Abstract
Background
More than half of all congenital deformities can be detected in utero. The initial surgical correction is of paramount importance for the achievement of good long-term results with low surgical morbidity and mortality.
Methods
Selective literature review and expert opinion.
Results
Congenital deformities are rare, and no controlled trials have been performed to determine their optimal treatment. In this article, we present the prenatal assessment, treatment, and long-term results of selected types of congenital deformity. Congenital diaphragmatic hernia (CDH) affects one in 3500 live-born infants, while esophageal atresia affects one in 3000 and small-bowel atresia one in 5000 to 10 000. If a congenital deformity is detected and its prognosis can be reliably inferred from a prenatal assessment, the child should be delivered at a specialized center (level 1 perinatal center). The associated survival rates are 60–80% after treatment for CDH and well over 90% after treatment for esophageal or small-bowel atresia. Despite improvements in surgical correction over the years, complications and comorbidities still affect 20–40% of the treated children. These are not limited to surgical complications in the narrow sense, such as recurrence, postoperative adhesions and obstruction, stenoses, strictures, and recurrent fistulae, but also include pulmonary problems (chronic lung disease, obstructive and restrictive pulmonary dysfunction), gastrointestinal problems (dysphagia, gastro-esophageal reflux, impaired intestinal motility), and failure to thrive. Moreover, the affected children can develop emotional and behavioral disturbances. Minimally invasive surgery in experienced hands yields results as good as those of conventional surgery, as long as proper selection criteria are observed.
Conclusion
Congenital deformities should be treated in recognized centers with highly experienced interdisciplinary teams. As no randomized trials of surgery for congenital deformities are available, longitudinal studies and registries will be very important in the future.
Correcting congenital deformities is a challenge for pediatric surgeons. Up until 1940, successful surgical repair of congenital deformities was rare; the techniques of pediatric anesthesia and neonatal and pediatric intensive care were inadequate. The enormous progress made since then in pediatric intensive medicine and anesthesia, and improved surgical techniques, make it possible today to repair almost any malformation, and patient survival (with the exception of those with congenital diaphragmatic hernia) is almost taken for granted (1, 2). The requirement in terms of the quality of the repair, both on the part of those affected and of their doctors, has moved on from simple survival to improved quality of life (2– 4, e1, e2). An essential prerequisite for this is multidisciplinary teams and standardized follow-up continuing into adulthood (3, 5). Malformations are rare; evidence-based treatment recommendations above the level of expert opinion do not exist (1, e3). With low case numbers, randomized studies are problematic and difficult to carry out for statistical reasons as well as for ethical reasons, because surgical correction is the only way to ensure survival (4– 7). Reliable longitudinal studies only describe small cohorts (1, 3, 4, 6, 7, e3). Results based on registry data show continuous improvement in the quality of care accompanied by a fall in treatment complications and comorbidity in high-volume centers, thus reinforcing the demand for centralization (1, 3, 4, 6, 7, e3).
The present review, which is based on an up-to-date selective literature review (to December 2014), describes the prenatal diagnosis, treatment, and long-term outcome of selected congenital deformities.
Methods
The present article deals with the main principles of current methods of treatment and relates only to congenital diaphragmatic hernia (CDH) and esophageal and small-bowel atresia. It is the result of a selective literature search (focused on the years 2010–2014) and includes the authors’ own experiences. The analysis is based on expert opinions and takes into account recent results in prenatal diagnosis, treatment, and long-term outcomes. Any relevant meta-analyses found were included in the analysis.
Pathogenesis of congenital deformities
Behind every malformation lies a genetic disorder, which is usually never completely clarified (6, e4– e7). Since most deformities are sporadic, the risk of recurrence in consecutive pregnancies is low (e2). The lack of evidence for Mendelian genetic transmission, coupled with indications that environmental or epigenetic factors may be in play, suggest a multifactorial process (e8). In 20% of cases at most, malformations are associated with syndromes (8, e8– e10). No environmental influences have been described for the malformations discussed in this article.
Prenatal diagnosis
The ultrasound scans around gestational weeks (GW) 10 and 22 have a screening function for congenital deformities and allow the place and mode of delivery to be decided. A qualified prenatal sonographer (at least DEGUM [Deutsche Gesellschaft für Ultraschall in der Medizin] level II) can identify direct or indirect indicators of deformities early on, and these must be very closely followed over the course of the pregnancy (6, 9, e8, e11, e12). Important signs relating to the three deformities are a low lung-to-head ratio (LHR), a small or absent gastric sac associated with polyhydramnios, and a distended gastric and duodenal sac or small-bowel dilatation transitioning into a hypoplastic large bowel. An MRI scan of mother and fetus will provide a greater level of detail (6, 9– 12, e13). Interdisciplinary advice and counseling of the parents about the extent of the malformation, treatment options, and prognosis are standard medical practice (13). Alternative treatment methods and intrauterine interventions, if any, are discussed within the team and with the parents, and initiated if necessary (4, 6, e14). Delivery should if possible take place in a center with demonstrable experience in the treatment of the deformity, in order to allow the best possible repair (4).
Congenital diaphragmatic hernia
In 80% of cases, CDH is a left-sided defect in the diaphragm with prolapse of abdominal organs into the thoracic space and, if the defect is large, pronounced ipsilateral pulmonary hypoplasia (e4). Pulmonary hypoplasia, a hypoplastic left heart ventricle (in patients with large defects), and persistent pulmonary hypertension of the newborn (PPHN), with retention of the fetal circulation and a right–left shunt via the patent ductus arteriosus, lead to marked respiratory distress (13). Despite treatment in intensive care, survival is only 40% to 60% worldwide, or up to 80% in specialist centers (4, 7). Early intrauterine diagnosis is important (Table 1). Prognostic factors associated with a poor outcome are:
Table 1. Prognosis of congenital diaphragmatic hernia _from GW 32 (from [6]).
| Prognostic values for the pregnancy _from GW 32 onwards | |
|---|---|
| LHR (ultrasound) reference value | LHR 1.8–3.0 |
| Probability that ECMO will be required | LHR <1.2 |
| Probability of death (100%) | LHR <0.9 |
| Bilateral lung volume on MRI | Volume 70 mL |
| Probability that ECMO will be required | Volume <25 ml |
| Probability of death (100%) | Volume <9ml |
GW = gestational week
LHR = lung-to-head ratio
ECMO = extracorporeal membrane oxygenation
MRI = magnetic resonance imaging
Early detection (<GW 25)
Intrathoracic parts of the liver
Small lung volume
Poor ventricular function
Prenatal diagnosis can anticipate the need for specialist treatment and thus for delivery in a specialist center (9, 13, e15). Attempts at fetal surgical repair remain unsuccessful. On the other hand, attempts have been made to stimulate lung growth by trapping the pulmonary fluid secretions inside the lungs through temporary blockage of the fetal trachea using a minimally invasively introduced balloon (fetal endoscopic tracheal occlusion, FETO). The timing of the intervention, duration of tracheal occlusion, and the value of the procedure are still under debate (1, 6, 14, e15– e17).
Embryology, development, and incidence
The diaphragm develops between GW 4 and GW 8 by the formation of pleuroperitoneal folds in the coelom. This process takes longer dorsally and on the left side, and for this reason the defects are dorsolateral in more than 90% of cases and left-sided in more than 80%. Maternal vitamin A deficiency significantly increases the incidence of CDH, although the exact retinol/cholesterol pathway is unclear (15, e4– e9).
The prevalence of CDH is around 1:3500 births (200–250 babies per year in Germany). A hidden mortality rate (stillbirths) of 34,9% has been assumed (e18). Seventy percent are sporadic, 18% occur as part of multiple deformities, and 10% occur as part of a syndrome (13).
