A WOX gene, TAB1/WUS, plays a critical role in the initiation of the axillary meristem development in rice.
Abstract
Axillary shoot formation is a key determinant of plant architecture. Formation of the axillary shoot is regulated by initiation of the axillary meristem or outgrowth of the axillary bud. Here, we show that rice (Oryza sativa) TILLERS ABSENT1 (TAB1; also known as Os WUS), an ortholog of Arabidopsis thaliana WUS, is required to initiate axillary meristem development. We found that formation of the axillary meristem in rice proceeds via a transient state, which we term the premeristem, characterized by the expression of OSH1, a marker of indeterminate cells in the shoot apical meristem. In the tab1-1 (wus-1) mutant, however, formation of the axillary meristem is arrested at various stages of the premeristem zone, and OSH1 expression is highly reduced. TAB1/WUS is expressed in the premeristem zone, where it shows a partially overlapping pattern with OSH1. It is likely, therefore, that TAB1 plays an important role in maintaining the premeristem zone and in promoting the formation of the axillary meristem by promoting OSH1 expression. Temporal expression patterns of WUSCHEL-RELATED HOMEOBOX4 (WOX4) indicate that WOX4 is likely to regulate meristem maintenance instead of TAB1 after establishment of the axillary meristem. Lastly, we show that the prophyll, the first leaf in the secondary axis, is formed from the premeristem zone and not from the axillary meristem.
INTRODUCTION
Plant architecture such as shoots and inflorescences are greatly influenced by the branching pattern (reviewed in Wang and Li, 2008; Domagalska and Leyser, 2011). Shoot branches grow from axillary buds, which are derived from the axillary meristems formed at the axil of leaf primordia. Branch formation is regulated at two developmental stages: initiation of the axillary meristem and outgrowth of the axillary bud. The latter event, which is induced by derepression of bud dormancy, is regulated by a coordinated action of phytohormones such as auxin, strigolactone, and cytokinin (reviewed in Domagalska and Leyser, 2011). Whereas there has been rapid progress in our understanding of bud outgrowth, the mechanism of axillary meristem initiation is insufficiently understood at present.
Genetic studies have revealed that initiation of the axillary meristem is regulated by genes such as MONOCULM1 (MOC1), LAX PANICLE1 (LAX1), and LAX2 in rice (Oryza sativa) (Komatsu et al., 2003; Li et al., 2003; Oikawa and Kyozuka, 2009; Tabuchi et al., 2011); barren stalk1 in maize (Zea mays) (Gallavotti et al., 2004); LATERAL SUPPRESSOR (LAS), REGULATOR OF AXILLARY MERISTEMS, and REGULATOR OF AXILLARY MERISTEM FORMATION (ROX) in Arabidopsis thaliana (Greb et al., 2003; Keller et al., 2006; Müller et al., 2006; Yang et al., 2012); and Lateral suppressor (Ls) and Blind in tomato (Solanum lycopersicum) (Schumacher et al., 1999; Schmitz et al., 2002). Most of these genes encode transcription factors belonging to the GRAS, MYB, and basic/helix-loop-helix families and are required for the early steps of axillary meristem initiation. These genes are expressed in distinct patterns in the axil of leaf primordia, for example, in the region where the axillary meristem initiates or the boundary region that discriminates the developing axillary meristem from other tissues. Recently, it has been demonstrated that auxin depletion and cytokinin signaling in the leaf axil are required for axillary meristem formation in Arabidopsis (Wang et al., 2014a, 2014b).
The shoot branch in rice is called a tiller, and rice propagates vegetatively by tillering (reviewed in Wang and Li, 2008; Pautler et al., 2013). Therefore, understanding the mechanism of tiller formation in rice is important not only for basic biological knowledge but also for agricultural improvement. A tiller is formed from an axillary bud (tiller bud). Unlike in Arabidopsis and tomato, where the axillary meristem forms on the adaxial side of the leaf, rice generates the axillary bud on the culm (stem) in the leaf axil (Li et al., 2003; Oikawa and Kyozuka, 2009). In rice moc1, lax1, and lax2 mutants, the tiller is absent or highly reduced, and the branching of the inflorescence (panicle) is compromised (Komatsu et al., 2003; Li et al., 2003; Oikawa and Kyozuka, 2009; Tabuchi et al., 2011). The genes responsible for these mutations play crucial roles in initiating the axillary meristem. OSH1, which is a marker of meristematic cells and a homolog of Arabidopsis SHOOT MERISTEMLESS (STM) and maize Knotted1 (Jackson et al., 1994; Long et al., 1996), is expressed in the region where the axillary meristem develops later in the axils of wild-type plants. In the lax1 mutant and the lax2 moc1 double mutant, however, the expression of OSH1 is strongly reduced, suggesting that these genes are required to initiate or maintain undifferentiated cell fate at a very early stage of axillary development (Oikawa and Kyozuka, 2009; Tabuchi et al., 2011).
In some plants, an axillary bud is accompanied by a prophyll (Esau, 1977), although this organ is not seen in Arabidopsis. The prophyll is the first leaf formed in a new axis and differs from foliage leaves both in shape and arrangement. In grasses, the prophyll is formed as a two-keeled organ, which is also distinct from a foliage leaf (Arber, 1923; Sharman, 1945; Bossinger et al., 1992). Concerning the ear shoot of maize, developmental and clonal analyses suggest that a two-keeled prophyll is formed via congenital fusion of two leaf primordia and that prophyll formation is closely associated with axillary meristem development (Uhrig et al., 1997; Johnston et al., 2010). In rice, the vegetative axillary bud is composed of the axillary meristem, the primordia of foliage leaves, and the prophyll that encloses them (Hoshikawa, 1989). The foliage leaf primordia are initiated from the axillary meristem, and the mechanism of this leaf initiation in Arabidopsis is well understood (reviewed in Barton, 2010; Aichinger et al., 2012). The genetic mechanism of axillary meristem formation is also gradually being elucidated in rice, as described above. However, it remains unknown how the prophyll differentiates and how the axillary meristems and the prophyll coordinately develop into axillary buds not only in rice but also in other grasses.
After the meristem is established, the axillary meristem functions as the shoot apical meristem (SAM) of the secondary shoot; the stem cells in the SAM are maintained by the CLAVATA-WUSCHEL (CLV-WUS) negative feedback loop in Arabidopsis (Mayer et al., 1998; Schoof et al., 2000; reviewed in Ha et al., 2010; Aichinger et al., 2012). WUS, a transcriptional regulator, promotes stem cell identity, whereas CLV signaling represses WUS expression. WUS protein moves from the organizing center, where it is expressed, to the stem cell region and promotes the expression of CLV3, which ultimately represses WUS expression, in addition to promoting stem cell identity (Yadav et al., 2011). In rice, CLV-like genes, known as FLORAL ORGAN NUMBER genes, are involved in the negative regulation of meristem maintenance, as seen in maize (Taguchi-Shiobara et al., 2001; Suzaki et al., 2004, 2006; Bommert et al., 2005). By contrast, little is known about the genes responsible for positive regulation in both rice and maize (reviewed in Pautler et al., 2013). Recently, WUSCHEL-RELATED HOMEOBOX4 (WOX4), a WOX gene related to the rice WUS ortholog (Os WUS), has been shown to be involved in the activity of the SAM in rice (Ohmori et al., 2013).
