Abstract
Purpose
A paucity of data exists on the insignificant disease potentially suitable for active surveillance among men with intermediate-risk prostate cancer. We tried to identify pathologically insignificant disease and its preoperative predictors in men who underwent radical prostatectomy for intermediate-risk prostate cancer.
Methods
We analyzed data of 1630 men who underwent radical prostatectomy for intermediate-risk disease. Total tumor volume data were available in 332 men. We examined factors associated with classically-defined pathologically insignificant cancer (organ-confined disease with total tumor volume ≤ 0.5 ml with no Gleason pattern 4 or 5) and pathologically favorable cancer (organ-confined disease with no Gleason pattern 4 or 5) potentially suitable for active surveillance. Decision curve analysis was used to assess clinical utility of a multivariable model including preoperative variables for predicting pathologically unfavorable cancer.
Results
In the entire cohort, 221 of 1630 (13.6%) total patients had pathologically favorable cancer. Among 332 patients with total tumor volume data available, 26 (7.8%) had classically-defined pathologically insignificant cancer. Between threshold probabilities of 20% and 40%, decision curve analysis demonstrated that using multivariable model to identify active surveillance candidates would not provide any benefit over simply treating all men who have intermediate-risk disease with radical prostatectomy.
Conclusions
Although a minority of patients with intermediate risk disease may harbor pathologically favorable or insignificant cancer, currently available conventional tools are not sufficiently able to identify those patients.
Keywords: prostate, prostatic neoplasms, insignificant, intermediate-risk, prediction
Introduction
Increasingly popular as a management option for localized prostate cancer (PCa), the main purpose of active surveillance (AS) is to avoid or delay radical therapy in men with low-risk PCa without missing the window of opportunity for cure. Starting on AS, patients are also provided with more time for decision-making on treatment approach. Although the rationale for AS is well established, no consensus has been reached regarding the optimal candidates for AS. Moreover, the application of AS may be influenced by availability of other therapeutic options. Eligibility criteria for AS vary considerably amongst different institutions, with some allowing the inclusion of men with intermediate-risk PCa [1-4].
In an effort to extend the benefits of AS to more patients, some investigators have suggested broadening the inclusion criteria for AS, with one investigator proposing a strategy that initially would include as many men with PCa as is reasonable and during follow-up would narrow down based on biopsy and/or clinical findings [5,6]. Despite the lack of long-term follow-up, recent reports have shown that AS may have a role in the management of selected men with intermediate-risk PCa, demonstrating consistency in the proportion of men with intermediate-risk PCa who continue on AS and the high rates of overall and disease-specific survival [2-4]. The guidelines on PCa management published by the National Comprehensive Care Network (NCCN) include AS as an option for men with intermediate-risk PCa in patients with a life expectancy of less than 10 years [7].
As appealing as potential advantages of AS may seem, men with intermediate-risk PCa are considered to have a significantly higher risk of progression during AS than those with low-risk disease. Moreover, intermediate-risk PCa has been known to be a heterogeneous disease [8]. Although recent findings as aforementioned have led to speculation that a proportion of intermediate-risk disease may be indolent enough to undergo AS, a paucity of data exists on the identification of indolent or insignificant disease potentially suitable for AS among men with intermediate-risk PCa regardless of life expectancy [2-4]. To assess the feasibility and safety of AS in intermediate-risk PCa, such data is necessary. Thus, we sought to identify pathologically insignificant disease and its preoperative predictors in men who underwent immediate radical prostatectomy (RP) for intermediate-risk PCa.
Patients and methods
After obtaining institutional review board approval, we reviewed the database of 5350 men who underwent RP for PCa from January 2000 to December 2009 at Memorial Sloan-Kettering Cancer Center. When the patients were stratified into NCCN recurrence risk groups, 2205 were observed to have intermediate-risk disease (clinical stage T2b–T2c, biopsy Gleason score 7, or PSA level 10–20 ng/ml) preoperatively [9]. Patients with missing data (n = 387) and/or history of preoperative treatment (transurethral resection of the prostate [n = 17], radiation therapy [n= 33], 5α-reductase inhibitor [n = 60], chemotherapy [n = 6], or hormone therapy [n = 87]) were excluded, A total of 1630 patients were included in our study, of which 332 were found to have total tumor volume (TTV) data available.
