Abstract
Background
Selective 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists are reported to have potent antiemetic effects for postoperative nausea and vomiting (PONV). The purpose of this study was to prospectively evaluate the efficacy of palonosetron, granisetron, and ramosetron for the prevention of PONV in patients undergoing laparoscopic gynecologic surgery.
Methods
In this prospective, randomized observational study, 105 healthy female patients who were undergoing laparocopic hystectomy under general anaesthesia were enrolled (clinical trial number: NCT01752374, www.clinicaltrials.gov). Patients were divided into three groups: the palonostron (0.075 mg i.v.; n = 35), the granisetron group (3 mg i.v.; n = 35), and the ramosetron group (0.3 mg i.v.; n = 35). The treatments were given before the end of surgery. The incidence of PONV, severity of nausea/vomiting, and the use of rescue antiemetic requirements during the first 48 h after surgery were evaluated.
Results
The overall incidence of PONV was 33.3 % for this series. The number of complete responders at 48 h after the surgery was 21 (60.0 %) for palonosetron, 24 (68.6 %) for granisetron, and 26 (71.4 %) for ramosetron, representing no statistical difference (P = 0.086).
Conclusions
There were no significant differences in the overall incidence of postoperative nausea and vomiting and complete responders for palonosetron, granisetron and ramosetron group.
Trial registration
Clinical trial number: NCT01752374, www.clinicaltrials.gov.
Background
Laparoscopic surgeries are the second most common cause of postoperative nausea and vomiting (PONV), a frequent and disturbing complication of surgery and anesthesia [1]. The incidence of PONV after gynecological laparoscopy is reported to be nearly 80 % [2] and can result in prolonged hospital stay and recovery times.
Numerous antiemetics have been studied to prevent and treat PONV after laparoscopic abdominal surgery, including antihistamines, anticholinergics and benzamide [3–5]. However, these agents can cause undesirable side effects such as sedation, dry mouth and hypotension.
Selective serotonin 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists have a well-established role in the prophylaxis and treatment of PONV due to their efficacy and fewer side effects compared to other antiemetics [6]. Most 5-HT3 receptor antagonist research has focused on ondansetron, and the antiemetic efficacy of these compounds has been well established for the prevention and treatment of chemotherapy-induced emesis, as well as for PONV [6, 7].
Granisetron selectively blocks the 5-HT3 receptor with a relatively short half-life of 4 to 9 h. Ramosetron is a recently developed selective 5-HT3 receptor antagonist, which exhibits a significantly greater binding affinity for 5-HT3 receptors and a slower dissociation rate compared to older 5-HT3 receptor antagonists, resulting in more potent and longer effects. Palonosetron is a second-generation 5-HT3 receptor antagonist with an even higher receptor binding affinity, and a prolonged mean half-life of about 40 h [8]. We hypothesized that long acting palonosetron treatment would be more effective in lowering the incidence of PONV, compared to treatment with granisetron and ramosetron. The purpose of this study was to prospectively evaluate the efficacy of palonosetron, granisetron, and ramosetron in the prevention of PONV in patients undergoing laparoscopic gynecologic surgery.
Methods
Inclusion criteria
The study proposal was finalized and approved by Gachon University, Gil Hospital institutional review board before the investigation was initiated. Written informed consent was acquired from all participants before enrollment in the study. This investigator-initiated, prospective, non-blinded, randomized controlled trial was performed at Gachon University, Gil Hospital between November 2011 and June 2013. Using a random number table, patients were randomly assigned to receive palonosetron (n = 35), ramosetron (n = 35), or granisetron (n = 35; Fig. 1).
Fig. 1.
