Abstract
Background/Objectives:
Increased adipose tissue mass closely associates with the development of insulin resistance and type 2 diabetes mellitus. Previously, we reported that CREB3L4 expressed in adipose tissue negatively regulates adipogenesis, and Creb3l4 knockout mice fed a high-fat diet for 16 weeks showed fat cell hyperplasia, with improved glucose tolerance and insulin sensitivity. These mice did not show significant weight gain and fat mass. Because fat diet or aging is known to be associated with the development of obesity, we examined the effects of Creb3l4 gene subjected to low-fat diet (LFD) or aging process on body composition and obesity risk.
Subjects/Methods:
We fed Creb3l4 knockout mice a low-fat diet for 16 weeks (LFD group) or chow diet for over 1 year (aged group) and observed various metabolic parameters in the LFD-fed and aged Creb3l4 knockout mice.
Results:
LFD-fed and aged Creb3l4 knockout mice showed significant weight gain and adiposity, impaired glucose tolerance and decreased insulin sensitivity, compared with wild-type mice.
Conclusions:
Creb3l4 has a critical role in metabolic phenotypes and a better understanding of its function may provide improved insight into the etiology of diabetes and other metabolic disorders.
Introduction
Western societies have adopted a lifestyle with low physical activity and overconsumption of energy-rich food,1 resulting in a substantial increase in the incidence of obesity accompanied by impaired glucose and lipid metabolism.1, 2, 3 In general, it is accepted that expansion of adipose tissue and lipid accumulation in insulin-sensitive tissues (for example, skeletal muscle and liver) closely relates to the development of metabolic diseases.4 In particular, increased adipose tissue mass closely associates with the development of insulin resistance and type 2 diabetes mellitus.5 In addition, increased adiposity is a hallmark of aging and a source of chronic inflammation, which further accelerates the aging process. This vicious cycle of aging, visceral fat accumulation and inflammation often leads to metabolic dysfunction.6, 7 Thus understanding the molecular mechanism(s) of adipose tissue expansion in the context of transcriptional regulation could be pivotal to combat metabolic syndromes, including obesity and type 2 diabetes mellitus.
The endoplasmic reticulum and Golgi transmembrane protein CREB3L4 (cAMP responsive element binding protein 3-like 4) is mainly expressed in human prostate tissue and mouse testis.8, 9 Specifically, CREB3L4a, also known as Atce1/Tisp40a, is the major form of CREB3L4 expressed in mouse testis8 and is highly homologous with mouse CREB3 (identity 62%, similarity 72%).10 Deletion of the transmembrane domain of CREB3L4 results in nuclear accumulation regulated by endoplasmic reticulum stress.11 Moreover, whole-body knockout of Creb3l4 (Creb3l4 KO) results in abnormal epididymal sperm nuclei.12 CREB3L4 is also known to have an important role in the development of prostate cancer and is more highly expressed in cancerous than in non-cancerous prostate cells.13 However, the functional role of CREB3L4 in insulin-sensitive tissues such as adipose tissue, skeletal muscle or liver is not well known.
Recently, we reported that CREB3L4 could regulate adipocyte differentiation.14 Upon CREB3L4 downregulation, adipocyte differentiation occurs even when cultured with a minimal hormonal inducer.14 In addition, we showed that Creb3l4 KO mice fed a high-fat diet (HFD) did not show significant differences in body weight or fat mass, as compared with wild-type (WT) controls.14 However, KO mice undergo fat cell hyperplasia owing to increased adipogenesis, with improved glucose tolerance and increased insulin sensitivity.14
To examine whether the process of aging and low-fat diet (LFD) influences metabolic phenotypes, we explored the effects of an LFD or aging on the metabolic profiles of Creb3l4 KO mice. Both LFD (LFD group) and aged Creb3l4 KO mice fed chow diet (aged group) were prone to body weight gain with increased adiposity. These mice also exhibited impaired glucose tolerance and decreased insulin sensitivity, as compared with WT control mice.
