Abstract
Developing biomarkers for detecting acetaminophen (APAP) toxicity has been widely investigated. Recent studies of adults with APAP-induced liver injury have reported human serum microRNA-122 (miR-122) as a novel biomarker of APAP-induced liver injury. The goal of this study was to examine extracellular microRNAs (miRNAs) as potential biomarkers for APAP liver injury in children. Global levels of serum and urine miRNAs were examined in three pediatric subgroups: 1) healthy children (n=10), 2) hospitalized children receiving therapeutic doses of APAP (n=10) and 3) children hospitalized for APAP overdose (n=8). Out of 147 miRNAs detected in the APAP overdose group, eight showed significantly increased median levels in serum (miR-122, −375, −423-5p, −30d-5p, −125b-5p, −4732-5p, −204-5p, and −574-3p), compared to the other groups. Analysis of urine samples from the same patients had significantly increased median levels of four miRNAs (miR-375, −940, −9-3p and −302a) compared to the other groups. Importantly, correlation of peak serum APAP protein adduct levels (an indicator of the oxidation of APAP to the reactive metabolite N-acetylpara-quinone imine) with peak miRNA levels showed that the highest correlation was observed for serum miR-122 (R=0.94; p<0.01) followed by miR-375 (R=0.70; p=0.05). Conclusion: Our findings demonstrate that miRNAs are increased in children with APAP toxicity and correlate with APAP protein adducts, suggesting a potential role as biomarkers of APAP toxicity.
Keywords: Acetaminophen, Pediatric, Urine, microRNA, DILI
Introduction
Acetaminophen (APAP) is one of the most commonly used drugs for pain and fever in adults and children (1, 2). The drug is generally considered to be safe when administered at doses recommended by the manufacturer. However, APAP overdose is a very common cause of acute liver failure (ALF) in adults, and accounts for ~ 14% of ALF in children (3). Since the etiology of ALF for up to 40% of pediatric cases is unknown, it is possible that undiagnosed APAP overdose is responsible for some of these indeterminate ALF cases (1). While liver injury itself is detected by increases in serum transaminase and bilirubin, the current approach for diagnosing APAP poisoning relies on a history of APAP exposure and quantitation of APAP in peripheral blood within the first 24 h after the overdose (ie., the Rumack nomogram). Limitations of this approach have been well-described and more sensitive, mechanism-based biomarkers are needed (4-6). Early biomarkers, which correctly identify individuals with toxicity, could be important to detect patients at risk for developing ALF.
MicroRNAs (miRNAs) show promise as possible new biomarkers of disease and injury (8). miRNAs are typically 21–23 nucleotides long and regulate gene expression by binding to the 3′ untranslated regions (3′UTR) of their target mRNAs (9, 10). Since miRNAs can be detected in body fluids, they are appealing as noninvasive biomarker candidates (11). Our laboratory previously reported that urinary miRNA profiles were altered in rats after administration of hepatotoxic doses of acetaminophen or carbon tetrachloride (12). Studies in the mouse model of APAP toxicity found that miR-122 and miR-192, both found at high levels in liver tissue, are potential liver injury biomarkers (13). Compared to alanine aminotransferase (ALT), miR-122 is liver specific and represents over 70% of the total liver miRNAs (14). Recent clinical studies support the increase of serum miR-122 under conditions of hepatotoxicity in patients with APAP overdose (15-19). Elevations of miR-122 have also been reported in heparin-induced liver necrosis (20), liver steatosis (21) and in hepatitis B and C infections (22-24). It is important to note that it these studies did not address the usefulness of measuring serum miR-122 levels in children exposed to high doses of APAP.
In this investigation, global miRNA levels were examined using small RNA sequencing of serum samples and human miRNA PCR array analysis on urine samples from three pediatric subgroups. The subgroups were 1) children with no recent APAP exposure, 2) hospitalized children receiving APAP for treatment of pain and fever, and 3) children with APAP overdose. We evaluated the hypothesis that miRNA expression profiles may have diagnostic potential for APAP toxicity in children.
Method
Study population and design
The study was approved by the institutional review board of the University of Arkansas for Medical Sciences. Following informed consent and assent when age appropriate, blood and urine samples were collected from study subjects (n=28). There were three subject groups: 1) control group, defined as healthy children with no use of APAP in the preceding 14 days (N=10); 2) APAP therapeutic group, defined as hospitalized children receiving APAP per standard of care (N=10); and 3) APAP overdose group, defined as children requiring hospitalization for treatment of APAP overdose (N=8). Clinical information on study subjects included gender, weight, and dose or doses of APAP received (Group 2) or ingested (Group 3), reported as mg/kg. A single sample was collected in Groups 1 and 2, while multiple samples were collected from some patients in Group 3. Blood samples were centrifuged immediately after collection, and serum and urine samples were stored at −80°C until further analysis.
Serum ALT and APAP protein adducts quantification
Serum ALT levels were quantified in the clinical laboratory of Arkansas Children's Hospital. Serum APAP protein adduct levels were quantified through a high-performance liquid chromatography with electrochemical detection assay as previously described (25-27).
