Abstract
Purpose
To identify risk factors associated with outcome in children with metastatic rhabdomyosarcoma in a large cohort of patients
Patients and Methods
Pooled data were obtained from 788 patients treated in nine studies performed by European and American cooperative groups. Clinical factors, including age, histology, site of primary, and site(s) and number of sites of metastatic disease, were correlated with event-free survival (EFS) and overall survival (OS).
Results
Seven hundred eighty-eight patients were eligible for analysis. The 3-year OS and EFS were 34% (SE, 1.7) and 27% (SE, 1.6), respectively. By univariate analysis, 3-year EFS was significantly and adversely influenced by age, alveolar histology, location of primary tumor in unfavorable site (defined as extremity and “other” sites), presence of three or more sites of metastatic disease, and the presence of bone or bone marrow involvement. By multivariate analysis, EFS was strongly correlated to all factors except histology. Relative risks were 1.6 for age younger than 1 year or at least 10 years, 1.4 for unfavorable site of primary tumor, 1.4 for bone or bone marrow involvement, 1.4 for three or more metastatic sites. EFS was 50% for patients without any of these four adverse factors and was respectively 42%, 18%, 12%, and 5% in patients with one, two, three, or four factors (P < .0001).
Conclusion
This analysis identified subsets of patients with metastatic rhabdomyosaroma with different outcomes to current therapy and offers a strategy to define patient candidates for experimental approaches to treatment.
INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in childhood. During the last 30 years, the introduction of multimodal therapy has resulted in a significant improvement in survival, with a cure rate of approximately 70% for patients with localized disease.1-3 Unfortunately, at least 15% of children with RMS present with metastatic (Intergroup Rhabdomyosarcoma Study [IRS] Group IV) disease, and their prognosis has not improved significantly in the last 15 years. Despite the development of more intensive therapies, the overall cure rate remains below 30%.2,4-11 However, data from several studies have suggested that clinical outcomes for children with metastatic RMS are not uniformly poor,5,7,10-12 but because of the rarity of these patients, most of these studies were not sufficiently large enough to undertake robust evaluation of prognostic factors. During the last 20 years, collaboration among the international research groups treating children with RMS has increased and several pooled analyses were conducted, increasing the knowledge about RMS at specific sites.13,14 The same groups therefore pooled their data again to analyze prognostic factors in a large cohort of children with metastatic RMS.
PATIENTS AND METHODS
Patient Population
Analyses were performed on data derived from nine studies from three international cooperative groups: Intergroup Rhabdomyosarcoma Study Group (IRS-III, IRS-IV-Pilot, IRS-IV, IRS-V, IRS-D9501), Italian Group (RMS4.99), International Society of Pediatric Oncology (SIOP) MMT Group (SIOP-MMT84, SIOP-MMT98), and European Intergroup (MMT89-91). The overall study population consisted of 788 children with metastatic RMS treated between 1984 and 2000. Patients with isolated regional lymph node involvement were not considered to have metastatic disease for the purpose of this analysis.
All patients had received histologic confirmation of tumor. Given that arrangements for central pathology review had existed within each collaborative group, diagnoses were not specifically re-reviewed for this analysis. All patients received conventional multiagent chemotherapy built on a backbone of alkylating agents (cyclophosphamide or ifosfamide), vincristine, and dactinomycin. Some patients received other drugs, depending on the research group and specific protocol. In all studies, local therapy (surgical resection and/or radiotherapy) was delivered between 3 and 5 months after the beginning of chemotherapy.