Apart from maintenance of the SAM, WOX genes play diverse roles in plant development, such as embryogenesis, vascular differentiation, lateral organ patterning, and maintenance of the root apical meristem (reviewed in Hirakawa et al., 2011; Lau et al., 2012; Nakata and Okada, 2013), in both Arabidopsis and rice. In addition, phylogenetic and evolutionary studies have added to our understanding of the function of WOX genes not only in angiosperms but also in gymnosperms (Nardmann et al., 2007, 2009; Lin et al., 2013; Nardmann and Werr, 2013)
Although much progress has been made in elucidating the role of the WOX genes, the function of WUS orthologs is still unclear in monocots. In grasses, spatial expression patterns of the WUS orthologs Os WUS and Zm WUS have been analyzed, and their developmental roles have been discussed (Nardmann and Werr, 2006). As yet, however, functional analyses using mutants and transgenic plants have not been reported.
With the aim of elucidating the function of Os WUS, in this article we analyzed knockout mutants of Os WUS, which we renamed TILLERS ABSENT1 (TAB1). Plants with loss of function of TAB1/WUS exhibited no tiller formation in the vegetative phase and partial defects in branching of the panicle in the reproductive phase. Our analysis revealed that rice TAB1 is required for initiation of the axillary meristem but not for maintenance of the SAM and suggested that TAB1 acts in a transient state during axillary meristem formation. Furthermore, our work uncovered the developmental origin of the prophyll.
RESULTS
The tab1/wus Mutant Produces No Tiller
Os WUS was reported to be an ortholog of Arabidopsis WUS based on sequence similarity (Nardmann and Werr, 2006) and encodes a similar protein containing a homeodomain, WUS box, and EAR motif (Supplemental Figure 1). To elucidate the function of Os WUS, we isolated a mutant by the TILLING (for targeting induced local lesions in genomes) approach. This mutant had a nucleotide substitution at the splice site of the first intron, resulting in premature termination of the protein in the intron. RT-PCR analysis confirmed that a misspliced transcript containing the first intron was produced (Supplemental Figure 2). Because tab1 was inherited as a recessive trait, protein function is likely lost in this mutant.
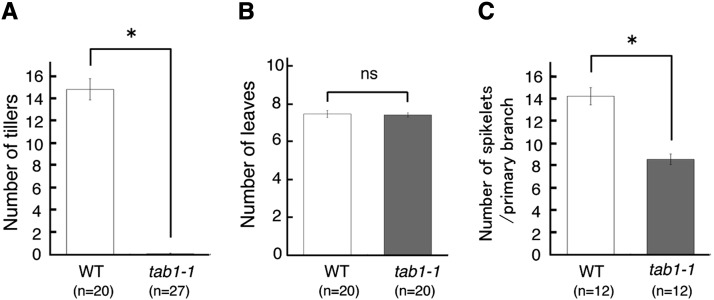
Phenotypic observation revealed that this mutant produced no tillers even in 2-month-old shoots, suggesting that axillary shoot formation is compromised (Figures 1A, 1B, and 2A). To avoid confusion, we named this mutant tab1 because the tab1 phenotype was unrelated to wus phenotypes (meaning bushy) in Arabidopsis. Unlike the Arabidopsis wus mutant, which shows premature termination of the SAM after generating a few leaves, the rice tab1-1 mutant produced the same number of leaves as wild-type rice did, suggesting that the activity of the SAM was not compromised (Figure 2B). In the reproductive phase, only a single panicle with short branches was formed in tab1-1 (Figures 1C and 1D). Spikelet development was also compromised (Figures 1G and 1H; Supplemental Figure 3). The defect in spikelet development was associated with a reduction in the expression of OSH1, which marks undifferentiated cells in the meristem (Figures 1I and 1J). The number of spikelets, most of which had morphological defects, was reduced in tab1-1 (Figures 1K, 1L, and 2C).
Figure 1.
Phenotypes of the tab1-1 Mutant.
(A) and (B) Shoot phenotypes of 2-month-old plants.
(C) and (D) Phenotypes of panicles.
(E) and (F) Complementation of tab1-1. Shown are a plant producing tillers (arrowheads) 2 months after regeneration (E) and a panicle with normal branches and spikelets (F). All six regenerated shoots with the TAB1 genomic sequence showed phenotypes similar to the wild type.
(G) and (H) Phenotypes of spikelets.
(I) and (J) Expression pattern of OSH1.
(K) and (L) Phenotypes of branches.
rg, rudimentary glume; sl, sterile lemma; sm, spikelet meristem. Bars in (A) to (F), (K), and (L) = 2 cm; bars in (G) and (H) = 1 mm; bars in (I) and (J) = 50 μm.
Figure 2.
Comparison of Shoot Phenotypes between the Wild Type and tab1-1.
Comparison of the number of tillers, leaves, and spikelets per primary branch between the wild type and tab1-1 (2-month-old plants) is shown. Asterisks indicate significant differences from the wild type (Student’s t test, P < 1 × 10−21 [A], P < 1 × 10−3 [C]). ns, not significant. Error bars indicate se.
To confirm that these phenotypic abnormalities resulted from a reduction of TAB1 activity, we generated transgenic rice in which expression of the endogenous TAB1 gene was silenced. The resulting transgenic plants showed phenotypes similar to those of the tab1-1 mutant: namely, failure of tiller formation and defects in inflorescence and spikelet development (Supplemental Figure 4). We then introduced an 8.6-kb genomic segment of the wild type including the TAB1 coding region into the tab1 mutant. The resulting transformants showed complete rescue of the tab1 phenotypes, such as defects in tiller formation and spikelet development (Figures 1E and 1F). Thus, TAB1 is likely to be involved in axillary shoot formation and inflorescence development.
Axillary Bud Formation Is Defective in the tab1 Mutant
Because the defects in inflorescence development in tab1-1 were partial, we focused on the function of TAB1 in axillary bud formation in the vegetative phase.
First, we observed axillary buds in the wild type. When the leaves were removed, small axillary buds were detected at the basal region of the culm (stem) in 3-week-old seedlings (Figure 3A). By scanning electron microscopy analysis, a small flap-like leaf, the prophyll, was observed in the axil of wild-type seedlings (Figure 3D). Cross sections of the bud revealed that the axillary meristem and a few leaf primordia were enclosed by the prophyll, which had two keels with large vascular bundles (Figure 3G).
Figure 3.
Effect of tab1-1 Mutation on the Axillary Buds.
(A) to (C) Basal region of the culm of 3-week-old plants after the removal of leaves. Arrowheads indicate the axillary buds in the wild type (A) and abnormal buds in tab1-1 ([B] and [C]). Complete absence of an axillary bud (type I) and putative type II axillary buds are indicated by the dashed and solid lines, respectively.