Clinical symptoms, diagnosis, and initial treatment
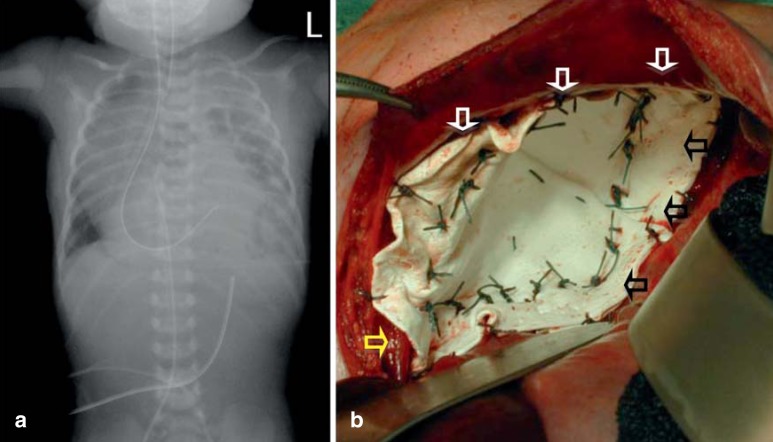
The main symptom is acute respiratory distress. Because air passes into the gastrointestinal system (meaning that mask ventilation is absolutely contraindicated), the hypoplastic lung does not expand and the respiratory distress rapidly intensifies (13). Other symptoms include a small abdomen, cyanosis, and signs of pulmonary hypertension. Chest radiograph shows the enterothorax with mediastinal displacement and pulmonary hypoplasia (Figure 1). Intestinal loops must not be mistaken for cysts and punctured (15).
Figure 1.
Radiological and intraoperative appearance of left-sided congenital diaphragmatic hernia.
a) Postnatal chest radiograph of a neonate with left-sided congenital diaphragmatic hernia. Note the left-sided enterothorax due to the defect in the diaphragm, with a barely visible left lung (hypoplasia), and the intrathoracic location of the gastric tube.
b) Intraoperative appearance after closure of a large, overlapping conical GoreTex patch. The conical shape has decreased the thoracic dead space and increased the abdominal space, reducing the risk of abdominal compartment syndrome. At the anterior margin the ventral diaphragmatic rim can be seen (white arrows, and medially the left crus of the diaphragm is visible attached to the esophagus (yellow arrow). Dorsally, the patch is attached around the ribs (black arrows)
Neonatal intensive care follows the guideline published by the EURO-CDH Consortium (a team of experts from large European centers with at least 10 cases a year) (13). If all neonatal ventilation options have been attempted and oxygenation is still inadequate with persistent hypercapnia and PPHN, extracorporeal membrane oxygenation (ECMO = artificial lung) must be considered (13). Surgical repair is performed only after hemodynamic stabilization has been achieved (4, 13).
Surgical repair
The technique used for surgery (open vs. thoracoscopic) depends on the size of the defect and on whether ECMO or some other form of ventilation is being used (1, 16). In babies with large defects where the diaphragmatic rim has remained incomplete, closure with a patch is obligatory and the risk of recurrence fundamentally higher (4, 15, 17, e19). The cardiopulmonary stability of a baby that has been on ECMO or is on high-frequency oscillatory ventilation (HFOV) is too poor for thoracoscopic repair to be carried out (1, 4, 17). The advantage of the abdominal approach is that the entire diaphragm remnant is in view, enabling secure anchoring of a patch. GoreTex Dualmesh has become internationally accepted as the best material for a patch (4, 7, 15, 16). Using absorbable material increases the recurrence rate significantly and is not recommended (1, 4, 6, 15, 17). The use of a conical patch reduces the dead space in the chest, increases the abdominal space, and reduces the recurrence rate (16). The advantage of thoracoscopy is the near-absence of scarring, but the disadvantage is the increased recurrence rate, up to 15% (1, 4, 6, 15, 17, e20).
Results and long-term outcome
Defects are classified by the Boston scale into four groups (A to D) and the associated mortality rates rise with the severity of the defect or concomitant malformations (from 2% in group A up to 61% in group D with concomitant malformations) (7, e21). Limiting variables for survival are defect size and PPHN; however, treatment complications, concomitant disease, and chronic lung disease continue to cause late morbidity into adulthood (Table 2) (2, 3, 12, 18– 20, e22– e44). Published data regarding recurrences are heterogeneous. Often, only the neonatal period or the first 6 months of life are considered, although 20% of recurrences occur after the second year of life (4, 7, 16, e21).
Table 2. Quality of life and morbidity of children and adults after repair of congenital diaphragmatic hernia. esophageal atresia. duodenal atresia. _or an abdominal wall defect (after [2]).
| Malformation | Mortality | Residual morbidity | Data in % | References |
|---|---|---|---|---|
| Congenital diaphragmatic hernia | 20–40% | Persistent pulmonary hypertension | 5–20% | (11. 12. 17. 20.e43) |
| Pulmonary hypoplasia | Up to 80% | (4. 15. e1) | ||
| Lung function disorders_(obstructive. restrictive. and mixed) | 40–85% | (1. 2. 4. 11. 12. 15. 18. 19. 27_ e4. e25. e27. e29. e46) | ||
| Recurrence due to patch separation | 5.4–50% | (4. 16.17. e2. e21. e23. e36–e38. e41. e77) | ||
| Chylothorax | 4.6% | (e40) | ||
| Failure to thrive | >60% | (19. 27.e35. e37. e42) | ||
| Chest wall deformities | 30% | (27. e37–39) | ||
| Emotional and behavioral disturbances | Up to 80% | (18. 19. e23– e26. e29. e33. e41. e46) | ||
| Esophageal atresia | 5–9% | Esophageal strictures | Up to 49% | (33. 34. e48. e53–e55. e60–e62. e67. e78) |
| Esophagotracheal fistula recurrence | Up to 4% | (e56. e57) | ||
| Impaired esophageal motility | 100% | (e64. e64. e79) | ||
| Gastroesophageal reflux. Barrett esophagus with risk of malignancy |
Up to 50% | (29. e48. e53–e55. e63–e70.e78) | ||
| Lung function disorders (obstructive. restrictive. and mixed) |
Up to 43% | (28. e34. e53. e54. e80. e81) | ||
| Recurrent upper airway infections | Up to 80% | (e44. e54. e80. e82. e83) | ||
| Tracheomalacia | Up to 80% | (e34. e54. e80. e82. e83) | ||
| Emotional and behavioral disturbances | Up to 80% | (e28–e31. e63–e65) | ||
| Small-bowel atresia | <5% | Bowel strictures | <5% | (36) |
| Motility disorders | <5% | (37. 39. 40) | ||
| Otherwise like the normal population | 100% | (38. e76) | ||
| Abdominal wall defect | <5% | Concomitant malformations | Up to 30% | (e32. e84. e85) |
| Otherwise like the normal population | 100% | (e32. e84. e85) |
Follow-up studies in children who underwent ECMO show cerebral morphological changes in two-thirds of the children (MRI) and a neurological deficit in 20% (e45). Longitudinal studies show airway disease, psychomotor retardation, chest deformities, gastroesophageal reflux, and failure to thrive persisting into adulthood (18, 19, e22, e46).
Esophageal atresia
Esophageal atresia (EA) involves an interruption of the continuity of the esophagus, with a blind pouch in the upper mediastinum and associated esophagotracheal fistula (90% Vogt IIIb). In the German-speaking countries, the Vogt classification dating from 1929 is widely used.
Within the past 50 years, mortality has been reduced from 60% to between 5% and 9% (21, e46). The first successful repair was carried out by Haight in 1941 and the first successful thoracoscopic repair by Lobe in 1999 (e47– e49). Prenatal diagnosis reveals up to 50% of cases of esophageal atresia (polyhydramnios with small gastric sac). From GW 28 onwards, imaging will show the upper blind pouch with the help of MRI. In 44.7% of cases this is an isolated malformation, in 9.6% of cases it occurs as part of VACTERL, and in 31.6% it is accompanied by other deformities. Chromosomal anomalies are found in 8.3% of those affected (8). The VACTERL association involves the co-occurrence of at least three of the following deformities:
Vertebral anomalies, anal atresia, cardiac defects, tracheoesophageal fistula and/or esophageal atresia, renal anomalies, and limb defects (8, 22).
Statistically significant factors for risk stratification of mortality in a series of 4168 cases of esophageal atresia were:
Birth weight <1500 g (OR = 4.5)
Surgery on first day of life (OR = 3.8)
Gestational age <GW 28 (OR = 2.2), and
Presence of ventricular septal defect (VSD) (OR = 3.8) (21).
Embryology, development, and incidence
Esophageal atresia results from failure of the esophagus to separate from the trachea during GW 3; exactly how this occurs is not clear (e8, e10).
