Light-mediated induction of RPT2 reduces the photosensitivity of phot1, which is required for second positive phototropism under bright light conditions.
Abstract
Living organisms adapt to changing light environments via mechanisms that enhance photosensitivity under darkness and attenuate photosensitivity under bright light conditions. In hypocotyl phototropism, phototropin1 (phot1) blue light photoreceptors mediate both the pulse light-induced, first positive phototropism and the continuous light-induced, second positive phototropism, suggesting the existence of a mechanism that alters their photosensitivity. Here, we show that light induction of ROOT PHOTOTROPISM2 (RPT2) underlies photosensory adaptation in hypocotyl phototropism of Arabidopsis thaliana. rpt2 loss-of-function mutants exhibited increased photosensitivity to very low fluence blue light but were insensitive to low fluence blue light. Expression of RPT2 prior to phototropic stimulation in etiolated seedlings reduced photosensitivity during first positive phototropism and accelerated second positive phototropism. Our microscopy and biochemical analyses indicated that blue light irradiation causes dephosphorylation of NONPHOTOTROPIC HYPOCOTYL3 (NPH3) proteins and mediates their release from the plasma membrane. These phenomena correlate closely with the desensitization of phot1 signaling during the transition period from first positive phototropism to second positive phototropism. RPT2 modulated the phosphorylation of NPH3 and promoted reconstruction of the phot1-NPH3 complex on the plasma membrane. We conclude that photosensitivity is increased in the absence of RPT2 and that this results in the desensitization of phot1. Light-mediated induction of RPT2 then reduces the photosensitivity of phot1, which is required for second positive phototropism under bright light conditions.
INTRODUCTION
Photosensory adaptation is the phenomenon by which the photosensitivity of photoreceptor signaling networks is altered in response to changes in light intensity. This phenomenon enables photoreceptor signaling networks to maintain a response over a broad range of light intensities (Galland, 1991; Wang and Montell, 2007). Mammalian optic organs adapt to dark and bright light conditions using rod cells that show high photosensitivity and cone cells that show low photosensitivity, respectively. In Arabidopsis thaliana, photoreceptors that are highly sensitivity to light, such as phytochrome A (phyA), cryptochrome2 (cry2), and phototropin1 (phot1), mediate photosensory adaptation. Under dark conditions, the expression of these photoreceptors is upregulated, resulting in increased photosensitivity, whereas under bright light conditions, these photoreceptors are degraded, thereby decreasing photosensitivity (Pedmale and Liscum, 2007; Franklin and Quail, 2010; Chaves et al., 2011). Other members of the photoreceptor family, such as phyB, cry1, and phot2, are expressed at constant levels under bright light conditions. In addition to photoreceptors, some signal transducers functioning downstream of the photoreceptors are required for establishing light adaptation systems (Galland, 1991; Wang and Montell, 2007; Hersch et al., 2014).
Phototropism, the growth response that orients plants in the direction of the light source to maximize light absorption, is regulated by phot1 and phot2 in Arabidopsis (Sakai and Haga, 2012; Christie and Murphy, 2013). Physiological analyses using blue light pulse irradiations indicate that, as the light intensity increases, hypocotyl curvatures of etiolated seedlings show a bell-shaped dose-response curve (Supplemental Figure 1; Poff et al., 1994). This is referred to as the first positive phototropism (Iino, 2001). A decrease in phototropic curvatures in the first positive phototropism is caused by the transient saturation of photoproducts involved in light perception and the signal transduction between the irradiated and shaded sides of the plant (Iino, 2001; Christie and Murphy, 2013). When the duration of blue light irradiation exceeds 10 to 30 min, the seedlings adapt to light environments and the phototropic response recovers after the refractory state (Supplemental Figure 1; Poff et al., 1994). This is referred to as the time-dependent, second positive phototropism (Iino, 2001). These phototropic responses are induced even by blue light intensities of <1 μmol m–2 s–1, conditions under which phot2 does not function (Sakai et al., 2001). The transition from first positive phototropism to second positive phototropism is relatively rapid, occurring more quickly than phot1 degradation (∼2 h; Roberts et al., 2011). It has been suggested that a conversion mechanism alters the sensitivity of phot1 signaling to light (Iino, 2001). Red light pretreatment affects several aspects of the phototropic response, such as enhancement of phototropic curvatures and desensitization to weak blue light in the first positive phototropism, appearance of the second positive phototropism, and reduction of the refractory period, which minimizes the lag period before the second positive phototropism. Even under red light pretreatment, the first positive phototropism was severely impaired in the phyA phyB double mutant, and the onset of second positive phototropism was delayed (Janoudi et al., 1997). Phy signaling appears to be involved in the light adaptation mechanism of phot1 signaling (Briggs, 2014).
NONPHOTOTROPIC HYPOCOTYL3 (NPH3) and ROOT PHOTOTROPISM2 (RPT2), both of which belong to the same NPH3/RPT2-like (NRL) family, are signal transducers that function in phototropism, and both proteins form a complex with phot1 that localizes to the inner surface of the plasma membrane (Motchoulski and Liscum, 1999; Sakai et al., 2000; Inada et al., 2004). A defect in NPH3 causes a complete loss of phototropism in Arabidopsis and rice (Oryza sativa) seedlings (Motchoulski and Liscum, 1999; Haga et al., 2005). NPH3 is phosphorylated under dark conditions and dephosphorylated by blue light irradiation in a phot1-dependent manner (Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008). It appears that phototropic responses depend on dephosphorylation of NPH3 and that NPH3 is active when dephosphorylated (Pedmale and Liscum, 2007). By contrast, the rpt2-1 mutant, which harbors a point mutation in the second exon splicing site that causes a frameshift in the following exon, exhibits a curious phenotype: phototropic curvatures of the mutant hypocotyls are induced by low fluence rate blue light, but the degree of curvature decreases as the light intensity increases (Sakai et al., 2000). Red and blue light-induced expression of RPT2 is regulated by both phys and crys (Sakai et al., 2000; Tsuchida-Mayama et al., 2010). Genetic analysis of phot1 rpt2 and phot2 rpt2 double mutants suggests that RPT2 is not necessary for phot2-mediated blue light signal transduction but is required for phot1-mediated photosensory adaptation responses (Inada et al., 2004). Because light-inducible RPT2 interacts with NPH3 in addition to phot1, RPT2 appears to modulate the function of phot1 and NPH3 and/or the interaction between phot1 and NPH3 in response to light (Inada et al., 2004).
In this study, we analyzed the phototropic responses of the rpt2 mutant to clarify the molecular mechanisms of RPT2-mediated photosensory adaptation. Under strong blue light and relatively weak blue light, the rpt2-2 null mutant showed severe phototropic impairment. We showed that the light-mediated induction of RPT2 is a critical step in the desensitization of a phot1-mediated light perception system, causing photosensory adaptation in Arabidopsis hypocotyls. Furthermore, our biochemical and cell biological analyses revealed that phot1-mediated dephosphorylation of NPH3 caused NPH3 proteins to detach from phot1 proteins and the plasma membrane, transiently resulting in an induction of the refractory period. In addition, we showed that RPT2 regulated the phosphorylation status of NPH3 proteins, the interaction between NPH3 and phot1, and the membrane localization of the phot1-NPH3 complex, strongly suggesting that these functions of RPT2 are required for photosensory adaptation.
RESULTS
RPT2 Is Necessary for the Appearance of Continuous Light-Induced Second Positive Phototropism
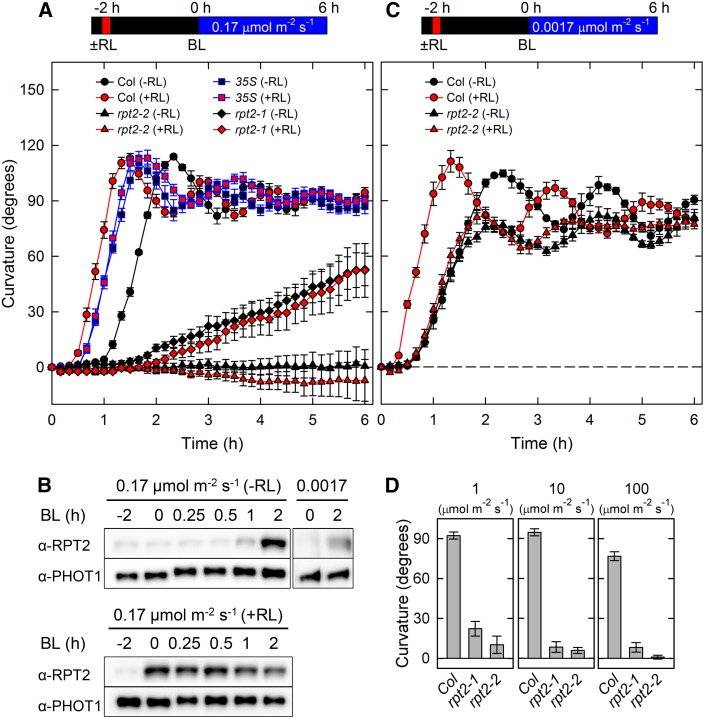
We hypothesized that the time required for light-mediated induction of RPT2 determines the length of the refractory state between the first and second positive phototropism. To test this hypothesis, we first investigated the time courses of the continuous light-induced second positive phototropism of Arabidopsis hypocotyls of the wild type, rpt2 mutants, and transgenic plants constitutively expressing RPT2 (35Spro:RPT2; Figure 1). When dark-grown seedlings were irradiated with unilateral blue light at 0.17 μmol m–2 s–1, the wild type showed phototropic curvatures around 70 min after the onset of exposure to blue light, and maximum curvatures appeared 140 min after the phototropic stimulation (Figure 1A). Immunoblot analysis showed that RPT2 expression was induced by 1 h of blue light treatment, and a high level of expression was observed after 2 h of light treatment in wild-type seedlings (Figure 1B), indicating that the timing of RPT2 expression corresponds with that of the phototropic responses. The rpt2-1 mutant, which was produced by ethyl methanesulfonate treatment (Sakai et al., 2000), requires a longer period to induce phototropic curvatures than the wild type does (Figure 1A). Furthermore, the hypocotyls bent gradually and the curvatures did not reach the horizontal position even after 6 h of blue light irradiation in the mutant. The rpt2-2 null mutant, which is a transposon insertion mutant (Inada et al., 2004), showed severe phototropic impairment (Figure 1A). By contrast, in transgenic plants expressing RPT2 driven by the cauliflower mosaic virus 35S promoter (CaMV 35Spro:RPT2), phototropic responses were observed 40 min after the onset of blue light irradiation, followed by maximum curvatures at around 100 min (Figure 1A). These results indicate that partial or severe impairment of RPT2 function delays or attenuates the responses of the second positive phototropism and that the constitutive expression of RPT2 significantly reduces the lag time by inducing the responses.
Figure 1.
Continuous Light-Induced Second Positive Phototropism in the rpt2 Mutants and the RPT2 Overexpression Line.