IRS Group Studies
In the IRS-III study (117 patients), children were randomly assigned to receive one of the following three combinations: vincristine, dactinomycin and cyclophosphamide (VAC); VAC with addition of doxorubicin and cisplatin; or VAC with addition of doxorubicin, cisplatin, and etoposide.1
In the IRS-IV pilot study, 112 patients received an up-front window of ifosfamide plus doxorubicin, followed by VAC.11 In the main IRS-IV study, 107 patients were randomly assigned to one of two up-front phase II window chemotherapy combinations: melphalan and vincristine, or ifosfamide and etoposide. This window phase was followed by VAC chemotherapy.4 Up-front phase II window studies were also used for IRS-V (38 children received topotecan alone) and D9501 (37 children received topotecan plus cyclophosphamide). All patients in the up-front window studies were evaluated for response after 6 weeks. Those who had no response or progressive disease proceeded to VAC alone, whereas those who had complete or partial response received a VAC regimen that incorporated the relevant window agent(s).15-17
SIOP-MMT Group Studies
Patients in the SIOP-84 study (n = 30) received chemotherapy consisting of ifosfamide, vincristine, and dactinomycin followed by second-line chemotherapy with doxorubicin and cisplatin if patients experienced partial response or progressive disease.18
Patients in the SIOP-MMT98 study (n = 127) were stratified into two groups. Standard-risk patients (defined as age < 10 years, with no bone or bone marrow involvement) received a 6 drug regimen of carboplatin, epirubicin, vincristine, ifosfamide, and etoposide (CEVAIE) for 27 weeks, but high-risk patients (defined as those age > 10 years or bone or bone marrow involvement) were entered onto a phase II window study with either carboplatin or doxorubincin before proceeding to a high-dose sequential monotherapy schedule incorporating cyclophosphamide, etoposide, and carboplatin, with autologous stem-cell rescue. All patients received local therapy as appropriate and then continued with a low-dose VAC maintenance chemotherapy.19
Italian Group RMS4-99 Study
Patients (n = 46) received three cycles (nine courses) of CEVAIE with local therapy followed by four high-dose drug combinations (cyclophosphamide/etoposide, thiotepa/melphalan, cyclophosphamide/thiotepa, melphalan) with stem-cell support, followed by six VAC maintenance cycles.20
European Intergroup MMT89-91 Studies
The European Intergroup conducted two consecutive studies. From 1989 to 1991, 57 patients from SIOP and Italian groups were treated with four cycles (12 courses) of CEVAIE. Local therapy was delivered as appropriate after maximum response, usually after two cycles of treatment. From 1991 to 1995, 117 patients were included in the second study. The subset of patients achieving complete remission after three cycles (nine courses) of CEVAIE received consolidation with a single course of high-dose melphalan with autologous stem-cell support.6,12
Statistical Analysis
Statistical analyses were performed at the Institut Gustave Roussy (Villejuif, France) using a general database management system. Survival curves were calculated by Kaplan-Meier method. Survival was calculated from the date of the start of treatment to the time of the last follow-up or death. Event-free survival (EFS) was calculated from the date of the start of treatment to the date of the first event, defined as tumor progression, relapse, or death as a result of any cause.
Pretreatment patient characteristics published as prognostic factors5,11,12,21 were evaluated with univariate analysis using the Kaplan-Meier method22 to calculate survival probabilities for EFS and overall survival (OS) at 3 years. Survival differences were compared using the log-rank test.23 Associations among variables were assessed with the χ2 test. To determine the independent prognostic significance of pretreatment factors on EFS, multivariate analysis was conducted using the Cox proportional hazards regression method.24 The variables correlated with EFS in univariate analysis were included in the model. A prognostic score was devised using the factors identified as prognostically significant for EFS by multivariate analysis. All calculations were performed with SAS software version 8.2 (2001; SAS Institute, Cary, NC). The date of analysis was September 2006, providing a minimum of 4 years from the date of study entry for the last patient included in the analysis.