(D) to (F) Scanning electron microscopy images of the region where the axillary bud is formed. Arrowheads indicate a normal prophyll in the wild type (D) and a highly reduced prophyll in tab1-1 (E). No prophyll-like structure is observed in (F).
(G) and (H) Cross sections of the axillary bud in the wild type (G) and a type II axillary bud in tab1-1 (H).
(I) to (L) Longitudinal sections of the axillary bud in the wild type ([I] and [J]) and an abnormal bud in tab1-1 ([K] and [L]). Note that the images in (I), (K), and (L) are shown at the same magnification.
(M) and (N) Schematic representations of tiller bud production in each leaf axil of the wild type and tab1-1 in 2-week-old seedlings (M) and 4-week-old seedlings (N). Each column stands for a single plant, and each row stands for a leaf axil in order from bottom to top. Green, normal axillary bud; dark green, elongated axillary bud after release from dormancy; yellow, absence of axillary bud (type I); gray, putative type II axillary bud; brown, type III axillary bud showing an abnormally elongated prophyll.
am, axillary meristem; lvb, large vascular bundle. Bars in (A) to (C) = 1 mm; bars in (G) to (L) = 200 μm; bars in (D) to (F) = 500 μm.
The phenotypes of the axillary bud in the tab1-1 mutant were roughly classified into three types, the formation of which depended on the age of the leaf that subtended the axillary bud (Figures 3M and 3N). First, no axillary buds were formed in the axils of younger leaves (type I; Figure 3B). In this type, no prophyll-like organ was detected even by scanning electron microscopy analysis (Figures 3E and 3F). Second, we observed abnormal axillary buds (type II; Figure 3B) that had a prophyll but did not have a meristem or leaf primordia (Figure 3H). This type of axillary bud in tab1-1 was indistinguishable from normal buds in the wild type by the naked eye (Figure 3B). Third, large leaf-like outgrowths were often observed in the axil of the fourth to sixth leaves (type III; Figure 3C). These outgrowths were similar to the elongated prophylls grown after the release of bud dormancy in the wild type (Supplemental Figure 5), suggesting that they resulted from precocious elongation of the prophylls in tab1-1. The distribution of the three types of tab1-1 axillary bud is schematically represented, together with leaf ages, in Figures 3M and 3N.
To gain further insight, we examined the axillary buds by making longitudinal sections. In the wild type, the axillary meristem, which exhibited a dome-like structure and was indistinguishable from the SAM, was observed together with several foliage leaves and the prophyll (Figures 3I and 3J). By contrast, a flattened structure (23 out of 27 apices examined) or a large protrusion (4 out of 27) was observed in type III tab1-1 axillary buds instead of the dome-like meristem, whereas no primordium of the foliage leaf was detected (Figures 3K and 3L). These observations suggest that meristem activity was severely compromised in type III axillary buds.
Taken together, these findings indicate that the lack of tiller in tab1-1 seems to result from a failure in axillary bud formation, but not in bud outgrowth, and that axillary meristem development is strongly disturbed in tab1-1.
TAB1 Seems to Be Required for the Early Developmental Stages of Axillary Meristem Formation
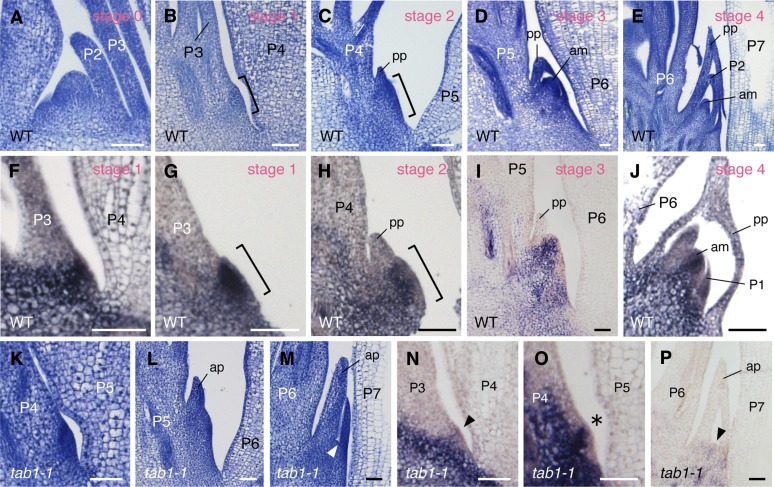
To determine which processes require TAB1 activity, we examined the developmental pattern of axillary bud formation. In the wild type, a bulge consisting of cytoplasm-dense cells first formed in the axil of the P4 leaf primordium (stage 1) and then protruded to create a cone-like structure (stage 2) (Figures 4A to 4C). OSH1 was strongly expressed throughout the bulge (stage 1) (Figures 4F and 4G) and in the central region of the cone-like structure (stage 2) (Figures 4H), suggesting that these regions have meristematic characteristics (accordingly, we termed them the “premeristem”). The dome and prophylls became gradually evident in subsequent stages (stage 3) (Figure 4D), and the axillary bud was established (stage 4) (Figure 4E). The expression of OSH1 was downregulated in the apical region of the cone-like structure (a putative prophyll primordium) (Figures 4H and 4I). The OSH1-expressing domain was restricted to a narrow part of the cone-like structure (Figure 4I) and ultimately confined to the dome of the axillary meristem, except for some expression in the pith (Figure 4J).
Figure 4.
Developmental Patterns of Axillary Buds in the Wild Type and tab1-1.
(A) to (E) Longitudinal sections of the shoot apex (A) and the region where the axillary buds initiate ([B] to [E]).
(F) to (J) Expression patterns of OSH1 during the formation of axillary buds in the wild type. Brackets indicate the premeristem zone.
(K) to (M) Longitudinal sections of the region where the axillary buds should form in tab1-1. Development of the premeristem zone was terminated in some plants ([K] and [L]), and an abnormally elongated prophyll was formed in others (M). The arrowhead indicates flattened tissue where the meristem dome would form in the wild type.
(N) to (P) Expression patterns of OSH1 in tab1-1. Arrowheads and the asterisk indicate very weak ([N] and [P]) and narrow (O) expression of OSH1.
am, axillary meristem; ap, abnormal prophyll; pp, prophyll. Bars = 50 μm.
The tab1-1 mutant showed termination of the premeristem zone at various developmental stages. For example, a bulge (stage 1) or a cone-like structure was still observed in the axil of older leaf primordium, P5 and P6, respectively (Figures 4K and 4L), whereas no dome-like structure was observed at a mature bud stage (Figure 4M). OSH1 expression was highly reduced or restricted to a narrower region in the premeristem zone and in the meristem dome (Figures 4N to 4P). These observations indicate that TAB1 activity is required in the initial stages of axillary meristem development, probably to maintain the undifferentiated state of cells in the premeristem zone by promoting OSH1 expression.