Serum and urine miRNA profiling
Total RNA was isolated from serum (50 μl) using the method described previously (12). The serum miRNA (minimum of 300 ng total RNA) profiling was conducted by Illumina high-throughput small RNA sequencing (HiSeq 2000, Illumina), according to the manufacturer's protocol. Four samples were not included in the analysis due to low RNA volume; therefore, a total of 36 serum RNA samples were sequenced, including: 1) control group, 9 samples from 9 subjects; 2) APAP therapeutic group, 8 samples from 8 subjects; and 3) APAP overdose group, 19 samples from 8 subjects. The Upper Quartile normalization method was used for data analysis (28, 29), where the counts were divided by the upper quartile of counts associated with their lane and multiplied by the mean upper quartile of counts across all the samples of the dataset. Absolute fold changes of > 2-fold at p < 0.05 were considered significant.
Total RNA was isolated from urine (300 μl) by the Trizol method (12), and 300 ng of RNA were used to generate cDNA. Whole genome profiling of urinary RNA samples was performed using human miRNome miRNA PCR arrays (MAH-3200E, Qiagen, Frederick, MD) covering 752 human miRNAs. These PCR arrays were run per the manufacturer's protocol (Qiagen) on a 7900 real-time PCR system (Applied Biosystems Inc., Foster, CA). Relative miRNA expression levels were determined with the ΔCt method per the manufacturer's recommendations. Three miRNAs (miR-7, miR-671-3p, and miR-943), showing low standard deviation, were selected to normalize the 752 miRNAs from PCR array. Fold changes of > 2-fold at p < 0.05 were used to select the significantly altered miRNAs. Hierarchical unsupervised clustering analysis (Heatmap) was performed as described previously (30); normalized counts (serum) or ΔCt values (urine) were used for these analyses.
Quantitative PCR validation
To validate the miRNA profiling results, TaqMan miRNA qRT-PCR assay (Applied Biosystems) was performed on selected miRNAs (miR-122 and miR-375) for the serum and urine samples and miR-940 levels were confirmed by SYBR Green Qiagen kit as the TaqMan assay was not available. This analysis was limited to miRNAs with a significant correlation (p<0.05) with APAP protein adducts. Non-human miRNA, ath-miR159a (Arabidopsis thaliana), was spiked into RNA samples as a control for extraction and amplification steps. Let-7d was used for normalization of serum samples based on the previous publication (15) and miR-671-3p was used for normalization of urine samples which was the most consistent urinary miRNA.
Statistical analysis
A non-parametric method (Kruskal–Wallis one-way analysis of variance by ranks) was used to determine whether there was a significant difference among the three subgroups. Dunn's method was used for all pairwise multiple comparisons. For correlation analysis between peak miRNA and peak APAP protein adduct, Pearson correlation test was performed in SigmaPlot (version 11.0, Systat Software Inc.), with p < 0.05 considered as statistically significant.
Results
Elevations of ALT and APAP protein adducts
Serum ALT and APAP protein adducts values are summarized in Table 1. Children in Groups 2 and 3 were older than those of Group 1. The median unit dose of APAP in the therapeutic group was 12.6 mg/kg (Range: 10 - 17.5 mg/kg) and the median daily dose was 17 mg/kg (Range: 10.2 – 28.5 mg/kg). The median reported total APAP exposure in overdose patients was 198.4 mg/kg (Range: 58.6-559.4 mg/kg). . Median values of ALT and APAP adducts were significantly (p< 0.05) higher in Group 3 than in the other groups. ).
Table 1.
Clinical and Laboratory Data of Study Subjects by Group
| Age | Gender (Male: Female) | APAP Dose(mg/kg) | ALT (IU/L) | APAP Protein Adducts (nmol/ml) | |
|---|---|---|---|---|---|
| 1 Healthy Control Group (N=10) | 10.5 (5-15) | 2:8 | 0 | 18 (10-37) | 0.01 (0 – 0.01) |
| 2 APAP Therapeutic Group (N=10) | 13.5 (5 -18) | 7:3 | 12.6 (10.0 -17.5) | 25 (6- 177) | 0.09 (0.01- 0.56) |
| 3 APAP Overdose Group (N=8) | 16.0 (14-17) | 5:3 | 198.4* (58.6 -559.4) | 1835* (29 – 9909) | 0.61* (0.10- 6.69) |
Note: Data presented as median (range).
p<0.05 by three way comparison.