Clinical data were obtained from the review of our database and medical records. Prostate volume was measured preoperatively by transrectal ultrasound (TRUS) or magnetic resonance imaging (MRI) using the widely recognized prolate ellipsoid formula [10]. In all patients, RP specimens were entirely submitted and whole-mounted for the pathologic analyses. As previously reported, RP specimens were uniformly processed, and TTV was measured by planimetry using Image-Pro Plus software, version 5.0.0.39 (Media Cybernetics, Inc., Rockville, MD, USA) in some patients [11].
Pathologically insignificant PCa was defined as organ-confined disease with TTV of ≤ 0.5 ml with no Gleason pattern 4 or 5 tumor foci [12]. Pathologically favorable PCa was defined as organ-confined disease with no Gleason pattern 4 or 5 tumor foci. Pathologically unfavorable PCa was defined as tumors that were non-organ-confined or having Gleason pattern 4 or 5 tumor foci.
We compared clinicopathologic features between patients who had TTV data available and those who did not via t-tests and chi-squared tests. The associations of preoperative variables (prostate volume, PSA at diagnosis, clinical stage, and biopsy Gleason score) with presence of pathologically insignificant cancer and pathologically favorable cancer were examined, respectively, via univariate and multivariate logistic regression models. Due to the limited number of patients with pathologically insignificant cancers among our subjects, only univariate logistic regression was used to assess the association between preoperative characteristics and the presence of pathologically insignificant cancer. The only exception was PSA, which was tested non-linearly using cubic splines. Kaplan-Meier method and log-rank test were used to assess postoperative biochemical recurrence (BCR)-free survival. Decision curve analysis was used to assess the clinical utility of the multivariable model for predicting pathologically unfavorable cancers and therefore identifying patients with intermediate-risk PCa who may be eligible for AS. Ten-fold cross-validation was used to correct for optimism. All analyses were performed using Stata 12.0 (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics are listed in Table 1. Of the 1630 patients with intermediate-risk PCa included in our study, 221 (13.6%) were found to have pathologically favorable cancer. Among the 332 patients with TTV data, 26 (7.8%) had pathologically insignificant cancer. When comparing patients with and without TTV data, the proportion of patients with pathologically favorable cancer was significantly higher in patients with TTV data (18% vs 12%, p = 0.007). Postoperative 5-year BCR-free survivals of patients with pathologically favorable cancer and those with pathologically insignificant cancer were 98.2% and 100%, respectively (p = 0.330). For patients with pathologically unfavorable PCa, 5-year BCR-free survival was 84.0%, significantly lower than the two groups (p < 0.0001).
Table 1.
Patient Characteristics (n=1,630)
| Median age at RP, yr (IQR) | 60 (55, 65) |
| Median BMI, kg/m2 (IQR) | 28.1 (25.9, 30.8) |
| Median prostate volume, ml (IQR) | 30.5 (23.3, 41.2) |
| Median PSA level, ng/ml (IQR) | 5.2 (4.1, 7.6) |
| Biopsy Gleason score (%) | |
| ≤ 6 | 300 (18) |
| 7 | 1330 (82) |
| Clinical T Stage (%) | |
| T1 | 867 (53) |
| T2 | 763 (47) |
| Pathologic Gleason score (%) | |
| 6 | 259 (16%) |
| 7 (3 + 4) | 938 (58%) |
| 7 (4 + 3) | 378 (23%) |
| 8-10 | 55 (3%) |
| Pathologic stage (%) | |
| pT2 | 1011 (62%) |
| pT3a | 511 (31%) |
| pT3b | 93 (6%) |
| pT3c | 2 (<1%) |
| pT4 | 13 (1%) |
| Pathologically favorable cancer (%) | 221 (13.6%) |
| Median tumor volume, ml (n=332 with TTV) (IQR) | 1.3 (0.7, 2.9) |
| Pathologically insignificant cancer (n=332 with TTV) (%) | 26 (7.8%) |
RP = radical prostatectomy; IQR= interquartile range; BMI = body mass index; PSA = prostate-specific antigen; TTV = total tumor volume
Analyzing only the patients with TTV data (n = 332), PSA (modeled with non-linear terms, p = 0.006), biopsy Gleason score (odds ratio [OR]: 0.16; 95% CI, 0.07, 0.36; p < 0.0001), and prostate volume (OR: 1.27 per 10-ml prostate volume; 95% CI, 1.12, 1.45; p = 0.0003) were observed to be significantly associated with pathologically insignificant cancer on univariate analyses. For example, for a man with a prostate volume of 35 ml, clinical stage T1 and biopsy Gleason 6, an increase in PSA from 4 ng/ml to 10 ng/ml corresponds to a 25% increase in the odds of having a pathologically unfavorable cancer. With only a minimal number of patients (7.8%) found to have pathologically insignificant cancer, multivariate analysis was not performed.