RESULTS
Patient Characteristics
The characteristics of the 788 patients included in the analysis are listed in Table 1. Median age at diagnosis was 8 years (range, 4 months to 20 years). Twenty patients were age younger than 1 year, 422 were 1 to 9 years, and 346 were 10 years or older.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients | 3-Year EFS (%) | SE | Relative Risk of Event* | Log-Rank Test (P) |
|---|---|---|---|---|---|
| Sex | |||||
| Males | 426 | 31 | 2.3 | 1 | .04 |
| Females | 362 | 22 | 2.2 | 1.2 | |
| Age, years | |||||
| ≤ 1 | 20 | 25 | 10 | 1.8 | < .0001 |
| 1-9 | 422 | 37 | 2.3 | 1 | |
| ≥ 10 | 346 | 15 | 2.0 | 1.8 | |
| Primary site | |||||
| Orbit | 4 | 0 | 0 | .02 | |
| Non-PM | 32 | 48 | 9.1 | 1 | |
| PM | 125 | 37 | 4.4 | 0.95 | |
| GU (bladder/prostate) | 55 | 36 | 6.5 | 0.96 | |
| GU (non-bladder/prostate) | 49 | 40 | 7.1 | 0.86 | |
| Limbs | 240 | 20 | 2.6 | 1.25 | |
| Other | 271 | 23 | 2.6 | 1.29 | |
| No primary | 12 | 8 | 8 | ||
| Primary site | |||||
| Orbit/Non-PM/PM/GU | 265 | 39 | 3 | 1 | .0002 |
| Limbs/other/no primary | 523 | 21 | 1.5 | 1.4 | |
| Regional nodal status | |||||
| No | 282 | 32 | 2.8 | 1 | .02 |
| Yes | 391 | 24 | 2.2 | 1.26 | |
| Unknown | 115 | 25 | 4.1 | 1.33 | |
| Pathology | |||||
| Nonalveolar | 390 | 37 | 2.5 | 1 | .0005 |
| Alveolar | 398 | 18 | 2.0 | 1.3 | |
| Bone marrow | |||||
| No | 483 | 34 | 2.2 | 1 | < .0001 |
| Yes | 292 | 14 | 2.1 | 1.7 | |
| Unknown | 13 | ||||
| Bones | |||||
| No | 512 | 32 | 2.1 | 1 | < .0001 |
| Yes | 262 | 15 | 2.3 | 1.6 | |
| Unknown | 14 | ||||
| Bones or bone marrow | |||||
| No | 370 | 37 | 2.5 | 1 | < .0001 |
| Yes | 399 | 17 | 1.9 | 1.6 | |
| Unknown | 19 | ||||
| Lung metastases | |||||
| Lung only | 145 | 42 | 4.2 | 1 | < .0005 |
| Outside the lung | 643 | 24 | 1.7 | 1.5 | |
| No. of sites of metastases | |||||
| ≤ 2 | 643 | 30 | 1.8 | 1 | < .0001 |
| ≥ 3 | 145 | 14 | 3.0 | 1.8 | |
| Period of treatment | |||||
| Before 1991† | 316 | 28 | 2.5 | 1.0 | NS |
| After 1991 | 472 | 26 | 2.0 | 1 |
Abbreviations: EFS, event-free survival; PM, parameningeal; GU, genitourinary.
The relative risk of an event is the ratio of observed events/expected events.
International Society of Pediatric Oncology trials MMT84, MMT89, Intergroup Rhabdomyosarcoma Study Group trials III, and IV P.
The most common site of metastasis was the lung (47%), and in 145 patients (18%), this was the only metastatic site. The second most common site of metastasis was bone marrow (38%). Other metastatic sites were bone (34%) and distant nodes (26%). Bone marrow involvement and bone metastases were strongly correlated.
Median follow-up of survivors was 78 months, with a range of 4 months to 17.9 years. Estimated 3-year OS and EFS for all patients were 34% (95% CI, 31% to 38%) and 27% (95% CI, 24% to 30%), respectively (Fig 1). There were no significant differences in EFS between European and American groups. The 3-year EFS was 24% (95% CI, 20% to 29%) for European groups and 29% (95% CI, 25% to 34%) for the American group.
Fig 1.
