Abstract
Purpose
Systemic chemotherapy has improved the survival of patients with hepatoblastoma (HB). INT-0098 Intergroup Liver Tumor Study demonstrated that patients with HB treated with either cisplatin/fluorouracil/vincristine (CFV) or cisplatin/doxorubicin (CD) had a similar survival. The Children's Oncology Group adopted the less toxic CFV as the standard regimen for treating HB. However, international cooperative groups still favor the CD combination. We therefore decided to revisit the role of doxorubicin for the treatment of HB.
Methods
Outcomes of patients with HB on the INT-0098 study were reviewed with an emphasis on the postevent survival time for both regimens to elucidate the role of doxorubicin in their retrieval.
Results
Sixty-four of the 173 randomly assigned patients had an event. Of these, 55 experienced progression or recurrence after initial treatment. Eleven (31%) of 36 patients treated with CFV were successfully retrieved with a doxorubicin-containing regimen and surgery and remain alive at last contact, whereas only one (6%) of 18 patients treated with CD was alive after retrieval therapy.
Conclusion
CFV is effective for stage I or II HB. Doxorubicin can be omitted as part of initial therapy in the majority of these patients, potentially limiting the long-term cardiac toxicities, without compromising outcome. Doxorubicin is effective in rescuing patients with recurrent disease after CFV and should be incorporated as a means of intensifying initial therapy for advanced-stage, nonmetastatic HB. Outcome of patients with metastatic disease at diagnosis is poor, and improving their survival will require new therapeutic approaches.
INTRODUCTION
The use of systemic chemotherapy has improved the survival of patients with hepatoblastoma (HB) by increasing tumor resectability, decreasing the development of metastasis, and reducing the incidence of local recurrence. Cisplatin has been identified as one of the most active agent in the treatment of HB.1,2 There are relatively few data on the efficacy of other single agents except for doxorubicin.3 A number of other chemotherapeutic agents (ifosfamide, etoposide, vincristine, fluorouracil, cyclophosphamide, and carboplatin) have been incorporated into standard regimens, thus making it difficult to determine the efficacy of any of these drugs as a single agent.4-6
The INT-0098 Intergroup Liver Tumor Study11 randomly assigned all patients except those with completely resected pure fetal histology to receive treatment with either cisplatin/fluorouracil/vincristine (CFV) or cisplatin/doxorubicin (CD). The 5-year event-free survival (EFS) for patients randomly assigned to CFV or CD was 57% and 69%, respectively (P = .09). However, 5-year overall survival was similar when the two regimens were compared (CFV, 69%; CD, 72%; P = .88). Although the EFS difference was not statistically significant, the types of events associated with the administration of each regimen were notably different. Patients randomly assigned to receive CFV experienced a higher cumulative incidence of disease progression (39%) compared with patients randomly assigned to receive CD (23%; P = .02). Treatment with CD was associated with an increased number of treatment complications and toxic deaths (five deaths resulting from complications v three such events in the CFV arm). On the basis of these results, the Children's Oncology Group (COG) adopted the CFV regimen as the standard for the treatment of children with HB. International cooperative groups have favored the use of cisplatin and doxorubicin for the treatment of HB,7-10 and because of these divergent treatment strategies, we decided to revisit the role of doxorubicin for the treatment of children with HB by reanalyzing the data from INT-0098.
METHODS
From August 1989 to December 1992, children (< 21 years of age) with previously untreated HB or hepatocellular carcinoma were enrolled onto the INT-0098 study. This report involves only HB patients and excludes those with stage I pure fetal histology. Details of the study design and requirements for eligibility have been described previously.11 The protocol was approved by the National Cancer Institute and the institutional review boards of the participating institutions. Informed consent was obtained from parents, patients, or both, as deemed appropriate, according to Department of Health and Human Services guidelines.
Stage of disease was determined by surgical and histologic criteria at the initial surgical intervention before the initiation of chemotherapy: stage I, complete gross resection with clear margins; stage II, gross total resection with microscopic residual disease at the margins of resection; stage III, gross total resection with nodal involvement or tumor spill or incomplete resection with gross residual intrahepatic disease; and stage IV, metastatic disease with either complete or incomplete resection. Patients with stage I disease who were further classified as having pure fetal histology were treated with surgical resection followed by four courses of pulse doxorubicin and are not considered in this report.