Global serum and urine miRNA analysis
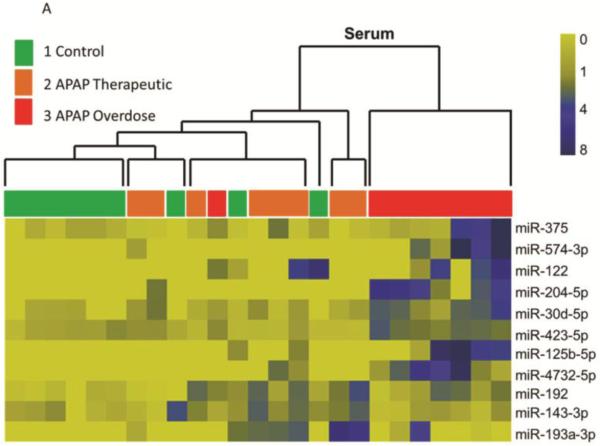
Small RNA sequencing (HiSeq 2000, Illumina) detected and quantified a total of 147 miRNAs in all serum samples (n=36). Comparison of the subgroups revealed that eight serum miRNAs (miR-122, −375, −423-5p, −30d-5p, −125b-5p, −4732-5p, −204-5p, and −574-3p) were increased more than 2-fold in samples from the APAP overdose group (Group 3) compared to the other subgroups (Table 2). Urinary miRNA profiling using the whole genome PCR array found that miR-375, miR-940, miR-9-3p and miR-302a were increased in Group 3 (Table 3) compared to the other two groups. miR-375 was increased in both urine and serum samples in Group 3 patients (Tables 2 and 3). While miR-122 was detected in urine, it was not statistically different among the groups (data not shown). To explore relationships between groups, hierarchical cluster analysis (HCA) was performed on the miRNA levels in individual samples. Since repeated measures were available for the Group 3 subjects (at multiple time points during the hospitalization), only the peak values were selected for HCA analysis. As shown in Figure 1A, levels of serum miRNAs could separate Group 3 (elevated ALT) from Groups 1 and 2 (normal to low ALT). This panel included eight up-regulated miRNAs (Table 2) and three down-regulated miRNAs (miR-192, miR-143-3p and miR-193a-3p) in Group 3 compared to Group 2. Similarly, a panel of 4 urinary miRNA (Table 3) partitioned the subjects into two clusters: Group 3 (elevated ALT) vs. Groups 1 and 2 (Figure 1B). The variability within Group 3 may be secondary to differences in the severity of or temporal stage of toxicity within this group, as was observed with the ALT and APAP protein adduct measurements.
Table 2.
Distribution of serum miRNAs in study groups
| miRNA ID | 1 Control | 2 APAP Therapeutic | 3 APAP Overdose |
|---|---|---|---|
| miR-122 | 0 (0-459.6) | 0 (0-243.6) | 96.5 (0-422.9) |
| miR-375 | 420.9 (0-698.0) | 120.3 (0-1883.7) | 927.3b (349.3-8349.7) |
| miR-423-5p | 15,785.7 (5,833.3-29,882.2) | 6,573.6 (5,691.1-25,723.4) | 45,282.7a,b (29,045.0-66,053.5) |
| miR-30d-5p | 0 (0-507.6) | 661.7 (165.2-1404.6) | 1829.5a (525.1-2701.4) |
| miR-125b-5p | 0 (0-65.2) | 0 (0-79.5) | 110.0 (0-446.4) |
| miR-4732-5p | 0 (0) | 0 (0-66.1) | 61.0a (0-238.7) |
| miR-204-5p | 0 (0) | 0 (0-24.1) | 46.6a (0-69.9) |
| miR-574-3p | 0 (0) | 0 (0-31.6) | 63.3 (0-358.0) |
Note: miRNA levels are expressed as counts by NGS platform and presented as median (range). For Dunn's pairwise comparison:
p<0.05 for Group 3 vs 1
p<0.05 for Group 3 vs 2.
Eight serum miRNAs (miR-122, miR-375, miR-423-5p, miR-30d-5p, miR-125b-5p, miR-4732-5p, miR-204-5p, and miR-574-3p) were increased in Group 3 (ANOVA, p<0.05).
Table 3.
Distribution of urinary miRNAs in study groups
| miRNA ID | 1 Control | 2 APAP Therapeutic | 3 APAP Overdose |
|---|---|---|---|
| miR-940 | 35.2 (9.4-97.3) | 25.3 (5.4-63.7) | 104.1b (19.9-634.4) |
| miR-375 | 3.1 (0.2-16.0) | 2.0 (0.2-6.2) | 10.7b (0.8-50.0) |
| miR-302a | 1.3 (0.2-117.6) | 0.8 (0.2-1.6) | 17.8a,b (1.7-295.5) |
| miR-9-3p | 135.3 (55.3-474.8) | 436.2 (96.7-834.5) | 1,186.3a (133.3-4,371.2) |
Note: The relative miRNA levels are expressed as relative concentration, normalized to three endogenous miRNAs (miR-7, miR-671-3p, and miR-943), and presented as median (range). For Dunn's pairwise comparison:
p<0.05 for Group 3 vs 1
p<0.05 for Group 3 vs 2.
One altered miRNA (miR-375) overlapped between serum and urine samples.
Figure 1. Clustering analysis of study subjects based on the pattern of altered miRNAs, the APAP overdose group is separated with others.