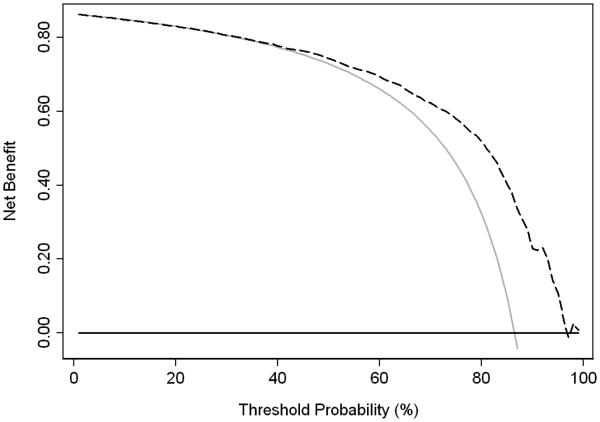
Among the 1630 total patients, PSA, prostate volume, clinical stage, and biopsy Gleason score were all found to be significantly associated with pathologically favorable cancer on multivariable analysis (Table 2). To assess the clinical utility of this multivariable model for the preoperative prediction of pathologically unfavorable cancer among our intermediate-risk PCa cohort, a decision curve was created (Fig. 1). We used threshold probabilities of 20% to 40%, considering that it would be difficult to justify performing RP on more than four men with insignificant disease for every one man appropriately treated for pathologically unfavorable disease. Between the threshold probabilities of 20% and 40%, we found that the multivariable model did not lead to greater clinical benefit than simply treating all patients, with none being offered AS. This finding is at least partly due to the relatively high rate (86%) of pathologically unfavorable cancer. A model would have to be nearly perfect to shift a substantial number of men from a mean risk of 86% to a risk lower than 20% to 40%. Meanwhile when we replaced the two variables of PSA and prostate volume with PSA density in multivariable model, no significant difference was observed in the clinical utility of the model.
Table 2.
Multivariate analysis of potential predictors for pathologically favorable cancers in intermediate-risk prostate cancer (n=1630)
| Predictors | OR | 95% CI | p-value |
|---|---|---|---|
| Clinical stage | 0.42 | 0.28, 0.63 | < 0.0001 |
| Biopsy Gleason score | 0.08 | 0.05, 0.11 | < 0.0001 |
| Prostate volume | 1.21 | 1.12, 1.30 | < 0.0001 |
| PSA | N/A* | N/A* | 0.003 |
OR = odds ratio; CI = confidence interval; PSA = prostate-specific antigen
Modeled with non-linear terms.
Fig. 1.
Decision curve for predicting pathologically unfavorable cancer. The black line at 0 represents treating no patients (active surveillance), while the gray line represents treating all patients. The dotted line represents treating patients based on the multivariable model.
Discussion
In this study, we observed that although only a very small number of patients who underwent RP for intermediate-risk PCa had pathologically insignificant cancer as classically defined, a non-negligible proportion of patients harbored pathologically favorable cancer potentially suitable for AS [12]. A multivariate model encompassing preoperative variables of PSA, prostate volume, clinical stage, and biopsy Gleason score was not observed to be clinically useful in the prediction of pathologically unfavorable cancers amongst patients with intermediate-risk PCa. As the interest in expanding the inclusion criteria for AS has been increasing, we believe that our findings may be considered clinically important because they lay the foundations for assessing the feasibility and safety of AS in intermediate-risk PCa.
As some have suggested that the classical definition of pathologically insignificant PCa is too stringent, Wolters et al. [13] has proposed an updated definition of pathologically insignificant PCa (organ-confined cancer with no Gleason 4 or 5 pattern and having a volume threshold < 1.3 cm3 for the index tumor and < 2.5 cm3 for the TTV) based on lifetime risk estimates of PCa diagnosis in screened and non-screened participants in a randomized screening trial and pathologic profiles of PCa found in corresponding RP specimens. In the same RP cohort, of which 57.5%, 22.5%, and 20.0% were low-risk, intermediate-risk, and high-risk PCa, respectively, authors found that nearly all (about 98%) of the organ-confined Gleason 6 PCa would be classified as insignificant cancer according to their updated definition of pathologically insignificant PCa [13]. A study comparing the eligibility criteria for AS of different institutions via a selected cohort of patients with biopsy Gleason 6 PCa showed that applying the updated definition of pathologically insignificant cancer rather than the classical Epstein definition increased the rate of insignificant cancer from 37% to 61% [1]. Only a minimal difference was observed between the updated definition of insignificant PCa (61%) and organ-confined Gleason ≤ 6 PCa without TTV restrictions (66%), consistent with the finding by Wolters et al. [1,13]. We hypothesize that in our cohort the proportion of men with organ-confined PCa without Gleason pattern 4 or 5, defined as pathologically favorable cancer, would be similar to that of patients meeting Wolters et al.’s updated definition of pathologically insignificant PCa.