Chemotherapy
INT-0098 study patients were randomly assigned to receive treatment with either CFV or CD. Each course of CFV consisted of intravenous (IV) cisplatin (90 mg/m2 for patients ≥1 year of age and 3 mg/kg for patients < 1 year of age) administered over 6 hours followed by IV hydration on day 0, and vincristine (1.5 mg/m2 IV push) and fluorouracil (600 mg/m2 IV push) on day 2. Each course of CD consisted of cisplatin (dose and administration as before) and continuous-infusion doxorubicin (20 mg/m2/d) administered for 96 consecutive hours and initiated during hydration 4 hours after completion of cisplatin. The initial chemotherapy phase consisted of four cycles. Patients with stage I or stage II HB who had no evidence of disease (complete remission) after four cycles of chemotherapy received no further therapy and entered follow-up. All other randomly assigned patients who did not have progressive disease were eligible for postinduction surgery I, if deemed feasible. If the tumor was completely resected or if there was no evidence of tumor at postinduction surgery I, the patient was to receive an additional two cycles of chemotherapy. If the surgery was not attempted or the tumor was not completely resected but either a partial response or stable disease was confirmed, the patient was to receive an additional four cycles of chemotherapy. Patients who received eight cycles of chemotherapy were eligible for postinduction surgery II, if deemed feasible.
Each cycle was administered at least 3 weeks apart, depending on recovery of peripheral neutrophil and platelet counts to at least 1,000 cells/μL and at least 100,000 cells/μL, respectively. Initial chemotherapy was delayed for at least 2 weeks for any patient in whom 50% of the liver or more was resected. Granulocyte colony-stimulating factor was not used during the first 30 months of the study, but the protocol was amended to permit its use during the last 11 months of the study.
Evaluation of Response
Complete response (no evidence of disease) was defined as no evidence of tumor by physical examination and imaging studies (computed tomography scans or magnetic resonance imaging) and normal serum alpha fetoprotein level for at least 4 weeks. Partial response was defined as a decrease of at least 50% in the sum of the products of the maximum perpendicular diameters of all measurable lesions, with no evidence of new lesions or progression in any lesion. Stable disease was defined as any response less than a partial response, without an increase in tumor size and without appearance of new lesions. Progressive disease was defined as a 25% or greater increase in the size of any lesion, appearance of new lesions, or a rising alpha fetoprotein level.
Statistical Design and Analysis
Study design.
Details of the study design and planned patient enrollment for INT-0098 have been described previously.11 The primary outcome measure for this study was EFS.
Outcome definitions.
EFS was defined as the period from the date chemotherapy was started until evidence of an EFS event (progressive disease, death, diagnosis of a second malignant neoplasm) or last contact, whichever occurred first. Overall survival (OS) was defined as the period from the date chemotherapy was started until death or last contact, whichever occurred first. A patient who died was considered to have experienced an OS event, regardless of the cause of death.
Postevent survival time (PEST) was defined as the period from the date of disease progression or recurrence until death or last contact, whichever occurred first. A patient who died was considered to have experienced an OS event, regardless of the cause of death. Patients who were alive at last contact were censored on that date.
Statistical methods.
Life-table estimates of survival time were calculated by the method of Kaplan and Meier,13 and the standard deviation of the Kaplan-Meier estimate of the survivor function at selected points was calculated using Greenwood's formula.14 Risk for death was compared across therapies and groups of patients using the log-rank statistic. For treatment comparisons, outcome was assigned to the randomized treatment, regardless of the therapy received.
Analytic strategy.
We undertook a review of the postrecurrence or postprogression outcome of patients enrolled onto INT-0098 to elucidate the role of chemotherapy (doxorubicin) in the retrieval of such patients. Only patients who experienced recurrence or progressive disease were considered for this analysis. We also reviewed the outcomes (EFS) of patients enrolled onto INT-0098 according to their initial stage and treatment randomization.