To examine the genetic relationships among genes known to regulate axillary meristem initiation, we performed in situ analyses of MOC1 and LAX1 expression (Komatsu et al., 2003; Li et al., 2003). MOC1 was expressed in the leaf axil from early stages in both the wild type and tab1-1 (Figures 5A and 5B). LAX1 was expressed in the boundary between the premeristem zone and upper tissues in both the wild type and tab1-1 (Figures 5C and 5D). At later stages, the expression pattern of LAX1 was also similar in tab1-1 and the wild type (Figures 5E and 5F). Thus, mutation of TAB1 did not affect the expression of MOC1 and LAX1.
Figure 5.
Expression Patterns of MOC1 and LAX1 during Axillary Bud Formation.
(A) and (B) Expression patterns of MOC1.
(C) to (F) Expression patterns of LAX1.
Bars = 50 μm.
TAB1 Is Expressed in the Premeristem Zone but Not in the Axillary Meristem
We examined the spatiotemporal expression patterns of TAB1 during axillary meristem formation. TAB1 transcript was first detected in the premeristem zone at stages 1 and 2 (Figures 6A and 6B). TAB1 was expressed in the apical and central parts of these zones (Figures 6A and 6B), and its expression domains were completely contained within the OSH1 expression domains (Figures 4G and 4H). Unlike OSH1, TAB1 expression disappeared at stage 3 (Figures 6C and 6D). Thus, the expression of TAB1 was transient during axillary meristem formation and was closely associated with development of the premeristem zone.
Figure 6.
Spatial Expression Patterns of TAB1 and WOX4 in the Wild Type.
(A) to (D) Expression of TAB1 in the premeristem zone and the axillary bud. No TAB1 expression is observed in developing (C) and established (D) axillary buds.
(E) to (G) Expression patterns of TAB1 (E), OSH1 (F), and WOX4 (G) in the SAM of a 4-week-old wild-type seedling.
(H) to (K) Expression of WOX4 in the premeristem zone ([H] and [I]) and the axillary bud ([J] and [K]). No WOX4 expression is observed in the premeristem zone ([H] and [I]).
Brackets indicate the premeristem zone. am, axillary meristem; pp, prophyll. Bars = 50 μm.
To confirm the absence of TAB1 expression in the established SAM, we performed in situ hybridization using probes for TAB1 and OSH1 in serial sections of the same shoot apex of seedlings. TAB1 transcript was not detected in the wild-type SAM, whereas OSH1 transcript was clearly observed in the serial section of the same shoot apex (Figures 6E and 6F). This result excluded the possibility that failure in the detection of TAB1 transcript resulted from the degradation of RNA due to poor tissue preparation. The absence of TAB1 transcription in the shoot apex was also confirmed by RT-PCR analysis (Supplemental Figure 6). By contrast, OSH1 was expressed in the tab1-1 SAM in a pattern similar to that observed in the wild-type SAM (Supplemental Figure 6), suggesting that loss of TAB1 function does not affect maintenance of the SAM. We also checked TAB1 expression during embryogenesis. No TAB1 transcripts were detected throughout embryogenesis, whereas OSH1 transcript was detected in the future meristem in the embryo 4 d after pollination and in the SAM in the mature embryo (Supplemental Figure 7). The lack of expression of TAB1 in the embryo was also supported by RT-PCR analysis (Supplemental Figure 6). These results suggest that TAB1 is unrelated to the formation of the SAM and its maintenance.
The disappearance of TAB1 in the established axillary meristem suggests that the other gene is required for maintenance of the axillary meristem. The most probable candidate is WOX4, a close paralog of TAB1 (Os WUS; Nardmann and Werr, 2006), which was recently found to be involved in maintenance of the SAM in rice (Ohmori et al., 2013). In situ analysis showed that WOX4 was expressed in the SAM and leaf primordia (Figure 6G), as described previously (Ohmori et al., 2013). During axillary meristem formation, WOX4 transcript was not detected in the premeristem zone (Figures 6H and 6I). By contrast, strong WOX4 expression was detected in the established axillary meristem, which was initiating leaf primordia (Figures 6J and 6K). The expression patterns of WOX4 in the established axillary bud and in the shoot apex in the main shoot were similar (Figures 6G and 6K). Thus, two related WOX genes, TAB1 and WOX4, are alternately expressed during development of the axillary meristem.
TAB1 Function in Developmental Processes Other Than Axillary Meristem Formation
We examined the effect of the tab1 mutation on developmental processes related to the formation and activity of the SAM. First, we investigated the effect on germination. More than 97% of seeds generated in the heterozygous plant germinated, and there was no difference in germination ability among seeds with different TAB1 genotypes (Supplemental Table 1), suggesting that the function of the SAM formed during embryogenesis is not affected by the tab1-1 mutation. Second, we examined the ability of tab1-1 to undergo shoot regeneration. Calli grown in regeneration medium were classified into four classes according to their efficiency of shoot formation (Supplemental Figures 8A to 8G). No difference in phenotypes was observed among calli with different TAB1 genotypes (Supplemental Figure 8H). Altogether, these observations suggest that TAB1 is not involved in the formation or maintenance of the SAM.
We examined the expression patterns of TAB1 during inflorescence development. TAB1 was expressed in the branch and spikelet meristems (Supplemental Figure 7). TAB1 transcripts were detected in the apical region of both meristems in a patchy pattern. The branch and spikelet meristems initiated the spikelet meristem and spikelet organs, respectively, indicating that these meristems were established. Thus, the expression patterns of TAB1 in the reproductive meristems differ from those of TAB1 in the vegetative meristems.
DISCUSSION
In this work, we have shown that the initial steps of axillary meristem formation in rice are regulated by TAB1, which encodes a homeobox protein orthologous to Arabidopsis WUS (Mayer et al., 1998). TAB1 seems to be required for maintaining the premeristem zone, a transient state comprising undifferentiated cells that will subsequently become the axillary meristem. TAB1 expression disappeared when the axillary meristem was almost established. After establishment, the axillary meristem seems to be maintained by functions of the closely related WOX4 gene. In addition, we have revealed that the prophyll is differentiated from the premeristem zone and is closely associated with the formation of the axillary meristem.
TAB1 Functions in the Premeristem Zone to Maintain Meristematic States
Our analyses suggest that TAB1 is required to initiate the formation of the axillary meristem in rice, because no normal axillary buds were formed in tab1-1. To describe the process of axillary meristem formation, we introduced a concept, the premeristem, to define a transient state of cell clusters that are subsequently incorporated into the axillary meristem, which has tunica-corpus organization within a dome-like structure. The premeristem zone is initially characterized by a small bulge consisting of cytoplasmically dense cells that express OSH1 (stage 1) (Figures 4B, 4G, and 7). The premeristem zone is subsequently restricted to a central region expressing OSH1 in a cone-like structure (stage 2) (Figures 4C, 4H, and 7), which later develops into the axillary meristem with a dome-like structure in the axillary bud.
Figure 7.
Schematic Representation of Axillary Bud Development in Rice.
Brackets indicate the premeristem zone. The expression domains of TAB1 and OSH1 are indicated with yellow and pink, respectively. am, axillary meristem.
