Abstract
Background and Objectives:
Tuberculosis (TB) continues to be a major health problem in developing countries like India. Abdominal TB is defined as an infection of the peritoneum, or hollow or solid abdominal organs with Mycobacterium tuberculosis (Mtb). The gastrointestinal tract is one of the most frequent sites of extrapulmonary involvement in TB. The present study was undertaken to evaluate the role of laboratory investigations in the diagnosis of abdominal TB.
Materials and Methods:
The study was conducted on 300 patients admitted to various departments of our hospital from November 2005 to October 2007. Detailed histories and thorough clinical examinations together with relevant hematological, biochemical, cytological, radiological, and histopathological investigations were carried out in suspected cases of Koch's abdomen.
Results:
Erythrocyte sedimentation rates with positive results were seen in 79.3% patients. Serological test enzyme-linked immunosorbent assay was performed on only 30 patients and was found to be positive for IgG, and IgM in 25 cases with a sensitivity of 83%. Thirteen out of 15 cases were positive for adenosine deaminase done on ascitic fluid. The results of the two patients who underwent Mtb polymerase chain reaction (PCR) were consistent with TB. Out of 21 image-guided fine-needle aspiration cytology (FNAC) cases, 10 (48%) of the positive cases showed caseating necrosis while 7 (33%) had noncaseous necrosis. Stain for acid-fast bacilli (AFB) was performed on all cases and was positive in 42 cases (38.8%). Lymph node biopsy was done in 95% of the cases.
Conclusions:
Serological investigations have a limited value, while PCR is a highly specific test. Since cost restricts its use, only two patients in our study could afford it. BACTEC is more sensitive and faster than culture techniques for the diagnosis of mycobacterial infections. FNAC is a reliable, cost effective alternative, and 81% diagnostic yield in the present study suggests that ultrasound guidance is a useful tool. Histopathological evaluation with positive AFB staining remains the gold standard for diagnosing abdominal TB. However, although the demonstration of AFB in aspirates and tissue sections is a definitive diagnostic method for TB, the positivity for AFB is variable.
Keywords: Abdominal, diagnosis, laboratory, tuberculosis
INTRODUCTION
Tuberculosis (TB) is prevalent all over the world especially in under-developed countries. Despite longstanding efforts to conquer TB, it continues to be a major health problem in developing countries like India. Abdominal TB is defined as an infection of the peritoneum, or hollow or solid abdominal organs with Mycobacterium tuberculosis (Mtb). Although, the lung is the most common site for primary TB infection, the gastrointestinal tract (GIT) is one of the most frequent sites of extrapulmonary involvement in TB.[1]
TB can involve any part of the GIT from the mouth to the anus. The peritoneum and the ileocaecal region are the most likely sites of infection and are involved in the majority of the cases by hematogenous spread, or by swallowing infected sputum from primary pulmonary TB.[1] The presenting symptoms vary from abdominal pain to acute intestinal obstruction often with constitutional symptoms. The diagnosis is usually made through a combination of radiologic, endoscopic, microbiologic, histologic, and molecular techniques. The clinical and radiological findings of gastrointestinal TB (GITB) often mimic diseases such as Crohn's disease, malignancy, and amoebiasis. Therefore, it sometimes takes a relatively long time for an accurate diagnosis of GITB to be made in clinical practice.[2]
The social and economic consequences of the disease have a profound effect on any country. Clinicians have the notion that GITB is infrequent in India. However, abdominal TB is far more common in India than is generally believed. India has featured among the 22 countries with high TB burden and has accounted for an estimated one-quarter (26%) of all TB cases worldwide.[3] The present study was undertaken to evaluate the role of laboratory investigations in the diagnosis of abdominal TB.
MATERIALS AND METHODS
The present study was conducted on 300 patients admitted to various departments of our hospital from November 2005 to October 2007. All suspected cases of Koch's abdomen, the diagnosis of which had been confirmed and had come for follow-up were included in the study, while those negative for TB or lost to follow-up were excluded. They were diagnosed as abdominal TB on the basis of a thorough clinical examination, investigations, pathological findings, and chemotherapeutic trial.