RESULTS
The 5-year EFS for all patients (excluding those with stage I pure fetal histology), treated with CFV was 57% (SE, 5%), and 69% (SE, 5%) for those treated with CD (P = .09). Five-year overall survival, however, was similar when the two regimens were compared (CFV, 69% [SE, 5%]; CD, 72% [SE, 5%]; P = .88).11 Sixty-four (37%) of the 173 eligible patients enrolled onto INT-0098, experienced an event at the time the data were obtained for analysis. Eight patients experienced death without disease progression as their first event, four as a result of liver failure (three CFV, one CD), two as a result of cardiotoxicity (all CD), one as a result of congestive heart failure secondary to tumor thrombus (CD), and one as a result of post–respiratory scyncylial virus pneumonia (CD). One patient developed a second malignancy, acute myeloid leukemia, after treatment with CD. Fifty-five patients experienced recurrence or progressive disease as their first event. Median follow-up time for event-free survivors was 5.7 years (range, 2.1 to 7.7 years). Thirty-nine patients initially received CFV and 25 received CD (Table 1). The distribution of sites of initial recurrence was similar across the two treatment groups (Table 2).
Table 1.
Initial Stage of Patients Enrolled Onto INT-0098 Who Experienced an Event
| Randomized Treatment Assignment | No. of Patients |
||||
|---|---|---|---|---|---|
| Stage I | Stage III | Stage IV | |||
| CFV | 3 | 18 | 18 | ||
| CD | 1 | 12 | 12 | ||
Abbreviations: CFV, cisplatin/fluorouracil/vincristine; CD, cisplatin/doxorubicin.
Table 2.
Sites of Recurrence According to Initial Stage and Randomized Treatment Regimen (n = 55)
| Randomized Treatment Assignment | Liver | Lung | Liver and Lung | Liver or Lung + Other |
|---|---|---|---|---|
| CFV | ||||
| Stage I | — | 2 | — | 1 |
| Stage III | 9 | 1 | 3 | 2 |
| Stage IV | 11 | 2 | 3 | 1 |
| Total | ||||
| No. | 20 | 5 | 6 | 5 |
| % | 55 | 14 | 17 | 14 |
| CD | ||||
| Stage III | 6 | 1 | 2 | 1 |
| Stage IV | 5 | 2 | 1 | 1 |
| Total | ||||
| No. | 11 | 3 | 3 | 2 |
| % | 58 | 16 | 16 | 10 |
Abbreviations: CFV, cisplatin/fluorouracil/vincristine; CD, cisplatin/doxorubicin.
Patients With Recurrence or Progressive Disease
Of the 55 patients who experienced recurrence or progressive disease as their first event, 36 initially received CFV and 19 received CD. All of the 36 patients who were initially treated with CFV received a doxorubicin-containing regimen at the time of relapse, most commonly a combination of cisplatin and doxorubicin. Of these, 13 (36%) had at least a partial response and were subsequently rendered disease free by surgical resection. Eleven (31%) of 36 patients remained alive at last contact. None of the 19 patients initially treated with CD received doxorubicin for recurrence. They were treated with ifosfamide and etoposide with or without addition of carboplatin. Only one patient was subsequently rendered disease free by such retrieval chemotherapy followed by surgical resection. All of the patients who were alive at last contact (median follow-up, 8.2 years) had complete surgical resection of residual disease. The risk of death after disease recurrence for patients initially enrolled in the CFV arm was significantly reduced when compared with patients receiving CD (Fig 1). In aggregate, the estimated 3-year PEST was 22% (SE, 6%). The estimated 3-year PEST according to regimen was 28% for patients initially treated with CFV (SE, 8%) and 11% for patients treated with CD (SE, 7%; P = .02). PEST according to initial stage and randomly assigned treatment is presented in Table 3.
Fig 1.
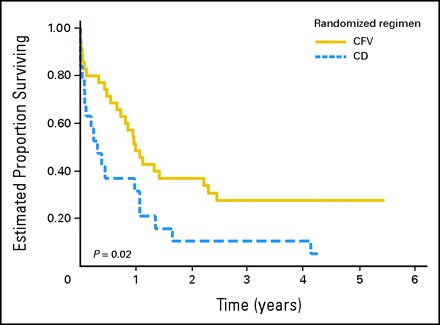
Postevent survival time according to initial treatment regimen. CD, cisplatin/doxorubicin; CFV, cisplatin/fluorouracil/vincristine.
Table 3.