Materials and methods
Animal experiments and KO mice
All animal experiments were approved by the Institutional Animal Care and Use Committee of Yonsei University Cmollege of Medicine (Protocol no. 2011-0199, 2011-0315, 2012-0255). All mice were housed under standardized conditions of humidity, temperature (22–24 °C) and a 12-h light/12-h dark cycle. The male mouse (11 weeks, n=4–5) strains db/m+(C57BLKS/J lar-m+/Leprdb), db/db (C57BLKS/J lar-Leprdb/Leprdb), lean and ob/ob (C57BL/6J Ham Slc-ob/ob) (Shizuoka laboratory, Hamamatsu, Japan) were fed a regular chow diet. The Creb3l4 KO mouse has been described previously,12 and Creb3l4 KO mouse embryos were purchased from RIKEN (The Institute of Physical and Chemical Research, Ibaraki, Japan) with permission from Dr Nojima. New born pubs of Creb3l4 KO mice and WT littermates were randomly selected by genotyping PCR using specific primer. Creb3l4 KO mice and their WT littermates were 10–11-week old and weighed approximately 23 g; the mice were fed a 10% LFD (D12450B, Research Diets, New Brunswick, NJ, USA) over 16 weeks. The body weight of mice was assessed every weekly. To observe an effect of aging process, the aged WT and aged Creb3l4 KO mice (n=3–6) were fed a regular chow diet for up to 85 weeks.
Glucose and insulin tolerance tests
For oral glucose tolerance tests, mice were fasted for 16 h and then orally administered glucose (20% wt/vol.) using a feeding tube (2 g kg−1 body weight), with blood glucose levels monitored at each time point. For insulin tolerance tests, mice were fasted for 6 h and then given an intraperitoneal injection of insulin (0.75 U kg−1 body weight; Humulin R, Eli Lilly, Indianapolis, IN, USA). Blood glucose levels were monitored (One TOUCH Sure Step, Life Scan, Milpitas, CA, USA) at the time points indicated in the figure legends. The areas under the curve of glucose were calculated during the course of the tests.
Phenotypic evaluation of mice
WT male and Creb3l4 KO male mice were fed a 10% LFD for 18 weeks. Plasma insulin and adiponectin levels were then measured using enzyme-linked immunosorbent assay kits (ALPCO Immunoassays, Salem, NH, USA). Plasma resistin was measured using MAGPIX (Luminex, Austin, TX, USA) with MILLIPLEX MAP mouse magnetic beads (Merck Millipore Corp., St Charles, MO, USA).
Quantitative real-time PCR
Total RNA was isolated from mouse adipose tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, and cDNA was generated using a reverse transcription system (ImProm-II Reverse Transcription System; Promega, Madison, WI, USA). Quantitative real-time PCR was performed using a real-time PCR system (Step One, Applied Biosystems, Foster City, CA, USA), according to the manufacturer's protocol. Changes in mRNA levels were calculated using the comparative Ct method.15 The relative amount of mRNA in each sample was normalized to transcript levels of the Rplp0 (36B4) ribosomal protein-encoding housekeeping gene. Primers used for real-time PCR were as follows: Creb3l4-F, 5′-ATATCTTCTCGACGGGATCCTT-3′ Creb3l4-R, 5′-TCCCTACCAGGAGATGTTTC; Cidec-F, 5′-TCCAGGACATCTTGAAACTT-3′ Cidec-R, 5′-GGCTTGCAAGTATTCTTCTGT-3′ Cd36-F, 5′-TGCACCACATATCTACCAAA-3′ Cd36-R, 5′-TTGTAACCCCACAAGAGTTC-3′ Pepck-F, 5′-ACACACACACATGCTCACAC-3′ Pepck-R, 5′-ATCACCGCATAGTCTCTGAA-3′ Fasn-F, 5′-TTTGCTGCCGTGTCCTTCTACC-3′ Fasn-R, 5′-ATGTGCACAGACACCTTCCCGT-3′ Nr1h3(Lxra)-F, 5′-GAGAAGCTGGTGGCTGCCCA-3′ Nr1h3(Lxra)-R, 5′-AGCTGTAGGAAGCCAGGGAG-3′ Cd11c-F, 5′-ACACAGTGTGCTCCAGTATGA-3′ Cd11c-R, 5′-GCCCAGGGATATGTTCACAGC-3′ Emr1(F4/80)-F, 5′-CTTTGGCTATGGGCTTCCAGTC-3′ Emr1(F4/80)-R, 5′- GCAAGGAGGACAGAGTTTATCGTG-3′ Rplp0-F, 5′-TGGCCAATAAGGTGCCAGCTGCTG-3′ and Rplp0-R, 5′-CTTGTCTCCAGTCTTTATCAGCTGCAC-3′.