In the tab1-1 mutant, the formation of a bulge of cytoplasmically dense cells suggests that the initial step in axillary meristem formation occurs. However, axillary meristem formation then terminates at various stages of the premeristem state. Thus, TAB1 appears to be required for the progression of axillary meristem formation in the premeristem zone after entry into this process. TAB1, like OSH1, was expressed in the premeristem zone, although the TAB1-expressing domain was restricted to its upper region. Expression of OSH1 was reduced or restricted to a narrower region in the premeristem zone in tab1-1, suggesting that TAB1 is required for the proper expression of OSH1. Therefore, it is likely that TAB1 is involved in maintaining the meristematic properties of the premeristem zone by promoting OSH1 and in ensuring the progression of axillary meristem formation.
Two WOX Genes Switch during Axillary Meristem Formation
Although TAB1 was expressed in the premeristem zone, its expression disappeared in the axillary meristem after its establishment. By contrast, WOX4 was expressed in the established meristem, but no or very weak expression was detected in the premeristem zone. Thus, expression was found to switch between two related WOX genes during axillary meristem formation in rice. As a result, TAB1 seems to function in a narrow developmental window. Nevertheless, the no-tiller phenotype of tab1-1 indicates that this transient function of TAB1 plays a critical role in establishing the axillary meristem in rice.
It is possible that, in the premeristem zone, a small group of cells might be specified as stem cells (see below), which are then subsequently incorporated into the axillary meristem. Considering that WUS is required for stem cell maintenance in the SAM in Arabidopsis (Mayer et al., 1998; reviewed in Ha et al., 2010; Aichinger et al., 2012), TAB1 may be involved in maintaining putative stem cells in the premeristem zone. It is possible that Arabidopsis WUS and rice TAB1 share a common ancestral function of general involvement in stem cell maintenance and that TAB1 was recruited to play a specific role to maintain the premeristem zone during the evolution of rice.
WOX4 is required for maintenance of the SAM, as we described previously (Ohmori et al., 2013). Here, we found that WOX4 also seems to play a role in maintaining the axillary meristem after it is almost established. As stated above, the related WOX genes are alternately expressed and seem to share the maintenance of meristematic cells during axillary meristem development in rice. Both TAB1 and WOX4 are members of the WUS clade of the WOX gene family and encode similar proteins containing the highly conserved homeodomain and the WUS domain (Nardmann and Werr, 2006; Zhang et al., 2010), suggesting that these two proteins have a similar biochemical function. It is possible that the functional difference between TAB1 and WOX4, as demonstrated by genetic analysis, is related to the timing of expression of the two genes during axillary formation. It will be of interest to know whether TAB1 and WOX4 share target genes and whether WOX4 expression in the premeristem zone can rescue the tab1 phenotype.
In the reproductive phase, inflorescence development was partially affected by the tab1 mutation: namely, the number of spikelets was reduced and spike development was compromised. These phenotypes appear to be associated with defects in the activity of the reproductive axillary meristems, such as the branch meristems and spikelet meristems (reviewed in Tanaka et al., 2013). TAB1 continued to be expressed in the branch meristem and spikelet meristem after their establishment. This is in contrast with the expression pattern of TAB1 in the axillary meristem. In addition, the number of primary branches is not reduced in tab1-1. Therefore, the function of TAB1 in reproductive development seems to be partially different from that in vegetative development. Elucidation of the function of TAB1 in inflorescence development remains a challenge for future studies.
Comparison of the Function and Expression Patterns of the WUS Orthologs
In Arabidopsis, WUS is expressed in specific cell types before the appearance of the SAM in embryos and in the organizing center in the postembryonic SAM (Mayer et al., 1998). The wus mutant shows similar defects in the SAM in the embryo and in the seedling (Laux et al., 1996; Mayer et al., 1998). Thus, the expression of WUS in both the embryonic and postembryonic SAM is consistent with the mutant phenotypes.
By contrast, no TAB1 transcript was detected in developing embryos or in the established SAM in rice seedlings. Leaf initiation, seed germination, and shoot regeneration are associated with SAM activities in rice (Nagasaki et al., 2007; Ohmori et al., 2013). These developmental processes were not affected by tab1-1 mutation. In addition, OSH1 was expressed in the SAM in tab1-1 as in the wild type. These observations suggest that TAB1 function is not related to SAM activities.
The absence of axillary shoots in tab1-1 is caused by a failure in axillary meristem initiation, the phenotype of which is consistent with TAB1 expression patterns in rice. By contrast, many adventitious shoots are formed in the Arabidopsis wus mutant and in transgenic Arabidopsis plants defective in WUS protein movement (Laux et al., 1996; Daum et al., 2014). This adventitious shoot formation in Arabidopsis is caused by the repeated initiation of extra meristems, although the meristems terminate prematurely (Laux et al., 1996). In their analysis of the Arabidopsis wus mutant, Laux et al. (1996) discuss that “shoot meristem initiation per se appears not to be affected in wus mutants.” Therefore, rice TAB1 and Arabidopsis WUS would seem to have different roles in the formation of the axillary meristem.
Alternatively, the difference in shoot phonotypes between rice and Arabidopsis may result from the function of both genes in the SAM. In the Arabidopsis wus mutant, adventitious shoots seem to form from extra axillary meristems, because apical dominance is defective owing to premature termination of the SAM. By contrast, the SAM is active in the rice tab1 mutant; as a result, apical dominance might repress the formation of axillary shoots in rice tab1, even though the axillary meristem is partially formed. It is possible that WUS is involved in maintaining the meristem during its formation in Arabidopsis. As discussed above, TAB1 seems to be involved in the maintenance of the premeristem zone in rice by promoting putative stem cells. In this view, the function of TAB1 in rice may not differ greatly from that of WUS in Arabidopsis. The difference in shoot phenotypes might be also related to the timing of the function of both genes: rice TAB1 acts in the very early stage of axillary meristem formation, whereas Arabidopsis WUS would act later.
In Arabidopsis, the axillary meristem is initiated at the base of the leaf axil, as indicated by the strong expression of STM (Long and Barton, 2000). STM is expressed uniformly in a cluster of cells, which become morphologically distinct to form a bump. This timing of the expression of Arabidopsis STM roughly resembles that of rice OSH1. However, the expression pattern of Arabidopsis WUS has not yet been demonstrated in this process. It will be interesting to know how WUS is involved in normal axillary meristem formation and how adventitious formation is promoted in the absence of WUS function in Arabidopsis.
TAB1 is expressed in the premeristem zone before the establishment of the axillary meristem, and its expression is partly localized to the upper region of the zone. This expression pattern is consistent with a previous study showing the expression of Os WUS (TAB1) in “emerging axillary meristems” (Nardmann and Werr, 2006). That study also showed that Os WUS (TAB1) is expressed in the SAM. In this study, however, we were unable to detect any TAB1 signals in the SAM in a number of in situ experiments using different probes. The absence of TAB1 transcript in the SAM was confirmed by RT-PCR analysis. One WUS ortholog, Zm WUS1, is expressed in the SAM in maize, whereas another ortholog, Zm WUS2, is expressed in other tissues. In the reproductive phase, both rice and maize WUS orthologs are expressed in other tissues (Nardmann and Werr, 2006). Genetic and functional analyses of the Zm WUS orthologs would provide important information about the conservation and specialization of WUS orthologs within the grass species and among plants in angiosperms.