3-Year PEST of 55 Patients With Relapse According to Their Initial Disease Stage and Randomized Regimen
| Stage | % |
||||||
|---|---|---|---|---|---|---|---|
| CFV |
CD |
||||||
| PEST | SE | PEST | SE | ||||
| I-II | 100 | 0 | * | ||||
| III | 19 | 11 | 10 | 10 | |||
| IV | 22 | 10 | 11 | 11 | |||
Abbreviations: PEST, postevent survival time; CFV, cisplatin/fluorouracil/vincristine; CD, cisplatin/doxorubicin.
There were no patients in the category.
Stage I Patients
The 5-year EFS for patients with stage I disease treated with CFV was 87%, and 95% for those treated with CD (Table 4). All three patients, whose respective disease progressed or recurred and were initially treated with CFV, are alive at last contact after receiving doxorubicin-containing therapy and surgery. One patient treated with CD died as a result of liver failure after initial surgical resection.
Table 4.
5-Year EFS According to Stage and Randomized Treatment Regimen
| Stage | % |
||||||
|---|---|---|---|---|---|---|---|
| CFV |
CD |
||||||
| 5-Year EFS | SE | 5-Year EFS | SE | ||||
| I | 87 | 7 | 95 | 5 | |||
| II | 100 | NA | 100 | NA | |||
| III | 60 | 7 | 68 | 7 | |||
| IV | 14 | 8 | 37 | 11 | |||
Abbreviations: EFS, event-free survival; CFV, cisplatin/fluorouracil/vincristine; CD, cisplatin/doxorubicin; NA, Greenwald estimate of the standard error cannot be calculated.
Stage II and III
The 5-year EFS for patients with stage III disease originally treated with CFV was 62%, and 71% for those treated with CD (Table 4). None of the patients with stage II disease experienced an event. Of the 18 stage III patients initially treated with CFV whose disease progressed or recurred, four (22%) are alive at last contact after receiving doxorubicin-containing therapy and surgery. None of the 12 patients initially treated with CD are alive.
Stage IV
The 5-year EFS for patients with stage IV disease treated with CFV was 14%, and 37% for those treated with CD (Table 4). Of the patients whose disease progressed or recurred, four (22%) of 18 patients initially treated with CFV are alive at last contact after receiving doxorubicin-containing therapy and surgery, whereas only one of the 12 patients initially treated with CD is alive.
DISCUSSION
On INT-0098, the long-term OS for CFV or CD was not significantly different for the combined group of patients with stage I or II disease or for the combined group of patients with stage III or IV HB.11 However, there were significant differences in the types of events and toxicities for the two regimens. Patients randomly assigned to receive CFV experienced a higher cumulative incidence of disease progression compared with patients randomly assigned to receive CD (P = .02).
Treatment with CFV provides an excellent outcome for patients with stage I and II disease, and this represents approximately one third of all HB patients. Furthermore, we demonstrated that an estimated 28% of patients whose disease progressed or recurred after initial treatment with CFV could be successfully rescued with a doxorubicin-containing regimen and surgery. All three recurrent patients with stage I non–pure fetal histology HB initially treated with CFV are alive without evidence of disease. Indeed, other investigators8 have demonstrated that anthracyclines can be omitted from initial therapy for patients with standard-risk HB without compromising outcome. This strategy spares at least one third of all newly diagnosed HB patients from exposure to the cardiotoxicity of anthracyclines. Most of these patients will be less than age 5 years at diagnosis. This investigation is a retrospective review, and the patients' postrecurrence therapy was not assigned through randomization.
Different investigators have used anthracycline-based regimens in patients with more advanced disease (stage III/IV) and obtained similar results. The German cooperative study HB9410 utilized a three-drug combination of ifosfamide, cisplatin and doxorubicin. Three-year EFS was 76% for patients with stage III disease and 21% for patients with stage IV disease. In the Japanese study for pediatric liver tumors, JPLT-1, conducted between 1991 and 1999, stage I and II patients received chemotherapy courses of cisplatin (40 mg/m2) and tetrahydropyranyl-doxorubicin (30 mg/m2). For stage IIIA, IIIB and IV patients, the doses of cisplatin and doxorubicin were increased to 80 mg/m2 and 30 mg/m2, respectively, for 2 days. OS and EFS for the entire cohort of 134 HB patients was 73% and 66%, respectively.15 In SIOPEL-2, the investigators of the Society of International Pediatric Oncology used a risk-adapted treatment strategy that segregated patients according to the PRETEXT (pretreatment extent of disease) staging system. Patients with high-risk disease received a chemotherapy regimen consisting of cisplatin alternating with carboplatin and doxorubicin. These patients are comparable with stage III and IV patients treated on INT-0098. The 3-year OS and progression-free survival for these patients were 53% and 48%, respectively.8 On the basis of these results, the optimal cisplatin-containing regimen for initial therapy for advanced stage HB can not be identified.