Demographic data with a detailed history and thorough clinical examinations were recorded. Relevant hematological, biochemical, cytological, radiological, and histopathological investigations were carried out. A routine hematological investigation such as complete blood count was done on a three-part differential counter and erythrocyte sedimentation rate (ESR) by Westergren method. Definitive diagnostic criteria included cytological and histopathological demonstration of epithelioid cell granulomas with or without caseous necrosis., demonstration of acid-fast bacilli (AFB) on Ziehl-Neelsen (ZN) staining, culture of ascitic fluid for AFB, imaging evidence indicative of abdominal TB, therapeutic response to chemotherapy and gross operative findings (wherever surgery was undertaken) suggestive of TB.
All relevant information was collected, and data were analyzed and the results drawn.
RESULTS
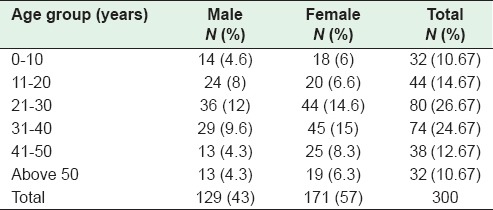
The study evaluated 300 patients, 192 cases of which were managed conservatively with appropriate anti-tuberculous treatment (ATT), while 108 were taken up for surgery. Age and sex distribution were analyzed, and a female preponderance of 171 (57%) females, and 129 (43%) males was noted. [Table 1]. The male: female ratio was 3:4. Age wise, comparative analysis showed that a maximum of 80 (26.6%) patients belonged to the 21–30 age group and a small number of cases toward the extremes of age that is 0–10 years and > 50 years. The overall mean age ± standard deviation (SD) was 29.6 ± 14.4 years, with the male mean age of 28.3 ± 11.28 years, and female mean age of 30.6 ± 13.22 years.
Table 1.
Distribution of cases according to age and sex
The clinical presentations of the patients varied. The important symptoms comprised abdominal pain, anemia, constipation, diarrhea, fever, weight loss, nausea, vomiting, and melena. Ninety-four percent of the patients presented with abdominal pain followed by anemia and constipation (62% each). Melena was the least common symptom presented only in 4% of cases.
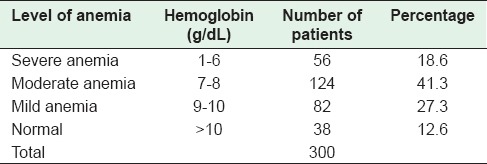
Routine hematological investigation was carried out, and levels of hemoglobin (Hb) were estimated in all the cases. Hb >10 g% was taken as normal and was evident in 12.6% cases only while 262 (87%) patients had anemia. Distribution of cases according to the level of anemia is shown on Table 2. Moderate anemia (WHO criteria)[4] was seen in 41.3% cases. Mean Hb ± SD was 8.0 ± 2.77 g%.
Table 2.
Levels of hemoglobin in the patients
ESRs were available in 111 cases only, with positive results in 79.3% patients (normal value - <15 mm/h in males and 20 mm/h in females). Mantoux test (Mx test) represents a dermal response to tuberculin antigen - an antibody reaction reflecting the immune response of the individual. A positive test result was taken when the induration was >10 mm [Table 3].
Table 3.
Distribution of Mantoux test results

Enzyme-linked immunosorbent assay (ELISA) was performed on only 30 patients and was found to be positive for IgG and IgM depending on the phase of TB in 25 cases, with a sensitivity of 83%. The limiting factor for ELISA was the cost of the test, which the patients could not afford. Ascites was seen in 38 cases, but adenosine deaminase (ADA) was done in 15 cases only, again due to the cost and unavailability of the test in the hospital. Taking 33 U/L as a cut-off, 13 of these were positive (87%), the mean value ± SD of ADA being 92.5 ± 47%. The only two patients who underwent Mtb polymerase chain reaction (PCR) were consistent for tuberculous infection. Bacteriological investigations were done on the tapped ascitic fluid. AFB staining and culture were available for 8 cases only. AFB staining was negative in all 8 cases, but culture positive in only 1. BACTEC, an automated system for growth and detection of mycobacteria was performed on 7 cases, the results of which were viewed on the 7th and 14th days. Positive results were obtained in 6 cases on the 7th day while for 1 case it took 14 days to get results. Fine-needle aspiration cytology (FNAC) was done on 21 patients. Eleven cases showed epithelioid cell granulomas [Figure 1] with caseous necrosis in 4 out of the 11 and noncaseous necrosis in 7 out of the 11 cases.