The incidence is 1:3000 (corresponding to 220 cases of esophageal atresia per year in Germany). In 50% to 70% of those affected, concomitant malformations are present.
Clinical symptoms, diagnosis, and initial treatment
Postnatally, neonates show frothing at the mouth and nose because they are unable to swallow the saliva. Aspiration and pneumonia result. Chest radiograph shows a gastric tube looped inside the blind pouch in the upper esophagus and an air-filled gastrointestinal tract in the presence of a (distal) esophagotracheal fistula. If fistula is absent, the abdomen is free of air (Vogt type II). Contrast imaging is not necessary. Clinical and radiological diagnostic investigations (abdominal ultrasound, echocardiography) will mostly identify any important concomitant malformations (cardiac, abdominal, renal, and extremities). The upper blind pouch is continuously suctioned. Repair is undertaken on the 2nd to 4th day of life after vital signs have been stabilized, taking account of any chromosomal and cardiac anomalies.
Surgical repair
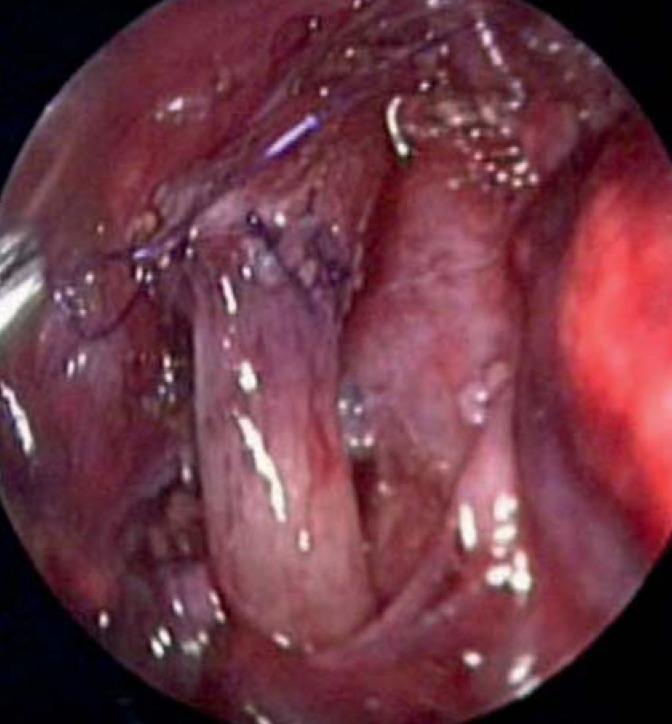
Preserving the original esophagus is the most important priority and is successfully achieved in 90% of all cases by primary anastomosis with closure of the esophagotracheal fistula. The preferred approach is through a right-sided thoracotomy (23). In the past 10 years, thoracoscopic repair has become standard in specialized centers (Figure 2). An international registry study showed that 10% of all cases are corrected by minimally invasive surgery and show treatment advantages compared to conventional surgery (Table 3) (24– 27, e50– e52). However, no randomized prospective study has been carried out on this.
Figure 2.
Thoracoscopically completed anastomosis in a child with esophageal atresia. Note the completed esophageal anastomosis; the purple sutures can be seen. Above and to the right, the esophagotracheal fistula oversewn at the trachea is visible
Table 3. Conventional versus minimally invasive repair of esophageal atresia.
| Study (year) | Leak after thoracotomy |
Stricture after thoracotomy |
Leak after thoracoscopy |
Stricture after thoracoscopy |
|---|---|---|---|---|
| Lugo (2008) | 14.3% | 14.3% | 19.2% | 50% |
| Al Tokhais (2008) | 17.4% | 8.7% | 13.6% | 18% |
| Allal (2009) | 0 | 21.4% | 0 | 23.5% |
| Szavay (2011) | 4% | 0 | 3.1% | 0 |
| Total | 8.7% | 8.7% | 9.3% | 21.6% |
Comparative meta-analyses of conventional (n = 97) versus thoracoscopic (n = 69) repair of esophageal atresia.
Operative times were similar (147 ± 20 minutes vs. 136 ± 31 minutes. p=ns). from (24)
Selection criteria for minimally invasive repair (with the aim of reducing morbidity and avoiding conversion to open repair) are birth weight <2000 g, long-gap esophageal atresia, and concomitant severe cardiac malformations. In addition to cosmetic advantages, thoracoscopy offers reduction of the morbidities associated with thoracotomy (rib fusion, scoliosis, winged scapula) (e51). The possible comorbidities of CO2 insufflation, leading to acidosis and cerebral damage, need to be evaluated (28). Several centers are investigating this question prospectively.
Placement of a transanastomotic tube, oral feeding starting between postoperative day 2 and 5, and contrast study on day 7 are recommended. Important complications are listed in the Box. Reoperation is required in 12% of patients.
Box. Significant complications of surgical repair of esophageal atresia.
All these are usually treated conservatively.
Long-gap esophageal atresia
The definition of long-gap esophageal atresia varies, and the distance between the upper and the lower blind pouch is given in centimeters (>2.5 cm) or number of thoracic vertebrae (>2). This is the form of esophageal atresia for which the debates over treatment are greatest, ranging from delayed anastomosis to esophageal replacement. Delayed anastomosis can be achieved through spontaneous growth of the ends of the esophagus or by means of an “elongation procedure.” The most controversial of these at present is the Foker method (transthoracic traction of both blind pouches). This sometimes requires several thoracotomies; the main problems are mediastinitis, leakage, and anastomotic stricture (30, 31).
Various techniques are available as alternatives to elongation for esophageal replacement (gastric pull-up, colon or small-bowel interposition [32, e58]). Each of these methods has associated complications and comorbidities. At present, gastric pull-up is the method of choice. Due to numerous severe complications, the “gastric tube” surgical method is no longer used. Colon interposition is problematic because it leads to functional problems and refractory halitosis. Ileum interposition can have a good functional result but is extremely demanding technically (32, e59).
Results and long-term outcome
Anastomotic strictures are associated with anastomoses sutured under tension or with leaks, and are the most frequent problem (9% to 45% of cases) (29, 33, 34, e48, e53– e55, e60, e61). The wide range of incidence rates is due to the fact that esophageal atresia takes many forms and treatment approaches differ. Intermittent dilation (1 to 15 dilations, with a 0.1% to 0.4% risk of esophageal perforation) is the treatment of choice and is successful in 58% to 96% of cases; 50% of all dilations show lasting success in the first 6 months. In 30% of cases multiple dilations are necessary because of persistent stenosis. If success has still not been achieved after 10 dilations, surgical reintervention is recommended (e60). To treat therapy-refractory strictures, local application of mitomycin-C and esophageal stent placement are still under debate (33, 34, e61, e62).
Long-term studies have documented dysphagia and gastroesophageal reflux (29, e63– e68). Forty percent of all children need secondary fundoplication (e69). Twenty percent of all patients show metaplasia (Barrett esophagus) (e70). Currently, only eight cases of esophageal carcinoma after repair of esophageal atresia have been described (e70). Pulmonary symptoms often persist (Table 2) (29, 35, e63– e67). Satisfactory long-term results occur even after a complex clinical course or esophageal replacement (2, 3). Gastric pull-up shows the best results (32, e59, e71, e72).
Small-bowel atresia (duodenum/small bowel)
Congenital defects of the continuity of the small bowel manifest as stenoses or atresia and can usually be diagnosed before birth (e73). The identification of any concomitant malformations has prognostic significance, especially in duodenal atresia (trisomy 21). Cystic fibrosis can occur in association with secondary small-bowel atresia in babies with meconium ileus (e74).
Embryology, development, and incidence
In 95% of cases small-bowel atresia is complete, occurring with a prevalence of 1:5000 to 1:10 000 live births; a third are in preterm neonates. Duodenal atresia occurs in 1:2500 to 1:5000 of neonates (36, 37).
The etiology of small-bowel atresia is unclear. There are two theories about how it arises:
Failure of the embryonic bowel to recanalize correctly
Selective obliteration of bowel segments due to vascular insufficiency (37).
For duodenal atresia, another possible cause in addition to failure to recanalize may be lack of rotation of the right pancreatic bud (annular pancreas) (37).