Hematoxylin and eosin staining
Liver, brown adipose tissue (BAT), epididymal white adipose tissue (WAT) and subcutaneous WAT were fixed with 10% neutral-buffered formalin, embedded in paraffin and sectioned. Hematoxylin and eosin staining was then performed on these sections.
Body composition analysis
Body compositions of WT and Creb3l4 KO mice were determined using non-invasive quantitative magnetic resonance relaxometry on an EchoMRI-900 (Echo Medical Systems, Houston, TX, USA) at the Phenogenomic Research Center, Woo Jung BSC, Inc., Korea. Scans were then performed by placing animals in a thin-walled plastic cylinder (3-mm thick, 4.5-cm inner diameter, based on mouse body weight), which was inserted into a mouse cylindrical sensory antenna, the A100, to limit movement. All quantitative magnetic resonance measurements were made during the light phase (0700–1900 hours). The accumulation factor used was set for extra-high precision ( × 3), resulting in a scan time of approximately 2.5 min.
Statistical analysis
Data are represented as means±s.e.ms. The LFD experiment was repeated at least three times. For aging study, experiment was performed once. All data sets were analyzed for statistical significance using nonparametric Mann–Whitney tests or Student's T-tests. All P-values <0.05 were considered significant. Statistical analyses were carried out using SPSS (IBM SPSS statistics ver. 20; IBM Corp., Armonk, NY, USA).
Results
Creb3l4 KO mice fed an LFD show increased adipocyte hypertrophy and obesity
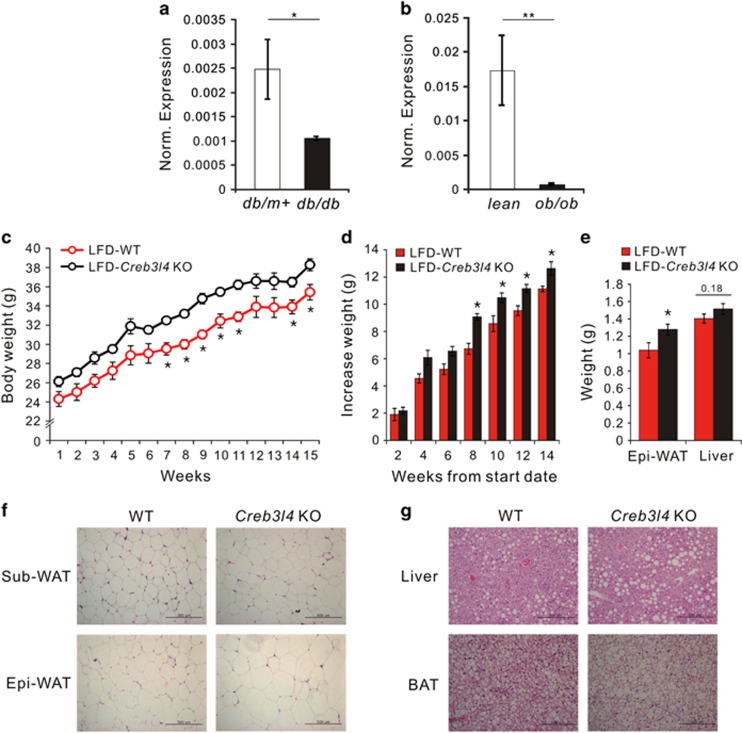
Because Creb3l4 is known to be expressed in WAT,14 we examined the mRNA levels of Creb3l4 in WAT of ob/ob and db/db mice to test whether obesity correlated with Creb314 gene expression. Creb3l4 mRNA levels in WAT of these mice were lower than those in the WT controls (Figures 1a and b). The reduced Creb3l4 expression in WAT of these mice led us to explore a cause–effect relationship between CREB3L4 and the development of adiposity.