Figure 1.
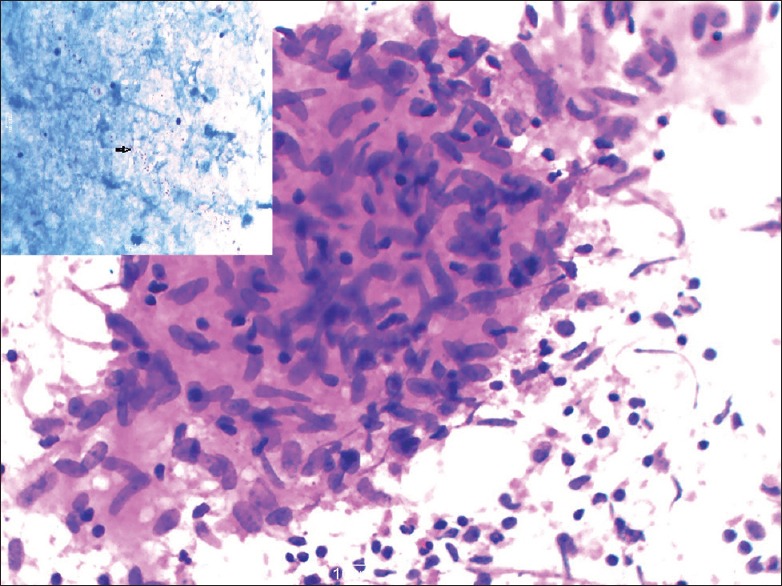
Fine-needle aspiration cytology smears from cervical lymph node showing epithelioid cell granulomas. Insert shows acid-fast bacilli (Giemsa, Ziehl–Neelsen stain, ×40)
Out of 21 image guided FNAC cases, 81% yielded diagnostic material. Ten (48%) of the positive cases showed caseating necrosis, while 7 (33%) had noncaseous necrosis [Figure 2].
Figure 2.
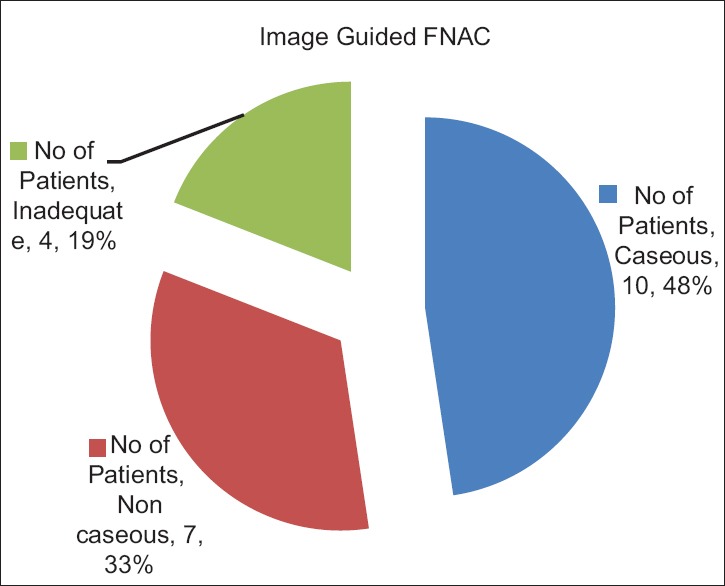
Distribution of image guided fine-needle aspiration cytology cases
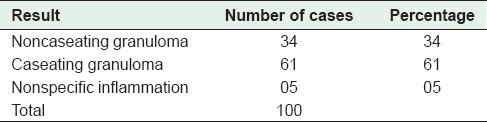
Surgical intervention was needed in 36% (108/300) of the cases and specimens were sent to the laboratory for histopathological evaluation. Surgical specimens included bowel only (8, 7.4%), bowel with mesenteric lymph nodes (86, 79.6%), and lymph nodes only (14, 13%). Lymph node biopsies were then sent in 100 cases. The distribution of cases is depicted in Tables 4 and 5.