Intensification of therapy by augmenting the dose of existing agents16,17 has been demonstrated to be efficacious in the treatment of other pediatric tumors. The results of COG studies and other cooperative group studies for patients with advanced HB (stage III) remain suboptimal. We therefore believe that exploration of intensification of therapy for advanced-stage HB is still warranted. Intensification of therapy by alternating platinum analogs resulted in an increased the risk of an adverse outcome for children with unresectable or metastatic HB.12 We believe that intensification should be done by the addition of effective agents to the standard regimen of cisplatin, fluorouracil, and vincristine. Results of our analysis demonstrated that doxorubicin is an effective agent in the treatment of newly-diagnosed HB and in the retrieval of patients who have recurred after being treated with a non–anthracycline-containing regimen. Although these results were based on a small number of patients in of retrospective review, we think this agent should be included in combinations for front-line chemotherapy of this patient population. Its role in the initial therapy of advanced-stage disease should also be readdressed.
Gonzalez-Crussi et al18 have suggested that HB is to the liver as Wilms tumor is to the kidney and that the lessons learned in the management of Wilms' tumor can be applied to the treatment of HB. For both blastomas, histology and stage play a role in prognosis. Surgical resection is critical for survival, but is complicated by the fact that liver tumor patients often cannot undergo the type of initial complete resection possible for patients with unilateral Wilms' tumor. More advanced-stage disease, either local residual disease, metastatic disease at diagnosis, or recurrent disease, requires more intense chemotherapy. In these situations doxorubicin plays an important role in the treatment of Wilms' tumor patients, and its efficacy should be readdressed for patients with advanced-stage HB and for patients with recurrence who have not received doxorubicin as part of their initial therapy.
INT-0098 utilized doxorubicin administration as a continuous infusion over 96 hours to decrease peak plasma concentration in hopes of decreasing cardiotoxicity, and also increased the dose per course from 60 mg/m2 to 80 mg/m2. We recommend doxorubicin not be administered in the dose and schedule used in INT-0098, which was a modification of the previously used regimen combining cisplatin with doxorubicin administered by IV bolus (30 mg/m2/d) for 2 consecutive days19 because we observed two deaths related to cardiac toxicity while patients were receiving protocol therapy. Furthermore, this dose and schedule of doxorubicin was associated with enhanced marrow and mucosal toxicity. Should doxorubicin again be used for patients with advanced-stage or recurrent HB, protracted infusions of the drug should be avoided. Further, the following maneuvers may decrease its toxicity: routine administration of hematopoietic growth factors such as granulocyte colony-stimulating factor to ameliorate myelosuppression, lower dose per course and lower cumulative dose to reduce risk of cardiotoxicity, and shorter infusion time to decrease GI toxicity.