Figure 1.
Effects of an LFD on Creb3l4 KO mice. Real-time PCR analysis of Creb3l4 mRNA levels in epididymal white adipose tissue (Epi-WAT) from db/db (a) and ob/ob (b) mice. The mRNA expression levels were normalized to those of Rplp0 (36B4) as a control. (c) Body weight and (d) increases in body weight of WT and Creb3l4 KO mice observed for 15 weeks on an LFD. (e) Weight and increased weight of Epi-WAT and liver in LFD-fed WT and Creb3l4 KO mice. (f) Hematoxylin and eosin (H&E) staining of subcutaneous white adipose tissue (Sub-WAT) and Epi-WAT from WT and Creb3l4 KO mice. (g) H&E stain of the liver and BAT. Scale bar, 500 μm. Values are expressed as means±s.e.ms., n=4–5 in panels (a–d), n=12–14 in panel (e), *P<0.05, **P<0.01.
To observe the effect(s) of CREB3L4 on whole body metabolism, we fed Creb3l4 KO mice an LFD and observed body weight changes over 16 weeks. LFD induced a significant gain in body weight (Figures 1c and d) in Creb3l4 KO mice that associated with increased weight of epididymal WAT, as compared with their WT littermates (Figure 1e). Although the food intake was not significantly different between WT and Creb3l4 KO mice, the Creb3l4 KO mice were likely to consume more food (Supplementary Figure S1a). Liver weight was not significantly different between these mice (Figure 1e). The size of subcutaneous and epididymal fat cells in Creb3l4 KO mice were larger than those in WT mice (Figure 1f). In addition, lipid accumulation in the liver and BAT increased in Creb3l4 KO mice (Figure 1g). These results suggest that Creb3l4-null mice fed an LFD incur adipocyte hypertrophy and obesity, with lipid accumulation in the liver and BAT, in addition to increased adipose tissue weight.
Creb3l4 KO mice fed an LFD exhibit impaired glucose tolerance and decreased insulin sensitivity
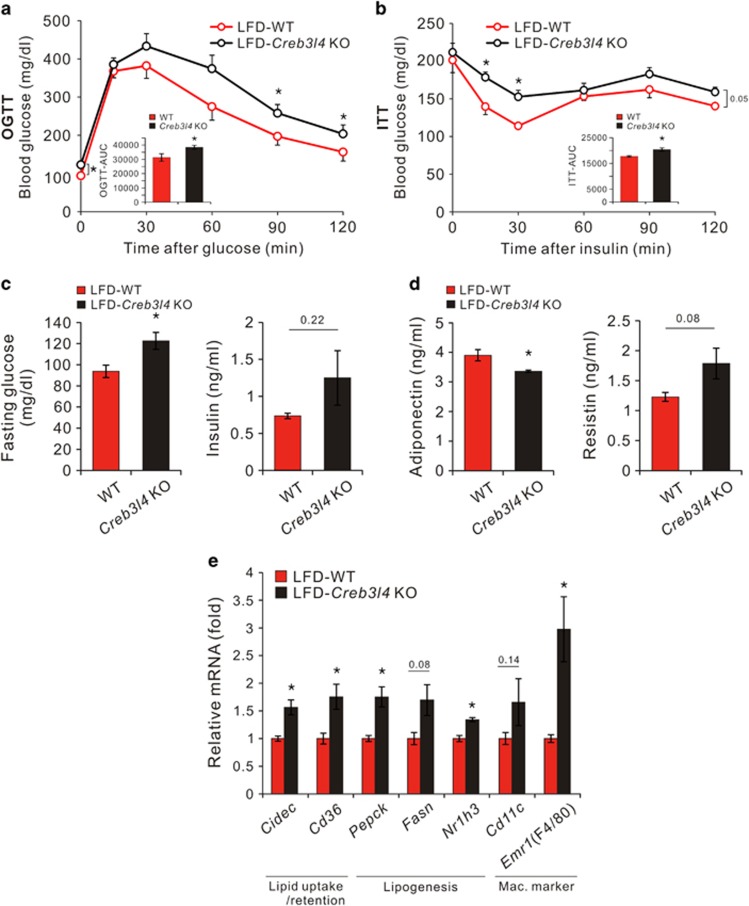
Because obesity leads to insulin resistance, we assessed glucose tolerance and insulin sensitivity in WT and Creb3l4 KO mice. Creb3l4 KO mice fed an LFD (LFD-Creb3l4 KO) showed impaired glucose tolerance and insulin sensitivity, as compared with WT littermate mice (Figures 2a and b).
