Moore JC, De Ruiter PC, Hunt HW, Coleman DC, Freckman DW. Microcosms and soil ecology: critical linkages between field studies and modeling food webs. Ecology. 1996;77:694–705. [Google Scholar]
- Petersen JE, Cornwell JC, Kemp M. Implicit scaling in the design of experimental aquatic ecosystems. Oikos. 1999;85:3–18. [Google Scholar]
- Odum EP. The mesocosm. Bioscience. 1984;34:558–562. [Google Scholar]
- Carpenter SR. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology. 1996;77:677–680. [Google Scholar]
- Novak RJ, Shroyer DA. Eggs, of Aedes triseriatus and Ae. hendersoni: a method to stimulate optimal egg hatch. Mosquito News. 1978;38:515–521. [Google Scholar]
- Livdahl TP. Competition within and between hatching cohorts of a treehole mosquito. Ecology. 1982;63:1751–1760. [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529–1546. [Google Scholar]
- Beehler J, Lohr S, DeFoliart G. Factors influencing oviposition in Aedes triseriatus (Diptera: Culicidae). Great Lakes Entomol. 1992;25:259–264. [Google Scholar]
- Jenkins B, Kitching RL, Pimm SL. Productivity, disturbance and food web structure at a local spatial scale in experimental container habitats. Oikos. 1992;65:249–255. [Google Scholar]
- Leonard PM, Juliano SA. Effect of leaf litter and density on fitness and population performance of the hole mosquito Aedes triseriatus. Ecol Entomol. 1995;20:125–136. [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996; 21:117–127. [Google Scholar]
- Barrera R, Medialdea V. Development time and resistance to starvation of mosquito larvae. J Nat Hist. 1996;30:447–458. [Google Scholar]
- Walker ED, Kaufman MG, Ayres MP, Riedel MH, Merritt RW. Effects of variation in quality of leaf detritus on growth of the eastern tree-hole mosquito, Aedes triseriatus (Diptera: Culicidae). Can J Zool. 1997;75:706–718. [Google Scholar]
- Heard SB. Pitcher-plant midges and mosquitoes: a processing chain commensalism. Ecology. 1994;75:1647–1660. [Google Scholar]
- Paradise CJ. Colonization and development of insects in simulated treehole habitats with distinct resource and pH regimes. Ecoscience. 1998;5:39–45. [Google Scholar]
- Laird M. The Natural History of Larval Mosquito Habitats. London, Academic Press. 1988.
- Savage H, Miller B. House mosquitoes of the USA, Culex pipiens complex. Wing Beats. 1995;6:8–9. [Google Scholar]
- Andersen JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, RA French, Garmendia AE, Van HJ Kruiningen. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science. 1999; 286:2331–2333. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- United States Centers for Disease Control (CDC) 2001. http://www.cdc.gov/ncidod/dvbid/westnile/insects.htm
- Matheson R. Handbook of the Mosquitoes of North America, 2nd edn. New York, Hafner Publishing Co. 1966.
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Sota T. Effects of capacity on resource input and the aquatic metazoan community structure in phytotelmata. Res Pop Ecol. 1996;38:65–73. [Google Scholar]
- Sota T. Microhabitat size distribution affects local difference in community structure: metazoan communities in treeholes. Res Pop Ecol. 1998;40:249–255. [Google Scholar]
- Daugherty MP, Juliano SA. Factors affecting the abundance of scirtid beetles in container habitats. J North Am Benth Soc. 2000;20:109–117. [Google Scholar]
- Bagarinao T. Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquatic Toxicol. 1992;24:21–62. doi: 10.1016/0166-445X(92)90015-F. [DOI] [Google Scholar]
- Hard JJ, Bradshaw WE, Malarkey DJ. Resource- and density-dependent development in treehole mosquitoes. Oikos. 1989;54:137–144. [Google Scholar]
- Paradise CJ. Effects of pH and resources on a processing chain interaction in simulated treeholes. J Anim Ecol. 2000; 69:651–658. doi: 10.1046/j.1365-2656.2000.00423.x. [DOI] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Drought and the organization of treehole communities. Oecologia. 1988;74:507–514. doi: 10.1007/BF00380047. [DOI] [PubMed] [Google Scholar]
- Aspbury AS, Juliano SA. Negative effects of habitat drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia. 1998;115:137–148. doi: 10.1007/s004420050500. [DOI] [PubMed] [Google Scholar]
- Paradise CJ. Abiotic and biotic factors controlling the structure of insect treehole communities. PhD Dissertation, University Park, PA, Pennsylvania State University. 1997.
- Carpenter SR. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- Paradise CJ, Kuhn KL. Effects of pH and leaf litter quantity on scirtid beetles inhabiting treeholes. Freshw Biol. 1999;41:43–49. doi: 10.1046/j.1365-2427.1999.00384.x. [DOI] [Google Scholar]
- Hawley WA. A high-fecudity aedine: factors affecting egg production of the western treehole mosquito, Aedes sierrensis. J Med Entomol. 1985;22:220–225. doi: 10.1093/jmedent/22.2.220. [DOI] [PubMed] [Google Scholar]
- Benjamin SN, Bradshaw WE. Body size and flight activity effects on male reproductive success in the pitcherplant mosquito (Diptera: Culicidae). Ann Entomol Soc Am. 1994;87:331–336. [Google Scholar]
- Kitching RL. Spatial and temporal variation in food webs in water-filled treeholes. Oikos. 1987;48:280–288. [Google Scholar]
- Warren PH, Spencer M. Community and food-web responses to the manipulation of energy input and disturbance in small ponds. Oikos. 1996;75:407–418. [Google Scholar]
- Wilton DP. Oviposition site selection by the tree-hole mosquito, Aedes triseriatus (Say). J Med Entomol. 1968;5:189–194. doi: 10.1093/jmedent/5.2.189. [DOI] [PubMed] [Google Scholar]
- Sota T, Mogi M, Hayamizu E. Habitat stability and the larval mosquito community in treeholes and other containers on a temperate island. Res Pop Ecol. 1994;36:93–104. [Google Scholar]
- Wallace JB, Eggert SL, Meyer JL, Webster JR. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science. 1997;277:102–104. doi: 10.1126/science.277.5322.102. [DOI] [Google Scholar]
- Neter J, Wasserman W, Kutner MH. Applied Linear Statistical Models: Regression, Analysis of Variance, and Experimental Designs, 3rd edn. Homewood, IL, Irwin. 1990.