Axillary Bud Formation and Prophyll Differentiation
In Arabidopsis, all leaves initiate from the newly formed axillary meristem, and no leaf-like organ is observed before the establishment of the axillary meristem (Long and Barton, 2000). By contrast, the axillary bud consists of the axillary meristem, foliage leaf primordia, and a prophyll in rice (Hoshikawa, 1989). Formation of the axillary bud proceeds via a complex process, in which each stage is characterized by distinct morphology and the expression pattern of OSH1. Axillary meristem initiation seems to start with the onset of OSH1 expression in the leaf axil before morphological change, as it does in Arabidopsis. Subsequently, OSH1 is expressed throughout the bulge formed on the culm (stage 1) and is then restricted to the central region of the cone-like structure (stage 2) (Figures 4 and 7). The cells expressing OSH1 seem to be incorporated into the axillary meristem. Thus, although distinct morphological changes are observed in axillary bud formation in rice, a similar mechanism is likely to underlie axillary meristem formation in both rice and Arabidopsis with regard to the fate determination of undifferentiated cells and the creation of the meristem.
Rice generates a prophyll (Hoshikawa, 1989), which is not seen in Arabidopsis. At stage 2, OSH1 was downregulated in the apical part of the cone-like structure. The prophyll is likely to be differentiated from this part, suggesting that this organ, unlike foliage leaves, is not a product of the axillary meristem. In addition, the origin of the prophyll suggests that prophyll development might be closely associated with the activity of the premeristem zone. The defect in the axillary bud in tab1-1 can be roughly classified into three types (I to III) mainly depending on the morphology of the prophyll. In tab1-1, development of the premeristem zone seems to initiate but soon terminates owing to the failure of its maintenance. The variable phenotype of the prophyll in tab1-1 may depend on the timing of termination of the premeristem. It is likely, therefore, that proper maintenance of the premeristem zone and formation of the prophyll are coordinately regulated during axillary bud formation.
The initiation of prophyll from the region where OSH1 is downregulated in the premeristem zone is similar to leaf initiation at the P0 site where OSH1 expression disappears in the SAM (Ohmori et al., 2013). Thus, it is possible that cell fate is partially specified in the premeristem zone even at stage 1: for example, specification of cell fate toward stem cells or cells to be differentiated into organs. Thus, the premeristem zone may have properties similar to those of the established meristem, although its shape is different from the dome-like structure of the SAM.
Genes Regulating Axillary Meristem Initiation in Rice
Mutants such as moc1, lax1, and lax2 exhibit defects in axillary meristem formation, resulting in no or reduced tiller formation (Komatsu et al., 2003; Li et al., 2003; Oikawa and Kyozuka, 2009; Tabuchi et al., 2011). In contrast with tab1, no enlargement of prophylls is observed in these mutants. This observation suggests that entry into the stage of the premeristem zone in the process of axillary meristem formation does not occur in these mutants. Therefore, the function of these genes would be prerequisite for the formation of the premeristem zone and would precede the function of TAB1. Consistent with this idea, in situ analysis showed that MOC1 and LAX1 were expressed in tab1-1 in a pattern similar to that in the wild type, suggesting that TAB1 acts downstream of MOC1 and LAX1 or in an independent pathway. MOC1 is expressed much earlier than LAX1, LAX2, and TAB1 in axillary meristem formation (Li et al., 2003; Oikawa and Kyozuka, 2009; Tabuchi et al., 2011). Thus, sequential or independent action of these genes would be responsible for the formation of axillary buds in rice.
In both Arabidopsis and tomato, LAS/Ls and ROX, which are orthologs of MOC1 and LAX1, respectively, play important roles in the formation of the axillary meristem (Schumacher et al., 1999; Greb et al., 2003; Yang et al., 2012). Thus, the genetic mechanism of axillary meristem formation appears to be conserved in eudicots and monocots. However, the Arabidopsis wus mutation does not cause defects in axillary shoot formation but rather enhances it (Laux et al., 1996), as described above; therefore, WUS function in rice seems to be different from that in dicots.
METHODS
Plant Materials
The tab-1 mutant of rice (Oryza sativa) was isolated by the TILLING approach using the mutant collections maintained by the National Bio-Resource Project (Suzuki et al., 2008; Satoh et al., 2010). Taichung65 (T65) was used as a wild-type strain for comparing phenotypes and for in situ analysis. Plants were generally grown in pots containing soil (outdoors). Transgenic plants were grown in an NK System BIOTRON (model LH-350S; Nippon Medical and Chemical Instruments).
RT-PCR Analysis
To detect the misspliced transcript in tab1-1, total RNAs were isolated from T65 and tab1-1 seedlings using TRIsure (BIOLINE) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 3 to 5 μg of total RNA using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) and the oligo(dT)15 primer. Next, 2 μL of each reverse transcription product was used for PCR with the primers 5′-GAGCAGATCAAGATCCTGCG-3′, 5′-ACACGCAGAAGCATCGATC-3′, and 5′-ACGTAGAAGCCCGGGAAAG-3′.
To examine TAB1 expression in different tissues, total RNAs were isolated from the tissue containing the SAM and leaf primordia, embryos, and young panicles by the same method as described above. First-strand cDNA synthesis and PCR were performed as described above. The primers used are as follows: 5′-GAGCAGATCCAGCGGATC-3′ and 5′-GTTGGCCTGATCCGTCTG-3′ for TAB1 and 5′-GCAGGACCTGGAGCTTCG-3′ and 5′-TTCTTGGTCCTCCTCAGAAGAG-3′ for OSH1.
Complementation Test
An 8.6-kb genomic fragment containing the 1-kb TAB1 coding sequence and the 3.6-kb upstream and 4-kb downstream sequences was isolated by digestion of the BAC clone OSJNBa 0084K01 (CUGI BAC/EST Resource Center, Clemson University) with BamHI and then introduced into a pBluescript SK+ plasmid (Agilent Technologies). After digestion with BamHI, the fragments were inserted into a pENTR 2B vector (Invitrogen) and then transferred into a pBI-Hm12-GW vector, which contains the Gateway rfC cassette (Invitrogen), by LR recombination (Yoshida et al., 2009). The recombinant plasmid was introduced into Agrobacterium tumefaciens EHA101 and transformed into calli derived from tab1-1 according to the method of Hiei et al. (1994).
RNA Interference Suppression
To make a construct for RNA interference, a partial sequence of TAB1 was amplified using the primers 5′-CACCACGCTCGACGTCAC-3′ and 5′-TCACATGGACCCTGCAGG-3′ and then cloned into a pENTR D-TOPO vector (Invitrogen). The fragment was transferred into a pANDA-EG1 vector by LR recombination. The recombinant plasmids were transformed into calli derived from T65 via Agrobacterium strain EHA101 according to the method of Hiei et al. (1994).