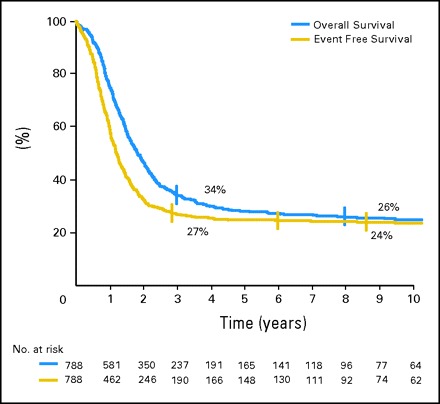
Overall survival and event-free survival of all 788 patients.
The following pretreatment clinical characteristics were considered potential prognostic factors for 3-year EFS (Table 1): sex, age (younger than age 1 year, 1 to 9 years, or 10 years or older), locoregional lymph node involvement (N0 v N1), histology (alveolar v non-alveolar), primary tumor site, presence or absence of bone or bone marrow metastases, and number of metastastic sites.
Patients younger than 1 year or 10 years old or older had a lower EFS than 1- to 9-year-old patients (25% and 15% v 36%; P < .0001). Site of primary tumor had an impact on EFS: patients with primary tumor localized in unfavorable sites (extremity and “other” sites) or with no identifiable primary site had a 21% EFS versus 39% for patients with orbital, head and neck, non-parameningeal, parameningeal, or genitourinary sites (P = .0002). EFS was significantly influenced by histology, with a lower EFS for alveolar RMS (18% v 37%; P = .0005). Regional node involvement negatively influenced EFS, which was 24% for N1 patients, compared with 32% for N0 patients (P = .02). EFS was significantly related to the number of metastatic sites present at the time of diagnosis. For patients with one or two metastatic sites at diagnosis, 3-year EFS was 30%, whereas it was 14% for patients with three or more metastatic sites (P < .0001).
EFS was negatively influenced by metastatic disease in bone marrow (14% v 34%; P < .0001), or bone (16% v 32%; P < .0001). Given that the presence of bone metastases was highly related to bone marrow involvement (P < .001), these two metastatic areas were combined in a single category for prognostic analysis. Patients with either bone or bone marrow involvement had lower EFS than patients without either bone or bone marrow metastases (17% v 37%; P < .0001). Patients with lung-only metastases fared better than patients with metastases in any other sites, possibly associated with lung metastases (42% v 24%; P < .0001). The other sites of metastases—liver, distant nodes, and others—had no impact on EFS. The period of treatment (before or after 1991) and the use of high-dose chemotherapy did not influence the outcome.
Cox regression analysis could be performed in 769 patients with complete data for all prognostic variables, confirming that several factors were independently and significantly correlated with improved EFS: favorable tumor sites (orbit, genitourinary, non–parameningeal, and parameningeal head and neck), absence of bone or bone marrow metastases, age between 1 and 9 years, and presence of two or fewer metastatic sites (Table 2). Histology, regional nodal status, and metastases to lungs only were not selected in the Cox model because these factors were strongly dependent on the all other prognostic factors (age, site of the primary, bone and bone marrow involvement, number of metastatic sites).
Table 2.
Cox Regression Model for Prognostic Factors on EFS: Multivariate Analysis Adjusted on Continent (United States and Europe; n = 769)
| Prognostic Factor | Relative Risk | 95% CI | Log-Rank Test (P) |
|---|---|---|---|
| Age, years | |||
| Favorable 1-9 | 1 | ||
| Unfavorable ≤ 1 or ≥ 10 | 1.6 | 1.4 to 1.9 | < .0001 |
| Site | |||
| Favorable | |||
| Orbit | 1 | ||
| Non-PM | |||
| PM | |||
| Bladder/prostate | |||
| Paratesticular/vagina | |||
| Unfavorable | 1.4 | 1.2 to 1.7 | .0003 |
| Limbs | |||
| Other | |||
| Bone or bone marrow involvement | |||
| No | 1 | ||
| Yes | 1.4 | 1.1 to 1.6 | .002 |
| No. of metastatic sites | |||
| ≤ 2 | 1 | ||
| ≥ 3 | 1.4 | 1.1 to 1.7 | .003 |
Abbreviations: EFS, event-free survival, PM, parameningeal.