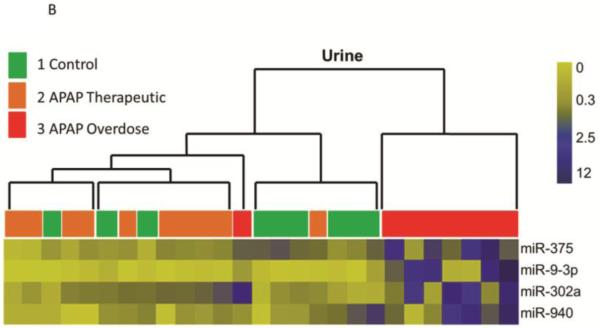
A. Heat map analysis showing normalized counts and a distinct serum miRNA pattern of the APAP overdose group. Group 1 (n=9) are indicated in green, Group 2 (n=8) are in orange and samples from Group 3 (n=8) are in red. B. Heat map analysis showing normalized miRNA levels and a distinct urinary miRNA pattern of the APAP overdose group. Group 1(n=9) are indicated in green, Group 2 (n=10) are in orange and samples from Group 3 (n=8) are in red.
Comparison of miRNAs to toxicology parameters
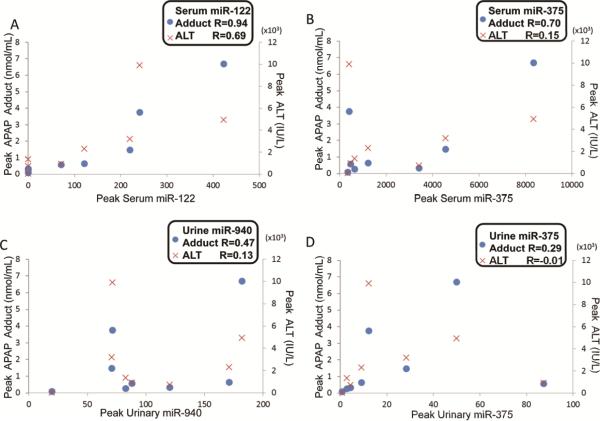
To further examine the relationship between miRNAs and toxicity, correlation analysis was performed among levels of APAP protein adducts, ALTand miRNAs in Group 3. Given that multiple measures were available from each patient, the peak APAP protein adducts or ALT values were used examine the relationship of these parameters to peak miRNA levels. As shown in Figure 2, there was a strong correlation between serum miR-122 and APAP protein adducts (R=0.94, p<0.001), whereas serum levels of miR-375 and APAP protein adducts were moderately correlated (R=0.70, p=0.05). Urinary levels of miRNAs (miR-940 and miR-375) were not correlated with APAP protein adducts levels or ALT. Interestingly, correlations between peak serum miRNAs levels and ALT values were not significant ( × symbol in Figure 2, R=0.69, p=0.06 for miR-122), In addition, APAP dose was not correlated with miRNAs, ALT or APAP protein adducts (data not shown).
Figure 2. Correlation of peak miRNA to APAP protein adducts or ALT, with p ≤ 0.05 was considered as statistically significant.
Correlation between miRNAs (x-axis) and APAP protein adducts (dot, left y-axis) or ALT levels (x-symbol, right y-axis) were determined by the Pearson method: serum miR-122 (A), serum miR-375 (B), urine miR-940 (C) and miR-375 (D).
Individual miRNA confirmation
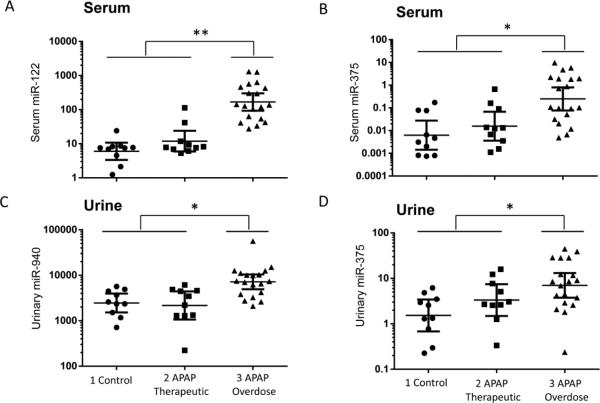
To confirm the expression of miRNAs in body fluids as biomarkers for APAP-induced liver injury in children, qRT-PCR was performed on individual miRNAs (serum miR-122/miR-375 and urine miR-940/miR-375) which were correlated with APAP protein adducts (Figure 2). As shown in Figure 3A, serum miR-122 showed significantly higher levels in Group 3 (p<0.01) compared to other groups (Groups 1 and 2). Serum miR-375 (Figure 3B) levels were also higher in Group 3 compared to other groups (p<0.05); however, the separation was not as good as that for miR-122. Urinary expression levels of miR-940 and miR-375 were significantly increased in Group 3 compared to Groups 1 and 2 (Figure 3C-D).
Figure 3. Increases of miRNAs from APAP overdose children confirmed by qRT-PCR.