A published review of the literature found that the incidence rates of insignificant PCa in RP specimens ranged from 2.3% to 25%, with higher rates reported in series that included only T1c and/or Gleason 6 PCa [14]. The authors suggest that differences in the selection of patients or the impact of screening practices may have contributed to the variations in the incidences of insignificant PCa. Meanwhile, a paucity of data exists on the incidence rate of pathologically insignificant disease in a cohort consisting only of intermediate-risk PCa. Compared with rates among low-risk patients or patients deemed eligible for AS according to the inclusion criteria of various institutions, the proportion of men in our intermediate-risk PCa cohort with insignificant PCa as defined classically was minimal. However, the rate of pathologically favorable cancer in our study, which we believe to be similar to that of pathologically insignificant cancer according to the updated definition, suggested that as many as 1 of 7 men with intermediate-risk disease undergoing RP may harbor insignificant disease, which would be difficult to consider negligible [13].
In our study, the model consisting of conventional variables PSA, prostate volume, clinical stage, and biopsy Gleason score was observed to offer no clinical benefit in the prediction of pathologically unfavorable cancer in men with intermediate-risk PCa. Since many patients had their diagnostic biopsy at other institutions before being referred to our institution, detailed biopsy findings, such as tumor length in biopsy cores, were often not available. Mostly analyzed among men with very low-risk PCa or low-risk PCa, to date the literature fails to offer a single superior predictive tool or model as a gold standard in the prediction of insignificant PCa [14]. The preoperative Epstein criteria have been reported to misclassify 16% to 42% of men who would have unfavorable pathologic features in RP specimens [12,14,15]. Several groups have put forward alternative predictive models or nomograms to enhance predictive accuracy, but most have been reported to be similar or only slightly superior to the Epstein criteria [16,17].
In the current study, we applied the endpoint of indolent pathology, defined classically or via updated definition, rather than metastasis-free or disease-specific survival. Since our endpoint can only be considered as a surrogate for long-term outcome, we admit that inherent limitations would exist regarding the interpretations of our findings. Recently, models combining clinical and biopsy data with MRI and MR spectroscopic imaging (MRSI) findings were found to be better than the clinical models for the prediction of insignificant PCa [18]. Also, efforts have been made to integrate state-of-the-art approach of analyzing genomic mutations in the prostate biopsy specimens to identify appropriate candidates for AS [19].
There are several potential limitations to our study. Not all patients with intermediate-risk PCa had TTV data. However, since the rate of pathologically favorable cancer was higher in men with TTV data than those without TTV data, it is reasonable to expect that the actual proportion of patients with pathologically insignificant cancer may not be higher than the observed rate among the patients with TTV data. Although we hypothesized that the rate of pathologically insignificant PCa according to the updated definition would be similar to the rate of pathologically favorable cancer we observed, pathologic analyses to calculate index tumor volume needs to be performed before we can apply the updated definition accurately to our intermediate-risk PCa cohort.
Conclusion
Although a minority of patients with intermediate risk disease may harbor pathologically favorable or insignificant cancer, currently available conventional tools are not sufficiently able to identify those patients. More efforts should be made to improve our ability to identify appropriate candidates for AS among men with intermediate risk PCa. Patients with intermediate-risk PCa should also be considered when planning prospective trials and investigations on the feasibility and safety of AS.
Acknowledgments
Funding/Support: Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers. Supported in part by funds provided by David H. Koch through the Prostate Cancer Foundation. Supported in part by NIH/NCI Cancer Center Support Grant to MSKCC under award number P30 CA008748.