Because the relative risk of event for each of the four factors identified by multivariate analysis was approximately 1.4 (range, 1.4 to 1.6), a prognostic score was devised with equal weighting, assigning a value of 1 for each factor present at diagnosis. The overall score was then applied to stratify patients into five groups on the basis of their prognostic scores. Eleven percent of patients had a prognostic score of 0, 31% had a score of 1, 30% had a score of 2, 19% had a score of 3, and 9% had a score of 4. EFS was significantly different across these groups: 50% at 3 years for patients without any of these four adverse factors and was respectively 42%, 18%, 12%, and 5% in patients with 1, 2, 3, or 4 factors (P < .0001; Fig 2).
Fig 2.
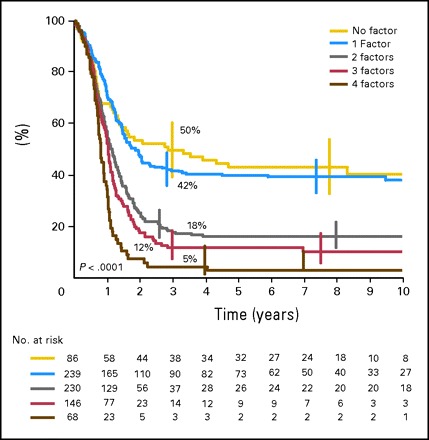
Event-free survival of patients according to number of unfavorable prognostic factors. The relative risks of event are respectively 1, 1.02, 1.9, 2.3, and 3.5.
The 12 patients with no identifiable primary tumor were classified as having a unfavorable primary site. Seven were 10 years or older. They all had bone marrow involvement and all but two had bone metastases. Eight had alveolar histology.
Two distinctly different prognostic subgroups were defined: patients with a prognostic score of < 1 represented 42% of the population and had 44% EFS (95% CI, 38% to 49%) whereas patients with a score > 2 represented 58% of the population and had 14% EFS (95% CI, 11% to 18%; P < .0001; Fig 3). Similar analyses (univariate and multivariate) were performed with overall survival as the end point and showed similar results. Because EFS was more stable than OS beyond 3 years, we elected to show the data for EFS.
Fig 3.
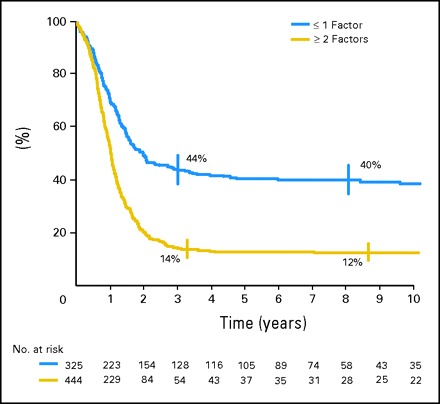
Event-free survival of patients according to risk score.
DISCUSSION
By contrast with the outcome for children with localized tumors, the prognosis of those with metastatic RMS has not substantially improved during the last 20 years. Five-year survival remains between 20% and 30% for the entire group,1,5,7-12 despite a high response rate to induction chemotherapy11,15,25,26 and the introduction of intensified treatment such as high-dose chemotherapy with stem-cell support.12,19,20,27,28 However, the factors that determine individual outcome are still a matter of discussion. This study aimed to define pretreatment characteristics which, when incorporated into a prognostic score, could stratify patients into risk groups on which to base treatment decisions. Patients with high risk of relapse and poor outcome with conventional treatment could be identified for front-line innovative therapies.