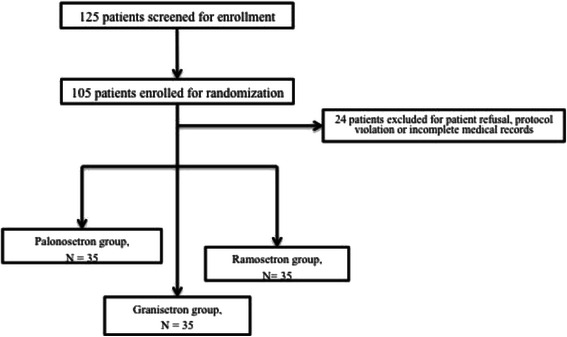
Patient allocation
Exclusion criteria
Exclusion criteria were as follows: Allergy to any of the experimental drugs, opioid dependence, a history of PONV and motion sickness, use of antiemetic medication within 24 h prior to surgery, pregnancy, inability to use the patient controlled analgesia (PCA) IV device or to comprehend the 10 cm visual analogue scales (VAS; 0, none; 10, maximum) for pain and nausea assessment, and unwillingness to be enrolled in the study.
Study protocol
All patients received intramuscular midazolam (0.05 mg/kg) and glycopyrrolate (0.2 mg) as premedication 1 h before anesthesia induction. In the operating room, the vital signs of the patient were continuously monitored using electrocardiogram, pulse oximetry, and measurement of noninvasive arterial pressure. Anesthesia was induced and maintained with propofol (target effect site concentration 2.5–3.5 μg/ml) and remifentanil (target effect site concentration 2.5–5.0 ng/ml) using a target controlled infusion (TCI) pump (Orchestra®, Fresenius Vial, Brezins, France). Rocuronium (0.6 mg/kg) was administered to facilitate tracheal intubation. Anesthesia was maintained with sevoflurane (0.5 ─5 %) and nitric oxide (50 %) throughout surgery.
All patients were ventilated using an S/5 Avance anesthetic machine (GE Healthcare, Madison, WI). After anesthesia induction, patients were mechanically ventilated with constant flow and I:E ratio of 1:2, and tidal volume (TV) set at 8 ml/kg of ideal body weight. Respiratory rate was adjusted to 8–20 breaths/min to maintain end-tidal carbon dioxide concentration (ETCO2) of 30–40 mmHg at 60 % inspired oxygen with air [9].
Pneumoperitoneum was established with a closed Veress needle technique, and the intra-abdominal pressure was maintained at 12–14 mmHg. After CO2 insufflation, patients were placed in the reverse Trendelenburg position at 20°. Laparoscopic gynecologic surgery was performed through two ports of 10 mm and two ports of 5 mm in the standard position with the legs closed [9].
Patients received a single dose of palonosetron 0.075 mg, granisetron 3 mg, or ramosetron 0.3 mg intravenously (IV) at the end of the surgery, prior to extubation. The experimental medications were prepared in identical 5 cc syringes and administered according to protocol by a nurse. For postoperative pain control, diclofenac 75 mg was administered intramuscularly (IM) at the start of wound closure, and repeated upon patient request. The incidence of nausea and vomiting was recorded during the assessment periods by ward nursing staff not blinded to the treatment drugs.
Nausea was defined as a subjectively unpleasant sensation associated with the urge to vomit. Incidences of vomiting included retching (defined as the labored, spastic, rhythmic contraction of the respiratory muscles without expulsion of the gastric contents) and actual vomiting (defined as the forceful expulsion of gastric contents from the mouth). A complete response to palonosetron, granisetron, and ramosetron was defined as an absence of PONV and no need for further rescue antiemetic drugs. A rescue antiemetic (10 mg metoclopramide) was administered IV upon patient request, if two or more episodes of PONV occurred during the study period, if nausea intensity increased from moderate to severe (VAS > 5) and on vomiting. The primary outcome was incidence of complete responders during the study period. Details of any other adverse effects such as headache and dizziness were also collected. All data were collected by a dedicated research nurse at 6, 24, and 48 h after surgery.
Statistical analyses
Sample size was calculated using two proportions power analysis on the basis of the primary outcome measure. It was estimated that 30 patients per group would be required for the power analysis (for a power of 80 % and a type 1 error of 5 %) to demonstrate a relative reduction of 25 % in complete response in each group 48 h after surgery. This calculation was based on previously published studies [10, 11]. Student’s t-test was used to compare the inter-group differences, and a chi-square test was used for categorical variables. P-values were corrected using the Bonferroni method. Values are expressed as counts or the mean ± standard deviation. P-values < 0.05 were considered statistically significant. All patients underwent laparoscopic hysterectomy under general anesthesia.