Serum miRNA levels, miR-122 (A) and miR-375 (B), from each group were reported as relative miRNA concentration, with the formula 2^-ΔCt normalized with Let-7d. Urinary miR-940 (C) and miR-375 (D) levels from each group were reported as relative concentration normalized with miR-671-3p. APAP overdose subgroup shows a significant median increase in the miRNA levels (* p < 0.05, ** p< 0.01).
Time course of miRNA, ALT and APAP protein adduct alterations
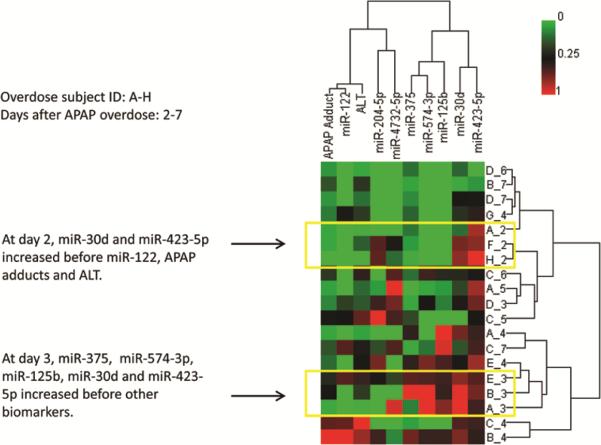
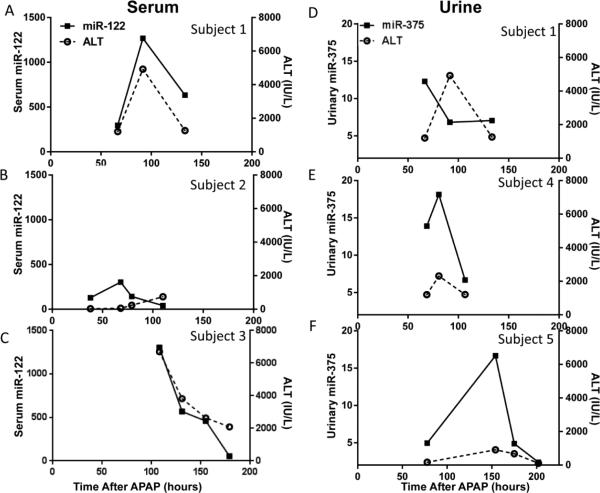
To further characterize the temporal changes of these miRNAs in body fluids, and compare them to those of established biomarkers (e.g., ALT), HCA was carried out on subjects with multiple time points. In Figure 4, each subject was assigned a unique letter of the alphabet (A-H) and each sample identified by the day after APAP ingestion (day 2 -7). The elevation of miR-122 was similar to APAP adduct and ALT, which had peaked at day 4, in subjects B and C (bottom of the cluster). However, miR-375 and other miRNAs peaked earlier, at day 3 (subjects A, B, E). Among the 8 subjects in the overdose group, selected individuals with at least 3 time course data are presented (serum: Figure 5 A-C; urine: Figure 5 D-F). The time course data covers the progression, peak and resolving toxicity. . Interestingly, for Subject 2 (Figure 5B and supplementary Figure 1) which displayed a mild ALT increase (peak value less than 1,000 IU/L), the level of serum miR-122 increased earlier and returned to baseline earlier than that of ALT. The levels of urinary miR-375 also showed temporal changes, with peaks coinciding with that of ALT changes for two of the three subjects. For subject 1 (Figure 5 A and D), for both serum and urine time course data, levels of urinary miR-375 both increased and returned to baseline at earlier times compared to those of serum miR-122 and ALT.
Figure 4. Clustering analysis of ALT, APAP adducts and miRNAs peak time from overdose subjects with multiple measures.
Overdose subjects were listed by alphabet (A-H) and days after APAP ingestion (day2 -7). For example, B_4 is sample collected from subject B at day 4 after APAP overdose. HCA analysis showing miR-122 peaks at similar time as ALT or APAP protein adducts, however, some miRNA levels precede those of ALT or APAP protein adducts.
Figure 5. Time courses of miRNAs and ALT in selected APAP overdose children.
Multiple samples were collected from Group 3 subjects, and the hours after APAP ingestion were recorded. The time course data covers different stages of toxicity: before or during the injury (before peak values) or during the resolution of toxicity (after peak). (A-C) Time course changes of serum miR-122 from individual APAP overdose child (n=3); (D-F) Time course changes of urinary miR-375 from individual APAP overdose child (n=3).
To further examine the relationship of the miRNAs to time course data, the time to reach peak measurement for each miRNA was compared to, APAP protein adducts and ALT. As shown in Figure 6, it appears that overall, these biomarkers, either from serum or urine, reached peak values in a similar range of time (92-110 hours) as ALT or APAP protein adducts.
Figure 6. Comparison of time to reach peak APAP protein adducts, ALT and miRNAs.