Table 4.
Distribution of cases on the basis of histopathological examination of bowel/bowel with lymph nodes
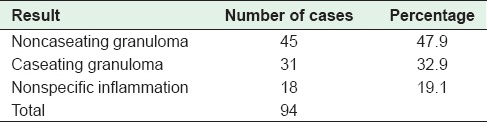
Table 5.
Distribution of cases on the basis of histopathological examination of mesenteric lymph nodes
On histopathology, both caseating and noncaseating granulomas with Langhans giant cells were seen [Figures 3 and 4]. Stain for AFB was performed on all cases, and 42 cases (38.8%) were positive. Lymph node biopsy was done in 95% of the cases. In the remaining 5% cases, a diagnosis of abdominal TB was made on clinical suspicion based on ATT trial and gross operative findings. All patients were kept on ATT for a period of 9 months. Of the 192 patients managed conservatively, 188 had an uneventful recovery, and 4 patients expired.
Figure 3.
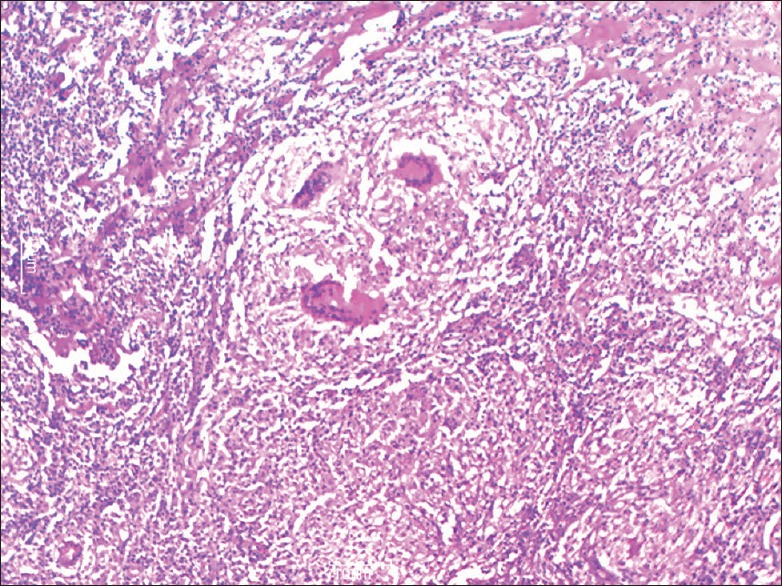
Microphotograph showing epithelioid cell granulomas in ileocaecal area with giant cells in a necrotic background (H and E, ×10)
Figure 4.