References
- 1.Iremashvili V, Pelaez L, Manoharan M, Jorda M, Rosenberg DL, Soloway MS. Pathologic prostate cancer characteristics in patients eligible for active surveillance: a head-to-head comparison of contemporary protocols. Eur Urol. 2012;62:462–468. doi: 10.1016/j.eururo.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Bul M, van den Bergh RC, Zhu X, Rannikko A, Vasarainen H, Bangma CH, Schröder FH, Roobol MJ. Outcomes of initially expectantly managed patients with low or intermediate risk screen-detected localized prostate cancer. BJU Int. 2012;110:1672–1677. doi: 10.1111/j.1464-410X.2012.11434.x. [DOI] [PubMed] [Google Scholar]
- 3.Godtman RA, Holmberg E, Khatami A, Stranne J, Hugosson J. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Göteborg randomised population-based prostate cancer screening trial. Eur Urol. 2013;63:101–107. doi: 10.1016/j.eururo.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Cowan JE, Hilton JF, Reese AC, Zaid HB, Porten SP, Shinohara K, Meng MV, Greene KL, Carroll PR. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29:228–234. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Kwast TH. The trade-off between sensitivity and specificity of clinical protocols for identification of insignificant prostate cancer. Eur Urol. 2012;62:469–471. doi: 10.1016/j.eururo.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed HU. Prostate cancer: time for active surveillance of intermediate-risk disease? Nat Rev Urol. 2013;10:6–8. doi: 10.1038/nrurol.2012.213. [DOI] [PubMed] [Google Scholar]
- 7.Mohler J, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D'Amico AV, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Kawachi MH, Kuettel M, Lee RJ, Macvicar GR, Malcolm AW, Miller D, Plimack ER, Pow-Sang JM, Richey S, Roach M, 3rd, Rohren E, Rosenfeld S, Small EJ, Srinivas S, Stein C, Strope SA, Tward J, Walsh PC, Shead DA, Ho M, National comprehensive cancer network Prostate cancer, version 1.2014. J Natl Compr Canc Netw. 2013;11:1471–1479. doi: 10.6004/jnccn.2013.0174. [DOI] [PubMed] [Google Scholar]
- 8.Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology. 2012;80:1075–1079. doi: 10.1016/j.urology.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 9.NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline®) Prostate cancer v4.2013. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 10.Hong SK, Poon BY, Sjoberg DD, Scardino PT, Eastham JA. Prostate size and adverse pathologic features in men undergoing radical prostatectomy. Urology. 2014;84:153–157. doi: 10.1016/j.urology.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udo K, Cronin AM, Carlino LJ, Savage CJ, Maschino AC, Al-Ahmadie HA, Gopalan A, Tickoo SK, Scardino PT, Eastham JA, Reuter VE, Fine SW. Prognostic impact of subclassification of radical prostatectomy positive margins by linear extent and Gleason grade. J Urol. 2013;189:1302–1307. doi: 10.1016/j.juro.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 13.Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RC, Hoedemaeker RF, van Leenders GJ, Schröder FH, van der Kwast TH. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011;185:121–125. doi: 10.1016/j.juro.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 14.Ploussard G, Epstein JI, Montironi R, Carroll PR, Wirth M, Grimm MO, Bjartell AS, Montorsi F, Freedland SJ, Erbersdobler A, van der Kwast TH. The contemporary concept of significant versus insignificant prostate cancer. Eur Urol. 2011;60:291–303. doi: 10.1016/j.eururo.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Lee MC, Dong F, Stephenson AJ, Jones JS, Magi-Galluzzi C, Klein EA. The Epstein criteria predict for organ-confined but not insignificant disease and a high likelihood of cure at radical prostatectomy. Eur Urol. 2010;58:90–95. doi: 10.1016/j.eururo.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Steyerberg EW, Roobol MJ, Kattan MW, van der Kwast TH, de Koning HJ, Schröder FH. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. 2007;177:107–112. doi: 10.1016/j.juro.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi H, Wang X, Ochiai A, Trpkov K, Yilmaz A, Donnelly JB, Davis JW, Troncoso P, Babaian RJ. A nomogram for predicting low-volume/low-grade prostate cancer: a tool in selecting patients for active surveillance. Cancer. 2007;110:2441–2447. doi: 10.1002/cncr.23055. [DOI] [PubMed] [Google Scholar]
- 18.van den Bergh RC, Ahmed HU, Bangma CH, Cooperberg MR, Villers A, Parker CC. Novel tools to improve patient selection and monitoring on active surveillance for low-risk prostate cancer: a systemic review. Eur Urol. 2014;65:1023–1031. doi: 10.1016/j.eururo.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Knezevic D, Goddard AD, Natraj N, Cherbavaz DB, Clark-Langone KM, Snable J, Watson D, Falzarano SM, Magi-Galluzzi C, Klein EA, Quale C. Analytical validation of the Oncotype DX prostate cancer assay – a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics. 2013;14:690. doi: 10.1186/1471-2164-14-690. [DOI] [PMC free article] [PubMed] [Google Scholar]