Scanning Electron Microscopy
Seedlings were fixed in 4% paraformaldehyde and 0.25% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.2, at 4°C for ∼24 h. Next, they were dehydrated in a graded ethanol series, and then ethanol was replaced with 3-methylbutyl acetate. Samples were dried at their critical point and sputter-coated with platinum. The samples were then observed using a scanning electron microscope (model JSM-820S; JEOL) at an accelerating voltage of 5 kV.
In Situ Hybridization
To make a probe for the TAB1 transcripts, partial cDNA fragments were amplified with the primers 5′-CGCATCGAGGGCAAGAAC-3′ and 5′-TCACATGGACCCTGCAGG-3′ and cloned into a pCRII vector (Invitrogen). To make a probe for the MOC1 transcript, partial cDNA fragments were amplified with the primers 5′-TTCCACTTCACCCCGCTCCTC-3′ and 5′-TGCCACGCTGAGACGGAGAG-3′ and cloned into a pCRII vector (Invitrogen). Next, RNA probes were transcribed with T7 or SP6 RNA polymerase using the above constructs as templates and were labeled with digoxigenin using the DIG Labeling Kit (Roche). OSH1 and LAX1 probes were prepared as described previously (Sato et al., 1996; Komatsu et al., 2003).
Plant tissues were fixed and dehydrated according to the protocols of Itoh et al. (2000) and embedded in Paraplast Plus (Oxford Labware) after replacement with xylene. Microtome sections (8 to 10 μm) were mounted on glass slides. In situ hybridization experiments and immunological detection of the signals were performed by the methods of Kouchi and Hata (1993).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL databases under the following accession numbers: AB218894 (Os WUS/TAB1), JF836159 (WOX4), AY242058 (MOC1), AB115668 (LAX1), and D16507 (OSH1).
Supplemental Data
Supplemental Figure 1. Alignment of amino acid sequences in Arabidopsis WUS, rice TAB1/WUS, and tab1-1.
Supplemental Figure 2. Mutation in the tab1-1 allele.
Supplemental Figure 3. Frequency of abnormal spikelets in four classes in the tab1-1 mutants.
Supplemental Figure 4. RNA silencing of TAB1.
Supplemental Figure 5. Similarity between the elongated prophyll and the large outgrowth in tab1-1.
Supplemental Figure 6. RT-PCR analysis and spatial expression pattern of OSH1 in the shoot apex.
Supplemental Figure 7. Spatial expression patterns of OSH1 and TAB1 in wild type.
Supplemental Figure 8. Effect of tab1 mutation on shoot regeneration.
Supplemental Table 1. Number of germinated seedlings for various TAB1 genotypes.
Supplementary Material
Acknowledgments
We thank David Jackson and Toshiro Ito for critical reading of the article. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (Grants 23248001 and 25113008 to H.-Y.H.) and the Japan Society for the Promotion of Science (Grant 13J05127 and a Research Fellowship for Young Scientists to W.T.).
AUTHOR CONTRIBUTIONS
W.T. and H.-Y.H. designed the research. W.T., Y.O., S.K., and H.-Y.H. performed the research. T.U., H.M., T.M., and T.K. developed the rice TILLING system. W.T. and H.-Y.H. wrote the article.
Glossary
- SAM
shoot apical meristem
- TILLING
targeting induced local lesions in genomes
- T65
Taichung65
Footnotes
Articles can be viewed online without a subscription.
References
- Aichinger E., Kornet N., Friedrich T., Laux T. (2012). Plant stem cell niches. Annu. Rev. Plant Biol. 63: 615–636. [DOI] [PubMed] [Google Scholar]
- Arber A. (1923). Leaves of the Gramineae. Bot. Gaz. 76: 374–388. [Google Scholar]
- Barton M.K. (2010). Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Dev. Biol. 341: 95–113. [DOI] [PubMed] [Google Scholar]
- Bommert P., Lunde C., Nardmann J., Vollbrecht E., Running M., Jackson D., Hake S., Werr W. (2005). thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine-rich repeat receptor-like kinase. Development 132: 1235–1245. [DOI] [PubMed] [Google Scholar]
- Bossinger G., Lundqvist U., Rohde W., Salamini F. (1992). Genetics of plant development in barley. In Barley Genetics, Vol. VI, Munck L., ed (Copenhagen: Munksgaard lnternational Publishers; ), pp. 989–1022. [Google Scholar]
- Daum G., Medzihradszky A., Suzaki T., Lohmann J.U. (2014). A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc. Natl. Acad. Sci. USA 111: 14619–14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska M.A., Leyser O. (2011). Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 12: 211–221. [DOI] [PubMed] [Google Scholar]
- Esau K. (1977). Anatomy of Seed Plants, 2nd ed. (New York: Wiley; ). [Google Scholar]
- Gallavotti A., Zhao Q., Kyozuka J., Meeley R.B., Ritter M.K., Doebley J.F., Pè M.E., Schmidt R.J. (2004). The role of barren stalk1 in the architecture of maize. Nature 432: 630–635. [DOI] [PubMed] [Google Scholar]
- Greb T., Clarenz O., Schafer E., Muller D., Herrero R., Schmitz G., Theres K. (2003). Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 17: 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C.M., Jun J.H., Fletcher J.C. (2010). Shoot apical meristem form and function. Curr. Top. Dev. Biol. 91: 103–140. [DOI] [PubMed] [Google Scholar]
- Hiei Y., Ohta S., Komari T., Kumashiro T. (1994). Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6: 271–282. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y., Kondo Y., Fukuda H. (2011). Establishment and maintenance of vascular cell communities through local signaling. Curr. Opin. Plant Biol. 14: 17–23. [DOI] [PubMed] [Google Scholar]
- Hoshikawa K. (1989). Growing the Rice Plant: An Anatomical Monograph. (Tokyo: Nobunkyo; ). [Google Scholar]
- Itoh J.-I., Kitano H., Matsuoka M., Nagato Y. (2000). Shoot organization genes regulate shoot apical meristem organization and the pattern of leaf primordium initiation in rice. Plant Cell 12: 2161–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D., Veit B., Hake S. (1994). Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120: 405–413. [Google Scholar]
- Johnston R., Candela H., Hake S., Foster T. (2010). The maize milkweed pod1 mutant reveals a mechanism to modify organ morphology. Genesis 48: 416–423. [DOI] [PubMed] [Google Scholar]
- Keller T., Abbott J., Moritz T., Doerner P. (2006). Arabidopsis REGULATOR OF AXILLARY MERISTEMS1 controls a leaf axil stem cell niche and modulates vegetative development. Plant Cell 18: 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu K., Maekawa M., Ujiie S., Satake Y., Furutani I., Okamoto H., Shimamoto K., Kyozuka J. (2003). LAX and SPA: Major regulators of shoot branching in rice. Proc. Natl. Acad. Sci. USA 100: 11765–11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H., Hata S. (1993). Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol. Gen. Genet. 238: 106–119. [DOI] [PubMed] [Google Scholar]
- Lau S., Slane D., Herud O., Kong J., Jürgens G. (2012). Early embryogenesis in flowering plants: Setting up the basic body pattern. Annu. Rev. Plant Biol. 63: 483–506. [DOI] [PubMed] [Google Scholar]
- Laux T., Mayer K.F.X., Berger J., Jürgens G. (1996). The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96. [DOI] [PubMed] [Google Scholar]