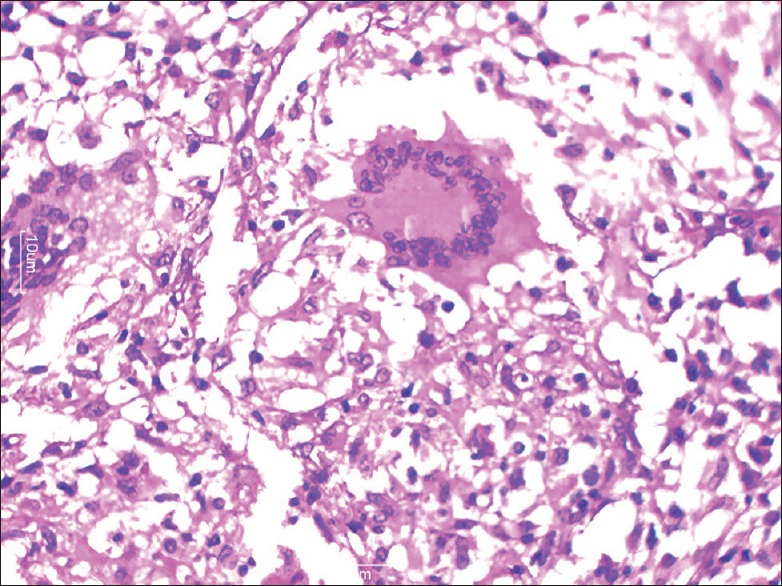
Microphotograph showing Langhan's giant cell (H and E, ×40)
DISCUSSION
TB is a chronic granulomatous disease caused by M. tuberculosis. In spite of the good progress made in treatment and prophylaxis, it still is a major global health problem. The GIT is the sixth most common site of extrapulmonary infection.[5,6] Although any part of the GIT can be involved, the ileocecal region is the most frequent site. The etiology of intestinal TB is both Mtb and bovis.[7]
A wide age range was seen in our study, the majority of cases (26.6%) being between 21 and 30 years of age with a mean age of 29.6 years. Other authors have also reported wide variations in age as well as in mean age and sex distribution.[7,8] A female preponderance was noted in the present study which does not accord with other studies in which there has been male predominance.[9,10]
The clinical presentations of abdominal TB vary ranging from acute symptoms to chronic or acute on chronic manifestations. Most patients have constitutional symptoms of fever, pain, diarrhea, constipation, alternating constipation, and diarrhea, weight loss, anorexia, and malaise[1,9,10] as we found in our study. Ninety-four of patients of the present study presented with abdominal pain, a feature that agrees with other studies.[11,12,13]
Routine hematological investigation revealed anemia (Hb <10 g%) in 87% patients, out of whom 18.6% suffered severe anemia (Hb <1–6 g%) and 41.4% with moderate anemia. Anemia was reported in 64% patients in a study from Nepal[13] while a study from Pakistan by Abro et al. revealed 81%, anemic patients.[11] All chronic infections including TB can cause anemia.[14] Various pathogeneses have been suggested in TB-associated anemia, but most studies have shown the suppression of erythropoiesis by inflammatory mediators as the cause of the anemia.[15,16] Nutritional deficiency and malabsorption syndrome can deepen the severity of anemia.[17,18] Although present in many other clinical ailments prevalent in tropical countries especially parasitic infestations, anemia is a nonspecific important parameter in abdominal TB. Similar to Basu et al., the mean value of Hb, in our study was 8.00 g%.[19]
Another routine hematological investigation, ESR, was evaluated in 111 patients in the present study, and was found to be elevated in 79.3% of the cases. A 77% prevalence, comparable to ours was reported by Niaz and Muhammad.[20] ESR is a nonspecific significant parameter that is raised in almost all patients with chronic ailments, as well as, in abdominal TB. Both of these parameters are considered nonspecific changes useful in validating other pathological and clinical findings.[1]
Mx test represents a dermal response to tuberculin antigen-an antibody reaction reflecting the immune response of the individual. Although a weak serological test with low specificity and negative predictive value, it is commonly used as a screening test in developing countries like India. In our study, Mx test was positive in 84.8% of the patients who underwent the test. This was higher than other studies.[21,22] The difference could be attributed to the technique in administering the standard dose and the interpretation of results. ELISA was done in 30 patients only, 83% of whom showed sensitivity. This is a costly test that our patient population could not afford. Another drawback is that ELISA remains positive even after therapy. The response to mycobacteria is variable, and its reproducibility is poor.[23] Hence, the value of immunological tests remains undefined in clinical practice. The conclusion is that no serological investigation is perfect, and it is unlikely that serological tests alone will provide the diagnosis in all cases. However, in areas where TB is common, these tests may support a clinical and radiological diagnosis in the absence of histological and microbiological confirmation.[24]
ADA is an aminohydrolase that converts adenosine to inosine. This enzyme activity is more in T-lymphocytes. ADA is increased in the tuberculous ascitic fluid as a result of the stimulation of T-cells by mycobacterial antigens.[1,25] In the present study, ascites was seen in 38 patients, with ADA levels available in 15 cases only. Thirteen patients were positive with 33 μ/l as a cut-off. Levels of ADA in tuberculous ascites are significantly higher than those in cirrhotic or malignant ascites.[25] PCR for Mtb is known to be a highly specific test although variation in sensitivity is high and mainly dependent on the source of specimen used.[26,27,28] Only two patients in the present study underwent PCR testing probably because of nonavailability or the cost of the test.