In the first three IRS studies (IRS-I, II, and III), children with metastatic RMS had a 5-year disease-free survival of 20%, 27%, and 32%, respectively.1,8,9 Other studies have analyzed prognostic factors in subsequent IRS trials. Sandler et al11 reported the results of patients with metastatic disease treated in the IRS-IV pilot study, which included a window phase of ifosfamide and doxorubicin, and found the following favorable prognostic factors in univariate analysis: embryonal histology, genitourinary primary site, and absence of nodal disease and or bone marrow involvement. Breneman et al5 analyzed pretreatment risk factors among metastatic patients enrolled onto the main IRS-IV study who were included in a window study incorporating either ifosfamide and etoposide or vincristine and melphalan, each followed by VAC. They identified several prognostic factors that negatively influenced failure-free survival (FFS) by univariate analysis: alveolar or undifferentiated histology, metastatic disease in distant lymph nodes, and multiple metastatic sites. Bone involvement seemed to negatively influence failure-free survival, though this value did not reach statistical significance. According to multivariate analysis, the only factor that correlated statistically significantly with improved FFS was the presence of two or fewer metastatic sites. Although the influence of histology did not achieve statistical significance in multivariate analysis, this study identified a subset of patients with embryonal histology and two or fewer metastatic sites who had a 40% failure-free survival.5 Unexpectedly, this pooled analysis showed that histology subtype, which is an important predictor of outcome among patients with nonmetastatic disease, is not an independent significant determinant of FFS. Main reason for this is the tight correlation with other prognostic factors (age, site of the primary, bone/bone marrow involvement).
The proportion of patients with alveolar histology increased with periods of the studies on both sides of the Atlantic Ocean (and in the same proportion), and overall the proportion of patients with alveolar histology was similar in Europe and in the United States (49% v 52%). However, this change in the definition of histology with time does not explain the absence of impact of histology, given that analyses were performed after adjusting for the treatment period.
In both the European Intergroup Studies (MMT4-89 and MMT4-91), univariate analysis identified the following unfavorable prognostic factors for EFS: primary tumor in parameningeal, extremity, or other sites; age younger than 1 year and older than 9 years; bone or bone marrow metastases; and multiple metastases and multiple sites of metastases. Multivariate analysis identified unfavorable site, bone or bone marrow involvement, and unfavorable age as independently adverse factors.12 The analysis of 2343 patients treated from 1983 to 1997 in the IRS studies found that infants (< 1 year) have the highest risk of treatment failure, followed by adolescents, and that age is associated independently with outcome, even after accounting for group, stage, and tumor histology.21 These findings led to the proposition that there were two subgroups with clearly different outcomes: patients with fewer than two unfavorable factors, who had 5-year EFS of 40%, and patients with two or more unfavorable factors, who had 5-year EFS of 7.5%.12 The current study, which pooled the data from the two European Intergroup studies with other studies, confirmed these prognostic factors, except the parameningeal primaries, which have a prognosis similar to other head and neck tumors. The larger number of patients in the present analysis also identified another prognostically independent factor—the number of metastatic sites.
In view of the poor survival of the patients with metastatic RMS and the need for new chemotherapy strategies, the IRSG (and since then, the Soft Tissue Sarcoma Committee of the Children's Oncology Group) have successively evaluated eight different new agents or new agent combinations in phase II window studies in metastatic patients. These data have recently been summarized by Lager et al.15 The data show that observed response rates to two cycles of chemotherapy are very encouraging, with response rates ranging from 41% to 55%, but that the incorporation of agents evaluated in the window study into subsequent standard therapy did not have any impact on OS. It is clear, therefore, that early sensitivity to chemotherapy in patients with metastatic RMS does not necessarily translate into cure.
The role of high-dose chemotherapy with stem-cell support on ultimate outcome is still questionable, given that only three studies incorporated high-dose chemotherapy in their design, and used different conditioning regimens for subset of patients in a nonrandomized way.12,19,20 Other studies failed to show a benefit of this kind of approach.27,28 A number of potential novel approaches are being investigated in preclinical studies.