Figure 2.
Creb3l4 KO mice show impaired glucose tolerance and decreased insulin sensitivity. (a, b) Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) responses (0–120 min) performed after 14 and 15 weeks of LFD feeding, respectively (c, d) Fasting glucose, plasma insulin, adiponectin and resistin levels from WT or Creb3l4 KO mice fed an LFD for 16 weeks. (e) Relative expression of adipogenic genes, macrophage marker genes and lipogenenic genes in epididymal white adipose tissue, normalized to the housekeeping gene Rplp0. Values are expressed as means±s.e.ms., n=6–7 in panels (a) and (c), n=4–5 in panels (b, d and e), *P<0.05 for Creb3l4 KO versus WT mice.
Fasting glucose and insulin levels were also increased in LFD-Creb3l4 KO mice, typical signs of insulin resistance (Figure 2c). However, plasma lipid profiles, including triglycerides, total cholesterol and non-esterified free fatty acid levels, were not significantly different between these groups (Supplementary Figure S1b). Because adipose tissue is known to control systemic glucose homeostasis by secreting adipokines, we measured adipokine plasma levels. The LFD-Creb3l4 group showed decreased serum adiponectin, but increased resistin levels, compared with levels in WT mice (LFD-WT). These results suggest that LFD-Creb3l4 mice possess an altered adipokine profile that would aggravate insulin sensitivity (Figure 2d). Moreover, the expression of the cell death-inducing DFFA-like effector c (Cidec) and Cd36, both related to lipid uptake/retention, was also increased in adipose tissue of LFD-Creb3l4 KO, as were the mRNA levels of the lipogenic genes phophoenolpyruvate carboxykinase (Pepck), fatty acid synthase (Fasn) and nuclear receptor subfamily 1, group H, member 3 (Nr1h3, lxra) (Figure 2e).
Obesity is known to be a state of chronic low-grade inflammation with infiltration of adipose tissue macrophages. Adipose tissue macrophages are characterized by the presence of F4/80+ and CD11b+, with subtypes M1 and M2 classified by the presence (M2) or absence (M1) of CD11c.16 As shown in Figure 2e, adipose tissue macrophages, particularly the M2 subtype, strongly infiltrated the epididymal fat in LFD-Creb3l4 KO mice, as compared with LFD-WT mice. These results are consistent with data showing increased fat mass and adipocyte size (hypertrophy) in LFD-Creb3l4 KO mice (Figures 1c and g). These findings further suggest that the WAT of Creb3l4-deficient mice, when fed an LFD, may undergo increased fat mass that impairs glucose tolerance and decreases insulin sensitivity. In addition, lipid accumulation in the liver and BAT may also contribute to defects in glucose tolerance and insulin sensitivity (Figure 1g).