The absolute diagnosis of TB is based on the demonstration of AFB, the concentration of which is high in caseation and necrotic debris while its yield is low in ascitic fluid. AFB was tried on the ascitic fluid in 8 cases and was negative in all. The yield of organisms on smear and culture is low. Staining for AFB is positive in < 3% of cases.[1] Radiometric BACTEC is a technique specific for mycobacterial growth, detecting the presence of mycobacteria on the basis of metabolism rather than visible growth. Mycobacteria in clinical samples can be detected in half the time compared to conventional culture methods. BACTEC is substantially more sensitive, efficient and faster than LJ medium in the laboratory diagnosis of mycobacterial infections.[29] Despite its high sensitivity, it cannot be carried out in all cases because of financial constraints. A sensitivity of 85.1% and 100% was seen on 7th and 14th day, respectively, results which were comparable to those of other authors who reported 87% and 96% positivity on the 2 days.[30] However, Amarapurkar et al. concluded that although BACTEC for culture enhances the speed of diagnosis, the sensitivity of these techniques in the diagnosis of GITB was poor because of the paucibacillary status.[28]
FNAC has been shown to be a safe, quick, reliable, and cost effective alternative for obtaining tissue for pathological evaluation. We conducted image guided (ultrasound guided) FNAC on a limited number of patients, with a diagnostic yield in 81% of the cases. Our results were lower than other studies, which was probably due to wrong technique or poor visualization of the lesion. Image guided FNAC has facilitated the easy collection of cellular material with greater accuracy.[31] The yield reported was much higher in other studies. Cytology demonstrated epithelioid cell granulomas with or without caseous necrosis. ZN stain demonstrated AFB mainly in cases with extensive necrosis.[32] Ultrasound guided FNAC is an effective, useful method which eliminates the need for surgical biopsy and provides sufficient information for the initiation of therapy.
Histopathological evaluation remains the gold standard for diagnosis. It remains the cornerstone for the diagnosis of GITB in under-developed countries where serological and molecular methods are too expensive for the patient population. Surgical intervention was needed in 108 cases, and specimens were sent for histopathological evaluation to the laboratory. Surgical specimens included bowel only, bowel with mesenteric lymph nodes, and lymph nodes only specimens. Lymph node biopsy was used in the diagnosis of 95% of the cases, and in the diagnosis of 81% cases of the bowel specimens. Variably sized tuberculous granulomas, initially localized in the mucosa or the Peyer's patches, characteristically tend to be confluent. They are often seen just beneath the mucosa mainly in the submucosal layer. However, many sections may show only nonspecific chronic inflammation and no granulomas[1] as seen in 19% of the cases of the present study. Mesenteric lymph nodes may be enlarged, matted, and may show characteristic epithelioid cell granulomas with caseation. This is especially common in patients who have had antituberculous therapy for some time.[33] Demonstration of AFB in tissue sections is a definitive diagnostic method for TB. AFB positivity is variable and ranges from 5% to 40.5%.[12,34,35] In our study, it was 38.8%. Thus, even though it is an important diagnostic test for TB, the sensitivity of AFB detection in tissues is very low.[12] Consequently, the absence of AFB does not necessarily rule out TB. The majority of patients in the present study were managed conservatively, and surgical intervention was required only in cases with complications.