The observation that RMS cell lines express vascular endothelial growth factor (VEGF) and that blocking VEGF receptor 1 antibody inhibits VEGF signaling and delays RMS proliferation29,30 has made antiangiogenic treatment an attractive option. Observation of dysregulation of the mammalian target of rapamycin pathway31 and expression of both epidermal growth factor receptor and erbB-2 in RMS32 suggest that molecularly targeted agents may have a future role in combination with chemotherapy. This kind of new approach is urgently needed for patients who are identified as being at high risk of treatment failure using the prognostic scoring system derived from this analysis. This score will be adopted for the stratification of patients with metastatic disease in the coming European study planned by the European Pediatric Soft Tissue Sarcoma Group. High-risk patients (with two or more risk factors) will be included in investigational treatment strategies.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTSOF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Odile Oberlin, Annie Rey, Gianni Bisogno, Michael C.G. Stevens, William H. Meyer, Modesto Carli, James R. Anderson
Provision of study materials or patients: Annie Rey, Elizabeth Lyden, Gianni Bisogno, James R. Anderson
Collection and assembly of data: Annie Rey
Data analysis and interpretation: Odile Oberlin, Annie Rey, Elizabeth Lyden, Michael C.G. Stevens, Modesto Carli, James R. Anderson
Manuscript writing: Odile Oberlin, Annie Rey, Michael C.G. Stevens
Final approval of manuscript: Odile Oberlin, Annie Rey, Elizabeth Lyden, Gianni Bisogno, Michael C.G. Stevens, William H. Meyer, Modesto Carli, James R. Anderson
Footnotes
Presented in poster form at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
REFERENCES
- 1.Crist W, Gehan EA, Ragab AH, et al: The third Intergroup Rhabdomyosarcoma Study. J Clin Oncol 13:610-630, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Koscielniak E, Harms D, Henze G, et al: Results of treatment for soft tissue sarcoma in childhood and adolescence: A final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol 17:3706-3719, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Stevens MC, Rey A, Bouvet N, et al: Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: Third study of the International Society of Paediatric Oncology–SIOP malignant mesenchymal tumor 89. J Clin Oncol 23:2618-2628, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Breitfeld PP, Lyden E, Raney RB, et al: Ifosfamide and etoposide are superior to vincristine and melphalan for pediatric metastatic rhabdomyosarcoma when administered with irradiation and combination chemotherapy: A report from the Intergroup Rhabdomyosarcoma Study Group. J Pediatr Hematol Oncol 23:225-233, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Breneman JC, Lyden E, Pappo AS, et al: Prognostic factors and clinical outcomes in children and adolescents with metastatic Rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol 21:78-84, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Carli M, Colombatti R, Oberlin O, et al: High-dose melphalan with autologous stem-cell rescue in metastatic rhabdomyosarcoma. J Clin Oncol 17:2796-2803, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Koscielniak E, Rodary C, Flamant F, et al: Metastatic rhabdomyosarcoma and histologically similar tumors in childhood: A retrospective European multi-center analysis. Med Pediatr Oncol 20:209-214, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Maurer HM, Beltangady M, Gehan EA, et al: The Intergroup Rhabdomyosarcoma Study I: A final report. Cancer 61:209-220, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Maurer HM, Gehan EA, Beltangady M, et al: The Intergroup Rhabdomyosarcoma Study-II. Cancer 71:1904-1922, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Raney RB Jr, Tefft M, Maurer HM, et al: Disease patterns and survival rate in children with metastatic soft-tissue sarcoma. A report from the Intergroup Rhabdomyosarcoma Study (IRS)-I. Cancer 62:1257-1266, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Sandler E, Lyden E, Ruymann F, et al: Efficacy of ifosfamide and doxorubicin given as a phase II “window” in children with newly diagnosed metastatic rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. Med Pediatr Oncol 37:442-448, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Carli