Clinical symptoms, diagnosis, and initial treatment
The typical prenatal presentation of small-bowel atresia shows the dilated stomach and variably dilated bowel loops. Depending on the level at which the obstruction has occurred, the mother experiences a pathological increase in the quantity of amniotic fluid (polyhydramnios) during the pregnancy. Duodenal atresia manifests as a classical widening of the stomach and duodenal bulb (“double bubble”) (e73). Newborns with small-bowel atresia present with bile-stained vomit (always highly pathological) (37). In babies with duodenal atresia the upper abdomen is domed forward and the lower abdomen is flat. In those with small-bowel atresia, the abdomen protrudes; the lower the atresia site, the greater the protrusion. Peritonism indicates a complication such as volvulus or peritonitis (after perforation).
Abdominal radiograph shows the atresia as a typical “double bubble” appearance (duodenal atresia) or multiple air–fluid levels in the bowel (small-bowel atresia). Surgery is performed electively on the 2nd to 4th day of life (36, 37). Relevant concomitant malformations are ruled out or repaired as needed, not least in order to prevent volvulus (malrotation).
Surgical repair
Repair of duodenal atresia requires a right upper abdominal laparotomy (or, optionally, laparoscopy) (e75) and consists of duodenoduodenostomy in the form of a diamond-shaped bypass anastomosis. If a windsock web is present, this is resected with preservation of the major duodenal papilla.
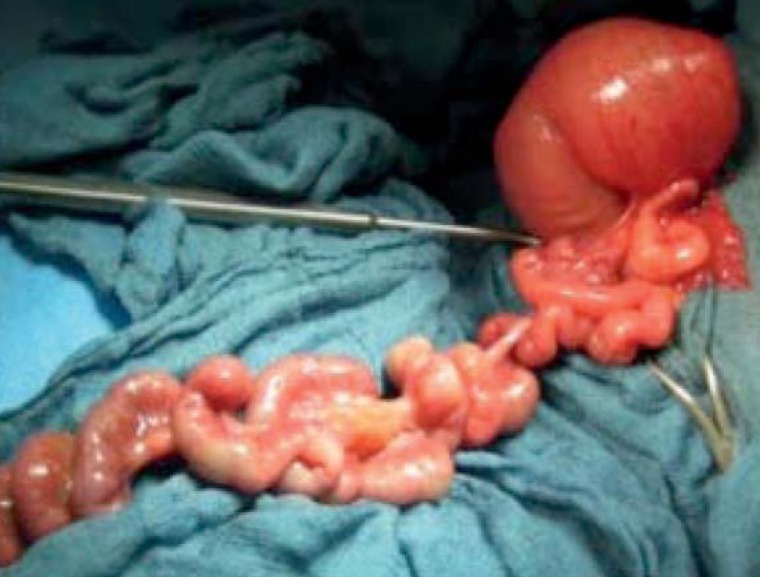
The incision for repair of small-bowel atresia is periumbilical or median. Repair of small-bowel atresia consists in resection of the atretic segment with anastomosis. “Apple-peel” small bowel (Figure 3) is a particular challenge, as in this syndrome significant parts of the small bowel are atretic and proper bowel function starts only after a long delay, leading to functional and actual short-bowel syndrome. In children with impaired bowel perfusion, volvulus, meconium ileus, or peritonitis, a double-barreled enterostomy is placed.
Figure 3.
Appearance of type IIIb (apple-peel) small-bowel atresia. Children with apple-peel malformation (IIIb) and multiple atresias (IV) have the poorest prognosis. Note the central vessel around which the small bowel winds like pared apple peel, making bowel perfusion very fragile
Results and long-term outcome
On the whole, the prognosis of small-bowel atresia is good (mortality <10%) (38). Postoperative complications include anastomotic leakage, stenosis, and infections. Prolonged impaired gastrointestinal motility is associated with secondary changes in innervation and the absence of interstitial cells of Cajal (39, 40). The long-term prognosis is determined by concomitant malformations or disease and is significantly poorer in patients with cystic fibrosis. The length of small bowel remaining has relevance for the severity or otherwise of short-bowel syndrome, if present (e76).
Conclusion
High rates of detection during prenatal diagnostic procedures mean that many deformities are identified early. The initial surgical treatment has a significant influence on long-term outcome, and for this reason treatment should if possible always be carried out in centers with demonstrable expertise and multidisciplinary teams, so as to reduce mortality and morbidity to a minimum. There is a role for minimally invasive surgery in carrying out repairs, so long as the selection criteria listed above are observed. Prospective studies—ideally multicenter or registry studies—are needed to provide a research basis for care provision in the future.
Key Messages.
Congenital malformations are rare.
Prenatal detection is extremely important and allows the interdisciplinary team time to prepare the parents, explaining the extent of the malformation and its possible treatment and prognosis.
The quality of the initial surgical treatment strongly affects the long-term outcome.
Despite the good repair procedures available, in most cases morbidity persists into adulthood.
Prospective registry studies and health services research are needed if the quality of care is to be further improved.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professors Rolle, Wessel, and Fuchs declare that no conflict of interest exists.
References
- 1.Hall NJ, Eaton S, Pierro A. The evidence base for neonatal surgery. Early Hum Dev. 2009;85:713–718. doi: 10.1016/j.earlhumdev.2009.08.058. [DOI] [PubMed] [Google Scholar]
- 2.Glinianaia SV, Embleton ND, Rankin J. A systematic review of studies of quality of life in children and adults with selected congenital anomalies. Birth Defects Res A Clin Mol Teratol. 2012;94:511–520. doi: 10.1002/bdra.23030. [DOI] [PubMed] [Google Scholar]
- 3.Ijsselstijn H, van Beelen NW, Wijnen RM. Esophageal atresia: long-term morbidities in adolescence and adulthood. Dis Esophagus. 2013;26:417–21. doi: 10.1111/dote.12059. [DOI] [PubMed] [Google Scholar]
- 4.Losty PD. Congenital diaphragmatic hernia: where and what is the evidence? Semin Pediatr Surg. 2014;23:278–282. doi: 10.1053/j.sempedsurg.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 5.van den Hout L, Tibboel D, Vijfhuize S, et al. The VICI-trial: high frequency oscillation versus conventional mechanical ventilation in newborns with congenital diaphragmatic hernia: an international multicentre randomized controlled trial. BMC Pediatr. 2011;11 doi: 10.1186/1471-2431-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deprest J, De Coppi P. Antenatal management of isolated congenital diaphragmatic hernia today and tomorrow: ongoing collaborative research and development. J Pediatr Surg. 2012;47:282–290. doi: 10.1016/j.jpedsurg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Lally KP, Lally PA, Van Meurs KP, et al. Treatment evolution in high-risk congenital diaphragmatic hernia: ten years’ experience with diaphragmatic agenesis. Ann Surg. 2006;244:505–513. doi: 10.1097/01.sla.0000239027.61651.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen RN, Calzolari E, Husby S, Garne E. Oesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Arch Dis Child. 2012;97:227–232. doi: 10.1136/archdischild-2011-300597. [DOI] [PubMed] [Google Scholar]
- 9.Kilian AK, Schaible T, Hofmann V, Brade J, Neff KW, Busing KA. Congenital diaphragmatic hernia: predictive value of MRI relative lung-to-head ratio compared with MRI fetal lung volume and sonographic lung-to-head ratio. AJR Am J Roentgenol. 2009;192:153–158. doi: 10.2214/AJR.08.1082. [DOI] [PubMed] [Google Scholar]
- 10.Ethun CG, Fallon SC, Cassady CI, et al. Fetal MRI improves diagnostic accuracy in patients referred to a fetal center for suspected esophageal atresia. J Pediatr Surg. 2014;49:712–715. doi: 10.1016/j.jpedsurg.2014.02.053. [DOI] [PubMed] [Google Scholar]