Results
All patients completed the study. Patient characteristics and basic operative data are presented in Table 1. A total of 125 patients were screened to assess their eligibility for this trial (Fig. 1) and 105 patients were enrolled in the study. The groups were comparable with respect to age, weight, duration of surgery, and ASA score. The overall incidence of PONV was 19.3 %. The incidence of nausea was highest during the first 6 h (total incidence of 18.1 %) and decreased throughout the study period.
Table 1.
Patient characteristics (n = 105)
| Palonosetron group, n = 35 | Granisetron group, n = 35 | Ramosetron group, n = 35 | P-value | |
|---|---|---|---|---|
| Age, year | 51.5 ± 16.3 | 52.5 ± 15.7 | 48.2 ± 12.1 | 0.42 |
| Height (cm) | 155.3 ± 3.1 | 157.1 ± 6.1 | 157.2 ± 3.3 | 0.65 |
| Weight (kg) | 60.1 ± 4.9 | 59.3 ± 5.1 | 56.3 ± 4.3 | 0.55 |
| Operation Time(min) | 100.2 ± 34.1 | 99.4 ± 25.3 | 92.6 ± 24.9 | 0.46 |
| Anesthesia Time(min) | 128.1 ± 47.5 | 123.5 ± 35.1 | 120.1 ± 23.0 | 0.63 |
| Fluid Admin(mL) | 522.1 ± 61.2 | 644 ± 33.3 | 538 ± 53.9 | 0.75 |
| ASA class I/II | 23/12 | 24/11 | 26/9 | 0.67 |
| Comorbid dis. | 0.56 | |||
| Hypertension | 7 | 8 | 8 | |
| Diabetes | 3 | 3 | 4 | |
| Othera | 1 | 0 | 1 |
aOther comorbid diseases include: Fatty liver, Rheumatoid arthritis
The number of complete responders at 48 h after the surgery was 21 (60.0 %) for palonosetron, 24 (68.6 %) for granisetron, and 26 (74.3 %) for ramosetron. The differences between the groups were not statistically significant (P = 0.086, Table 2). All three groups were comparable across the time intervals examined.
Table 2.
Number of nausea and vomiting over time in the three study groups (n = 105)
| Palonosetron group, n = 35 (%) | Granisetron group, n = 35 (%) | Ramosetron group, n = 35 (%) | P-value | |
|---|---|---|---|---|
| 0-6 h | ||||
| Nausea | 6 (17.1) | 5 (14.2) | 6 (17.1) | 0.87 |
| Vomiting | 1 (2.9) | 0 (0) | 1 (2.9) | N/A |
| Rescue drug | 2 (5.7) | 1 (2.9) | 1 (2.9) | 0.69 |
| 6-24 h | ||||
| Nausea | 3 (8.6) | 4 (11.4) | 4 (11.4) | 0.73 |
| Vomiting | 1 (2.9) | 0 (0) | 0 (0) | N/A |
| Rescue drug | 1 (2.9) | 0 (0) | 0 (0) | N/A |
| 24-48 h | ||||
| Nausea | 2 (5.7) | 1(2.9) | 1 (2.9) | 0.43 |
| Vomiting | 0 (0) | 0 (0) | 0 (0) | N/A |
| Rescue drug | 0 (0) | 0 (0) | 0 (0) | N/A |
| No. complete responder | 21, 60.0 % | 24, 68.6 % | 26,74.3 % | 0.086 |
N/A; not applicable due to small number of samples
The number of subjects in each of the three study groups experiencing at least one episode of vomiting within the three time intervals is shown in Table 2. More subjects suffered vomiting in the early phase (0–6 h) of postoperative period in the palonosetron and ramosetron groups compared with the granisetron group, which had no vomiting during this period. However, this difference was not statistically significant.
