Abstract
The sweetpotato whitefly (WF), Bemisia tabaci, is a major pest that damages a wide range of vegetable crops in Malaysia. WF infestation is influenced by a variety of factors, including previous infestation of the host plant by other insect pests. This study investigated the effects of previous infestation of host chilli plants by the green peach aphid (Myzus persicae) on the olfactory behavioural response of B. tabaci, using free-choice bioassay with a Y-tube olfactometer. We analysed volatile organic compounds (VOCs) emitted by non-infested and M. persicae-infested chilli plants using solid-phase microextraction and gas chromatography–mass spectrometry. Our results showed that female WFs preferred non-infested to pre-infested plants. Collection and analysis of volatile compounds emitted by infested plants confirmed that there were significant increases in the production of monoterpenes (cymene; 1,8-cineole), sesquiterpenes (β–cadinene, α-copaene), and methyl salicylate (MeSA) compared to non-infested plants. Our results suggest that host plant infestation by aphids may induce production of secondary metabolites that deter B. tabaci from settling on its host plants. These results provide important information for understanding WF host selection and dispersal among crops, and also for manipulating WF behaviour to improve IPM in chilli.
The whitefly (WF) Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is an economically important pest in agricultural ecosystems. Bemisia tabaci damages a wide variety of crops by feeding on cell sap and by transmitting viral diseases1. The first record of B. tabaci in Malaysia was in 19352, where it was reported on chilli (Capsicum annuum L.), soybean (Glycine max (L.) Merr.), and okra (Abelmoschus esculentus (L.) Moench) in lowland areas. Although it was initially an unimportant agricultural pest, WF has recently become destructive to many host plants, including brinjal (Solanum melongena L.), tomato (Solanum lycopersicum L.), and chilli3. Feeding by adults and nymphs causes chlorotic leaf spots, leaf fall, and reduced plant growth. Honeydew produced by feeding nymphs coats leaf surfaces, and can result in reduced photosynthetic potential when colonized by moulds. Under heavy infestation, plant height, number of internodes, and quality and quantity of yield can be adversely affected4. Crop losses of up to 50% have been attributed to this pest in Malaysia3.
Damage caused by B. tabaci is compounded by its ability to transmit more than 100 viruses, 90% of which belong to the genus Begomovirus5. Insecticides have been used extensively to eliminate WF infestations3. However, B. tabaci has become resistant to a number of pesticide active ingredients6 and the chemicals have adverse effects on the natural enemies that control WF populations. For example, avermectin insecticides efficiently control the proliferation of WF larvae on brinjal and tomato, but are toxic to the predator Macrolophus caliginosus Wagner (Heteroptera: Miridae), which feeds on WFs7.
WF infestation occurs on several host plants in Malaysia and severe infestations of B. tabaci on plants offer opportunities to study interspecific interactions with other herbivores8. Numerous studies on competition have concluded that previous feeding by one species induces nutritional and allelochemical changes in the host plant that adversely affect the performance of other species that subsequently feed on the same host9,10,11,12. Interspecific competition between B. tabaci and other herbivores has been investigated by Inbar et al. (1999), who reported that first-instar cabbage loopers, Trichoplusia ni (Hubner), switched to the WF-free sides of collard leaves that were previously infested with B. tabaci. Negative effects on host preference and performance of Liriomyza trifolii Burgess and Liriomyza sativae Blanchard were observed in the presence of B. tabaci on tomato, pumpkin (Cucurbita pepo L.), and cucumber (Cucumis sativus L.)13,14.
There is abundant evidence that heavy feeding by sap-consuming insects induces long-term reductions in plant quality15,16 that could diminish the performance of later-colonizing species17. For example, aphid feeding on wheat seedlings induced chemical changes in the plants that subsequently repelled other aphid species18. However, reciprocal effects of aphid infestation on WF preferences have not been extensively studied. As such this work aimed to investigate the effects of chilli defence responses induced by pre-infestation with aphids on B. tabaci host selection, and to explore the fundamental mechanisms underlying the interspecific interactions between B. tabaci, aphids and the host plant. This knowledge will be useful for improving plant protection programs and to increase understanding of induced defenses in plants and their effects on other organisms.
Results
Olfactory Bioassay
In olfactory response bioassays, female B. tabaci were found to significantly prefer VOCs from non-infested chilli plants over VOCs from pre-infested plants (t = 2.31, P < 0.05) (Table 1, Exp. 1). Similarly, when WF females were given a choice between VOCs from pre-infested chilli plants and clean air, they showed a significant preference for clean air (t = 4.43, P < 0.05) (Exp. 2). However, no significant difference in preference was observed between non-infested chilli plants and clean air (t = 2.00, P > 0.05) (Exp. 3). These results show that VOCs released by pre-infested chilli plants play an important role in mediating the attraction of female B. tabaci.