(A) Time-course analysis of continuous light-induced phototropism. Two-day-old dark-grown seedlings (Columbia [Col; circles], rpt2-2 [triangles], rpt2-2 transgenic plants harboring 35Spro:RPT2 [35S; squares], and rpt2-1 [diamonds]) were pretreated with (red symbols) or without (black symbols) overhead red light (RL) at 20 μmol m–2 s–1 for 2 min. The hypocotyls were then irradiated with unilateral blue light (BL) at 0.17 μmol m–2 s–1 for 6 h, during which time the hypocotyl curvatures were determined at 10-min intervals. The data shown are means ± se from eight seedlings.
(B) Effects of light irradiation on levels of RPT2. Dark-grown seedlings were pretreated with (top panel) or without (bottom panel) overhead red light for 2 min. After 2 h, seedlings were continuously irradiated with unilateral blue light at 0.17 or 0.0017 μmol m–2 s–1. The shoots were harvested at the indicated time points and used to prepare crude microsomal insoluble proteins. Proteins (6 μg) in each fraction were separated on 7.5% SDS-PAGE gels, followed by immunoblotting with anti-RPT2 and anti-PHOT1 antibodies. Time 0 corresponds to the onset of unilateral blue light treatment.
(C) Time-course analysis of continuous light-induced phototropism at 0.0017 μmol m–2 s–1. The data shown are means ± se from eight seedlings. Other details are as described in Figure 1A.
(D) Hypocotyl phototropism induced by strong blue light. Dark-grown seedlings were irradiated with unilateral blue light for 6 h at 1, 10, and 100 μmol m–2 s–1. The data shown are means ± se from 10 seedlings.
When wild-type plants were irradiated with a very weak blue light (0.0017 μmol m–2 s–1), phototropic responses were induced sooner than when irradiated with weak blue light (0.17 μmol m–2 s–1) (Figures 1A and 1C). On the other hand, the rpt2-2 mutants showed slightly decreased, but pronounced, curvatures and a normal lag time in response to unilateral blue light at a very low fluence rate (0.0017 μmol m–2 s–1; Figure 1C). These results indicate that RPT2 is not necessary for photosensory adaptation and a shortening of the lag time under very weak blue light conditions. Because RPT2 (i.e., the time for the RPT2 induction) was not required for the second positive phototropism under very weak blue light conditions, the lag time under very weak blue light conditions appeared to be shorter than that under weak blue light conditions. By contrast, phototropic defects were more severe in the rpt2 mutants under higher blue light intensities (Figure 1D). These results indicate that RPT2 is critical for responses to higher intensities of blue light but not to very low intensities, as has been described previously (Sakai et al., 2000; Inada et al., 2004), and that failure to adapt to high-intensity blue light is much more severe in rpt2-2 than in rpt2-1. Therefore, rpt2-2 and rpt2-1 alleles were likely null and leaky, respectively. Although our previous immunoblot analysis did not reveal RPT2 expression in the rpt2-1 mutant (Inada et al., 2004), the transcript levels of RPT2 in the rpt2-1 mutant determined by quantitative RT-PCR or RNA gel blotting were similar to those in the wild type. By contrast, no RPT2 expression was detected in the rpt2-2 mutant (Supplemental Figure 2 and Supplemental Table 1). Truncated forms of RPT2 proteins with reduced function may be expressed at low levels in the rpt2-1 mutant if alternative start codons following the mutation at residue Trp-24 are used (e.g., a codon encoding the RPT2 residue Met-49; Sakai et al., 2000).
Previous studies have reported that red light pretreatment reduces the lag time to induce phototropic responses in Arabidopsis hypocotyls and accelerates phototropic curvature rates (Hangarter, 1997; Haga and Sakai, 2012; Haga et al., 2014). When the red light pretreatment was applied for 2 min before 2 h of phototropic stimulation, the lag time, in addition to the period required for the induction of maximum curvatures, became significantly shorter, and the phototropic curvature rates were accelerated in wild-type hypocotyls (Figures 1A and 1C). Immunoblot analysis showed that red light pretreatment considerably enhanced levels of RPT2 proteins 2 h after the pretreatment, which corresponds to time 0 (i.e., the onset of blue light irradiation; Figure 1B). Furthermore, the red light pretreatment did not affect the phototropic responses in rpt2-1, rpt2-2, and transgenic plants harboring 35Spro:RPT2 (Figures 1A and 1C). Therefore, induction of RPT2 expression reduces the lag time and increases the rate of phototropic curvature following red light pretreatment in the second positive phototropism.
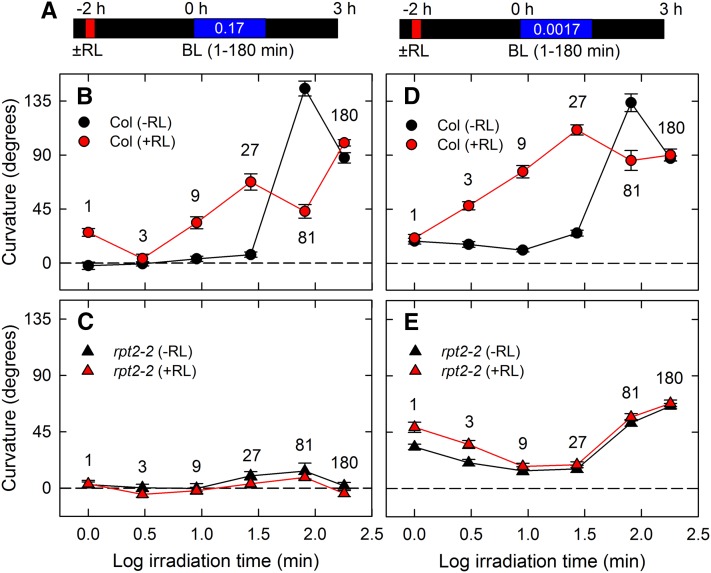
To examine how many minutes the seedlings require to establish the adaptation to phototropic stimulation, we next analyzed the effects of irradiation time on phototropic curvature when irradiating plants for 1 to 180 min with blue light (Figure 2). When seedlings were irradiated with 0.17 μmol m–2 s–1 blue light in the absence of red light pretreatment, a 1- to 27-min irradiation followed by dark incubation did not induce phototropic responses in the wild-type seedlings (Figures 2A and 2B, black symbols). When seedlings were irradiated with blue light for 81 min followed by dark incubation, there was overshooting of the phototropic curvature at around 140°, and 180 min of continuous irradiation induced phototropic curvatures that were nearly in the horizontal position (Figure 2B, black symbols). On the other hand, the rpt2-2 mutant did not show any significant phototropic curvatures (Figure 2C), suggesting that the second positive phototropism requires recovery periods of longer than 27 min after the start of phototropic stimulation in an RPT2-dependent manner. When wild-type seedlings were pretreated with red light to induce RPT2 expression (Figure 2B, red symbols), a 1-min blue light pulse irradiation resulted in phototropic responses, which presumably corresponds to the first positive phototropism, a 3-min irradiation did not result in any curvatures, which may correspond to the refractory state, and a 9-min irradiation induced phototropic curvatures, which corresponds to the second positive phototropism. Red light pretreatment did not have a similar effect on rpt2-2 (Figure 2C). These impairments were clear when the data presented in Figure 2 were replotted as fluence-response curves (Supplemental Figure 3). Therefore, RPT2 expression induced by red light pretreatment does not eliminate the appearance of the refractory state but can reduce the duration of the refractory state significantly, resulting in immediate induction of second positive phototropism.
Figure 2.
Time-Dependent Second Positive Phototropism in the rpt2-2 Mutant.
Dark-grown seedlings were pretreated with or without overhead red light (RL). After 2 h, the hypocotyls were stimulated with unilateral blue light (BL). The hypocotyl curvatures were determined 3 h after the onset of blue light.
(A) Experimental scheme for time-dependent second positive phototropism.
(B) to (E) Time-dependent second positive phototropism. The dark-grown seedlings were pretreated with (red symbols) or without (black symbols) overhead red light, and they were irradiated with unilateral blue light at 0.17 μmol m–2 s–1 ([B] and [C]) and 0.0017 μmol m–2 s–1 ([D] and [E]) for 1, 3, 9, 27, 81, and 180 min. The data shown are means ± se from 11 to 16 seedlings.
Under irradiation with a very weak blue light (0.0017 μmol m–2 s–1), adaptation to continuous phototropic stimulation occurred much more readily; in the wild type, the second positive phototropism was slightly induced, even by 27 min of irradiation in the absence of red light pretreatment (Figure 2D, black symbols), and the refractory state was almost absent with red light pretreatment (Figure 2D, red symbols). The lag period of 20 min under the conditions of continuous blue light with red light pretreatment (Figure 1C) probably indicates the time required for establishing asymmetrical growth caused by an auxin gradient following the perception of phototropic signals rather than the time required for escaping the refractory state and establishing the photosensory adaptation. In the case of the rpt2-2 mutant, a 27-min irradiation was not effective in inducing second positive phototropism without red light pretreatment, and the red light pretreatment did not reduce the length of the refractory state (Figure 2E). It was unclear whether reduction of the second positive phototropic responses induced by 27 and 81 min of blue light in the rpt2-2 mutant resulted from a delay in the appearance of second positive phototropism by deletion of RPT2 or from the attenuation of phototropic curvatures. Interestingly, in the rpt2-2 hypocotyls, 1- and 3-min irradiations caused larger curvatures than in the wild type, and red light preirradiation enhanced the hypocotyl curvatures. These results suggest that RPT2 functions as a negative regulator of first positive phototropism under very weak blue light conditions and that enhancement of the first positive phototropic curvatures by red light pretreatment is mediated by components other than RPT2. These results are in agreement with our recent finding that the PINOID (PID)/WAVY ROOT GROWTH (WAG) family, which regulates auxin transporter PIN-FORMED (PIN) proteins, is an essential element of phytochrome-mediated enhancement of first positive phototropism (Haga et al., 2014).