M, Colombatti R, Oberlin O, et al: European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: Final results and analysis of prognostic factors. J Clin Oncol 22:4787-4794, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Oberlin O, Rey A, Anderson J, et al: Treatment of orbital rhabdomyosarcoma: Survival and late effects of treatment-results of an international workshop. J Clin Oncol 19:197-204, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Raney B, Anderson J, Jenney M, et al: Late effects in 164 patients with rhabdomyosarcoma of the bladder/prostate region: A report from the international workshop. J Urol 176:2190-2194, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Lager JJ, Lyden ER, Anderson JR, et al: Pooled analysis of phase II window studies in children with contemporary high-risk metastatic rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol 24:3415-3422, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Pappo AS, Lyden E, Breneman J, et al: Up-front window trial of topotecan in previously untreated children and adolescents with metastatic rhabdomyosarcoma: An intergroup rhabdomyosarcoma study. J Clin Oncol 19:213-219, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Walterhouse DO, Lyden ER, Breitfeld PP, et al: Efficacy of topotecan and cyclophosphamide given in a phase II window trial in children with newly diagnosed metastatic rhabdomyosarcoma: A Children's Oncology Group study. J Clin Oncol 22:1398-1403, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Flamant F, Rodary C, Rey A, et al: Treatment of non-metastatic rhabdomyosarcomas in childhood and adolescence: Results of the second study of the International Society of Paediatric Oncology—MMT84. Eur J Cancer 34:1050-1062, 1998 [DOI] [PubMed] [Google Scholar]
- 19.McDowell H, Foot A, Bergeron C, et al: Metastatic rhabdomyosarcoma report: SIOP trial MMT98. Pediatr Blood Cancer 45:411, 2005. (abstr) [Google Scholar]
- 20.Bisogno G, Rossi L, Ferrari A, et al: Sequential high dose chemotherapy in children with metatstatic soft tissue sarcoma. Med Pediatr Oncol 41:278, 2003. (abstr) [Google Scholar]
- 21.Joshi D, Anderson JR, Paidas C, et al: Age is an independent prognostic factor in rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Pediatr Blood Cancer 42:64-73, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EM, Meyer P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 23.Mantel N: Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163-170, 1966 [PubMed] [Google Scholar]
- 24.Cox D: Regression models and life-tables. J R Stat Soc B 34:187-220, 1972 [Google Scholar]
- 25.Frascella E, Pritchard-Jones K, Modak S, et al: Response of previously untreated metastatic rhabdomyosarcoma to combination chemotherapy with carboplatin, epirubicin and vincristine. Eur J Cancer 32A:821-825, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Pappo AS, Lyden E, Breitfeld P, et al: Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: The Children's Oncology Group. J Clin Oncol 25:362-369, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Walterhouse DO, Hoover ML, Marymont MA, et al: High-dose chemotherapy followed by peripheral blood stem cell rescue for metastatic rhabdomyosarcoma: The experience at Chicago Children's Memorial Hospital. Med Pediatr Oncol 32:88-92, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Weigel BJ, Breitfeld PP, Hawkins D, et al: Role of high-dose chemotherapy with hematopoietic stem cell rescue in the treatment of metastatic or recurrent rhabdomyosarcoma. J Pediatr Hematol Oncol 23:272-276, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Gee MF, Tsuchida R, Eichler-Jonsson C, et al: Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene 24:8025-8037, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Onisto M, Slongo ML, Gregnanin L, et al: Expression and activity of vascular endothelial growth factor and metalloproteinases in alveolar and embryonal rhabdomyosarcoma cell lines. Int J Oncol 27:791-798, 2005 [PubMed] [Google Scholar]
- 31.Petricoin EF, III, Espina V, Araujo RP, et al: Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer Res 67:3431-3440, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ganti R, Skapek SX, Zhang J, et al: Expression and genomic status of EGFR and ErbB-2 in alveolar and embryonal rhabdomyosarcoma. Mod Pathol 19:1213-1220, 2006 [DOI] [PubMed] [Google Scholar]