- 11.Schaible T, Busing KA, Felix JF, et al. Prediction of chronic lung disease, survival and need for ECMO therapy in infants with congenital diaphragmatic hernia: additional value of fetal MRI measurements? Eur J Radiol. 2012;81:1076–1082. doi: 10.1016/j.ejrad.2011.02.060. [DOI] [PubMed] [Google Scholar]
- 12.van den Hout L, Reiss I, Felix JF, et al. Risk factors for chronic lung disease and mortality in newborns with congenital diaphragmatic hernia. Neonatology. 2010;98:370–380. doi: 10.1159/000316974. [DOI] [PubMed] [Google Scholar]
- 13.Reiss I, Schaible T, van den Hout L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology. 2010;98:354–364. doi: 10.1159/000320622. [DOI] [PubMed] [Google Scholar]
- 14.Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924. doi: 10.1056/NEJMoa035005. [DOI] [PubMed] [Google Scholar]
- 15.Keijzer R, Puri P. Congenital diaphragmatic hernia. Semin Pediatr Surg. 2010;19:180–185. doi: 10.1053/j.sempedsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Loff S, Wirth H, Jester I, et al. Implantation of a cone-shaped double-fixed patch increases abdominal space and prevents recurrence of large defects in congenital diaphragmatic hernia. J Pediatr Surg. 2005;40:1701–1705. doi: 10.1016/j.jpedsurg.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Tsao K, Lally PA, Lally KP. Minimally invasive repair of congenital diaphragmatic hernia. J Pediatr Surg. 2011;46:1158–1164. doi: 10.1016/j.jpedsurg.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Cammen-van Zijp MH, Gischler SJ, Mazer P, van Dijk M, Tibboel D, Ijsselstijn H. Motor-function and exercise capacity in children with major anatomical congenital anomalies: an evaluation at 5 years of age. Early Hum Dev. 2010;86:523–528. doi: 10.1016/j.earlhumdev.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Gischler SJ, van der Cammen-van Zijp MH, Mazer P, et al. A prospective comparative evaluation of persistent respiratory morbidity in esophageal atresia and congenital diaphragmatic hernia survivors. J Pediatr Surg. 2009;44:1683–1690. doi: 10.1016/j.jpedsurg.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Safavi A, Synnes AR, O’Brien K, Chiang M, Skarsgard ED, Chiu PP. Multi-institutional follow-up of patients with congenital diaphragmatic hernia reveals severe disability and variations in practice. J Pediatr Surg. 2012;47:836–841. doi: 10.1016/j.jpedsurg.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Tashiro J, Allan BJ, et al. A nationwide analysis of clinical outcomes among newborns with esophageal atresia and tracheoesophageal fistulas in the United States. J Surg Res. 2014;190:604–612. doi: 10.1016/j.jss.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Garabedian C, Vaast P, Bigot J, et al. [Esophageal atresia: prevalence, prenatal diagnosis and prognosis] J Gynecol Obstet Biol Reprod (Paris) 2014;43:424–430. doi: 10.1016/j.jgyn.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Laberge JM, Blair GK. Thoracotomy for repair of esophageal atresia: not as bad as they want you to think! Dis Esophagus. 2013;26:365–371. doi: 10.1111/dote.12053. [DOI] [PubMed] [Google Scholar]
- 24.Borruto FA, Impellizzeri P, Montalto AS, et al. Thoracoscopy versus thoracotomy for esophageal atresia and tracheoesophageal fistula repair: review of the literature and meta-analysis. Eur J Pediatr Surg. 2012;22:415–419. doi: 10.1055/s-0032-1329711. [DOI] [PubMed] [Google Scholar]
- 25.Rothenberg SS. Thoracoscopic repair of esophageal atresia and tracheoesophageal fistula in neonates, first decade’s experience. Dis Esophagus. 2013;26:359–364. doi: 10.1111/dote.12054. [DOI] [PubMed] [Google Scholar]
- 26.Szavay PO, Zundel S, Blumenstock G, et al. Perioperative outcome of patients with esophageal atresia and tracheo-esophageal fistula undergoing open versus thoracoscopic surgery. J Laparoendosc Adv Surg Tech A. 2011;21:439–443. doi: 10.1089/lap.2010.0349. [DOI] [PubMed] [Google Scholar]
- 27.Zani A, Eaton S, Hoellwarth ME, et al. International survey on the management of esophageal atresia. Eur J Pediatr Surg. 2014;24:3–8. doi: 10.1055/s-0033-1350058. [DOI] [PubMed] [Google Scholar]
- 28.Bishay M, Giacomello L, Retrosi G, et al. Hypercapnia and acidosis during open and thoracoscopic repair of congenital diaphragmatic hernia and esophageal atresia: results of a pilot randomized controlled trial. Ann Surg. 2013;258:895–900. doi: 10.1097/SLA.0b013e31828fab55. [DOI] [PubMed] [Google Scholar]
- 29.Rintala RJ, Pakarinen MP. Long-term outcome of esophageal anastomosis. Eur J Pediatr Surg. 2013;23:219–225. doi: 10.1055/s-0033-1347912. [DOI] [PubMed] [Google Scholar]
- 30.Nasr A, Langer JC. Mechanical traction techniques for long-gap esophageal atresia: a critical appraisal. Eur J Pediatr Surg. 2013;23:191–197. doi: 10.1055/s-0033-1347916. [DOI] [PubMed] [Google Scholar]
- 31.Sroka M, Wachowiak R, Losin M, et al. The Foker technique (FT) and Kimura advancement (KA) for the treatment of children with long-gap esophageal atresia (LGEA): lessons learned at two European centers. Eur J Pediatr Surg. 2013;23:3–7. doi: 10.1055/s-0033-1333891. [DOI] [PubMed] [Google Scholar]
- 32.Spitz L. Esophageal replacement: overcoming the need. J Pediatr Surg. 2014;49:849–852. doi: 10.1016/j.jpedsurg.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Levesque D, Baird R, Laberge JM. Refractory strictures post-esophageal atresia repair: what are the alternatives? Dis Esophagus. 2013;26:382–387. doi: 10.1111/dote.12047. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J RL, Huo J, Zhu Z, Liu D. The use of retrievable fully covered self-expanding metal stent in refractory postoperative restenosis of benign esophageal stricture in children. J Pediatr Surg. 2013;48:2235–2240. doi: 10.1016/j.jpedsurg.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Beucher J, Wagnon J, Daniel V, et al. Long-term evaluation of respiratory status after esophageal atresia repair. Pediatr Pulmonol. 2013;48:188–194. doi: 10.1002/ppul.22582. [DOI] [PubMed] [Google Scholar]
- 36.Best KE, Tennant PW, Addor MC, et al. Epidemiology of small intestinal atresia in Europe: a register-based study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F353–358. doi: 10.1136/fetalneonatal-2011-300631. [DOI] [PubMed] [Google Scholar]
- 37.Adams SD, Stanton MP. Malrotation and intestinal atresias. Early Hum Dev. 2014;90:921–925. doi: 10.1016/j.earlhumdev.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Stollman TH, de Blaauw I, Wijnen MH, et al. Decreased mortality but increased morbidity in neonates with jejunoileal atresia; a study of 114 cases over a 34-year period. J Pediatr Surg. 2009;44:217–221. doi: 10.1016/j.jpedsurg.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 39.Gfroerer S, Metzger R, Fiegel H, Ramachandran P, Rolle U. Differential changes in intrinsic innervation and interstitial cells of Cajal in small bowel atresia in newborns. World J Gastroenterol. 2010;16:5716–5721. doi: 10.3748/wjg.v16.i45.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gfroerer S, Fiegel H, Ramachandran P, Rolle U, Metzger R. Changes of smooth muscle contractile filaments in small bowel atresia. World J Gastroenterol. 2012;18:3099–3104. doi: 10.3748/wjg.v18.i24.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Frenckner BP, Lally PA, Hintz SR, Lally KP. Prenatal diagnosis of congenital diaphragmatic hernia: how should the babies be delivered? J Pediatr Surg. 2007;42:1533–1538. doi: 10.1016/j.jpedsurg.2007.04.016. [DOI] [PubMed] [Google Scholar]