RPT2 Regulates Photosensitivity during Hypocotyl Phototropism
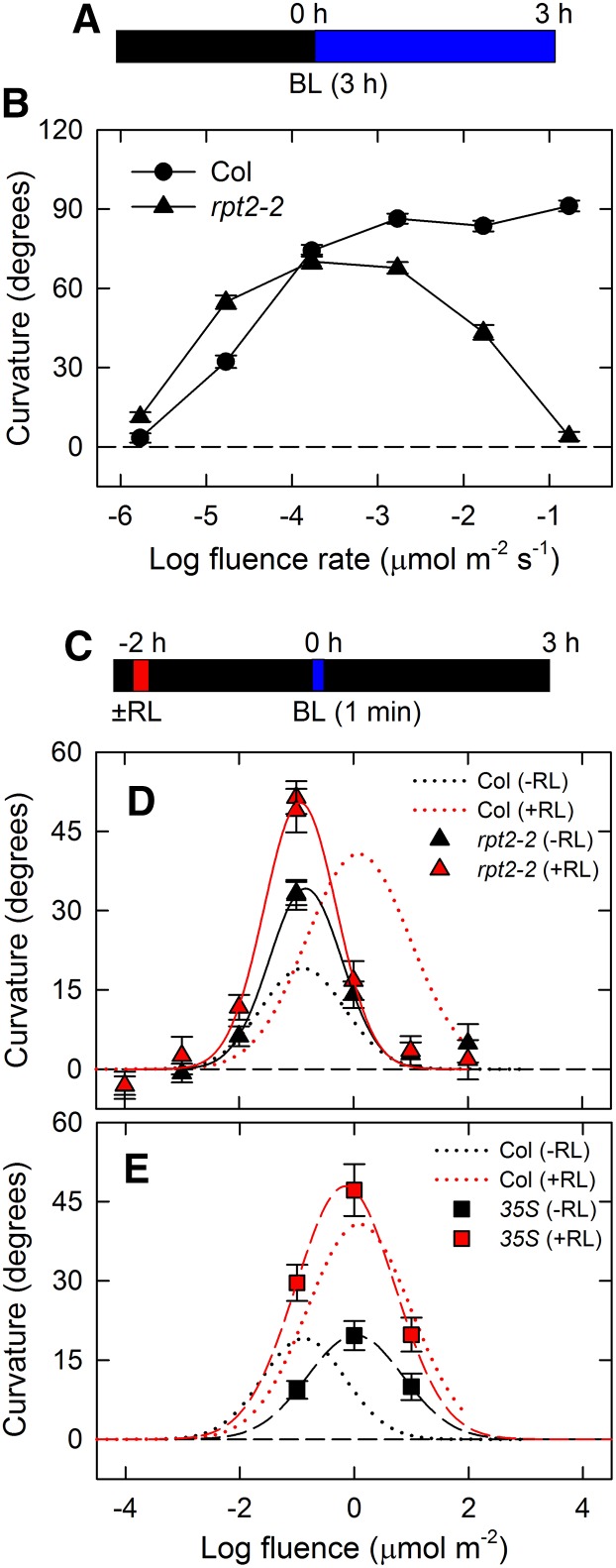
Our results indicated that RPT2 is necessary for adaptation to blue light conditions, which suggests that RPT2 regulates photosensitivity during hypocotyl phototropism. Therefore, we investigated the fluence-response relationship for phototropic responses in the rpt2-2 mutant (Figure 3). When wild-type seedlings were irradiated with continuous blue light for 3 h, a fluence rate of 1.7 × 10−6 μmol m–2 s–1 had no effect, whereas a fluence rate at 1.7 × 10−3 μmol m–2 s–1 almost caused hypocotyls to reach the horizontal position (Figures 3A and 3B). Interestingly, the rpt2-2 mutant exhibited higher and lower sensitivities than the wild type at a fluence rate of 1.7 × 10−5 μmol m–2 s–1 and fluence rates of 1.7 × 10−3 μmol m–2 s–1 or more, respectively (Figure 3B). This suggested that an absence of RPT2 causes hyperphotosensitivity, leading to an increase in hypocotyl curvature at a fluence rate of 1.7 × 10−5 μmol m–2 s–1 and a decrease at fluence rates of 1.7 × 10−3 μmol m–2 s–1 or more through desensitizing phototropic stimulation in the continuous light-induced second positive phototropism.
Figure 3.
Fluence-Response Curves of Continuous Light-Induced Phototropism and Pulse-Induced First Positive Phototropism in the rpt2-2 Mutant.
Dark-grown seedlings were pretreated with or without overhead red light (RL). After 2 h, the hypocotyls were stimulated with unilateral blue light (BL). The hypocotyl curvatures were determined 3 h after the onset of blue light.
(A) Experimental scheme for continuous light-induced phototropism.
(B) Fluence rate-response curves of continuous light-induced phototropism. Dark-grown seedlings were irradiated with unilateral blue light for 3 h. The data shown are means ± se from 38 to 54 seedlings.
(C) Experimental scheme for pulse-induced first positive phototropism.
(D) and (E) Fluence-response curves of pulse-induced phototropism in the rpt2-2 mutant (D) and rpt2-2 transgenic plants harboring 35Spro:RPT2 (35S) (E). Hypocotyls were stimulated with unilateral blue light at various fluences with (red symbols) or without (black symbols) red light pretreatment. The data shown are means ± se from 16 seedlings. The fitted curves obtained from Columbia (Col) data (Haga and Sakai, 2012) are reproduced as dotted lines, for comparison.
We next analyzed the fluence-response relationship of the first positive phototropism induced by a 1-min blue light pulse in the rpt2-2 mutant (Figure 3C). Previous studies showed that a typical bell-shaped curve appears in the pulse-induced first positive phototropism in the wild type and that red light pretreatment enhances the phototropic curvatures and shifts the peak of the fluence-response curve to higher fluences (Figure 3D; Haga and Sakai, 2012). Although the fluence-response curves of the rpt2-2 mutant showed that the fluence dependency is similar to that of the wild type, maximum curvatures of the mutant were higher than those of the wild type when the seedlings were not pretreated with red light (Figure 3D). On the other hand, while red light pretreatment enhanced the phototropic curvature in the rpt2-2 mutant, the pretreatment did not shift the fluence-response curve in the mutant (Figure 3D). In transgenic plants constitutively expressing RPT2 (35Spro:RPT2), the shift of the fluence-response curve was observed even when the seedlings were not pretreated with red light, although the pretreatment enhanced the phototropic curvatures in the transgenic plants harboring 35Spro:RPT2 (Figure 3E). Furthermore, the phototropic curvatures induced by a pulse of blue light at 0.1 μmol m–2 in transgenic plants harboring 35Spro:RPT2 were lower than those in the wild type when the seedlings were not pretreated with red light. These results indicate that RPT2 functions as a negative regulator in the first positive phototropism under relatively low fluence conditions (such as 0.1 μmol m–2) and that its constitutive expression in etiolated seedlings decreases photosensitivity. Furthermore, our results indicate that expression of RPT2 influences the deetiolation of seedlings and desensitizes photoreceptors to low fluence light and that RPT2 functions in red light-mediated photosensory adaptation but not in red light-mediated enhancement of the phototropic responses. Therefore, suppression of RPT2 expression under dark conditions enhances photosensitivity, and the light-mediated induction of RPT2 expression promotes photosensory adaptation, resulting in the appearance of the second positive phototropism, which enables plants to adapt to a wide range of light conditions in nature.
phot1-Mediated Internalization of NPH3 Is Regulated by RPT2
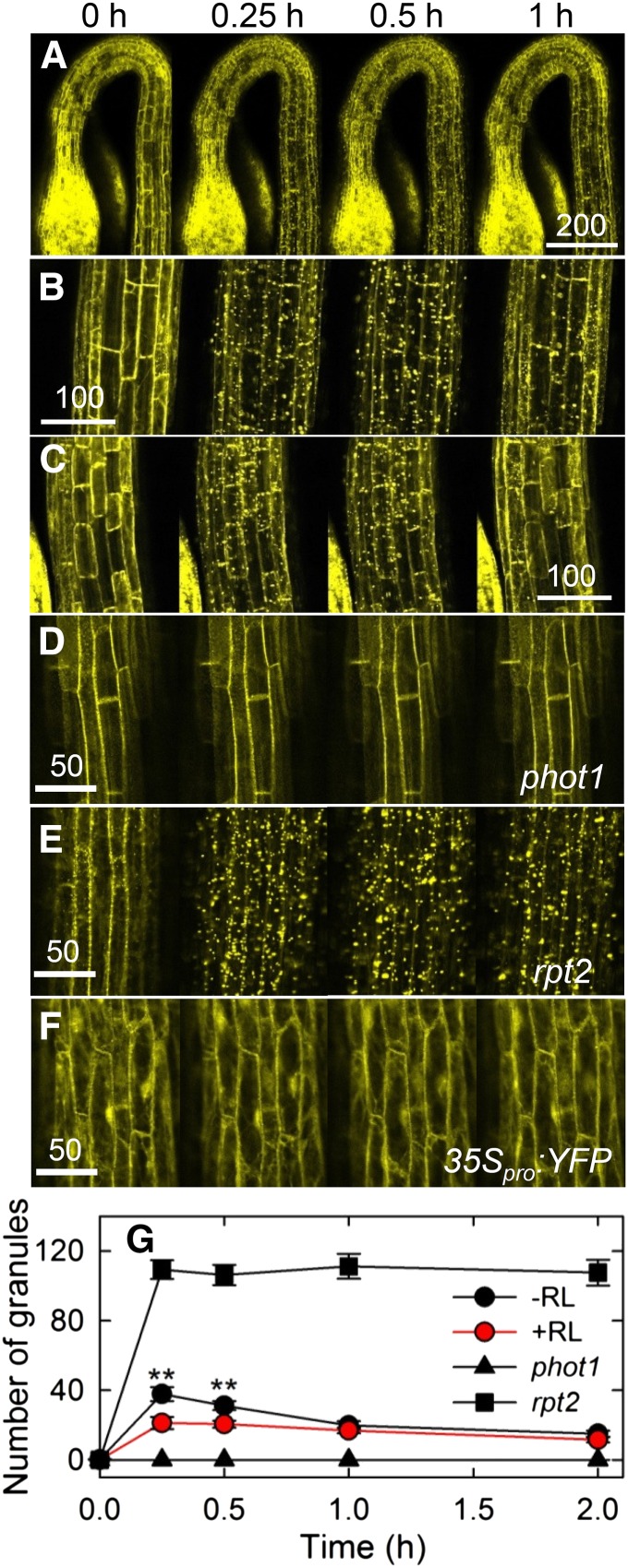
When we next analyzed the expression patterns of NPH3 proteins in pulse-induced first positive phototropism using nph3-102 transgenic plants harboring 35Spro:YFP-NPH3, we noticed alterations in the subcellular localization of yellow fluorescent protein (YFP)-NPH3 proteins in response to light irradiation (Figure 4). The severe impairment of hypocotyl phototropism in the nph3-102 mutant was complemented in the transgenic plants (Supplemental Figure 4). When the transgenic plants were grown under complete darkness, most YFP signal was detected uniformly around the cellular peripheral regions (Figures 4A and 4B), supporting the previous results that NPH3 proteins are localized to the plasma membrane (Motchoulski and Liscum, 1999). Intriguingly, blue light irradiation at a low fluence rate (0.17 μmol m–2 s–1) attenuated the YFP signal originally detected around the cellular peripheral regions, and some YFP signal was observed in the cytosol (Figure 4B). YFP signal detached from the plasma membrane formed small grains, suggesting that YFP-NPH3 proteins formed aggregates. Furthermore, the aggregated YFP particles became larger with an increase in the duration of blue light irradiation. Following 1 h of irradiation, the amount of YFP aggregates was reduced, and the YFP signals were once more observed around the peripheral regions of the cells. As transgenic plants harboring 35Spro:YFP did not show such alterations (Figure 4F), these results indicate that blue light irradiation leads to a transient release of NPH3 proteins from the plasma membrane.
Figure 4.
Effects of Light Treatments on the Localization of NPH3 Proteins.