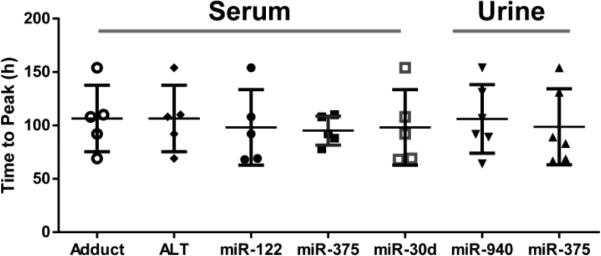
Time to reach peak measure for each miRNA was compared to known hepatotoxicity biomarkers (APAP protein adduct and ALT). The peak times were similar, with median in the range of 92-110 hours (n=6).
Discussion
The advancement of high-throughput, large-scale technologies, such as next generation deep sequencing, has significantly expanded the field of biomarker research. With the discovery of stable miRNAs in various body fluids, increasing attention has been paid to the use of extracellular miRNAs as potential biomarkers for informing early diagnosis, choice of treatment and monitoring of disease progression or recovery (11, 31, 32). A time course study in the mouse model of APAP hepatotoxicity showed that the level of many plasma miRNAs inversely correlated with the level of hepatic miRNAs, indicating that for these miRNAs, hepatic injury caused the release of miRNAs into the circulation system (13). Recent studies in adults confirmed the increase of serum miR-122 in patients with APAP poisoning (15-18). However, serum miR-122 is not specific to APAP-induced liver injury and can be observed with other causes of drug induced liver injury (20) and viral infection (23, 33). The hypothesis of this study was that a panel of extracellular miRNAs can be used as biomarkers to indicate APAP overdose in children and separate patients exposed to therapeutic dose vs. overdose situations. To our knowledge, this is the first attempt to use small RNA sequencing for global miRNAs profiling to discover extracellular miRNA-based biomarkers for APAP overdose in the pediatric population.
Using small RNA sequencing, up to 147 serum miRNAs exhibited altered levels and 8 miRNAs were significantly increased in children with APAP overdose (Table 2). Serum miRNA profiles in Group 3 were distinct when compared to the other groups (Groups 1 and 2) (Figure 1). Furthermore, two serum miRNAs, miR-122 and miR-375, were strongly correlated with peak serum APAP protein adducts. As expected, serum miR-122 levels were significantly elevated in APAP overdose patients with almost undetectable levels in the Groups 1 and 2 (Table 2). For urine samples, a panel of 4 increased miRNAs (miR-375, miR-940, miR-9-3p and miR-302a) was identified in the APAP overdose group. Interestingly, miR-375 was elevated in both serum and urine samples in Group 3.
In adults, previous studies found that miR-122 increased earlier (18) and returned to baseline while ALT remained high (16, 17). These previous observations suggest that extracellular miRNA has a shorter half-life and will provide earlier and possibly more accurate indication of APAP-induced liver injury. Interestingly, the clustering analysis suggested that the changes of miR-122 followed the rise in ALT and APAP protein adducts, while other miRNAs preceded (Figure 4). These divergent findings for miR-122 time course changes could be explained by the heterogeneity of the patients and the variance in the timing of sample collection. Therefore, additional studies in wider cohorts of APAP-induced liver injury should be conducted to test miRNAs’ prognostic value.
Human studies of miRNAs in APAP toxicity have primarily focused on miR-122 (16). Other liver-rich miRNAs, such as miRNA-125b-5p and miR-192-5p have been reported to increase in APAP-treated mice (34). In this study, a significant elevation of miR-192-5p was noted in the APAP therapeutic group. Our current study found several miRNAs that have not been reported before: miR-375 and miR-940. The function of miR-940 is still unclear. Interestingly, miR-375 has previously been associated with pancreatic diseases (35), lung development (36), breast and gastric cancer (37, 38). The gene targets of miR-375 play important roles in insulin secretion (39) and cell proliferation (36). It is likely that miRNAs discovered in this study interact with key molecules in cell death and proliferation; therefore, it is not surprising to see that some of the altered miRNAs have been reported to be associated with other human diseases. In addition, a recent study conducted in adults with APAP hepatotoxicity suggested that the highest elevations of circulating miRNAs did not come from liver tissue (40).
Urinary biomarkers may provide a more specific, sensitive and consistent indicator of liver injury than existing biomarkers (12). One potential limitation of urinary miRNAs is that not all serum miRNAs are excreted into the urine due to different “filtering” processes and urine has far fewer detectable miRNAs than other human body fluids (31). The main advantages of using urinary biomarkers are that the sample collections are non-invasive and multiple time-series measurements are easy to obtain (41). In addition, such samples can be collected in a relatively large amount, which is a limitation for serum miRNA quantification.