- e2.Jancelewicz T, Vu LT, Keller RL, et al. Outcomes of multigestational pregnancies affected by congenital diaphragmatic hernia. J Pediatr Surg. 2010;45:1753–1758. doi: 10.1016/j.jpedsurg.2010.04.006. [DOI] [PubMed] [Google Scholar]
- e3.Legrand C, Michaud L, Salleron J, et al. Long-term outcome of children with oesophageal atresia type III. Arch Dis Child. 2012;97:808–811. doi: 10.1136/archdischild-2012-301730. [DOI] [PubMed] [Google Scholar]
- e4.Rottier R, Tibboel D. Fetal lung and diaphragm development in congenital diaphragmatic hernia. Semin Perinatol. 2005;29:86–93. doi: 10.1053/j.semperi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- e5.Beurskens LW, Schrijver LH, Tibboel D, et al. Dietary vitamin A intake below the recommended daily intake during pregnancy and the risk of congenital diaphragmatic hernia in the offspring. Birth Defects Res A Clin Mol Teratol. 2013;97:60–66. doi: 10.1002/bdra.23093. [DOI] [PubMed] [Google Scholar]
- e6.Cooper MK, Wassif CA, Krakowiak PA, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- e7.Lam WW, Kirk J, Manning N, Reardon W, Kelley RI, Fitzpatrick D. Decreased cholesterol synthesis as a possible aetiological factor in malformations of trisomy 18. Eur J Med Genet. 2006;49:195–199. doi: 10.1016/j.ejmg.2005.05.011. [DOI] [PubMed] [Google Scholar]
- e8.Fragoso AC, Tovar JA. The multifactorial origin of respiratory morbidity in patients surviving neonatal repair of esophageal atresia. Front Pediatr. 2014;2 doi: 10.3389/fped.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Veenma DC, de Klein A, Tibboel D. Developmental and genetic aspects of congenital diaphragmatic hernia. Pediatr Pulmonol. 2012;47:534–545. doi: 10.1002/ppul.22553. [DOI] [PubMed] [Google Scholar]
- e10.Brosens E, Eussen H, van Bever Y, et al. VACTERL association etiology: the impact of de novo and rare copy number variations. Mol Syndromol. 2013;4:20–26. doi: 10.1159/000345577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Heling KS, Wauer RR, Hammer H, Bollmann R, Chaoui R. Reliability of the lung-to-head ratio in predicting outcome and neonatal ventilation parameters in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2005;25:112–118. doi: 10.1002/uog.1837. [DOI] [PubMed] [Google Scholar]
- e12.Odibo AO, Najaf T, Vachharajani A, Warner B, Mathur A, Warner BW. Predictors of the need for extracorporeal membrane oxygenation and survival in congenital diaphragmatic hernia: a center’s 10-year experience. Prenat Diagn. 2010;30:518–521. doi: 10.1002/pd.2508. [DOI] [PubMed] [Google Scholar]
- e13.Heling KS, Bollmann R, Wauer RR, Hammer H, Chaoui R. Der Stellenwert der Lung-to-head-Ratio in der Prognoseeinschätzung bei Feten mit isolierter Zwerchfellhernie. Z Geburtsh Neonatal. 2003;207:89–166. [Google Scholar]
- e14.van den Hout L, Schaible T, Cohen-Overbeek TE, et al. Actual outcome in infants with congenital diaphragmatic hernia: the role of a standardized postnatal treatment protocol. Fetal Diagn Ther. 2011;29:55–63. doi: 10.1159/000322694. [DOI] [PubMed] [Google Scholar]
- e15.Busing KA, Kilian AK, Schaible T, Debus A, Weiss C, Neff KW. Reliability and validity of MR image lung volume measurement in fetuses with congenital diaphragmatic hernia and in vitro lung models. Radiology. 2008;246:553–561. doi: 10.1148/radiol.2462062166. [DOI] [PubMed] [Google Scholar]
- e16.Diemert A, Diehl W, Glosemeyer P, Deprest J, Hecher K. Intrauterine surgery—choices and limitations. Dtsch Arztebl Int. 2012;109:603–638. doi: 10.3238/arztebl.2012.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e17.Deprest JA, Nicolaides K, Gratacos E. Fetal surgery for congenital diaphragmatic hernia is back from never gone. Fetal Diagn Ther. 2011;29:6–17. doi: 10.1159/000322844. [DOI] [PubMed] [Google Scholar]
- e18.Skari H, Bjornland K, Haugen G, Egeland T, Emblem R. Congenital diaphragmatic hernia: a meta-analysis of mortality factors. J Pediatr Surg. 2000;35:1187–1197. doi: 10.1053/jpsu.2000.8725. [DOI] [PubMed] [Google Scholar]
- e19.Stolar CJH DP. Congenital diaphragmatic hernia and eventration. In: Grosfeld JL, O’Neil JA, Fonkalsrud EW, et al., editors. Pediatric Surgery 2006. Philadelphia: Mosby Elsevier; 2006. pp. 931–954. [Google Scholar]
- e20.Jancelewicz T, Langer JC, Chiang M, Bonnard A, Zamakhshary M, Chiu PP. Thoracoscopic repair of neonatal congenital diaphragmatic hernia (CDH): outcomes after a systematic quality improvement process. J Pediatr Surg. 2013;48:321–325. doi: 10.1016/j.jpedsurg.2012.11.012. discussion 5. [DOI] [PubMed] [Google Scholar]
- e21.Tsai J, Sulkowski J, Adzick NS, Hedrick HL, Flake AW. Patch repair for congenital diaphragmatic hernia: is it really a problem? J Pediatr Surg. 2012;47:637–641. doi: 10.1016/j.jpedsurg.2011.11.054. [DOI] [PubMed] [Google Scholar]
- e22.van den Hout L, Sluiter I, Gischler S, et al. Can we improve outcome of congenital diaphragmatic hernia? Pediatr Surg Int. 2009;25:733–743. doi: 10.1007/s00383-009-2425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e23.Peetsold MG, Heij HA, Nagelkerke AF, et al. Pulmonary function and exercise capacity in survivors of congenital diaphragmatic hernia. Eur Respir J. 2009;34:1140–1147. doi: 10.1183/09031936.00181408. [DOI] [PubMed] [Google Scholar]
- e24.Peetsold MG, Vonk-Noordegraaf A, Heij HH, Gemke RJ. Pulmonary function and exercise testing in adult survivors of congenital diaphragmatic hernia. Pediatr Pulmonol. 2007;42:325–331. doi: 10.1002/ppul.20579. [DOI] [PubMed] [Google Scholar]
- e25.Bouman NH, Koot HM, Tibboel D, Hazebroek FW. Children with congenital diaphragmatic hernia are at risk for lower levels of cognitive functioning and increased emotional and behavioral problems. Eur J Pediatr Surg. 2000;10:3–7. doi: 10.1055/s-2008-1072314. [DOI] [PubMed] [Google Scholar]
- e26.Chen C, Jeruss S, Chapman JS, et al. Long-term functional impact of congenital diaphragmatic hernia repair on children. J Pediatr Surg. 2007;42:657–665. doi: 10.1016/j.jpedsurg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- e27.Poley MJ, Stolk EA, Tibboel D, Molenaar JC, Busschbach JJ. Short term and long term health related quality of life after congenital anorectal malformations and congenital diaphragmatic hernia. Arch Dis Child. 2004;89:836–841. doi: 10.1136/adc.2002.016543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e28.Peetsold MG, Heij HA, Deurloo JA, Gemke RJ. Health-related quality of life and its determinants in children and adolescents born with oesophageal atresia. Acta Paediatr. 2010;99:411–417. doi: 10.1111/j.1651-2227.2009.01579.x. [DOI] [PubMed] [Google Scholar]