(A) Localization of NPH3 proteins without red light pretreatment. Dark-grown transgenic nph3 plants harboring 35Spro:YFP-NPH3 were stimulated with blue light at 0.17 μmol m–2 s–1, and YFP signals were observed with a microscope at the indicated times after the onset of blue light irradiation. Bar = 200 μm.
(B) and (C) Effects of red light pretreatment on the localization of NPH3 proteins. The transgenic plants were pretreated with (C) or without (B) overhead red light and then stimulated with blue light at 0.17 μmol m–2 s–1. Time 0 corresponds to the onset of blue light irradiation. Bars = 100 μm.
(D) Localization of NPH3 proteins in the phot1 mutant. Dark-grown phot1 mutants harboring 35Spro:YFP-NPH3 were stimulated with blue light at 0.17 μmol m–2 s–1. Bar = 50 μm.
(E) Localization of NPH3 proteins in the rpt2-2 mutant. Dark-grown rpt2-2 mutants harboring 35Spro:YFP-NPH3 were stimulated with blue light at 0.17 μmol m–2 s–1. Bar = 50 μm.
(F) Distribution of YFP fluorescence. Dark-grown Columbia seedlings harboring 35Spro:YFP were stimulated with blue light at 0.17 μmol m–2 s–1. Bar = 50 μm.
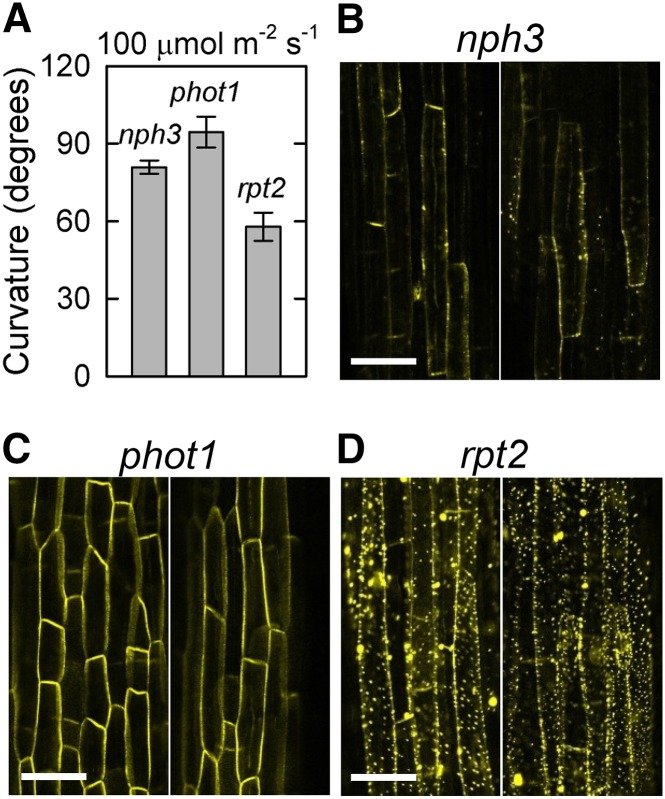
(G) Quantification of YFP granules. The nph3, phot1, and rpt2-2 mutants harboring 35Spro:YFP-NPH3 were pretreated with or without overhead red light (RL) and then stimulated with blue light at 0.17 μmol m–2 s–1. Time 0 corresponds to the onset of blue light irradiation. The number of YFP granules of >1 μm diameter was counted after blue light irradiation. The data shown are means ± se from 8 to 18 seedlings. Double asterisks indicate statistically significant differences compared with red light pretreatment (Student’s t test, P < 0.01).
Although red light pretreatment alone did not influence the membrane localization of NPH3 proteins, the blue light-induced aggregation of NPH3 proteins was partially prevented by the pretreatment (Figure 4C). Quantitative analysis confirmed the effects of blue light on the aggregation of NPH3 proteins and the inhibitory effects of red light pretreatment (Figure 4G). These results suggest not only that a reduction of phototropic responses in the first positive phototropism correlates with a release of NPH3 proteins from the plasma membrane but also that the appearance of the second positive phototropism correlates with a redistribution of NPH3 proteins to the plasma membrane. These results also suggest that the desensitization of the first positive phototropism mediated by red light pretreatment is closely related to the suppression of blue light-induced aggregation of NPH3 proteins.
Our previous studies indicated that phot1 inhibits continuous light-induced phototropism in the absence of RPT2 (Sakai et al., 2000; Inada et al., 2004). We hypothesized that activation of phot1 concurrently induces the first positive phototropism and the release of NPH3 proteins from the plasma membrane, resulting in a reduction of phototropic responses. We also hypothesized that the absence of RPT2 fails to redistribute NPH3 proteins to the plasma membrane, resulting in failure of the second positive phototropism. To examine this possibility, we observed the distribution patterns of YFP-NPH3 in phot1 and rpt2 mutants. As expected, a blue light stimulus did not induce any significant alterations of YFP signals in the phot1 mutant (Figures 4D and 4G), and the rpt2 mutant showed exaggerated blue light-induced granulation of YFP signals (Figures 4E and 4G). These results suggest that the blue light-induced generation of NPH3 aggregates is mediated by phot1 proteins and that phot1-mediated aggregation of NPH3 is attenuated by RPT2.
Subsequently, we examined the effects of a very weak blue light and a strong blue light on YFP-NPH3 distribution changes in nph3, rpt2, and phot1 mutant backgrounds. The generation of YFP-NPH3 aggregates was attenuated under blue light irradiation at 0.001 μmol m–2 s–1 for 6 h but not under strong blue light irradiation at 100 μmol m–2 s–1 in nph3 (Figure 5). A strong blue light irradiation at 100 μmol m–2 s–1, which activates another phot, phot2 (Sakai et al., 2001), did not induce the generation of YFP-NPH3 aggregates in the phot1 mutant (Figure 5B). This suggests that phot2 does not have the ability to induce the release of NPH3 proteins from the plasma membrane. This result appeared to be consistent with the phototropic responses of the phot1 mutant under such light conditions (Sakai et al., 2000; Inada et al., 2004). In the rpt2 mutant, weak blue light irradiation, which induces phototropic responses in the mutant, partially attenuated the generation of YFP-NPH3 aggregates and partially eliminated YFP-NPH3 signal from the cellular peripheral regions (Figure 5A). However, aggregation was greater in rpt2 than in nph3 and phot1. Furthermore, strong blue light irradiation enhanced the formation of YFP aggregates (Figure 5B). These results also indicated that detachment of NPH3 proteins from the plasma membrane due to the production of NPH3 aggregates corresponds to an impairment of phototropic responses.
Figure 5.
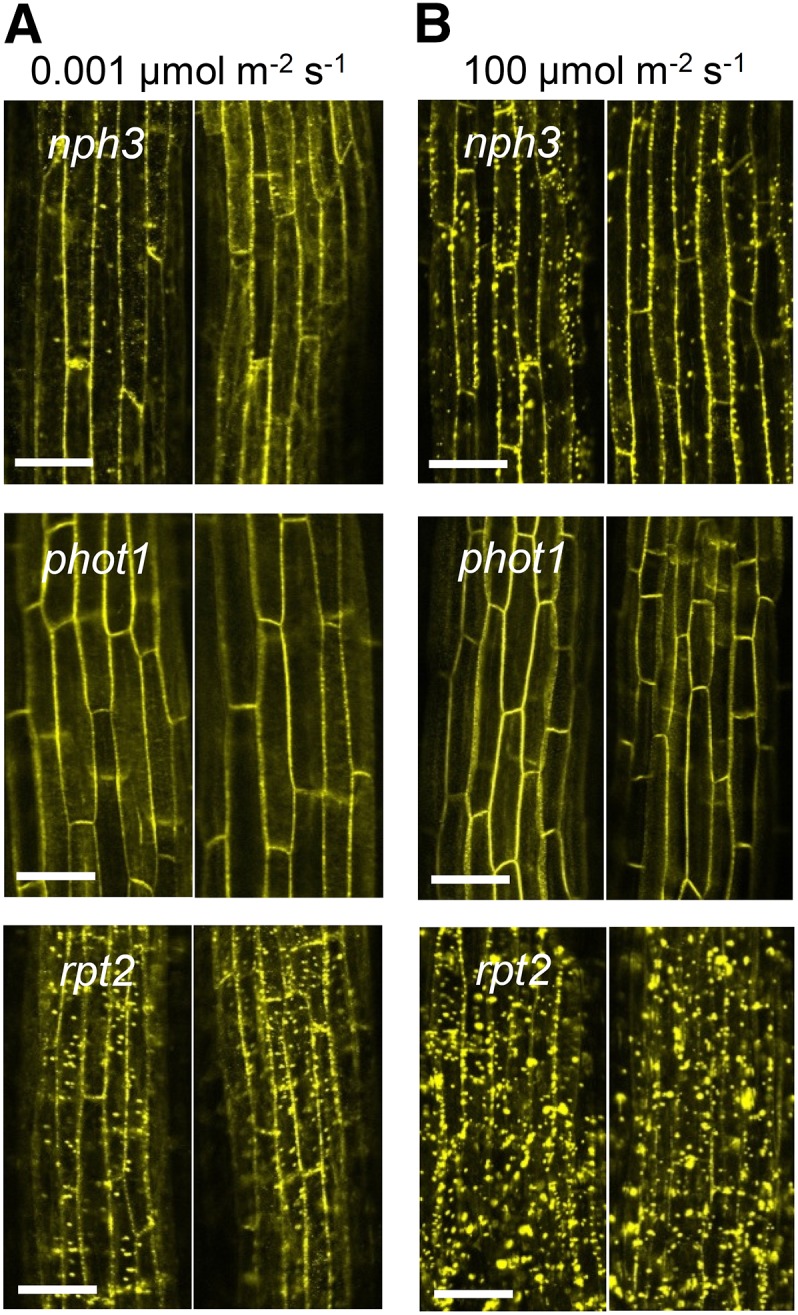
Effects of Blue Light Intensity on Granulation of NPH3.
(A) Effects of very weak blue light on NPH3 granulation. The nph3, phot1, and rpt2-2 mutants harboring 35Spro:YFP-NPH3 were stimulated with blue light at 0.001 μmol m–2 s–1 for 6 h, and two representative images are shown for each. Bars = 50 μm.
(B) Effects of strong blue light on NPH3 granulation. The nph3, phot1, and rpt2-2 mutants harboring 35Spro:YFP-NPH3 were stimulated with blue light at 100 μmol m–2 s–1 for 6 h, and representative images are shown for each. Bars = 50 μm.
Next, we examined the effects of a prolonged blue light irradiation at 100 μmol m–2 s–1 for 24 h on the phototropic response and the distribution pattern of YFP-NPH3 in the rpt2-2 mutant (Figure 6). To prevent the seedlings and agar medium from drying out during prolonged irradiation, seedlings were grown along the surface of a vertically oriented agar plate. Under these conditions, phototropic responses were partially recovered in the rpt2-2 mutant and YFP-NPH3 signals were present around the cellular peripheral regions (Figures 6A and 6B). This result suggests that prolonged irradiation leads to partial recovery of the redistribution of NPH3 proteins to the plasma membrane in the absence of functional RPT2. The YFP-NPH3 signals were markedly attenuated in nph3, but not in phot1 and rpt2, under this light condition (Figures 6B to 6D). A prolonged blue light irradiation at high fluence rates might destabilize NPH3 proteins in a phot1- and RPT2-dependent manner.