This study provides the first published serum and urine miRNA profiles for APAP poisoning in children. Importantly, this work serves as a proof of concept that the alteration in extracellular miRNA could be a useful biomarker to timely represent the hepatocellular injury. One major limitation of our study is that a small number of subjects were analyzed. This decreased the power of the study and larger studies will be required to confirm the findings. In addition, Group 3 included samples collected at various time points after the APAP overdose; whereas, a single time point was used for Groups 1 and 2. APAP overdose subjects present in multiple stages of APAP poisoning, such as: 1) before injury has occurred, 2) during the peak of toxicity and 3) during the resolution of toxicity. . Hence, there are diverse miRNA alteration patterns within Group 3 (Figure 1B), which limited our ability to fully characterize temporal patterns among biomarkers. . The 4 urinary miRNAs identified in the current study do not overlap with the previous rat study (12). A major difference is that all overdosed subjects of our current study received N-acetyl-cysteine (NAC) treatment. NAC is the standard-of-care treatment for APAP overdose; it serves as a precursor for GSH synthesis and reduces oxidative stress. This may explain why a relatively small number of miRNAs were identified in the current study compared to our previous rat study.
Supplementary Material
In summary, this study identified altered miRNA patterns in serum and urine from children, which are potential biomarkers of APAP toxicity. Furthermore, the elevation of serum miR-122 in the pediatric population is similar to that reported for adults with APAP poisoning. The data herein suggest that multiple serum miRNAs associate with liver injury, but the most notable appear to be miR-122 and miR-375 as they exhibited a high correlation with the level of serum APAP protein adducts. Urinary miR-940 and miR-375 were also elevated in subjects overdosed with APAP. In addition, the time course analyses provide a basis for future studies to validate miRNA as useful prognostic biomarkers in clinical setting. Qualifying these results on a larger cohort may provide stable and sensitive miRNA biomarkers of hepatotoxicity. Additional studies of liver injury not caused by APAP will be required to determine the biomarkers’ specificity for APAP-mediate liver injury. In summary, this study identified multiple miRNAs in serum and urine that are potentially altered by APAP exposure which may prove useful as APAP-specific biomarkers or, more likely, biomarkers of liver injury in general.
Highlights.
Serum miR-122 and miR-375 levels were increased in children with APAP overdose
Urine levels of miR-375 and miR-940 were increased in the APAP overdose group
Peak serum miR-122 levels were correlated with peak serum APAP protein adducts
Acknowledgements
The authors are indebted to their medical and nursing colleagues as well as the children and their parents who agreed to take part in this study. We would like to thank to Lee Howard, the Arkansas Children's Hospital clinical research coordinator in this study.
Financial disclosure: This study was funded in part by a grant (R01 DK75936 to LPJ) from the National Institutes of Diabetes, Digestive and Kidney Diseases. James is part owner of Acetaminophen Toxicity Diagnostics, LLC and has a patent pending for the development of a commercial assay for measurement of acetaminophen protein adducts. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Abbreviations
- miRNA
microRNA
- DILI
drug induced liver injury
- APAP
acetaminophen
- ALT
alanine aminotransferase
- ALF
acute liver failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The information in these materials is not a formal dissemination of information by U.S. Food and Drug Administration (FDA) or Arkansas Biosciences Institute and does not represent agency position or policy.
References
- 1.James LP, Capparelli EV, Simpson PM, Letzig L, Roberts D, Hinson JA, Kearns GL, et al. Acetaminophen-associated hepatic injury: evaluation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin Pharmacol Ther. 2008;84:684–690. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algren DA. Review of N-Acetylcysteine for the treatment of acetaminophen (paracetamol) toxicity in pediatrics. Geneva: 2008. [Google Scholar]
- 3.Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, Dhawan A, et al. Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. The Journal of Pediatrics. 2006;148:652–658.e652. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGill MR, Cao M, Svetlov A, Sharpe MR, Williams CD, Curry SC, Farhood A, et al. Argininosuccinate synthetase as a plasma biomarker of liver injury after acetaminophen overdose in rodents and humans. Biomarkers. 2014;19:222–230. doi: 10.3109/1354750X.2014.897757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGill MR, Li F, Sharpe MR, Williams CD, Curry SC, Ma X, Jaeschke H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch Toxicol. 2014;88:391–401. doi: 10.1007/s00204-013-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92:761–794, viii. doi: 10.1016/j.mcna.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 9.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 10.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 11.Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: A new source of biomarkers. Mutat Res. 2011;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Greenhaw J, Shi Q, Su Z, Qian F, Davis K, Mendrick DL, et al. Identification of urinary microRNA profiles in rats that may diagnose hepatotoxicity. Toxicol Sci. 2012;125:335–344. doi: 10.1093/toxsci/kfr321. [DOI] [PubMed] [Google Scholar]