- e29.Bouman NH, Koot HM, Hazebroek FW. Long-term physical, psychological, and social functioning of children with sophageal atresia. J Pediatr Surg. 1999;34:399–404. doi: 10.1016/s0022-3468(99)90485-2. [DOI] [PubMed] [Google Scholar]
- e30.Faugli A, Bjornland K, Emblem R, Novik TS, Diseth TH. Mental health and psychosocial functioning in adolescents with esophageal atresia. J Pediatr Surg. 2009;44:729–737. doi: 10.1016/j.jpedsurg.2008.09.027. [DOI] [PubMed] [Google Scholar]
- e31.Ludman L, Spitz L. Quality of life after gastric transposition for oesophageal atresia. J Pediatr Surg. 2003;38:53–57. doi: 10.1053/jpsu.2003.50009. discussion. [DOI] [PubMed] [Google Scholar]
- e32.Ginn-Pease ME, King DR, Tarnowski KJ, Green L, Young G, Linscheid TR. Psychosocial adjustment and physical growth in children with imperforate anus or abdominal wall defects. J Pediatr Surg. 1991;26:1129–1135. doi: 10.1016/0022-3468(91)90688-p. [DOI] [PubMed] [Google Scholar]
- e33.Koivusalo A, Pakarinen M, Vanamo K, Lindahl H, Rintala RJ. Health-related quality of life in adults after repair of congenital diaphragmatic defects—a questionnaire study. J Pediatr Surg. 2005;40:1376–1381. doi: 10.1016/j.jpedsurg.2005.05.037. [DOI] [PubMed] [Google Scholar]
- e34.Banjar H HZ, Mohamed G. Long-term pulmonary consequences of esophageal atresia and tracheoesophageal fistula. Curr Pediatr Res. 2005;9:51–55. [Google Scholar]
- e35.Chiu P, Hedrick HL. Postnatal management and long-term outcome for survivors with congenital diaphragmatic hernia. Prenat Diagn. 2008;28:592–603. doi: 10.1002/pd.2007. [DOI] [PubMed] [Google Scholar]
- e36.Clark RH, Hardin WD, Jr., Hirschl RB, et al. Current surgical management of congenital diaphragmatic hernia: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1998;33:1004–1009. doi: 10.1016/s0022-3468(98)90522-x. [DOI] [PubMed] [Google Scholar]
- e37.Jancelewicz T, Chiang M, Oliveira C, Chiu PP. Late surgical outcomes among congenital diaphragmatic hernia (CDH) patients: why long-term follow-up with surgeons is recommended. J Pediatr Surg. 2013;48:935–941. doi: 10.1016/j.jpedsurg.2013.02.005. [DOI] [PubMed] [Google Scholar]
- e38.Jancelewicz T, Vu LT, Keller RL, et al. Long-term surgical outcomes in congenital diaphragmatic hernia: observations from a single institution. J Pediatr Surg. 2010;45:155–160. doi: 10.1016/j.jpedsurg.2009.10.028. discussion 60. [DOI] [PubMed] [Google Scholar]
- e39.Kotecha S, Barbato A, Bush A, et al. Congenital diaphragmatic hernia. Eur Respir J. 2012;39:820–829. doi: 10.1183/09031936.00066511. [DOI] [PubMed] [Google Scholar]
- e40.Levy SM, Lally PA, Lally KP, Tsao K. The impact of chylothorax on neonates with repaired congenital diaphragmatic hernia. J Pediatr Surg. 2013;48:724–729. doi: 10.1016/j.jpedsurg.2012.11.035. [DOI] [PubMed] [Google Scholar]
- e41.Peetsold MG, Huisman J, Hofman VE, Heij HA, Raat H, Gemke RJ. Psychological outcome and quality of life in children born with congenital diaphragmatic hernia. Arch Dis Child. 2009;94:834–840. doi: 10.1136/adc.2008.156158. [DOI] [PubMed] [Google Scholar]
- e42.Pierog A, Aspelund G, Farkouh-Karoleski C, et al. Predictors of low weight and tube feedings in children with congenital diaphragmatic hernia at 1 year of age. J Pediatr Gastroenterol Nutr. 2014;59:527–530. doi: 10.1097/MPG.0000000000000454. [DOI] [PubMed] [Google Scholar]
- e43.Pierro M, Thebaud B. Understanding and treating pulmonary hypertension in congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2014;19:357–363. doi: 10.1016/j.siny.2014.09.008. [DOI] [PubMed] [Google Scholar]
- e44.Sistonen SJ, Pakarinen MP, Rintala RJ. Long-term results of esophageal atresia: Helsinki experience and review of literature. Pediatr Surg Int. 2011;27:1141–1149. doi: 10.1007/s00383-011-2980-7. [DOI] [PubMed] [Google Scholar]
- e45.Harrington KP, Goldman AP. The role of extracorporeal membrane oxygenation in congenital diaphragmatic hernia. Semin Pediatr Surg. 2005;14:72–76. doi: 10.1053/j.sempedsurg.2004.10.028. [DOI] [PubMed] [Google Scholar]
- e46.Sulkowski JP, Deans KJ, Asti L, Mattei P, Minneci PC. Using the Pediatric Health Information System to study rare congenital pediatric surgical diseases: development of a cohort of esophageal atresia patients. J Pediatr Surg. 2013;48:1850–1855. doi: 10.1016/j.jpedsurg.2013.02.062. [DOI] [PubMed] [Google Scholar]
- e47.Haight C. Congenital atresia of the esophagus with tracheoesophageal fistula. Surg Gynecol Obstet. 1947;84:504–506. [PubMed] [Google Scholar]
- e48.Allal H, Kalfa N, Lopez M, et al. Benefits of the thoracoscopic approach for short- or long-gap esophageal atresia. J Laparoendosc Adv Surg Tech A. 2005;15:673–677. doi: 10.1089/lap.2005.15.673. [DOI] [PubMed] [Google Scholar]
- e49.Haight CTH. Congenital atresia of the esophagus with tracheoesophageal fistula: extrapleural ligation of fistula and end to end anastomosis of esophageal ends. Surg Gynecol Obstet. 1943;76:672–688. [Google Scholar]
- e50.Al Tokhais T, Zamakhshary M, Aldekhayel S, et al. Thoracoscopic repair of tracheoesophageal fistulas: a case-control matched study. J Pediatr Surg. 2008;43:805–809. doi: 10.1016/j.jpedsurg.2007.12.015. [DOI] [PubMed] [Google Scholar]
- e51.Lawal TA, Gosemann JH, Kuebler JF, Gluer S, Ure BM. Thoracoscopy versus thoracotomy improves midterm musculoskeletal status and cosmesis in infants and children. Ann Thorac Surg. 2009;87:224–228. doi: 10.1016/j.athoracsur.2008.08.069. [DOI] [PubMed] [Google Scholar]
- e52.Rothenberg SS. Thoracoscopic repair of esophageal atresia and tracheo-esophageal fistula in neonates: evolution of a technique. J Laparoendosc Adv Surg Tech A. 2012;22:195–199. doi: 10.1089/lap.2011.0063. [DOI] [PubMed] [Google Scholar]
- e53.Lan LC, Wong KK, Lin SC, et al. Endoscopic balloon dilatation of esophageal strictures in infants and children: 17 years’ experience and a literature review. J Pediatr Surg. 2003;38:1712–1715. doi: 10.1016/j.jpedsurg.2003.08.040. [DOI] [PubMed] [Google Scholar]
- e54.Holcomb GW, 3rd, Rothenberg SS, Bax KM, et al. Thoracoscopic repair of esophageal atresia and tracheoesophageal fistula: a multi-institutional analysis. Ann Surg. 2005;242:422–428. doi: 10.1097/01.sla.0000179649.15576.db. discussion 8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e55.Patkowski D RK, Jaworski W, Zielinska M, Siejka G, Konsur K, Czernik J. Thoracoscopic repair of tracheoesophageal fistula and esophageal atresia. J Laparoendosc Adv Surg Tech A. 2009;19:19–22. doi: 10.1089/lap.2008.0139.supp. [DOI] [PubMed] [Google Scholar]
- e56.Lugo B, Malhotra A, Guner Y, Nguyen T, Ford H, Nguyen NX. Thoracoscopic versus open repair of tracheoesophageal fistula and esophageal atresia. J Laparoendosc Adv Surg Tech A. 2008;18:753–756. doi: 10.1089/lap.2007.0220. [DOI] [PubMed] [Google Scholar]
- e57.Huang J, Tao J, Chen K, et al. Thoracoscopic repair of oesophageal atresia: experience of 33 patients from two tertiary referral centres. J Pediatr Surg. 2012;47:2224–2227. doi: 10.1016/j.jpedsurg.2012.09.011. [DOI] [PubMed] [Google Scholar]