Figure 6.
Effects of a Long Blue Light Irradiation on Granulation of NPH3.
(A) Hypocotyl phototropism induced by long blue light. The transgenic nph3, phot1, and rpt2 mutants harboring 35Spro:YFP-NPH3 were stimulated with blue light at 100 μmol m–2 s–1 for 24 h, and hypocotyl curvatures were measured. The data shown are means ± se from 10 to 23 seedlings.
(B) to (D) Distribution of NPH3 proteins. The nph3 (B), phot1 (C), and rpt2-2 (D) mutants harboring 35Spro:YFP-NPH3 were stimulated with blue light at 100 μmol m–2 s–1 for 24 h, and two representative images are shown for each. Bars = 50 μm.
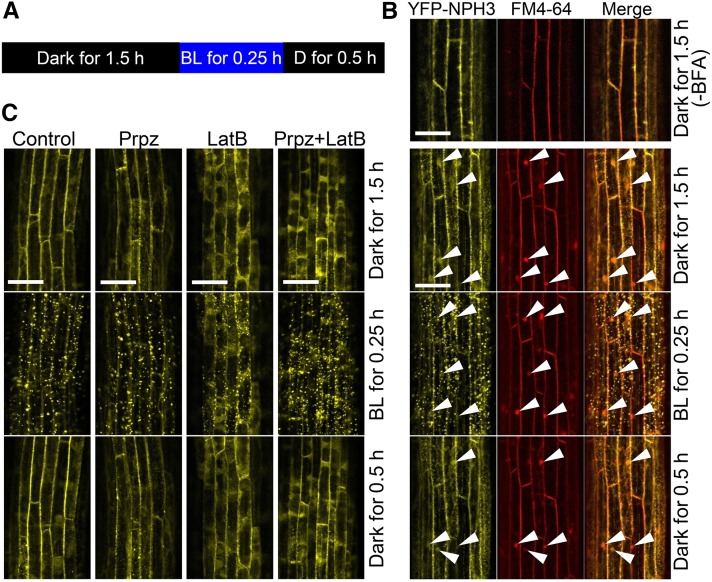
Next, we examined whether the alternation of YFP-NPH3 distribution patterns between the plasma membrane and the cytosol and/or granules depended on endocytosis and exocytosis, similar to PIN auxin efflux carrier proteins (Dhonukshe et al., 2007; Kleine-Vehn et al., 2009). We did so using the guanine nucleotide exchange factors for the ADP-ribosylation factor inhibitor brefeldin A (BFA) (Figure 7), which inhibits BFA-sensitive exocytosis and leads to the formation of intracellular vesicles called BFA compartments (Steinmann et al., 1999; Geldner et al., 2001). Under dark conditions (Figure 7A), BFA treatment caused the formation of BFA compartments, which were visualized using the membrane-staining agent FM4-64, but not of YFP-NPH3 aggregates in dark-grown nph3 transgenic plants harboring 35Spro:YFP-NPH3 (Figure 7B). Blue light irradiation at 0.1 μmol m–2 s–1 for 0.25 h caused striking aggregation of YFP-NPH3 signals, and subsequent dark incubation for 30 min restored the membrane localization of YFP-NPH3 proteins. BFA did not attenuate blue light-induced aggregation of YFP-NPH3 signals and the recovery of membrane localization by the following dark incubation (Figure 7B). We then analyzed the colocalization of YFP-NPH3 aggregates with intracellular vesicles, visualized by FM4-64, and YFP signal was detected even at sites that were not stained with FM4-64, but not in the BFA compartments. We also examined the effects of propyzamide and latrunculin B, which are microtubule- and actin-destabilizing drugs, respectively. The dark-grown nph3 transgenic plants were treated with propyzamide and/or latrunculin B (Figure 7C), both of which partially attenuated phototropic responses during the early stage of the response and elongation growth of hypocotyls (Supplemental Figure 5). Although the drugs, especially latrunculin B, promoted the localization of YFP signals to the cytosol, blue light-induced aggregation of YFP signals and recovery from aggregation during a subsequent dark incubation were not severely impaired by those chemicals. Thus, neither vesicle trafficking systems nor cytoskeleton systems are involved in the blue light-mediated alteration of NPH3 distribution patterns.
Figure 7.
Effects of Inhibitors on Blue Light-Induced Granulation of NPH3.
(A) Experimental scheme. Dark-grown nph3 seedlings harboring 35Spro:YFP-NPH3 were treated with chemicals for 1.5 h under complete darkness at 60 rpm and stimulated with blue light (BL) at 0.1 μmol m–2 s–1 for 0.25 h. The seedlings were incubated again under complete darkness (D) for 0.5 h.
(B) Effects of BFA. Concentrations of BFA and FM4-64 were 250 and 50 μM, respectively. White arrowheads show the positions of BFA bodies. Bars = 50 μm.
(C) Effects of propyzamide (Prpz) and/or latrunculin B (LatB). Concentrations of propyzamide and latrunculin B were 100 and 30 μM, respectively. Bars = 50 μm.
Dephosphorylation of NPH3 Proteins Is Mediated by RPT2
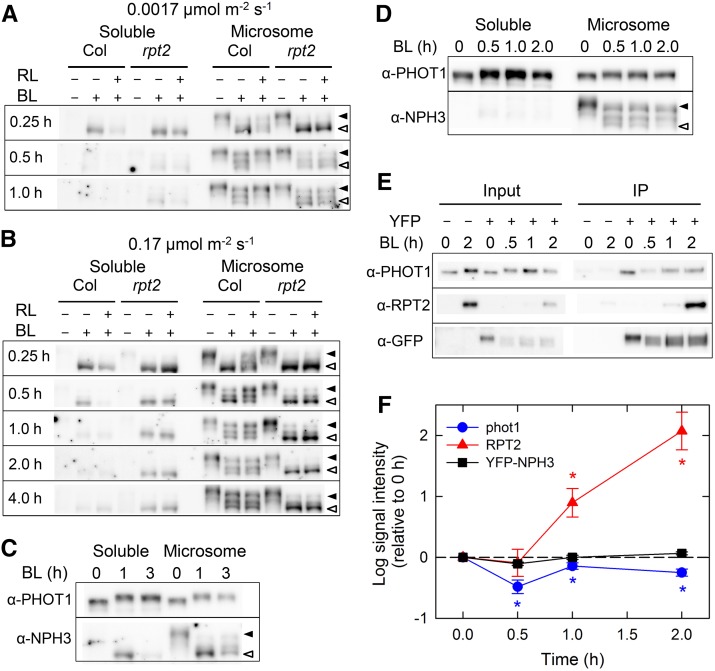
We found that blue light alters the localization of NPH3 proteins and that this alteration is regulated by phot1 and RPT2 but not by phot2. Because the dephosphorylation of NPH3 proteins in response to blue light irradiation occurs in a phot1- but not a phot2-dependent manner (Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008), we hypothesized that the phosphorylation status of NPH3 determines its subcellular localization. If this is the case, we would expect to find abnormalities in the phosphorylation status of NPH3, as well as in its subcellular localization, in the rpt2-2 mutant. To examine this possibility, we investigated the effects of the rpt2 mutation on the blue light-induced dephosphorylation of NPH3 proteins by observing the alteration of molecular weights of NPH3 proteins in an immunoblot analysis (Figure 8), as reported previously (Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008). Most NPH3 proteins extracted from dark-grown seedlings were found in a crude microsomal membrane fraction, indicating that NPH3 proteins are localized mainly to plasma membranes under dark conditions (Figures 8A and 8B), as reported previously (Motchoulski and Liscum, 1999). When wild-type seedlings were irradiated with unilateral blue light at a very low fluence rate (0.0017 μmol m–2 s–1), we observed three types of NPH3 proteins with different mobility, presumably reflecting variations in the dephosphorylation status of NPH3 proteins (slightly, moderately, and excessively dephosphorylated) (Figure 8A). Those distinct bands of NPH3 proteins were not detected in previous studies (Motchoulski and Liscum, 1999; Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008). A preparation of the microsomal membrane fraction enhanced the detection of bands of different molecular weights. Excessively dephosphorylated NPH3 proteins accumulated after 15 min (0.25 h) of irradiation, and the accumulation gradually decreased, resulting in an even distribution of NPH3 proteins with different phosphorylation status after 1 h of irradiation (Figure 8A). A transient accumulation of excessively dephosphorylated NPH3 proteins was also observed in the wild type when the seedlings were stimulated with unilateral blue light at a low fluence rate (0.17 μmol m–2 s–1) for 15 and 30 min (Figure 8B, 0.25 and 0.5 h). It is important to note that some of the excessively dephosphorylated NPH3 proteins were detected in the soluble cytosolic fraction in addition to the crude microsomal fraction (Figures 8A and 8B). Furthermore, red light pretreatment prevented the accumulation of the excessively dephosphorylated NPH3 proteins induced by blue light irradiation of both very low and low fluence rates as well as the formation of YFP-NPH3 aggregates. Such a tendency was more clearly observed when the protein samples were analyzed on a per-shoot basis (Supplemental Figure 6). This suggests that the dephosphorylation status of NPH3 proteins affects the membrane localization of the proteins.
Figure 8.
Blue Light-Induced Alteration of NPH3 Proteins in the rpt2 Mutant.
After light treatments, soluble proteins (soluble) and crude microsomal proteins (membrane) were prepared from the shoots of dark-grown seedlings. Proteins (6 μg) in each fraction were separated on 7.5% SDS-PAGE gels, followed by immunoblotting with anti-NPH3 antibody. The closed and open arrowheads indicate the positions of slightly and excessively dephosphorylated NPH3 proteins, respectively.
(A) and (B) Effects of red light (RL) pretreatment. Dark-grown seedlings were pretreated with or without red light and irradiated with continuous unilateral blue light (BL) at 0.0017 μmol m–2 s–1 (A) or 0.17 μmol m–2 s–1 (B) for the indicated periods.
(C) and (D) Effects of blue light on the phosphorylation status of phot1 and NPH3 proteins in the rpt2-1 mutant (C) and rpt2-2 transgenic plants harboring 35Spro:RPT2 (D). Dark-grown seedlings were irradiated with continuous unilateral blue light at 0.17 μmol m–2 s–1 for the indicated periods.
(E) Effects of light treatments on the interaction of NPH3 with phot1 and RPT2 in vivo. Dark-grown transgenic nph3 plants harboring 35Spro:YFP-NPH3 were irradiated with continuous unilateral blue light at 0.17 μmol m–2 s–1 for the indicated periods. The seedlings were harvested and used to prepare crude microsomal proteins. The crude extracts were immunoprecipitated (IP) with GFP agarose beads and separated on 7.5% SDS-PAGE gels, followed by immunoblotting with anti-PHOT1, anti-RPT2, and anti-GFP antibodies. A 1:200 dilution of crude microsomal proteins used for immunoprecipitation was also electrophoresed as a control for phot1 and RPT2 proteins.