CONCLUSIONS
Intestinal TB is a common extrapulmonary manifestation of TB. The onset usually is insidious, a considerable number of patients presenting with nonspecific features. Patients usually present with abdominal pain and diagnosis is made through a combination of hematological, microbiologic, histologic, and molecular techniques. Eighty-seven percent of the patients with TB had anemia and 79.3% had elevated ESR suggesting that in endemic areas, strong clinical suspicion together with positive nonspecific laboratory investigations such as anemia and raised ESR are also an indication of TB. Serological investigations have a limited value while PCR is a highly specific test though its use could be restricted because of cost. Only two patients in our study could afford the test. Microbiological investigation such as BACTEC is a substantially more sensitive, efficient, and fast method than culture techniques in the laboratory diagnosis of Mycobacterial infections. FNAC is a safe, quick, reliable and cost effective alternative, and 81% diagnostic yield in the present study suggests that ultrasound guidance is a very useful tool in the armamentarium of diagnostic investigations. Histopathological evaluation with positive AFB staining remains the gold standard for diagnosing abdominal TB. However, although the demonstration of AFB in aspirates and tissue sections is a definitive diagnostic method for TB, the positivity for AFB is variable.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120:305–15. [PubMed] [Google Scholar]
- 2.Hulnick DH, Megibow AJ, Naidich DP, Hilton S, Cho KC, Balthazar EJ. Abdominal tuberculosis: CT evaluation. Radiology. 1985;157:199–204. doi: 10.1148/radiology.157.1.4034967. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SK, Mohan A. Tuberculosis: From an incurable scourge to a curable disease - Journey over a millennium. Indian J Med Res. 2013;137:455–93. [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Vitamin and Mineral Nutrition Information System. (WHO/NMH/NHD/MNM/11.1) Geneva: World Health Organization; 2011. [Last accessed on 2012 Dec 01]. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf . [Google Scholar]
- 5.Chong VH, Lim KS. Gastrointestinal tuberculosis. Singapore Med J. 2009;50:638–45. [PubMed] [Google Scholar]
- 6.Bolukbas C, Bolukbas FF, Kendir T, Dalay RA, Akbayir N, Sokmen MH, et al. Clinical presentation of abdominal tuberculosis in HIV seronegative adults. BMC Gastroenterol. 2005;5:21. doi: 10.1186/1471-230X-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasgupta A, Singh N, Bhatia A. Abdominal tuberculosis: A histopathological study with special reference to intestinal perforation and mesenteric vasculopathy. J Lab Physicians. 2009;1:56–61. doi: 10.4103/0974-2727.59700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishore P, Chandrsekhar T, Palaian S. Diagnosing abdominal tuberculosis: A retrospective study from Nepal. Internet J Gastroenterol. 2007. p. 6. Available from: http:// http://print.ispub.com/api/0/ispub-article/6147 .
- 9.Uygur-Bayramicli O, Dabak G, Dabak R. A clinical dilemma: Abdominal tuberculosis. World J Gastroenterol. 2003;9:1098–101. doi: 10.3748/wjg.v9.i5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radzi M, Rihan N, Vijayalakshmi N, Pani SP. Diagnostic challenge of gastrointestinal tuberculosis: A report of 34 cases and an overview of the literature. Southeast Asian J Trop Med Public Health. 2009;40:505–10. [PubMed] [Google Scholar]
- 11.Abro A, Siddiqui FG, Akhtar S, Memon AS. Spectrum of clinical presentation and surgical management of intestinal tuberculosis at tertiary care hospital. J Ayub Med Coll Abbottabad. 2010;22:96–9. [PubMed] [Google Scholar]
- 12.Tripathi PB, Amarapurkar AD. Morphological spectrum of gastrointestinal tuberculosis. Trop Gastroenterol. 2009;30:35–9. [PubMed] [Google Scholar]