Table 1. Olfactory response of B. tabaci females in olfactometer experiments given a choice between VOCs from chilli plants: (1) pre-infested by aphids and non-infested; (2) pre-infested and clean air; and, (3) non-infested and clean air.
| Experiment | Proportion (±SE) of WF females responding | P-value | |
|---|---|---|---|
| 1 | Pre-infested | Non-infested | 0.041 |
| 0.352 ± 0.063b | 0.647 ± 0.63a | ||
| 2 | Pre-infested | Clean Air | 0.011 |
| 0.332 ± 0.037b | 0.667 ± 0.037a | ||
| 3 | Non-infested | Clean Air | 0.58 |
| 0.610 ± 0.055a | 0.389 ± 0.055a | ||
Means (±SE) with different letters in each row significantly differ, using t-tests (for dependent-samples) (P < 0.05).
Free-choice bioassay
There was a significant difference in the number of WFs settling between treatment plants, but not between the times of response to host plants (Table 2 and Figure 1). No significant interaction between treatment and time was found (Table 2). Significantly more WFs were found on non-infested than on pre-infested chilli plants regardless of time after release (Figure 2).
Table 2. Results of two-way ANOVA for mean numbers of B. tabaci settling on treatment plants at different times after release in free-choice bioassay.
| Source | df | Sum of squares | F-value | P-value |
|---|---|---|---|---|
| Time | 3 | 19.35 | 0.87 | P > 0.05 |
| Plants (Treatment) | 1 | 172.22 | 7.72 | P < 0.05 |
| Time × plants | 3 | 7.292 | 0.33 | P > 0.05 |
| Error | 39 | 22.30 |
Figure 1. Mean (±SE) numbers of B. tabaci females settled on non-infested and pre-infested chilli plants at different times (1, 2, 3, 4 h) after WF release under free-choice bioassay, using two-way ANOVA.
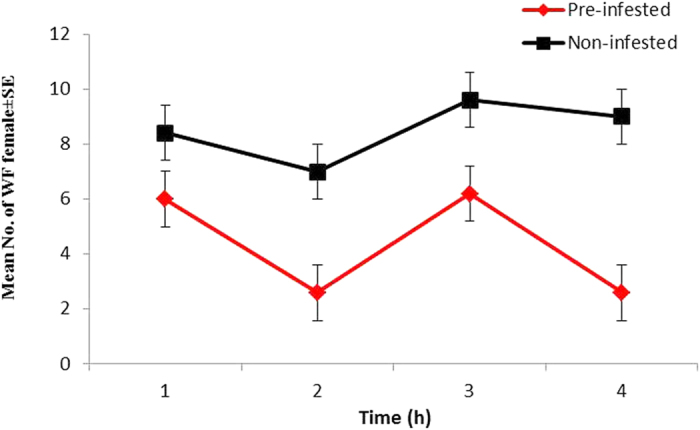
Figure 2. Total mean (±SE) number of settled B. tabaci females in free-choice bioassay with non-infested and pre-infested chilli plants.
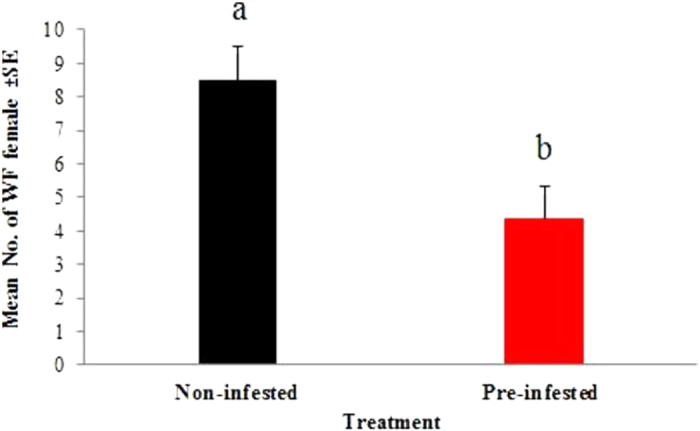
Different letters indicate a significant difference at (P < 0. 05). Each bar represents the mean of 5 replicates each assessed at 4 time intervals, using two-way ANOVA.
Volatile organic chemicals released by pre-infested and non-infested chilli plants
In total, 34 compounds were detected from pre-infested and non-infested chili plants, including terpenoids (monoterpenes, triterpenes, and sesquiterpenes), ketones, aldehydes, esters, hydrocarbons, fatty acids, and esters (Table 3). We confirmed that differences exist in volatile emissions of plants pre-infested with aphids compared to non-infested plants. The monoterpene emissions from pre-infested plants were significantly higher than non-infested plants in the following compounds: cymene (t = −5.66, df = 4, P = 0.005); and 1,8-cineole (t = 4.71, df = 4, P = 0.042). Emissions of some sesquiterpenes were also significantly higher in pre-infested, than non-infested, plants, including α-copaene (t = 4.50, df = 4, P = 0.045) and β-cadinene (t = 55, df = 4, P < 0.000). In addition, pre-infested plants released one ester compound (methyl salicylate) that was absent from the headspace of non-infested plants. In contrast, two different volatile compounds, eicosane and α-humulene, were released at higher levels from headspace samples of non-infested plants compared to pre-infested plants. On the other hand, no clear quantitative differences were observed between plant treatments in aldehydes, fatty acids, ketones, triterpenes and hydrocarbons (Table 3).