Aged-Creb3l4 KO mice fed chow diet show increased adiposity with impaired glucose tolerance and decreased insulin sensitivity
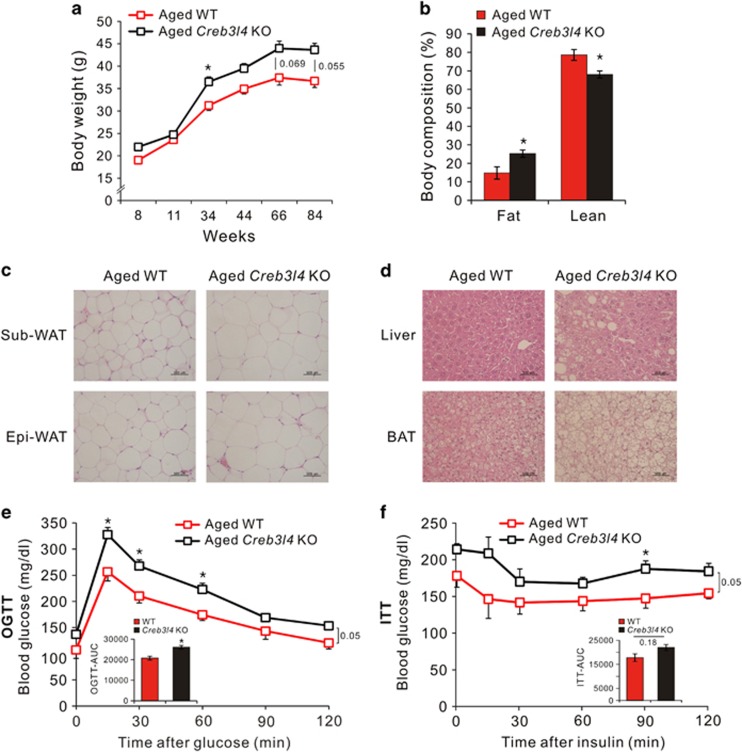
Aging is an important risk factor for metabolic disorders, including obesity, impaired glucose tolerance and type 2 diabetes.7, 17 To further investigate an effect of CREB3L4 on age-related adiposity, both WT and Creb3l4 KO mice were fed a chow diet for up to 85 weeks, resulting in significantly increased body weight of the aged-Creb3l4 KO mice, compared with the body weight of aged-WT mice (Figure 3a). The fat composition of Creb3l4 KO mice also increased (Figure 3b), as did fat cell size, in aged-Creb3l4 KO mice, compared with aged-WT mice (Figure 3c). Lipid accumulation in the liver and BAT also increased in aged-Creb3l4 KO mice (Figure 3d). Aged-Creb3l4 KO mice also showed impaired glucose tolerance and decreased insulin sensitivity, compared with their WT littermates (Figures 3e and f), a phenomenon we also observed in LFD-fed mice.
Figure 3.
Aged-Creb3l4 KO mice fed a chow diet show increased adiposity with impaired glucose tolerance and decreased insulin sensitivity. (a) Body weight changes in WT and Creb3l4 KO mice fed a chow diet for 84 weeks. (b) Body composition (% body weight) analysis of WT and Creb3l4 KO mice. (c) Hematoxylin and eosin (H&E) stain of subcutaneous white adipose tissue (Sub-WAT) and epididymal white adipose tissue (Epi-WAT) from aged-WT and aged-Creb3l4 KO mice (d) H&E stain of the liver and BAT from aged-WT and aged-Creb3l4 KO mice. Oral glucose tolerance test (OGTT) (e) and insulin tolerance test (ITT) (f) were performed after 90 weeks glucose intake and 92 weeks insulin adminstration, respectively. Values are expressed as means±s.e.ms., n=3–6 in panels (a, b, e and f). *P<0.05 for Creb3l4 KO versus WT.