- Li X., et al. (2003). Control of tillering in rice. Nature 422: 618–621. [DOI] [PubMed] [Google Scholar]
- Lin H., Niu L., McHale N.A., Ohme-Takagi M., Mysore K.S., Tadege M. (2013). Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc. Natl. Acad. Sci. USA 110: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J., Barton M.K. (2000). Initiation of axillary and floral meristems in Arabidopsis. Dev. Biol. 218: 341–353. [DOI] [PubMed] [Google Scholar]
- Long J.A., Moan E.I., Medford J.I., Barton M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69. [DOI] [PubMed] [Google Scholar]
- Mayer K.F., Schoof H., Haecker A., Lenhard M., Jürgens G., Laux T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. [DOI] [PubMed] [Google Scholar]
- Müller D., Schmitz G., Theres K. (2006). Blind homologous R2R3 Myb genes control the pattern of lateral meristem initiation in Arabidopsis. Plant Cell 18: 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki H., Itoh J., Hayashi K., Hibara K., Satoh-Nagasawa N., Nosaka M., Mukouhata M., Ashikari M., Kitano H., Matsuoka M., Nagato Y., Sato Y. (2007). The small interfering RNA production pathway is required for shoot meristem initiation in rice. Proc. Natl. Acad. Sci. USA 104: 14867–14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata M., Okada K. (2013). The leaf adaxial-abaxial boundary and lamina growth. Plants 2: 174–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardmann J., Werr W. (2006). The shoot stem cell niche in angiosperms: Expression patterns of WUS orthologues in rice and maize imply major modifications in the course of mono- and dicot evolution. Mol. Biol. Evol. 23: 2492–2504. [DOI] [PubMed] [Google Scholar]
- Nardmann J., Werr W. (2013). Symplesiomorphies in the WUSCHEL clade suggest that the last common ancestor of seed plants contained at least four independent stem cell niches. New Phytol. 199: 1081–1092. [DOI] [PubMed] [Google Scholar]
- Nardmann J., Reisewitz P., Werr W. (2009). Discrete shoot and root stem cell-promoting WUS/WOX5 functions are an evolutionary innovation of angiosperms. Mol. Biol. Evol. 26: 1745–1755. [DOI] [PubMed] [Google Scholar]
- Nardmann J., Zimmermann R., Durantini D., Kranz E., Werr W. (2007). WOX gene phylogeny in Poaceae: A comparative approach addressing leaf and embryo development. Mol. Biol. Evol. 24: 2474–2484. [DOI] [PubMed] [Google Scholar]
- Ohmori Y., Tanaka W., Kojima M., Sakakibara H., Hirano H.-Y. (2013). WUSCHEL-RELATED HOMEOBOX4 is involved in meristem maintenance and is negatively regulated by the CLE gene FCP1 in rice. Plant Cell 25: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T., Kyozuka J. (2009). Two-step regulation of LAX PANICLE1 protein accumulation in axillary meristem formation in rice. Plant Cell 21: 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautler M., Tanaka W., Hirano H.Y., Jackson D. (2013). Grass meristems I: Shoot apical meristem maintenance, axillary meristem determinacy and the floral transition. Plant Cell Physiol. 54: 302–312. [DOI] [PubMed] [Google Scholar]
- Sato Y., Hong S.K., Tagiri A., Kitano H., Yamamoto N., Nagato Y., Matsuoka M. (1996). A rice homeobox gene, OSH1, is expressed before organ differentiation in a specific region during early embryogenesis. Proc. Natl. Acad. Sci. USA 93: 8117–8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Matsusaka H., Kumamaru T. (2010). Use of N-methyl-N-nitrosourea treatment of fertilized egg cells for saturation mutagenesis of rice. Breed. Sci. 60: 475–485. [Google Scholar]
- Schmitz G., Tillmann E., Carriero F., Fiore C., Cellini F., Theres K. (2002). The tomato Blind gene encodes a MYB transcription factor that controls the formation of lateral meristems. Proc. Natl. Acad. Sci. USA 99: 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H., Lenhard M., Haecker A., Mayer K.F.X., Jürgens G., Laux T. (2000). The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644. [DOI] [PubMed] [Google Scholar]
- Schumacher K., Schmitt T., Rossberg M., Schmitz G., Theres K. (1999). The Lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc. Natl. Acad. Sci. USA 96: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman B.C. (1945). Leaf and bud initiation in the Gramineae. Bot. Gaz. 106: 269–289. [Google Scholar]
- Suzaki T., Sato M., Ashikari M., Miyoshi M., Nagato Y., Hirano H.-Y. (2004). The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 131: 5649–5657. [DOI] [PubMed] [Google Scholar]
- Suzaki T., Toriba T., Fujimoto M., Tsutsumi N., Kitano H., Hirano H.-Y. (2006). Conservation and diversification of meristem maintenance mechanism in Oryza sativa: Function of the FLORAL ORGAN NUMBER2 gene. Plant Cell Physiol. 47: 1591–1602. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Eiguchi M., Kumamaru T., Satoh H., Matsusaka H., Moriguchi K., Nagato Y., Kurata N. (2008). MNU-induced mutant pools and high performance TILLING enable finding of any gene mutation in rice. Mol. Genet. Genomics 279: 213–223. [DOI] [PubMed] [Google Scholar]
- Tabuchi H., et al. (2011). LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell 23: 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi-Shiobara F., Yuan Z., Hake S., Jackson D. (2001). The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes Dev. 15: 2755–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka W., Pautler M., Jackson D., Hirano H.Y. (2013). Grass meristems II: Inflorescence architecture, flower development and meristem fate. Plant Cell Physiol. 54: 313–324. [DOI] [PubMed] [Google Scholar]
- Uhrig H., Marocco A., Döring H.-P., Salamini F. (1997). The clonal origin of the lateral meristem generating the ear shoot of maize. Planta 201: 9–17. [Google Scholar]
- Wang Q., Kohlen W., Rossmann S., Vernoux T., Theres K. (2014a). Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. Plant Cell 26: 2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li J. (2008). Molecular basis of plant architecture. Annu. Rev. Plant Biol. 59: 253–279. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang J., Shi B., Yu T., Qi J., Meyerowitz E.M., Jiao Y. (2014b). The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell 26: 2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R.K., Perales M., Gruel J., Girke T., Jönsson H., Reddy G.V. (2011). WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev. 25: 2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Wang Q., Schmitz G., Müller D., Theres K. (2012). The bHLH protein ROX acts in concert with RAX1 and LAS to modulate axillary meristem formation in Arabidopsis. Plant J. 71: 61–70. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Suzaki T., Tanaka W., Hirano H.-Y. (2009). The homeotic gene long sterile lemma (G1) specifies sterile lemma identity in the rice spikelet. Proc. Natl. Acad. Sci. USA 106: 20103–20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zong J., Liu J., Yin J., Zhang D. (2010). Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar. J. Integr. Plant Biol. 52: 1016–1026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.