Table 3. Quantities of major volatile compounds released by non-infested and aphid pre-infested chilli plants through headspace sampling by SPME.
| Volatile compound | RT | Non-infested | Pre-infestation by aphids | P-value |
|---|---|---|---|---|
| Monoterpenes | ||||
| α-Pinene | 7.73 | 0.060 ± 0.045 | 0.006 ± 0.006 | 0.386 |
| β-Pinene | 9.23 | 0.023 ± 0.023 | 0.006 ± 0.003 | 0.525 |
| Limonene | 10.85 | 0.410 ± 0.22 | 0.217 ± 0.068 | 0.173 |
| p-Cymene | 10.6 | 0.016 ± 0.008 | 0.043 ± 0.003 | 0.005 |
| Geranylacetone | 23.23 | 0.323 ± 0.117 | 0.137 ± 0.078 | 0.155 |
| β-2-Carene | 11.65 | 0.076 ± 0.076 | 0.010 ± 0.005 | 0.250 |
| y-Terpineol | 16.21 | 0.00 | 0.130 ± 0.045 | 0.051 |
| α-Terpineol | 16.13 | 0.00 | 1.11 ± 1.11 | 0.211 |
| Terpinen-4-ol | 15.63 | 0.00 | 0.050 ± 0.050 | 0.211 |
| Camphor | 14.71 | 0.00 | 0.403 ± 0.143 | 0.053 |
| 1,4-Cineole | 9.96 | 0.00 | 0.050 ± 0.050 | 0.374 |
| 1,8-Cineole | 10.7 | 0.00 | 0.056 ± 0.012 | 0.021 |
| Borneol | 15.45 | 0.00 | 0.140 ± 0.049 | 0.052 |
| Triterpenes | ||||
| Squalene | 56.18 | 1.690 ± 0.88 | 0.263 ± 0.206 | 0.251 |
| Sesquiterpenes | ||||
| α-Humulene | 23.55 | 0.723 ± 0.078 | 0.00 | 0.012 |
| (E)-Caryophyllene | 22.73 | 0.82 ± 0.79 | 1.44 ± 1.20 | 0.459 |
| Copaene | 21.26 | 0.157 ± 0.123 | 0.010 ± 0.006 | 0.360 |
| β -Cadinene | 25.08 | 0.00 | 0.366 ± 0.006 | 0.000 |
| α-Copaene | 21.21 | 0.00 | 0.143 ± 0.044 | 0.045 |
| Aldehydes | ||||
| Nonanal | 13.16 | 0.243 ± 0.158 | 0.217 ± 0.217 | 0.792 |
| Decanal | 11.96 | 0.433 ± 0.069 | 0.357 ± 0.251 | 0.716 |
| Octanal | 10.01 | 0.00 | 0.143 ± 0.143 | 0.423 |
| Ketones | ||||
| 5-Hepten-2-one,6-methyl | 9.41 | 0.070 ± 0.032 | 0.00 | 0.161 |
| Fatty acids | ||||
| Hexadecanoic acid | 35.16 | 0.237 ± 0.109 | 0.00 | 0.645 |
| Hydrocarbons | ||||
| Eicosane | 37.71 | 0.513 ± 0.062 | 0.073 ± 0.038 | 0.009 |
| Tetradecane | 21.7 | 0.197 ± 0.068 | 0.653 ± 0.470 | 0.452 |
| Hexacosane | 48.45 | 4.99 ± 2.0 | 2.62 ± 2.6 | 0.081 |
| Tridecane | 21.56 | 0.286 ± 0.072 | 0.1000 ± 0.005 | 0.123 |
| Undecane | 12.76 | 0.297 ± 0.061 | 0.290 ± 0.051 | 0.954 |
| Dodecane | 15.85 | 0.297 ± 0.073 | 0.170 ± 0.085 | 0.331 |
| Heptacosane | 45.58 | 3.04 ± 1.03 | 1.56 ± 1.56 | 0.605 |
| Pentadecane | 24.35 | 0.086 ± 0.052 | 0.033 ± 0.033 | 0.582 |
| Decane | 9.3 | 0.250 ± 0.056 | 0.296 ± 0.066 | 0.663 |
| Ester | ||||
| Methyl salicylate | 16.06 | 0.00 | 0.056 ± 0.012 | 0.042 |
RT = retention time.
Each value represents the mean peak areas (±SE) of 3 replicates. P values in boldface indicate significant differences between the means for non-infested and pre-infested plants in that row, at α = 0.05, using t-tests (one-tailed; two-tailed).