Although the up-front use of doxorubicin in patients enrolled on INT-009811 imparted an EFS benefit for patients with stage IV HB, the overall outcome continues to be dismal. New therapeutic strategies need to be evaluated to improve the poor outcome in patients with metastatic disease.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTSOF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Marcio H. Malogolowkin, Howard M. Katzenstein, Mark Krailo
Provision of study materials or patients: Marcio H. Malogolowkin, John J. Quinn, Marleta Reynolds, Jorge A. Ortega
Collection and assembly of data: Marcio H. Malogolowkin, Mark Krailo, Zhengjia Chen, Marleta Reynolds, Jorge A. Ortega
Data analysis and interpretation: Marcio H. Malogolowkin, Mark Krailo, Zhengjia Chen, John J. Quinn, Jorge A. Ortega
Manuscript writing: Marcio H. Malogolowkin, Mark Krailo, John J. Quinn, Jorge A. Ortega
Final approval of manuscript: Marcio H. Malogolowkin, Howard M. Katzenstein, Mark Krailo, Zhengjia Chen, John J. Quinn, Marleta Reynolds, Jorge A. Ortega
Footnotes
Supported by Grant No. CA 98543 from the Children's Oncology Group.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Douglass EC, Green AA, Wrenn E, et al: Effective cisplatin (DDP) based chemotherapy in the treatment of hepatoblastoma. Med Pediatr Oncol 13:187-190, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Black CT, Cangir A, Choroszy M, et al: Marked response to preoperative high-dose cis-platinum in children with unresectable hepatoblastoma. J Pediatr Surg 26:1070-1073, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Neglia JP, Woods WG: Continuous infusion doxorubicin in the treatment of primary hepatic malignancies of childhood. Cancer Treat Rep 70:655-657, 1986 [PubMed] [Google Scholar]
- 4.Katzenstein HM, London WB, Douglass EC, et al: Treatment of unresectable and metastatic hepatoblastoma: A pediatric oncology group phase II study. J Clin Oncol 20:3438-3444, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Fuchs J, Bode U, von Schweinitz D, et al: Analysis of treatment efficiency of carboplatin and etoposide in combination with radical surgery in advanced and recurrent childhood hepatoblastoma: A report of the German Cooperative Pediatric Liver Tumor Study HB 89 and HB 94. Klin Padiatr 211:305-309, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Evans AE, Land VJ, Newton WA, et al: Combination chemotherapy (vincristine, adriamycin, cyclophosphamide, and 5-fluorouracil) in the treatment of children with malignant hepatoma. Cancer 50:821-826, 1982 [DOI] [PubMed] [Google Scholar]
- 7.Suita S, Tajiri T, Takamatsu H, et al: Improved survival outcome for hepatoblastoma based on an optimal chemotherapeutic regimen: A report from the Study Group for Pediatric Solid Malignant Tumors in the Kyushu Area. J Pediatr Surg 39:195-198, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Perilongo G, Shafford E, Maibach R, et al: Risk-adapted treatment for childhood hepatoblastoma: Final report of the second study of the International Society of Paediatric Oncology—SIOPEL 2. Eur J Cancer 40:411-421, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Pritchard J, Brown J, Shafford E, et al: Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: A successful approach-results of the first prospective study of the international society of pediatric oncology. J Clin Oncol 18:3819-3828, 2000 [DOI] [PubMed] [Google Scholar]
- 10.von Schweinitz D, Byrd DJ, Hecker H, et al: Efficiency and toxicity of ifosfamide, cisplatin and doxorubicin in the treatment of childhood hepatoblastoma: Study Committee of the Cooperative Paediatric Liver Tumour Study HB89 of the German Society for Paediatric Oncology and Haematology. Eur J Cancer 33:1243-1249, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Ortega JA, Douglass EC, Feusner JH, et al: Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol 18:2665-2675, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Malogolowkin MH, Katzenstein H, Krailo MD, et al: Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma. J Clin Oncol 24:2879-2884, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 14.Kalbfleisch JD and Prentice RL: The Statistical Analysis of Failure Time Data. New York, NY, John Wiley & Sons, 1980
- 15.Sasaki F, Matsunaga T, Iwafuchi M, et al: Outcome of hepatoblastoma treated with the JPLT-1 (Japanese Study Group for Pediatric Liver Tumor) Protocol-1: A report from the Japanese Study Group for Pediatric Liver Tumor. J Pediatr Surg 37:851-856, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Cheung NV, Heller G: Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J Clin Oncol 9:1050-1058, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Cushing B, Giller R, Cullen JW, et al: Randomized comparison of combination chemotherapy with etoposide bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: A pediatric intergroup study—Pediatric Oncology Group 9049 and Children's Cancer Group 8882. J Clin Oncol 22:2691-2700, 2004 [DOI] [PubMed] [Google Scholar]
- 18.González-Crussi F, Upton MP, Maurer HS: Hepatoblastoma: Attempt at characterization of histologic subtypes. Am J Surg Pathol 6:599-612, 1982 [PubMed] [Google Scholar]
- 19.Quinn JJ, Altman AJ, Robinson HT, et al: Adriamycin and cisplatin for hepatoblastoma. Cancer 56:1926-1929, 1985 [DOI] [PubMed] [Google Scholar]