- 13.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, et al. miR-122, a Mammalian Liver-Specific microRNA, is Processed from hcr mRNA and MayDownregulate the High Affinity Cationic Amino Acid Transporter CAT-1. RNA Biology. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 15.Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, French NS, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 17.Starkey Lewis PJ, Merz M, Couttet P, Grenet O, Dear J, Antoine DJ, Goldring C, et al. Serum microRNA Biomarkers for Drug-Induced Liver Injury. Clin Pharmacol Ther. 2012;92:291–293. doi: 10.1038/clpt.2012.101. [DOI] [PubMed] [Google Scholar]
- 18.Thulin P, Nordahl G, Gry M, Yimer G, Aklillu E, Makonnen E, Aderaye G, et al. Keratin-18 and microRNA-122 complement alanine aminotransferase as novel safety biomarkers for drug-induced liver injury in two human cohorts. Liver Int. 2013 doi: 10.1111/liv.12322. [DOI] [PubMed] [Google Scholar]
- 19.Krauskopf J, Caiment F, Claessen SM, Johnson KJ, Warner RL, Schomaker SJ, Burt DA, et al. Application of High-Throughput Sequencing to Circulating microRNAs Reveals Novel Biomarkers for Drug-induced Liver Injury. Toxicol Sci. 2014 doi: 10.1093/toxsci/kfu232. [DOI] [PubMed] [Google Scholar]
- 20.Harrill AH, Roach J, Fier I, Eaddy JS, Kurtz CL, Antoine DJ, Spencer DM, et al. The Effects of Heparins on the Liver: Application of Mechanistic Serum Biomarkers in a Randomized Study in Healthy Volunteers. Clin Pharmacol Ther. 2012;92:214–220. doi: 10.1038/clpt.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su TH, Liu CH, Liu CJ, Chen CL, Ting TT, Tseng TC, Chen PJ, et al. Serum microRNA-122 level correlates with virologic responses to pegylated interferon therapy in chronic hepatitis C. Proc Natl Acad Sci U S A. 2013;110:7844–7849. doi: 10.1073/pnas.1306138110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arataki K, Hayes CN, Akamatsu S, Akiyama R, Abe H, Tsuge M, Miki D, et al. Circulating microRNA-22 correlates with microRNA-122 and represents viral replication and liver injury in patients with chronic hepatitis B. Journal of Medical Virology. 2013;85:789–798. doi: 10.1002/jmv.23540. [DOI] [PubMed] [Google Scholar]
- 24.Laterza OF, Scott MG, Garrett-Engele PW, Korenblat KM, Lockwood CM. Circulating miR-122 as a potential biomarker of liver disease. Biomark Med. 2013;7:205–210. doi: 10.2217/bmm.12.107. [DOI] [PubMed] [Google Scholar]
- 25.Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of Acetaminophen-Protein Adducts in Mouse Liver and Serum and Human Serum after Hepatotoxic Doses of Acetaminophen Using High-Performance Liquid Chromatography with Electrochemical Detection. Drug Metabolism and Disposition. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 26.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metabolism and Disposition. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 27.James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, et al. Pharmacokinetics of Acetaminophen-Protein Adducts in Adults with Acetaminophen Overdose and Acute Liver Failure. Drug Metabolism and Disposition. 2009;37:1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bullard J, Purdom E, Hansen K, Dudoit S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinformatics. 2010;11:94. doi: 10.1186/1471-2105-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dillies M-A, Rau A, Aubert J, Hennequet-Antier C, Jeanmougin M, Servant N, Keime C, et al. A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis. Briefings in Bioinformatics. 2013;14:671–683. doi: 10.1093/bib/bbs046. [DOI] [PubMed] [Google Scholar]
- 30.Fang H, Harris SC, Su Z, Chen M, Qian F, Shi L, Perkins R, et al. ArrayTrack: an FDA and public genomic tool. Methods Mol Biol. 2009;563:379–398. doi: 10.1007/978-1-60761-175-2_20. [DOI] [PubMed] [Google Scholar]
- 31.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salminen WF, Yang X, Shi Q, Mendrick DL. Using microRNA as Biomarkers of Drug-Induced Liver Injury. J Mol Biomark Diagn. 2011;2:119. [Google Scholar]
- 33.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, et al. MicroRNA Expression, Survival, and Response to Interferon in Liver Cancer. New England Journal of Medicine. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, van Obberghen E. miR-375 Targets 3′ -Phosphoinositide – Dependent Protein Kinase-1 and Regulates Glucose-Induced Biological Responses in Pancreatic β -Cells. Diabetes. 2008;57:2708–2717. doi: 10.2337/db07-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Huang C, Reddy Chintagari N, Bhaskaran M, Weng T, Guo Y, Xiao X, et al. miR-375 regulates rat alveolar epithelial cell trans-differentiation by inhibiting Wnt/β-catenin pathway. Nucleic Acids Research. 2013 doi: 10.1093/nar/gks1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Souza Rocha Simonini P, Breiling A, Gupta N, Malekpour M, Youns M, Omranipour R, Malekpour F, et al. Epigenetically Deregulated microRNA-375 Is Involved in a Positive Feedback Loop with Estrogen Receptor α in Breast Cancer Cells. Cancer Research. 2010;70:9175–9184. doi: 10.1158/0008-5472.CAN-10-1318. [DOI] [PubMed] [Google Scholar]
- 38.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, et al. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 39.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, MacDonald PE, Pfeffer S, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 40.Ward J, Kanchagar C, Veksler-Lublinsky I, Lee RC, McGill MR, Jaeschke H, Curry SC, et al. Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1412608111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Salminen W, Schnackenberg L. Current and emerging biomarkers of hepatotoxicity. Current Biomarker Findings. 2012;2:43–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.