Discussion
Our results demonstrate that aphid infestation influenced the release of volatile compounds from chilli plants, which in turn influenced the behavioural response of female WF to host plants. The strongest evidence was provided by the olfactometer experiments, in which the WFs had no visual or physical contact with the plants and significantly preferred the odour of non-infested chilli plants (Table 1). This preference was also reflected in the results of free-choice bioassay, where WFs chose non-infested plants more often than pre-infested plants (Figure 2).
The ability of female B. tabaci to discriminate between non-infested chilli plants and those pre-infested by aphids suggests that the response of WF to aphid-infested plants was affected by volatile compounds released by the plants. During probing of the leaves aphids puncture virtually all mesophyll cells on their path to a major vein of the phloem19. Salivary proteins injected by aphids while feeding on plants are known to be directly involved in triggering plant responses to insect herbivores20,21. Guerrieri et al. (1993)22 found that aphid infestation appeared to induce volatile emissions that repelled further infestation by other aphids. Similarly, resistance induced by the spider mite Tetranychus turkestani feeding on cotton seedlings reduced WF densities23.
The differences in preference of female WFs to pre-infested and non-infested chilli plants (Table 2) could be attributed to qualitative and quantitative differences in volatile compounds emitted from the plants. Some of these compounds may act as direct24 or indirect defences against specific herbivores25, and may influence the host-plant selection process of other herbivores26. For example, the large quantities of methyl salicylate (MeSA) that were detected in pre-infested chilli plants may have been induced by aphid infestation, as reported in other plant species such as lima bean, Arabidopsis thaliana, tomato, alfalfa, and soybean27,28,29,30,31,32. MeSA has been reported to be involved in plant defence, particularly in the elicitation of systemic acquired resistance (SAR)33. Zhu and Park (2005)31 and Pareja et al. (2009)32 identified MeSA as a good indicator of aphid feeding on soybean and alfalfa plants. Girling et al. (2008) also reported that aphid infestation induced release of MeSA in Arabidopsis thaliana (Brassicales: Brassicaceae). In another study, MeSA showed antifeedant activity against pine weevils34. Moreover, a positive electroantennogram response was shown when MeSA was applied to the antennae of Coccinella septempunctata (Coleoptera; Coccinellidae), and this predator and syrphid flies were attracted to MeSA-baited traps31. Our study also confirmed that aphid-infested chilli plants exhibited changes in the level of MeSA release that could be responsible for the effective resistance response of chilli plants against WFs; it may thus be advantageous for WFs to avoid chilli plants that produce this compound.
In general, plants infested with aphids showed altered terpene release profiles35,36, including induced release of α-pinene, β-pinene, cymene, α-phellandrene and d-limonene37. In our study, aphid infested chilli plants were shown to have increased release of volatile monoterpenes compared to non-infested plants (Table 3). Production of the monoterpenes cymene and 1,8-cineole was significantly increased in pre-infested chilli plants compared with non-infested plants. These compounds are known to be repellent to insects38. Recently, Yang et al. (2010)39 noted that ginger oil extract repelled adult WFs in a vertical olfactometer experiment. Repellent properties of this essential oil appear to be associated with a mixture of constituents including monoterpenes (1,8-cineole, phellandrene, camphene, α-pinene, myrcene, citral, and borneol), sesquiterpenes, aldehydes, and alcohols40,41,42,43. Similarly, B. tabaci has been reported to prefer cultivated tomato varieties over wild tomatoes, which was attributed to high levels of the monoterpenes p-cymene, g-terpinene, and b-myrcene being released by wild tomato plants44,45. 1,8-Cineole also showed significant antifeedant activity against Tribolium castaneum46. It is possible that WFs could respond to honeydew or other aphid-associated cues on pre-infested plants, however, bioassay conditions did not lead to noticeable honeydew production nor microbial growth. To our knowledge, there are no studies that report the volatile profiles of honeydew produced by M. persicae. However, twelve volatiles were found in honeydew produced by Megoura viciae, most of which were fermentation-associated products with a butane core47. None of these compounds, with the exception of limonene, was found in the volatile profile of pre-infested plants in our study. Limonene was found in both non-infested and pre-infested plant volatiles, but there was no significant difference in limonene production among plant types. Therefore, honeydew and associated aphid cues do not appear to have influenced WF behavior. These results support the conclusion that aphid infestation plays a role in volatile compound-induced resistance to WF infestation in chilli.
This study provides new evidence that infestation by aphids affects the defences of chilli plants against WFs by inducing the emission of various volatile compounds, which subsequently have an adverse (repellent) effect on B. tabaci females. Further study is needed to evaluate the selected VOCs for their behavioral effects on B. tabaci in order to determine which compounds have the most adverse effect on host plant selection. The data from such studies could enhance the IPM program for WFs by using commercially produced VOCs as repellents or attractant traps for WFs in the field.