Discussion
As accumulation of fat is known to be related to insulin resistance, obesity and metabolic syndrome, studies of associated transcription factor are critical to development of therapy. Based on previous studies which showed that inhibition of Creb3l4 induced adipogenesis,14 we examined whether Creb3l4 affects fat mass in mice fed an LFD and aged mice with chow diet. Increased fat mass, with hypertrophic WAT, was present in LFD-fed Creb3l4 KO mice and aged-Creb3l4 KO mice, which likewise exhibited impaired glucose tolerance and decreased insulin sensitivity, presumably caused by upregulated lipogenic genes in their epididymal WAT. Increased expression of lipogenic genes in the WAT of Creb3l4 KO mice could contribute to accumulation of triglyceride in adipocytes. Triglyceride content was also increased in the liver and BAT of LFD-Creb3l4 KO mice. There is no difference of energy expenditure and physical activity between WT and Creb3l4 KO mice (Supplementary Figure S2). But the Creb3l4 KO mice showed tendency to uptake more food (Supplementary Figure S1a), which may contribute to higher body fat contents in LFD-fed Creb3l4 KO mice than LFD-fed WT mice.
Furthermore, because Creb3l4 KO mice used in this study is whole-body KO, we could not rule out indirect effect of the absence of Creb3l4 on lipid homeostasis. Detailed relationship between lipogenesis and Creb3l4 gene needs further research. In contrast, HFD-fed Creb3l4 KO mice showed no significant differences in body weight or fat mass, with adipocyte hyperplasia improving glucose tolerance and insulin sensitivity, as compared with WT controls.14 However, these findings raise the question of why do adipocyte responses in Creb3l4 KO mice fed an LFD differ from those fed a HFD? Moreover, why do LFD-fed Creb3l4 KO mice showed decreased insulin sensitivity (with adipocyte hypertrophy), whereas HFD-fed Creb3l4 KO mice exhibit increased insulin sensitivity with adipocyte hyperplasia, as compared with WT mice? We speculated that the phenotype of HFD-Creb3l4 KO mice may arise owing to increasingly generated adipocyte precursors, arising from embryonic stem cells and their self-renewal, being recruited to WAT, resulting in adipocyte hyperplasia that improves glucose tolerance. Indeed, high dietary fat content is known to expand adipose tissue mass by recruiting white adipocytes from progenitor cells.18, 19 In addition, HFD-induced adipose tissue expansion is triggered by hypertrophy during the first month of HFD feeding, after which adipose tissue expands by formation of a large number of new fat cells (hyperplasia),20 which are known to increase glucose disposal and insulin sensitivity.21, 22, 23 At present, it is not clear whether LFD induces formation of new fat cell. However, as it was shown that chow diet did not induce adipogenesis,20 we speculate that the response and role of Creb3l4 may differ from food (diet) type in terms of adipogenesis, resulting in different body fat content, as manifested by opposite patterns of lipogenesis versus lipolysis, resulting in weight gain in the Creb3l4 KO mice fed HFD versus LFD diets, as compared with WT.
Indeed, because Creb3l4 is mainly expressed in the stromal vascular fraction, an adipose tissue subpopulation of various progenitor and immune cells,14 we could not exclude the possibility that Creb3l4 may have a role in the formation and recruitment of adipocyte precursors to WAT, based on diet (for example, LFD or HFD) that affects adipocyte size. These observations, together with the presence of Creb3l4 in the stromal vascular fraction in WAT, might explain another potential role of Creb3l4 in the transition of embryonic stem cells into preadipocytes, leading to the increased numbers of adipocytes observed in HFD-Creb3l4 KO mice. In addition, in contrast to the HFD group, the LFD regimen consisted of 35% sucrose, rather than fat. Fructose, derived from sucrose, can affect carbohydrate metabolism by changing intermediate glycolytic metabolites and gene expression patterns of metabolism-related enzymes.24 Moreover, high consumption of fructose causes hepatic steatosis and obesity, while also eliciting metabolic abnormalities such as insulin resistance, leptin resistance and hypertriglyceridemia.24, 25 Thus it is speculated that different phenotype observed in Creb3l4 KO mice, in response to different diets, might be due to the sucrose disaccharide contained in the LFD diet.