(F) Quantification of the interaction of NPH3 with phot1 and RPT2. The immunoprecipitated samples (see above) were measured with ImageJ (version 1.48; http://imageJ.nih.gov/ij). The data shown are means ± se from four experiments using biologically independent samples. Asterisks indicate statistically significant differences compared with time 0 (Student’s t test, P < 0.05).
In the rpt2-2 mutant, excessively dephosphorylated NPH3 proteins accumulated after 15 min of blue light stimulation at 0.0017 and 0.17 μmol m–2 s–1 and even after 30 min of stimulation at 0.17 μmol m–2 s–1, as observed in the wild type (Figures 8A and 8B). As expected, blue light stimulation at 0.17 μmol m–2 s–1, which did not induce hypocotyl phototropism in the rpt2-2 mutant (Figure 1A), promoted the accumulation of excessively dephosphorylated NPH3 proteins regardless of whether or not the mutant was subjected to red light pretreatment, even after 4 h of blue light irradiation (Figure 8B; Supplemental Figure 6). Furthermore, stimulation at a very low fluence rate for 1 h was not fully successful in altering the excessively dephosphorylated status of NPH3 proteins to a slightly dephosphorylated status in rpt2-2 (Figure 8A). A subset of the excessively dephosphorylated NPH3 proteins was detected in the soluble cytosolic fraction of rpt2-2 seedlings. In contrast with the rpt2-2 mutant, NPH3 in the rpt2-1 mutant displayed a slight shift from the excessively dephosphorylated status to the slightly dephosphorylated status (Figure 8C), and the accumulation of excessively dephosphorylated NPH3 proteins was suppressed in rpt2-2 transgenic plants harboring 35Spro:RPT2 (Figure 8D). On the other hand, the rpt2-1 mutation and constitutive expression of RPT2 did not affect the phosphorylation status or distribution of phot1 (Figures 8C and 8D). These results indicate that RPT2 is necessary for the recovery of NPH3 proteins from their state of excessive dephosphorylation and for the inhibitory effects of the red light treatment on the blue light-induced accumulation of the excessively dephosphorylated NPH3 proteins. Thus, RPT2-mediated regulation of the phosphorylation status of NPH3 proteins is closely related to the blue light-induced internalization of NPH3 proteins and affects the inducibility of phototropic responses.
We also analyzed the effects of red light pretreatment and rpt2 mutation on the blue light-induced modification of PHOT1 proteins (Supplemental Figure 7). Blue light irradiation is known to solubilize a subset of the membrane-localized PHOT1 proteins, and red light pretreatment prevents the solubilization of membrane-localized PHOT1 proteins (Han et al., 2008). However, under our experimental conditions using a phototropic analysis, in both the wild type and the rpt2-2 mutant, the light treatments did not clearly change the distribution of PHOT1 proteins into cytosolic and microsomal fractions, irrespective of the fluence rates of blue light (Supplemental Figure 7), nor did red light pretreatment influence the distribution of PHOT1 proteins. On the other hand, blue light-induced autophosphorylation of phot1 occurred normally in both the wild type and the rpt2-2 mutant regardless of whether or not the seedlings were subjected to red light pretreatment. Therefore, it is possible that the rpt2 mutation does not affect blue light-induced alterations of phosphorylation status and the localization of phot1 blue light photoreceptors during continuous light-induced phototropism under weak/low-intensity light conditions. Stabilization of the membrane localization of phot1 proteins by red light pretreatment may function predominantly under relatively strong blue light conditions, as used by Han et al. (2008).
Interaction of NPH3 with phot1 Is Transiently Attenuated by Blue Light Irradiation
Next, we investigated whether the phosphorylation status of NPH3 proteins affected the interactions with RPT2 and phot1 proteins in vivo using a coimmunoprecipitation assay. Under dark conditions (before blue light stimulation), YFP-NPH3 interacted with phot1 but not with RPT2 (Figure 8E), because RPT2 expression was low (Figure 1B). Interestingly, the interaction of NPH3 with phot1 was attenuated by a 30-min blue light irradiation, and its interaction was then partially recovered by a 1-h blue light irradiation (Figures 8E and 8F). On the other hand, NPH3 interacted with a small amount of RPT2 after 1 h of blue light irradiation and with a large amount of RPT2 after 2 h (Figures 8E and 8F), probably because RPT2 expression was induced by extended blue light irradiation (Figure 1B). We confirmed the light-induced expression of RPT2 and its membrane localization by microscopy analysis of transgenic plants harboring RPT2pro:RPT2-VENUS (Supplemental Figure 8). We found that the interaction between NPH3 and phot1 was transiently attenuated by blue light irradiation and that this interaction was recovered by a prolonged blue light irradiation together with the interaction between NPH3 and RPT2.
DISCUSSION
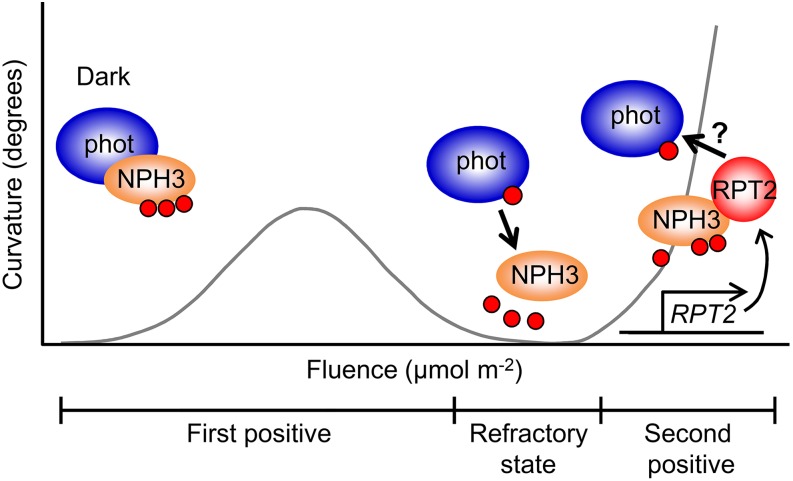
Based on our results here, we present a hypothetical model for photosensory adaptation in hypocotyl phototropism (Figure 9). The blue light photoreceptor phot1 can perceive a wide range of blue light intensities, from very low fluence rates (1.7 × 10−5 μmol m–2 s–1 or less) to high fluence rates (102 μmol m–2 s–1 or more), to induce phototropic responses in Arabidopsis hypocotyls (Figure 3B; Sakai et al., 2001). phot1 exhibits high photosensitivity in the absence of RPT2, but its high photosensitivity desensitizes phototropic responses under blue light conditions, resulting in the appearance of the refractory state following the fluence-dependent, first positive phototropism. Phosphorylated NPH3 protein forms a complex with phot1 under darkness, and blue light stimulation causes NPH3 to become excessively dephosphorylated and to dissociate from phot1 and the plasma membrane in a phot1-dependent manner. Dissociation between NPH3 and phot1 and/or the plasma membrane probably causes desensitization of the first positive phototropism, depending on light fluences. Seedlings express RPT2 under light conditions (Supplemental Figure 8; Sakai et al., 2000; Inada et al., 2004), and expression increases as the light intensity increases (Sakai et al., 2000). When phot1 and/or NPH3 associate with RPT2, the NPH3 proteins partially recover their phosphorylation status and associate with phot1 and the plasma membrane again, and the seedlings can once again recognize the direction of the light source under light conditions. Prior RPT2 expression due to red light pretreatment is thought to cause the establishment of phototropic systems that can acclimate to higher intensities of blue light and also to desensitize deetiolated seedlings to lower blue light intensities (Figures 1 to 3). Such molecular mechanisms would function in effector adaptation in phototropic responses, which participate in recovery from the refractory state, followed by immediate appearance of the second positive phototropism.
Figure 9.
Model for Phototropic Adaptation Mechanisms.
Phosphorylated NPH3 proteins interact with phot1 proteins under darkness. When the seedlings are irradiated with slightly strong blue light, in which the refractory state is induced, NPH3 proteins are dephosphorylated. The proteins are then detached from phot1 complexes, resulting in the formation of aggregates. Accordingly, the phot signal cannot be transduced efficiently downstream. Both red light and blue light upregulate the transcription of RPT2, accumulating RPT2 proteins gradually, and the RPT2 proteins bind to dephosphorylated NPH3 proteins. The RPT2-NPH3 complexes interact with phot1 proteins, and the phot signal is transduced through the RPT2-NPH3 complexes. Therefore, the RPT2-mediated regulation of blue light-induced dephosphorylation of NPH3 proteins is necessary for establishing the effector adaptation mechanism that separates the first positive and second positive phototropism. Small red circles represent phosphorylated phot1 and NPH3 proteins.
In this study, we showed that RPT2 is a key component for establishing phototropic systems that express the second positive phototropism (i.e., recover from the refractory state) through the phot1-NPH3 interaction. The roles of RPT2 in the first and second positive phototropism are summarized according to our results here (Table 1; Supplemental Table 2). In the first positive phototropism, RPT2 is necessary for the phytochrome-mediated desensitization of phototropic responses but not for the phytochrome-mediated enhancement of the responses (Table 1, Figures 3D and 3E). Because the PID/WAG family inhibits hypocotyl bending in the first positive phototropism (Haga et al., 2014), the phytochrome-mediated enhancement of the responses is probably caused by the suppression of PID/WAG expression by red light pretreatment (Haga and Sakai, 2012). On the other hand, RPT2 is necessary for the emergence of the second positive phototropism induced by higher blue light intensities (Figures 1 to 3; Supplemental Table 2). In addition, RPT2 increases the phototropic curvature rates and reduces the lag time before the second positive phototropism (Figure 1; Supplemental Table 2). The prior expression of RPT2 could shorten the refractory state, but it could not diminish the descending arm of the fluence-response curve of the pulse-induced first positive phototropism (Figure 3E). The first activation of LOV (light, oxygen, or voltage) domain(s) of phot1 seems to occur at the start of blue light irradiation in dark-grown seedlings, even if RPT2 regulates the light sensitivity of phot1. This activation probably causes the sensory adaptation of phot1 (Iino, 2001; Christie and Murphy, 2013). Thus, the activation of phot1 probably occurs at both the irradiated and shaded sides of the plant, and phot1 signaling activity does not differ between the irradiated and shaded sides.