Methods
Host plants
Chilli (Capsicum annuum var. Kulai) seeds were obtained from the Malaysian Agriculture Research & Development Institute (MARDI) Station, Jalan Kebun, Klang. Seeds were placed in distilled water for 8 days to germinate, after which they were placed in hydroponic solution (100.30 kg Ca (NO3)2, 790 g iron chelate, 2.63 kg K2HPO4, 5.83 kg KNO3, 5.13 kg MgSO4, 30 g H3BO3, 61 g MnSO4, 3.9 g CuSO4, 3.7 g (NH4)2MoO4, and 4.4 g ZnSO4 dissolved in 100 L water) in plant cups. The plant cups were placed into holes cut in a cylindrical piece of polystyrene through which the hydroponic solution flowed. The plants selected for the experiments were at least 30 d old and were at the nine- or ten-leaf stage.
Insect rearing
Green peach aphids, Myzus persicae (Sulzer), were established from apterous adult aphids collected from C. annuum plants grown in a glasshouse at MARDI. Myzus persicae individuals were reared and maintained on C. annuum plants in a growth chamber under controlled laboratory conditions (20 ± 2 °C; 60–70% relative humidity [RH]). The aphids were provided with new chilli plants weekly. Bemisia tabaci were collected in the field at MARDI and reared on C. annuum in insect-proof mesh cages (60 × 60 × 60 cm) in a greenhouse at 30–36 °C and at 50–60% RH. Newly emerged female WFs were collected and starved for 2 h before the beginning of each trial.
Pre-infestation of host plants
Four-week-old chilli seedlings were covered with plastic tubes (15 cm dia, 30 cm high) and a total 100 aphids per plant were carefully released into the tube with a fine brush. Chilli plants infested with adult aphids were held for 3 days in a growth chamber with environmental conditions of 20 ± 2 °C and a photoperiod of 16 h L: 8 h D. The aphids were then removed carefully with a paintbrush before the experiments. Non-infested plants were used as the control treatment, and were maintained under the same conditions but were not exposed to aphids.
Olfactory choice experiment
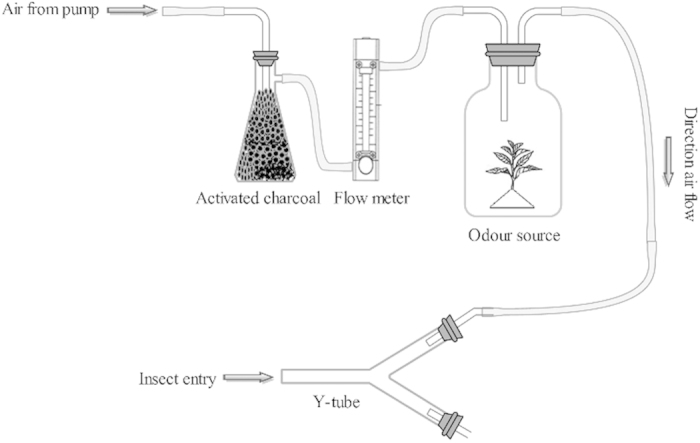
Olfactory-choice bioassays to assess B. tabaci responses to volatile organic compounds (VOCs) produced by chilli plants pre-infested with M. persicae were conducted using a Y-tube olfactometer, as previously described by Akol et al. (2003)48 (Figure 3) with some minor modifications in the size of Y-tube (0.8 cm i.d., 10-cm-long base, two 10-cm branches at a 45° angle from one another). Plants were placed inside two 3-L glass containers, one affixed to each arm of the olfactometer with silicone tubes. The VOCs were circulated through the system using pressure pumps (Cole-Parmer Air cadet vacuum/pressure station, Vernon Hills, Illinois, USA). Air was pumped through an active charcoal filter prior to passing through two flow meters, which channelled the air at 60 mL/min into the two glass containers, where it passed through the odour source, and then into the two arms of the olfactometer. Female WFs were exposed to all of the potential stimulus treatment pairs (Exp. 1: pre-infested with aphids vs. non-infested; Exp. 2: pre-infested with aphids vs. clean air; Exp. 3: non-infested vs. clean air). Individuals were released within the first centimetre of the olfactometer base tube and their responses were measured for 10 min. Insects that walked at least 4 cm into one of the arms and did not return after 15 s were considered to have made a final choice. Insects that did not make a decision within the 10-min limit were excluded from the results. Each experiment was repeated five times for each combination of stimulus pairs, and each replicate consisted of 10 adult female WF assayed individually (i.e. total n = 50 for each treatment). The assays were conducted at 24 °C and 65–75% RH. Assay equipment was washed using soap and water and thoroughly sterilized with cotton wool drenched in 70% ethanol between each replicate to avoid the possibility of contamination by odours left from previous replicates.
Figure 3. A schematic diagram of the Y-tube olfactometer, modified from Akol et al. (2003)48, used to test the effect of M. persicae feeding on behavioural response of B. tabaci.