- e58.Totonelli G, Maghsoudlou P, Fishman JM, et al. Esophageal tissue engineering: a new approach for esophageal replacement. World J Gastroenterol. 2012;18:6900–6907. doi: 10.3748/wjg.v18.i47.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e59.Gallo G, Zwaveling S, Groen H, Van der Zee D, Hulscher J. Long-gap esophageal atresia: a meta-analysis of jejunal interposition, colon interposition, and gastric pull-up. Eur J Pediatr Surg. 2012;22:420–425. doi: 10.1055/s-0032-1331459. [DOI] [PubMed] [Google Scholar]
- e60.Thyoka M, Timmis A, Mhango T, Roebuck DJ. Balloon dilatation of anastomotic strictures secondary to surgical repair of oesophageal atresia: a systematic review. Pediatr Radiol. 2013;43:898–901. doi: 10.1007/s00247-013-2693-2. quiz 896-7. [DOI] [PubMed] [Google Scholar]
- e61.Chapuy L, Pomerleau M, Faure C. Topical mitomycin-C application in recurrent esophageal strictures after surgical repair of esophageal atresia. J Pediatr Gastroenterol Nutr. 2014;59:608–611. doi: 10.1097/MPG.0000000000000352. [DOI] [PubMed] [Google Scholar]
- e62.Manfredi MA, Jennings RW, Anjum MW, Hamilton TE, Smithers CJ, Lightdale JR. Externally removable stents in the treatment of benign recalcitrant strictures and esophageal perforations in pediatric patients with esophageal atresia. Gastrointest Endosc. 2014;80:246–252. doi: 10.1016/j.gie.2014.01.033. [DOI] [PubMed] [Google Scholar]
- e63.Burgos L, Barrena S, Andres AM, et al. Colonic interposition for esophageal replacement in children remains a good choice: 33-year median follow-up of 65 patients. J Pediatr Surg. 2010;45:341–345. doi: 10.1016/j.jpedsurg.2009.10.065. [DOI] [PubMed] [Google Scholar]
- e64.Deurloo JA, Ekkelkamp S, Hartman EE, Sprangers MA, Aronson DC. Quality of life in adult survivors of correction of esophageal atresia. Arch Surg. 2005;140:976–980. doi: 10.1001/archsurg.140.10.976. [DOI] [PubMed] [Google Scholar]
- e65.Koivusalo A, Pakarinen MP, Turunen P, Saarikoski H, Lindahl H, Rintala RJ. Health-related quality of life in adult patients with esophageal atresia–a questionnaire study. J Pediatr Surg. 2005;40:307–312. doi: 10.1016/j.jpedsurg.2004.10.014. [DOI] [PubMed] [Google Scholar]
- e66.Ure BM, Slany E, Eypasch EP, Weiler K, Troidl H, Holschneider AM. Quality of life more than 20 years after repair of esophageal atresia. J Pediatr Surg. 1998;33:511–515. doi: 10.1016/s0022-3468(98)90100-2. [DOI] [PubMed] [Google Scholar]
- e67.Lemoine C, Aspirot A, Le Henaff G, Piloquet H, Levesque D, Faure C. Characterization of esophageal motility following esophageal atresia repair using high-resolution esophageal manometry. J Pediatr Gastroenterol Nutr. 2013;56:609–614. doi: 10.1097/MPG.0b013e3182868773. [DOI] [PubMed] [Google Scholar]
- e68.Koivusalo A, Pakarinen MP, Rintala RJ. The cumulative incidence of significant gastrooesophageal reflux in patients with oesophageal atresia with a distal fistula–a systematic clinical, pH-metric, and endoscopic follow-up study. J Pediatr Surg. 2007;42:370–374. doi: 10.1016/j.jpedsurg.2006.10.010. [DOI] [PubMed] [Google Scholar]
- e69.Tovar JA, Fragoso AC. Anti-reflux surgery for patients with esophageal atresia. Dis Esophagus. 2013;26:401–404. doi: 10.1111/dote.12063. [DOI] [PubMed] [Google Scholar]
- e70.Schneider A, Michaud L, Gottrand F. Esophageal atresia: metaplasia, Barrett. Dis Esophagus. 2013;26:425–427. doi: 10.1111/dote.12057. [DOI] [PubMed] [Google Scholar]
- e71.Dingemann C, Meyer A, Kircher G, et al. Long-term health-related quality of life after complex and/or complicated esophageal atresia in adults and children registered in a German patient support group. J Pediatr Surg. 2014;49:631–638. doi: 10.1016/j.jpedsurg.2013.11.068. [DOI] [PubMed] [Google Scholar]
- e72.Gupta L, Bhatnagar V, Gupta AK, Kumar R. Long-term follow-up of patients with esophageal replacement by reversed gastric tube. Eur J Pediatr Surg. 2011;21:88–93. doi: 10.1055/s-0030-1267240. [DOI] [PubMed] [Google Scholar]
- e73.Virgone C, D’Antonio F, Kalhil A, John R, Manzoli L, Giuliani S. Accuracy of prenatal ultrasound in detecting jejunal and ileal atresia: A systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2014;28:201–214. doi: 10.1002/uog.14651. [DOI] [PubMed] [Google Scholar]
- e74.Sweeney B, Surana R, Puri P. Jejunoileal atresia and associated malformations: correlation with the timing of in utero insult. J Pediatr Surg. 2001;36:774–776. doi: 10.1053/jpsu.2001.22958. [DOI] [PubMed] [Google Scholar]
- e75.van der Zee DC. Laparoscopic repair of duodenal atresia: revisited. World J Surg. 2011;35:1781–1784. doi: 10.1007/s00268-011-1147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e76.Burjonrappa SC, Crete E, Bouchard S. Prognostic factors in jejuno-ileal atresia. Pediatr Surg Int. 2009;25:795–798. doi: 10.1007/s00383-009-2422-y. [DOI] [PubMed] [Google Scholar]
- e77.Keijzer R, van de Ven C, Vlot J, et al. Thoracoscopic repair in congenital diaphragmatic hernia: patching is safe and reduces the recurrence rate. J Pediatr Surg. 2010;45:953–957. doi: 10.1016/j.jpedsurg.2010.02.017. [DOI] [PubMed] [Google Scholar]
- e78.Rintala RJ, Sistonen S, Pakarinen MP. Outcome of esophageal atresia beyond childhood. Semin Pediatr Surg. 2009;18:50–56. doi: 10.1053/j.sempedsurg.2008.10.010. [DOI] [PubMed] [Google Scholar]
- e79.Aspirot A, Faure C. Esophageal dysmotility: characterization and pathophysiology. Dis Esophagus. 2013;26:405–409. doi: 10.1111/dote.12058. [DOI] [PubMed] [Google Scholar]
- e80.Malmstrom K, Lohi J, Lindahl H, et al. Longitudinal follow-up of bronchial inflammation, respiratory symptoms, and pulmonary function in adolescents after repair of esophageal atresia with tracheoesophageal fistula. J Pediatr. 2008;153:396–401. doi: 10.1016/j.jpeds.2008.03.034. [DOI] [PubMed] [Google Scholar]
- e81.Stark Z, Patel N, Clarnette T, Moody A. Triad of tracheoesophageal fistula-esophageal atresia, pulmonary hypoplasia, and duodenal atresia. J Pediatr Surg. 2007;42:1146–1148. doi: 10.1016/j.jpedsurg.2007.01.044. [DOI] [PubMed] [Google Scholar]
- e82.Kovesi T, Rubin S. Long-term complications of congenital esophageal atresia and/or tracheoesophageal fistula. Chest. 2004;126:915–925. doi: 10.1378/chest.126.3.915. [DOI] [PubMed] [Google Scholar]
- e83.Peetsold MG, Heij HA, Nagelkerke AF, Deurloo JA, Gemke RJ. Pulmonary function impairment after trachea-esophageal fistula: a minor role for gastro-esophageal reflux disease. Pediatr Pulmonol. 2011;46:348–355. doi: 10.1002/ppul.21369. [DOI] [PubMed] [Google Scholar]
- e84.Koivusalo A, Lindahl H, Rintala RJ. Morbidity and quality of life in adult patients with a congenital abdominal wall defect: a questionnaire survey. J Pediatr Surg. 2002;37:1594–1601. doi: 10.1053/jpsu.2002.36191. [DOI] [PubMed] [Google Scholar]
- e85.van Eijck FC, Hoogeveen YL, van Weel C, Rieu PN, Wijnen RM. Minor and giant omphalocele: long-term outcomes and quality of life. J Pediatr Surg. 2009;44:1355–1359. doi: 10.1016/j.jpedsurg.2008.11.034. [DOI] [PubMed] [Google Scholar]