Table 1. Critical Components for First Positive Phototropism.
| First Positive Phototropism Components | Components |
|---|---|
| Ascending arm | phot1, NPH3 |
| Descending arm | phot1, NPH3 |
| Phytochrome-mediated enhancement | PINOID family |
| Phytochrome-mediated desensitization | RPT2 |
Our results here do not demonstrate how RPT2 regulates the light sensitivity of phot1 and the phosphorylation status of NPH3 proteins. A previous study reported that RPT2 proteins do not show E3 ubiquitin ligase activity in vitro, in contrast with NPH3 proteins (Roberts et al., 2011), and we could not detect any roles of RPT2 in the destabilization of PHOT1 and NPH3 proteins, at least during a 2-h irradiation. One possible regulatory mechanism is that the interaction between RPT2 and phot1 might alternate the photocycle of LOV domain(s) and/or the enzymatic activity of the kinase domain of phot1. If RPT2 accelerates the photocycle of LOV domain(s), phot1 returns to the steady state soon after blue light irradiation and requires further blue light irradiation for the activation. A decrease of the photosensitivity and/or enzymatic activity of phot1 may suppress the dephosphorylation of NPH3, due to a decrease of phot1 signaling or dissociation from autophosphorylated phot1. Another possibility is that RPT2 affects the ability of NPH3 to bind to other proteins that play roles in protein modification or in attachment to the plasma membrane. The interaction between RPT2 and NPH3 might inhibit or stimulate the physical interaction between protein phosphatases or kinases and NPH3 proteins. Alternatively, a reinforcement of the interaction of NPH3 with phot1 and/or the plasma membrane may indirectly influence the phosphorylation status of NPH3 proteins. These possibilities should be examined in future studies to better understand the functions of RPT2 and NPH3 in effector adaptation mechanisms during phototropic responses.
Blue light-induced autophosphorylation of phot proteins (Christie et al., 1998; Sakai et al., 2001) and the dephosphorylation of NPH3 proteins (Motchoulski and Liscum, 1999; Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008) are the early events of phot signaling involved in phototropic responses. Pedmale and Liscum (2007) proposed that NPH3 dephosphorylation is a trigger response for phototropic responses, because phot1 activation causes its dephosphorylation and treatment with protein phosphatase inhibitors simultaneously suppresses NPH3 dephosphorylation and phototropic responses. However, we have already indicated that NPH3 dephosphorylation is unnecessary, at least for the phot2-induced phototropic response, and that the effects of such phosphatase inhibitors is questionable because they also affect other signaling mechanisms to induce hypocotyl tropism downstream of NPH3 (Tsuchida-Mayama et al., 2008). This study suggests that nonphosphorylated NPH3 is an inactive form that cannot transduce phot signals to induce phototropic responses, because excessively dephosphorylated NPH3 proteins and suppression of phototropic responses appear together. On the other hand, slightly dephosphorylated NPH3 proteins were detected soon after the emergence of the second positive phototropism. This suggests that NPH3 proteins that are phosphorylated (i.e., in the active form) to some extent have the ability to induce phototropic responses. Although the results presented here support the physiological significance of NPH3 dephosphorylation in phototropic responses, the dephosphorylation events have implications that are more complex.
Our previous study identified three Ser residues in NPH3, Ser-212, Ser-222, and Ser-236, as sites that are dephosphorylated in response to phot1 activation (Tsuchida-Mayama et al., 2008). The transgenic nph3 mutant line TG16, in which four Ser residues in NPH3 are replaced with Ala (NPH3S212A/S222A/S232A/S236A), displayed an almost normal second positive phototropism (Supplemental Figure 9A; Tsuchida-Mayama et al., 2008). Because this study strongly suggests that fully dephosphorylated NPH3 is an inactive form that cannot transduce the phototropic signal necessary for induction of the second positive phototropism, it appears that further phosphorylation sites in addition to Ser-212, Ser-222, and Ser-236 are present in NPH3. The shift in molecular weight observed for NPH3 proteins from wild-type seedlings was also apparent for NPH3 proteins from the microsomal fraction of the TG16 line (Supplemental Figure 9B). This suggests that other phosphorylation sites with critical roles in phototropic responses are present in NPH3. Determination of such critical phosphorylation sites is necessary to understand the roles of NPH3 proteins in phot-mediated phototropic signaling.
Our microscopy and biochemical analyses demonstrated that blue light induces the internalization of NPH3 proteins from the plasma membrane to the cytosol. However, the reason for the aggregation of YFP-NPH3 in the cytosol should be carefully considered. It is already known that the membrane localization of green fluorescent protein (GFP)-fused NPH3 proteins is severely impaired by the elimination of a C-terminal region, causing aggregation of truncated GFP-NPH3 proteins (Inoue et al., 2008). Because BTB domain-containing proteins, including NPH3 and RPT2, often undergo oligomerization (Stogios et al., 2005), the phosphorylation status of NPH3 and/or the binding of RPT2 to NPH3 may modulate the oligomerization of NPH3 in the cytosol. Although our 35Spro:YFP-NPH3 constructs complement the aberrant phenotypes in nph3, and YFP expression itself by the 35Spro:YFP vector control did not cause any YFP aggregation, the overexpression of YFP-NPH3 proteins driven by the 35S promoter may artificially induce their aggregation in the cytosol. Thus, the question remains whether 35Spro:YFP-NPH3 constructs exhibit normal localization in the cytosol.
It has been reported that phot1 has negative functions in both phot2-mediated phototropic responses and leaf morphogenesis and that RPT2 is involved in phot1-mediated negative functions (Sakai et al., 2000; Harada et al., 2013). This study demonstrates that RPT2 partially attenuates the phot1-mediated internalization of NPH3 (Figures 4 and 5). Although NPH3 is necessary not only for phot1-mediated phototropic responses but also for phot2-mediated responses (Sakai et al., 2000; Inada et al., 2004), our investigation indicated that phot2 is not involved in blue light-induced alterations of the membrane localization of NPH3 proteins (Figures 5 and 6). Therefore, it is most likely that phot1 itself attenuates phot-mediated phototropic responses by regulating changes in the distribution of NPH3 proteins and that RPT2 partially inhibits phot1-mediated negative functions by preventing the internalization of NPH3 proteins. Because both RPT2 and NPH3 are involved in leaf-flattening and -positioning responses (Inoue et al., 2008; Harada et al., 2013), it appears that similar regulatory mechanisms function not only in phototropic responses but also in leaf morphogenesis. Leaf flattening and positioning in response to blue light were found to be more severe in the rpt2 mutant than in the nph3 mutant (Inoue et al., 2008; Harada et al., 2013). This not only indicates that RPT2 is critical for the regulation of leaf morphogenesis but also that components other than NPH3 are necessary for phot-mediated regulation of leaf positioning and flattening. NPH3 and RPT2 belong to the NRL family, which consists of 32 members in Arabidopsis (Pedmale et al., 2010). Therefore, it is possible that NRL homologs are involved in the regulation of leaf morphogenesis and that RPT2 may regulate their functions. Because this study suggested that RPT2 regulates the phosphorylation status of NPH3 proteins by interacting with them, it appears that a similar regulatory mechanism also regulates the functions of NRL homologs. Analyses that include multiple mutants of the NRL family should provide useful insights into phot-mediated signal transduction.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana seeds of rpt2-1 (Landsberg erecta background) and rpt2-2 (Columbia background) mutants were as described previously (Sakai et al., 2000; Inada et al., 2004). The rpt2-2 transgenic lines harboring 35Spro:RPT2 were prepared as described previously (Tsuchida-Mayama et al., 2010). The nph3-102 transgenic line expressing NPH3S212A/S222A/S232A/S236A (TG16) was as described previously (Tsuchida-Mayama et al., 2008). The CaMV 35Spro:YFP-NPH3 construct was generated using Gateway-based subcloning of NPH3 cDNA into the pH35YG binary vector (Yamaguchi et al., 2008). The construct was transformed into the nph3-102 mutant via the floral dip method using Agrobacterium tumefaciens-mediated transformation, as described previously (Clough and Bent, 1998). rpt2-2 and phot1, both of which harbored the CaMV 35Spro:YFP-NPH3 gene, were generated by crossing these mutants with nph3-102 transgenic lines.
For physiological experiments, etiolated Arabidopsis seedlings were prepared as described previously (Haga and Sakai, 2012). Briefly, Arabidopsis seeds were sown in 0.2-mL plastic tubes filled with 1.5% agar medium (Sakai et al., 2000), placed in a black plastic box, and kept at 4°C for 3 to 5 d. Following induction of germination, the prepared seeds were incubated for 2 d under complete darkness. Seedlings were selected based on the length of the hypocotyls (3 to 5 mm). During the experiments, seedlings were kept in a black plastic box, under high humidity, until needed. For immunoblot and microscopy analyses, the etiolated seedlings were grown along the surface of vertically oriented agar medium (Sakai et al., 2000; Haga and Sakai, 2013). Experimental manipulations were performed under dim green light.
Induction of Phototropism and Measurement of Curvature
For phototropic stimulation, selected seedlings were irradiated using a blue light-emitting diode light source (470 ± 30 nm, LED-B; Eyela) through two layers of blue filter (no. 72 film; Tokyo Butai Shomei). The fluence rate was controlled with neutral-density plastic filters (Fujifilm) and by changing the distance between the light source and the seedlings. The direction of the phototropic stimulation was perpendicular to the plane of the hook (Haga and Sakai, 2012). For pretreatment with red light, the seedlings were irradiated with overhead red light (660 ± 20 nm, LED-R; Eyela) at 20 μmol m–2 s–1 for 2 min.
Images of dark-grown seedlings were recorded just before phototropic stimulation and at 3 h after the onset of the stimulation with a digital camera (D5000; Nikon), from which a UV/infrared light cut filter was removed (IDAS Division, ICAS Enterprises) under infrared illumination (IRDR-110; Nissen Electronics). For time-course experiments, images of the seedlings were captured at 10-min intervals using the same equipment. The angles of the hypocotyls were measured with an e-Ruler (Haga and Sakai, 2012).
Immunoblot Analysis
Following the light treatments, shoots were harvested from ∼100 dark-grown seedlings and immediately dipped in liquid nitrogen. The frozen samples were ground to a fine powder with a mixer mill (TissueLyser II; Qiagen), and total crude proteins were extracted with 100 μL of extraction buffer (50 mM Tris-MES, pH 7.5, 300 mM sucrose, 150 mM NaCl, 10 mM potassium acetate, 5 mM EDTA, and a protease inhibitor mixture [Complete EDTA-free; Roche Diagnostics]). The extracts were centrifuged twice at 10,000g at 4°C for 10 min to remove cell debris, and the supernatants were centrifuged at 100,000g at 4°C for 75 min to isolate crude microsomal membranes as an insoluble protein fraction. The supernatants were mixed with a half-volume of 3× SDS gel loading buffer and used as the soluble protein fraction. The microsomal pellet was resuspended in extraction buffer containing 0.5% Triton X-100 by pipetting and mixed with 3× SDS gel loading buffer. The samples were then boiled and separated on 7.5% SDS-PAGE gels for subsequent immunoblot analysis. Anti-NPH3 antibody was prepared as described previously (Tsuchida-Mayama et al., 2008), and anti-PHOT1 antibody was purchased from Trans Genic. Anti-NPH3, anti-PHOT1, and horseradish peroxidase-conjugated anti-rabbit IgG antibodies were used as described previously (Inada et al., 2004; Tsuchida-Mayama et al., 2008). Horseradish peroxidase activity was detected using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) and imaged with an Image Quant LAS4000 Mini (GE Healthcare).