Free-choice bioassay
To investigate the preference of B. tabaci between plants pre-infested with aphids and non-infested plants in the presence of both olfactory and visual cues, release–recapture experiments were performed. Alternating non-infested and pre-infested chilli plants were arranged in a circle inside wooden cages covered with insect-proof nets (60 × 60 × 60 cm), equidistant from a central insect release point. There were 5 replicate cages, and each cage contained 10 plants (5 non-infested and 5 pre-infested with aphids). Light was provided by high-pressure sodium lamps and cages were maintained in laboratory conditions at 24 °C and 65% RH. Female whiteflies (n = 300 per cage) were starved for 2 h prior to release from glass vials at the centre of each cage. The numbers of whiteflies settled on the underside of 3 leaves per plant were counted at 1, 2, 3, and 4 h after release. One sample leaf was selected from each of three strata (upper, middle, lower) on the plant.
Collection and analysis of VOCs
The VOCs emitted by pre-infested and non-infested chilli plants were collected using a static-headspace sampling device with a solid-phase microextraction (SPME) fibre coated with polydimethylsiloxane/divinylbenzene (PDMS/DVB, 65 μm). Each plant sample with 9 to 10 leaves was enclosed in a 3 L glass container for 60 min, and the SPME fibre was extended into the headspace to collect volatiles for a fixed 30 min time period. The glass chambers contained large openings for easy insertion and removal of plant sample (Figure 4). After collection of volatile substances at (23 ± 1 °C and 60% ± 5% RH), the SPME fibre was inserted directly into a thermal desorption gas chromatograph-mass spectrometer (Shimadzu, GC-MS QP-2010 model), with a DB5-MS column (30 m × 25 mm × 0.25 μm film thickness). The fibre was left in the injector (on splitless mode) for 2 min at a final temperature of 250 °C (initial temperature of 40 °C for 5 min hold, and increased by 3 °C/min until reaching 250 °C). Helium (1 mL/min) was used as the GC carrier gas. The identification of separated compounds was conducted using a NIST 2008 spectral library, matching retention time and mass spectra with those of authentic standards. The relative quantities of each volatile compound were estimated based on its peak area shown by mass spectrometry. Three non-infested and three pre-infested chili plants were used in the analyses.
Figure 4. Static headspace sampling with (SPME) device.
(A) The plant sample was enclosed in a glass container for 60 min, with a broad opening for easy removal of the plant. (B) The fiber is mounted on a SPME fiber holder. (C) The fiber was injected through the septum of the sample container by pushing the plunger of the SPME fiber holder, and extended from the needle and exposed to volatiles for 30 min. After collection VOCs, the fiber was retracted into the needle and the SPME device removed from the container for GC-MS analysis.
Statistical analyses
Paired t-tests were used to compare the behavioural responses of female WFs to odour source pairs in the olfactometer assays. Free-choice bioassay data were analysed by two-way ANOVA, where treatment and time were independent variables, and number of female WF responding was the dependent variable. Differences in the total peak area of each VOC produced by pre-infested and non-infested chilli plants were analysed using unpaired two sample t -tests with the exception of one-tailed t- testing for zeros variances, at P < 0.05. All statistical analyses were conducted using the Minitab Statistical Package (Version 16).
Additional Information
How to cite this article: Saad, K. A. et al. Aphid-induced Defences in Chilli Affect Preferences of the Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Sci. Rep. 5, 13697; doi: 10.1038/srep13697 (2015).
Acknowledgments
We are grateful to the staff members of the Strategic Resources Research Centre of MARDI for generously providing the laboratory facilities for collection and analysis of VOCs. This study was supported by the research grants no 06-01-02-SF086 and GUP-2014-028 of Ministry of Sciences, Technology and Innovation (MOSTI) and Universiti Kebangsaan Malaysia Research grant (UKM- GUP), respectively.
Footnotes
Author Contributions K.A.S. performed the experiments and wrote the main manuscript text. I.A.B. helped interpret the data and with the discussion and revision of the paper. M.R.M.N., R.H.H. contributed through discussion and revision of the paper. All of the authors read and approved the final manuscript.
References
- Van Emden H. F. Host-plant resistance: Aphids as Crop Pests (eds , van Emden H. F. & Harrington R. ) Ch. 17, 447–468 (CABI, Wallingford, 2007). [Google Scholar]
- Corbett G. H. Malaysian Aleurodidae. J. Fed. Malay States Mus. 17, 722–825 (1935). [Google Scholar]
- Syed A. R., Sivapragasam A., Loke W. H. & Mohd. Roff M. N. Whiteflies infesting vegetables in Malaysia. In: Proceedings of the plant resource management seminar. Organized by MAPPS, DoA Sarawak and SIAS. p. 38–43 (2000).