Thus LFD and/or aged Creb3l4 KO mice showed increased adiposity with hypertrophic WAT, which may contribute to the development of impaired glucose tolerance and decreased insulin sensitivity. Because WAT expresses more Creb3l4 mRNA than other metabolic tissues,14 this metabolic phenotype may result from altered CREB3L4 action in WAT. However, in the absence of WAT-specific Creb3l4 KO mice, we cannot rule out that Creb3l4 deficiency in other tissues also contributes to the phenotype observed in these mice.
In summary, the aging process, or consumption of an LFD, in Creb3l4 KO mice showed different phenotypes than those fed a HFD. Thus, understanding the molecular mechanism of differential regulation of adiposity in Creb3l4 KO mice fed differing diet or aging may provide critical clues in combating metabolic syndromes associated with obesity.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2014R1A2A2A01004396 to YHA, NRF-2013R1A1A2060537 to THK) and Korea Mouse Phenotyping Project (2013M3A9D5072550) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Nutrition & Diabetes website (http://www.nature.com/nutd)
Supplementary Material
References
- Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Montgomery MK, Fiveash CE, Osborne B, Cooney GJ, Bell-Anderson K, et al. PPARalpha-independent actions of omega-3 PUFAs contribute to their beneficial effects on adiposity and glucose homeostasis. Sci Rep. 2014;4:5538. doi: 10.1038/srep05538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, et al. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Muzumdar RH. Pancreatic function, type 2 diabetes, and metabolism in aging. Int J Endocrinol. 2012;2012:320482. doi: 10.1155/2012/320482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Alfy M, Azzi L, Lessard J, Lavergne E, Pelletier M, Labrie C. Stage-specific expression of the Atce1/Tisp40alpha isoform of CREB3L4 in mouse spermatids. J Androl. 2006;27:686–694. doi: 10.2164/jandrol.106.000596. [DOI] [PubMed] [Google Scholar]
- Fujii T, Tamura K, Masai K, Tanaka H, Nishimune Y, Nojima H. Use of stepwise subtraction to comprehensively isolate mouse genes whose transcription is up-regulated during spermiogenesis. EMBO Rep. 2002;3:367–372. doi: 10.1093/embo-reports/kvf073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Ni X, Jiang M, Ma Y, Cheng H, Guo L, et al. Molecular cloning and characterization of a novel human cAMP response element-binding (CREB) gene (CREB4) J Hum Genet. 2002;47:373–376. doi: 10.1007/s100380200053. [DOI] [PubMed] [Google Scholar]
- Stirling J, O'Hare P. CREB4, a transmembrane bZip transcription factor and potential new substrate for regulation and cleavage by S1P. Mol Biol Cell. 2006;17:413–426. doi: 10.1091/mbc.E05-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamori I, Yomogida K, Ikawa M, Okabe M, Yabuta N, Nojima H. The testes-specific bZip type transcription factor Tisp40 plays a role in ER stress responses and chromatin packaging during spermiogenesis. Genes Cells. 2006;11:1161–1171. doi: 10.1111/j.1365-2443.2006.01013.x. [DOI] [PubMed] [Google Scholar]
- Labrie C, Lessard J, Ben Aicha S, Savard MP, Pelletier M, Fournier A, et al. Androgen-regulated transcription factor AIbZIP in prostate cancer. J Steroid Biochem Mol Biol. 2008;108:237–244. doi: 10.1016/j.jsbmb.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Kim TH, Jo SH, Choi H, Park JM, Kim MY, Nojima H, et al. Identification of Creb3l4 as an essential negative regulator of adipogenesis. Cell Death Dis. 2014;5:e1527. doi: 10.1038/cddis.2014.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, Patwari P, et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20:1049–1058. doi: 10.1016/j.cmet.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- Koo HY, Wallig MA, Chung BH, Nara TY, Cho BH, Nakamura MT. Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim Biophys Acta. 2008;1782:341–348. doi: 10.1016/j.bbadis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology. 2013;58:1632–1643. doi: 10.1002/hep.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.