- 13.Sharma YR, Roy PK, Hasan M. Abdominal tuberculosis - A study of 25 cases. Kathmandu Univ Med J (KUMJ) 2004;2:137–41. [PubMed] [Google Scholar]
- 14.Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16:87–96. doi: 10.1054/blre.2002.0193. [DOI] [PubMed] [Google Scholar]
- 15.Means RT., Jr Recent developments in the anemia of chronic disease. Curr Hematol Rep. 2003;2:116–21. [PubMed] [Google Scholar]
- 16.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 17.Schwenk A, Macallan DC. Tuberculosis, malnutrition and wasting. Curr Opin Clin Nutr Metab Care. 2000;3:285–91. doi: 10.1097/00075197-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Ramadan IT, Abdul-Ghaffar NU. Malabsorption syndrome complicating tuberculous peritonitis. Int J Tuberc Lung Dis. 1997;1:85–6. [PubMed] [Google Scholar]
- 19.Basu S, Ganguly S, Chandra PK. Clinical profile and outcome of abdominal tuberculosis in Indian children. Indian J Tuberc. 2003;50:151–6. [PubMed] [Google Scholar]
- 20.Niaz K, Muhammad A. Intestinal tuberculosis: Diagnostic dilemma. Prof Med J. 2010;17:532–7. [Google Scholar]
- 21.Ahmad Z, Amin SS. Role of tuberculin test, FNAC and ELISA in the diagnosis of tuberculous lymphadenitis. J Indian Acad Clin Med. 2003;4:292–5. [Google Scholar]
- 22.Wells AD, Northover JM, Howard ER. Abdominal tuberculosis: Still a problem today. J R Soc Med. 1986;79:149–53. doi: 10.1177/014107688607900307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor VK. Abdominal tuberculosis. Postgrad Med J. 1998;74:459–67. doi: 10.1136/pgmj.74.874.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapoor VK. Abdominal tuberculosis: The Indian contribution. Indian J Gastroenterol. 1998;17:141–7. [PubMed] [Google Scholar]
- 25.Dwivedi M, Misra SP, Misra V, Kumar R. Value of adenosine deaminase estimation in the diagnosis of tuberculous ascites. Am J Gastroenterol. 1990;85:1123–5. [PubMed] [Google Scholar]
- 26.Indian Council of Medical Research Bulletin. 2002;32:28–31. [Google Scholar]
- 27.Amarapurkar DN, Patel ND, Amarapurkar AD, Agal S, Baigal R, Gupte P. Tissue polymerase chain reaction in diagnosis of intestinal tuberculosis and Crohn's disease. J Assoc Physicians India. 2004;52:863–7. [PubMed] [Google Scholar]
- 28.Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn's disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14:741–6. doi: 10.3748/wjg.14.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itani LY, Cherry MA, Araj GF. Efficacy of BACTEC TB in the rapid confirmatory diagnosis of mycobacterial infections. A Lebanese tertiary care center experience. J Med Liban. 2005;53:208–12. [PubMed] [Google Scholar]
- 30.Venketaraman P, Herbert D, Paramasivan CN. Comparison of BACTEC radiometric method with conventional method for drug susceptibility testing. Indian J Med Res. 1997;101:100–10. [Google Scholar]
- 31.Parajuli S, Tuladhar A, Basnet RB. Ultrasound and computerised tomography guided fine needle aspiration cytology in dignosing intra-abdominal and intra-thoracic lesions. J Pathol Nepal. 2011;1:17–21. [Google Scholar]
- 32.Ahmad SS, Akhtar K, Akthar SS, Arif SH, Nasir A, Khalid M, et al. Ultrasound guided fine needle aspiration biopsy of gastrointestinal masses. J Cytol. 2007;24:73–7. [Google Scholar]
- 33.Hoon JR, Dockerty MB, Pemberton Jde J. Ileocecal tuberculosis including a comparison of this disease with nonspecific regional enterocolitis and noncaseous tuberculated enterocolitis. Int Abstr Surg. 1950;91:417–40. [PubMed] [Google Scholar]
- 34.Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005;37:351–6. doi: 10.1055/s-2005-861116. [DOI] [PubMed] [Google Scholar]
- 35.Patel N, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Gastrointestinal luminal tuberculosis: Establishing the diagnosis. J Gastroenterol Hepatol. 2004;19:1240–6. doi: 10.1111/j.1440-1746.2004.03485.x. [DOI] [PubMed] [Google Scholar]