- Pollard D. G. The identity of the cotton flea beetle of the Sudan. Ann. and Mag. Nat. Hist. 12, 713–717 (1955). [Google Scholar]
- Jones D. R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 109, 195–219 (2003). [Google Scholar]
- Horowitz A. R., Kontsedalov S., Khasdan V. & Ishaaya I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Int. Physiol. Biochim. 58, 216–225 (2005). [DOI] [PubMed] [Google Scholar]
- Mohd Rasdi Z., Che Salmah M. R., Abu Hassan A., Hamady D., Hamaseh A. & Ismail F. Field evaluation of some insecticides on whitefly (Trialeurodes vaporariorum) and predator (Macrolophus caliginosus) on brinjal and tomato plants. Asian J. Agric. Rural Dev. 2, 302–311 (2012). [Google Scholar]
- Rosell R. C., Blackmer J. L., Czosnek H. & Inbar M. Mutualistic and dependent relationships with other organisms. In: Bemisia: Bionomics and management of a global pest (eds. Stansly P. A. & Naranjo S. E. ) pp. 161–184 (Springer, Berlin, Germany, 2010). [Google Scholar]
- Petersen M. K. & Sandström J. P. Outcome of indirect competition between two aphid species mediated by responses in their common host plant. Funct. Ecol. 15, 525–534 (2001). [Google Scholar]
- Awmack C. S. & Leather S. R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844 (2002). [DOI] [PubMed] [Google Scholar]
- Bezemer T. M., Wagenaar R., Van Dam N. M. & Wäckers F. L. Interactions between above‐and belowground insect herbivores as mediated by the plant defense system. Oikos 101, 555–562 (2003). [Google Scholar]
- Denno R. F. & Kaplan I. Plant-mediated interactions in herbivorous insects: mechanisms, symmetry, and challenging the paradigms of competition past. Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, 19–50 (2007). [Google Scholar]
- Inbar M., Doostdar H., Leibee G. L. & Mayer R. T. The role of plant rapidly induced responses in asymmetric interspecific interactions among insect herbivores. J. Chem. Ecol. 25, 1961–1979 (1999). [Google Scholar]
- Zhang L. P., Zhang G. Y., Zhang W. J. & Liu Z. Interspecific interactions between Bemisia tabaci (Hem. Aleyrodidae) and Liriomyza sativae (Dipt. Agromyzidae). J. Appl. Entomol. 129, 443–446 (2005). [Google Scholar]
- McClure M. S. Competition between exotic species: scale insects on hemlock. Ecology 61, 1391–1401 (1980). [Google Scholar]
- Olmstead K. L., Denno R. F., Morton T. C. & Romeo J. T. Influence of Prokelisia planthoppers on the amino acid composition and growth of Spartina alterniflora. J. Chem. Ecol. 23, 303–321 (1997). [Google Scholar]
- Denno R. F., McClure M. S. & Ott. J. R. Interspecific interactions in phytophagous insects: competition revisited and resurrected. Annu. Rev. Entomol 40, 297–331 (1995). [Google Scholar]
- Quiroz A., Pettersson J., Pickett J. A., Wadhams L. J. & Niemeyer H. M. Semiochemicals mediating spacing behavior of bird cherry-oat aphid, Rhopalosiphum padi, feeding on cereals. J. Chem. Ecol. 23, 2599–2607 (1997). [Google Scholar]
- Walling L. L. Avoiding effective defences: strategies employed by phloem-feeding insects. Plant Physiol. 146, 859–866 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles P. W. Aphid saliva. Biol. Rev. Camb. Philos. 74, 41–85 (1999). [Google Scholar]
- De Vos M., Kim J. H. & Jander G. Biochemistry and molecular biology of Arabidopsis-aphid interactions. Bio. Essays 29, 871–883 (2007). [DOI] [PubMed] [Google Scholar]
- Guerrieri E., Pennacchio F. & Tremblay E. Flight behavior of the aphid parasitoid Aphidius ervi (Hymenoptera, Braconidae) in response to plant and host volatiles. Eur. J. Entomol. 90, 415–421 (1993). [Google Scholar]
- Agrawal A. A., Karban R. & Colfer R. G. How leaf domatia and induced plant resistance affect herbivores, natural enemies and plant performance. Oikos. 89, 70–80 (2000). [Google Scholar]
- Hildebrand J. D., Schaller M. D. & Parsons J. T. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J. Cell Biol. 123, 993–1005 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girling R. D., Hassall M., Turner J. G. & Poppy G. M. Behavioural responses of the aphid parasitoid Diaeretiella rapae to volatiles from Arabidopsis thaliana induced by Myzus persicae. Entomol. Exp. Appl. 120, 1–9 (2006). [Google Scholar]
- Delphia C. M., Mescher M. C. & De Moraes C. M. Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips. J. Chem. Ecol. 33, 997–1012 (2007). [DOI] [PubMed] [Google Scholar]
- James D. G. Field evaluation of herbivore-induced plant volatiles as attractants for beneficial insects: Methyl salicylate and the green lacewing Chrysopa nigricornis. J. Chem. Ecol. 29, 1601–1609 (2003). [DOI] [PubMed] [Google Scholar]