Confocal Laser Scanning Microscopy
YFP and VENUS signals were detected with a TCS-SP5 confocal laser scanning microscope (Leica Microsystems). The fluorescent signals were excited with an argon laser at 514 nm, and the spectral detector was set at 525 to 560 nm. FM4-64FX (Life Technology) signals were detected as described previously (Haga and Sakai, 2012). All scans were performed at a 2048 × 2048-pixel resolution with repeated scanning of two lines.
Immunoprecipitation Analysis
To examine the interaction of NPH3 with phot1 and RPT2 in vivo, we used nph3-102 transgenic plants harboring 35Spro:YFP-NPH3. The microsomal fraction was prepared from ∼300 dark-grown seedlings, and the fraction was incubated with GFP-Trap-A agarose beads (Chromotech) for 1 h at 4°C. After centrifugation at 800g for 20 s, the supernatant was discarded, and the agarose beads were washed with extraction buffer containing 0.2% Triton X-100. After centrifugation at 800g for 20 s, the supernatant was discarded, and the agarose beads were incubated with 2× SDS for 10 min at 95°C. The supernatant was used for immunoblot analysis using anti-PHOT1 and anti-RPT2 antibodies (Inada et al., 2004).
Transcript Analysis
Following light treatment, shoots were harvested from ∼100 dark-grown seedlings and immediately dipped in liquid nitrogen. The frozen samples were ground to a fine powder with a mixer mill (TissueLyser II; Qiagen), and total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen). The extracts were treated with RNase-free DNase (Qiagen) to remove DNA. Quantitative RT-PCR was performed using a PCR system (CFX96; Bio-Rad) with an iScript One-Step RT-PCR Kit (Bio-Rad). Triplicate PCRs were performed in each case, and three independent biological replicates were performed for each gene. The primers used are listed in Supplemental Table 1 (Muto et al., 2007), and 18S rRNA was amplified as an internal standard. RNA gel blot analysis was performed as described previously (Sakai et al., 2000).
Cloning of RPT2pro:RPT2-VENUS
The RPT2pro:RPT2-VENUS gene was constructed using the binary vector pZP221 (Hajdukiewicz et al., 1994). Approximately 5.4 kb of RPT2 genomic DNA covering 2.8 kb of the promoter sequence and its protein-coding regions and the VENUS gene were cloned into this vector. The construct was then introduced into the rpt2-2 mutant by Agrobacterium-mediated transformation (Clough and Bent, 1998). The T3 generation of transgenic lines, showing the complemented phototropic phenotypes in the hypocotyls and roots, was examined for VENUS fluorescence.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or the GenBank/EMBL data libraries under the following accession numbers: NPH3 (AT5G64330), PHOT1 (AT3G45780), and RPT2 (AT2G30520).
Supplemental Data
Supplemental Figure 1. Fluence-Response Curve for Phototropism.
Supplemental Figure 2. Expression of RPT2 mRNA in the rpt2-1 and rpt2-2 Mutants.
Supplemental Figure 3. Time-Dependent Second Positive Phototropism in the rpt2-2 Mutant.
Supplemental Figure 4. Complementation of the nph3-102 Mutant with 35Spro:YFP-NPH3.
Supplemental Figure 5. Effects of Propyzamide and Latrunculin B on Hypocotyl Phototropism and Growth.
Supplemental Figure 6. Distribution of NPH3 Proteins in Soluble Cytosolic and Microsomal Fractions per Shoot.
Supplemental Figure 7. Effects of Red Light Pretreatment on Blue-Light-Induced Modulation of phot1 Proteins in the rpt2-2 Mutant.
Supplemental Figure 8. Expression Patterns of RPT2.
Supplemental Figure 9. Blue-Light-Induced Dephosphorylation of Mutated NPH3 Proteins.
Supplemental Table 1. Gene-Specific Primers Used for Quantitative RT-PCR.
Supplemental Table 2. Critical Components for Second Positive Phototropism.
Supplementary Material
Acknowledgments
We thank the Sainsbury Laboratory at the John Innes Center for providing the rpt2-2 mutant and the ABRC for providing the nph3-102 mutant (Salk_110039). We also thank T. Demura and M. Yamaguchi for the pH35YG binary vector, Y. Niwa for the pTH2 vector, and T. Mitsui (Niigata University) for the use of the Bio-Rad CFX96 facility. This work was supported by JSPS KAKENHI Grant 26440134, by MEXT KAKENHI Grant 25120710, the Sasaki Environment Technology Foundation, and The Ito Foundation (to T.S.), and by JSPS KAKENHI Grant 24657027 (to K.H.).
AUTHOR CONTRIBUTIONS
K.H. and T.S. designed the research and wrote the article. K.H. performed the research and analyzed the data. T.T.-M. generated the RPT2pro:RPT2-YFP transgenic lines. M.Y. investigated phototropic responses in the rpt2 mutant.
Glossary
- BFA
brefeldin A
References
- Briggs W.R. (2014). Phototropism: Some history, some puzzles, and a look ahead. Plant Physiol. 164: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves I., Pokorny R., Byrdin M., Hoang N., Ritz T., Brettel K., Essen L.-O., van der Horst G.T.J., Batschauer A., Ahmad M. (2011). The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62: 335–364. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Murphy A.S. (2013). Shoot phototropism in higher plants: New light through old concepts. Am. J. Bot. 100: 35–46. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Reymond P., Powell G.K., Bernasconi P., Raibekas A.A., Liscum E., Briggs W.R. (1998). Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., Aniento F., Hwang I., Robinson D.G., Mravec J., Stierhof Y.D., Friml J. (2007). Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 17: 520–527. [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Quail P.H. (2010). Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland P. (1991). Photosensory adaptation in aneural organisms. Photochem. Photobiol. 54: 1119–1134. [Google Scholar]
- Geldner N., Friml J., Stierhof Y.D., Jürgens G., Palme K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428. [DOI] [PubMed] [Google Scholar]
- Haga K., Sakai T. (2012). PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol. 160: 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Sakai T. (2013). Differential roles of auxin efflux carrier PIN proteins in hypocotyl phototropism of etiolated Arabidopsis seedlings depend on the direction of light stimulus. Plant Signal. Behav. 8: e22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Hayashi K., Sakai T. (2014). PINOID AGC kinases are necessary for phytochrome-mediated enhancement of hypocotyl phototropism in Arabidopsis. Plant Physiol. 166: 1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Takano M., Neumann R., Iino M. (2005). The rice COLEOPTILE PHOTOTROPISM1 gene encoding an ortholog of Arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin. Plant Cell 17: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P., Svab Z., Maliga P. (1994). The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25: 989–994. [DOI] [PubMed] [Google Scholar]
- Han I.S., Tseng T.S., Eisinger W., Briggs W.R. (2008). Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 20: 2835–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter R.P. (1997). Gravity, light and plant form. Plant Cell Environ. 20: 796–800. [DOI] [PubMed] [Google Scholar]
- Harada A., Takemiya A., Inoue S., Sakai T., Shimazaki K. (2013). Role of RPT2 in leaf positioning and flattening and a possible inhibition of phot2 signaling by phot1. Plant Cell Physiol. 54: 36–47. [DOI] [PubMed] [Google Scholar]
- Hersch M., Lorrain S., de Wit M., Trevisan M., Ljung K., Bergmann S., Fankhauser C. (2014). Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl. Acad. Sci. USA 111: 6515–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. (2001). Phototropism in higher plants. In Photomovement: ESP Comprehensive Series in Photosciences, Vol. 1, Häder D., Lebert M., eds (Amsterdam: Elsevier; ), pp. 659–811. [Google Scholar]
- Inada S., Ohgishi M., Mayama T., Okada K., Sakai T. (2004). RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16: 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Kinoshita T., Takemiya A., Doi M., Shimazaki K. (2008). Leaf positioning of Arabidopsis in response to blue light. Mol. Plant 1: 15–26. [DOI] [PubMed] [Google Scholar]
- Janoudi A.K., Konjević R., Whitelam G., Gordon W., Poff K.L. (1997). Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thaliana seedlings. Physiol. Plant. 101: 278–282. [Google Scholar]
- Kleine-Vehn J., Huang F., Naramoto S., Zhang J., Michniewicz M., Offringa R., Friml J. (2009). PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell 21: 3839–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A., Liscum E. (1999). Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964. [DOI] [PubMed] [Google Scholar]
- Muto H., Watahiki M.K., Nakamoto D., Kinjo M., Yamamoto K.T. (2007). Specificity and similarity of functions of the Aux/IAA genes in auxin signaling of Arabidopsis revealed by promoter-exchange experiments among MSG2/IAA19, AXR2/IAA7, and SLR/IAA14. Plant Physiol. 144: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale U.V., Liscum E. (2007). Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J. Biol. Chem. 282: 19992–20001. [DOI] [PubMed] [Google Scholar]
- Pedmale, U.V., Celaya, R.B., and Liscum, E. (2010). Phototropism: Mechanisms and outcomes. The Arabidopsis Book 8: e0125, doi/10.1199/tab.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poff K.L., Janoudi A.-K., Rosen E.S., Orbović V., Konjević R., Fortin M.-C., Scott T.K. (1994). The physiology of tropisms. In Arabidopsis, Meyerowitz E.M., Somerville C.R., eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; ), pp. 639–664. [Google Scholar]
- Roberts D., Pedmale U.V., Morrow J., Sachdev S., Lechner E., Tang X., Zheng N., Hannink M., Genschik P., Liscum E. (2011). Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3NPH3. Plant Cell 23: 3627–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Haga K. (2012). Molecular genetic analysis of phototropism in Arabidopsis. Plant Cell Physiol. 53: 1517–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Kagawa T., Kasahara M., Swartz T.E., Christie J.M., Briggs W.R., Wada M., Okada K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98: 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Wada T., Ishiguro S., Okada K. (2000). RPT2: A signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T., Geldner N., Grebe M., Mangold S., Jackson C.L., Paris S., Gälweiler L., Palme K., Jürgens G. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318. [DOI] [PubMed] [Google Scholar]
- Stogios P.J., Downs G.S., Jauhal J.J., Nandra S.K., Privé G.G. (2005). Sequence and structural analysis of BTB domain proteins. Genome Biol. 6: R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida-Mayama T., Nakano M., Uehara U., Sano M., Fujisawa N., Okada K., Sakai T. (2008). Mapping of the phosphorylation sites on the phototropic signal transducer, NPH3. Plant Sci. 174: 626–633. [Google Scholar]
- Tsuchida-Mayama T., Sakai T., Hanada A., Uehara Y., Asami T., Yamaguchi S. (2010). Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J. 62: 653–662. [DOI] [PubMed] [Google Scholar]
- Wang T., Montell C. (2007). Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 454: 821–847. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Kubo M., Fukuda H., Demura T. (2008). Vascular-related NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J. 55: 652–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.