- Arimura G. I., Ozawa R., Nishioka T., Boland W., Koch T., Kühnemann F. & Takabayashi J. Herbivore‐induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J. 29, 87–98 (2002). [DOI] [PubMed] [Google Scholar]
- Ament K., Kant M. R., Sabelis M. W., Haring M. A. & Schuurink R. C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 135, 2025–2037 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., D’Auria J. C., Tholl D., Ross J. R., Gershenzon J., Noel J. P. & Pichersky E. An Arabidopsis thaliana gene for methyl salicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. The Plant J. 36, 577–588 (2003). [DOI] [PubMed] [Google Scholar]
- Zhu J. & Park K. C. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 31, 1733–1746 (2005). [DOI] [PubMed] [Google Scholar]
- Pareja M., Mohib A., Birkett M. A., Dufour S. & Glinwood R. T. Multivariate statistics coupled to generalized linear models reveal complex use of chemical cues by a parasitoid. Anim. Behav. 77, 901–909 (2009). [Google Scholar]
- Park S. W., Kaimoyo E., Kumar D., Mosher S. & Klessig D. F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113–116 (2007). [DOI] [PubMed] [Google Scholar]
- Borg-Karlson A. K., Nordlander G., Mudalige A., Nordenhem. H. & Unelius C. R. Antifeedants in the feces of the pine weevil Hylobius abietis: Identification and biological activity. J. Chem. Ecol. 32, 943–957 (2006). [DOI] [PubMed] [Google Scholar]
- Giorgi A., Panseri S., Masachchige Chandrika Nanayakkara N. & Chiesa L. HS-SPME-GC/MS analysis of the volatile compounds of Achillea collina: Evaluation of the emissions fingerprint induced by Myzus persicae infestation. J. Plant Biol. 55, 251–260 (2012). [Google Scholar]
- Verheggen F. J., Haubruge E., De Moraes C. M. & Mescher M. C. Aphid responses to volatile cues from turnip plants (Brassica rapa) infested with phloem-feeding and chewing herbivores. Arthropod-Plant Inte. 7, 567–57 (2013). [Google Scholar]
- Francis F., Vandermoten S., Verheggen F. J., Lognay G. & Haubruge E. Is (E)-β-farnesene the only volatile terpenoid in aphids? J Appl Entomol 129, 6–11 (2005). [Google Scholar]
- Park B. S., Choi W. S., Kim J. H., Kim K. H. & Lee S. E. Monoterpenes from thyme (Thymus vulgaris) as potential mosquito repellents. J Am Mosq Control Assoc. 21, 80–83 (2005). [DOI] [PubMed] [Google Scholar]
- Yang N. W., Li A. L., Wan F. H., Liu W. X. & Johnson D. Effects of plant essential oils on immature and adult sweetpotato whitefly, Bemisia tabaci biotype B. Crop Prot. 29, 1200–1207 (2010). [Google Scholar]
- Owolabi M. S., Oladimeji M. O., Labunmi L., Singh G., Marimuthu P. & Valery A. I. Composition and biological potentials of the essential oil of Zingiber officinal (Roscoe) from Nigeria. Bull. Pure Appl. Sci. 26, 113–119 (2007). [Google Scholar]
- Suekawa M., Ishige A., Yuasa K., Sudo K., Aburada M. & Hosoya E. Pharmacological studies on ginger. I. Pharmacological actions of pungent constituents, (6)-gingerol and (6)-shogaol. J. Pharm. 7, 836–848 (1984). [DOI] [PubMed] [Google Scholar]
- Tang W. & Eisenbrand G. Chinese drugs of plant origin. Chemistry, pharmacology, and use in traditional and modern medicine. 1st edn. Kaiserslautern. Berlin: Springer-Verlag (1992). [Google Scholar]
- Ukeh D. A., Birkett M. A., Pickett J. A., Bowman A. S. & Mordue Luntz A. J. Repellent activity of alligator pepper, Aframomum melegueta , and ginger, Zingiber officinale, against the maize weevil, Sitophilus zeamais. Phytochemistry 70, 751–758 (2009). [DOI] [PubMed] [Google Scholar]
- Simmons A. M. & Gurr G. M. Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric. For. Entomol 7, 265–27 (2005). [Google Scholar]
- Bleeker P. M., Diergaarde P. J., Ament K., Guerra J., Weidner M., Schütz S. & Schuurink R. C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 151, 925–935 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A. K., Prajapati V., Khanuja S. P. S. & Kumar S. Toxicity, feeding deterrence and effect of activity of 1,8-cineole from Artemisia annua on progeny production of Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 94, 979–983 (2001). [DOI] [PubMed] [Google Scholar]
- Leroy P. D., Heuskin S., Sabri A., Verheggen F. J., Farmakidis J., Lognay G., Thonart P., Wathelet J.-P., Brostaux Y. & Haubruge E. Honeydew volatile emission acts as a kairomonal message for the Asian lady beetle Harmonia axyridis (Coleoptera: Coccinellidae). J. Insect Sci. 19, 498–506 (2012). [Google Scholar]
- Akol A. M., Njagi P. G. N., Sithanantham S. & Mueke J. M. Effects of two neem insecticide formulations on the attractiveness, acceptability and suitability of diamondback moth larvae to the parasitoid, D. mollipla (Holmgren) (Hym.,Ichneumonidae). J. Appl. Entomol. 127, 325–331 (2003). [Google Scholar]