The incidence of most of the common adverse events, such as headache and dizziness, was similar among the three groups, and no clinically significant treatment-related adverse events were observed.
Discussion
In the present study, all three antiemetics were shown to be equally effective in preventing nausea and vomiting. There were no statistically significant differences in the number of complete responses between the three groups. A variety of 5-HT3 antagonists have been used to manage PONV [12]. It is generally accepted that all 5-HT3 antagonists have a similar mechanism of action (selective or competitive binding to 5-HT3 receptors) as well as comparable efficacy and safety profiles [5, 10]. This is the first direct comparison between these three antiemetics for their role in case of laparoscopic gynecologic surgery.
The reported incidence of PONV is 30 to 80 % within the first 24 h after laparoscopic surgery when no prophylactic antiemetic is administered [5, 3, 13]. The present study reports an overall incidence of 33.3 % within the first 48 h post-surgery, without any statistical difference between the three groups. The high incidence during the first 24 h of surgery may be explained by the central action of carbon dioxide (CO2), stretching of the peritoneum and diaphragm, and increased blood pressure in the peritoneal cavity after CO2 insufflation during laparoscopic surgery, as previously reported by Ryu et al. [3]. All these factors are considered to provoke nausea and vomiting by reducing the blood flow and releasing emetogenic substances, including serotonin [14, 15].
The reported efficacy of ramosetron for the prevention of chemotherapy-induced emesis is similar to that of granisetron [16]. However, ramosetron appears to have a longer duration of action; its effects last for 24 h after cisplatin-based chemotherapy [17]. Pal et al. [18] found that ramosetron is also more effective than palonosetron and ondansetron during the early postoperative period. However, we did not find any significant difference between patients treated with these compounds in the present study. Of note, we used remifentanil to achieve the desired analgesic effect. Remifentanil infusion during desflurane- or propofol-induced anesthesia facilitates early recovery without immensely increasing PONV, pain or the need for rescue medication after laparoscopic surgery [19].
We are unaware of any published study simultaneously evaluating the efficacy of palonosetron, granisetron, and ramosetron. Discrepancies exist between studies examining the effects of each 5-HT3 antagonist individually [20]. To the best of our knowledge, ours is the first study to evaluate the efficacy of palonosetron, granisetron, and ramosetron as prophylactics for preventing PONV in patients undergoing uniform laparoscopic gynecologic surgery. Aspinall and Goodman suggested that if active drugs are available, placebo-controlled trials are unethical because PONV is a very disturbing and distressing event that can occur after laparoscopic surgery [20]. In the current study, three commercially available 5-HT3 antagonists were used as antiemetics to prevent PONV after laparoscopic surgery. We found that the three agents yielded similar numbers of complete responses (i.e., no postoperative nausea or vomiting and no rescue antiemetic administration required) between 0 and 48 h after surgery.
We observed relatively similar numbers of complete responders in each group, in contrast with previously published reports (Table 3). The most frequently reported adverse events of 5-HT3 receptor antagonists are dizziness and headache [6]. The adverse events observed in our study were similar among all three groups of patients.
Table 3.
Results of studies evaluating outcomes of PONV patients undergoing laparoscopic surgery
| Authors | Prospective study | Number of patients enrolled | Year of study | Type of PONV | Complete responder |
|---|---|---|---|---|---|
| Incidence, (%) | |||||
| Yun et al. | No | 98 | 2010 | Az, On | 35-51 |
| Ryu et al. | No | 120 | 2010 | On, Ra | 23-40 |
| Fujii et al. | Yes | 120 | 1999 | Ra, Gr | 87-90 |
| Swaika et al. | Yes | 87 | 2011 | Ra, Pa | 38-66 |
| Current study | Yes | 105 | 2012 | Pa, Ra, On | 60-71 |
Az azasetron; On ondansetron; Pa palonostron; Ra ramosetron; Gr graniestron
The major limitations of the current study are: (1) We compared the efficacy of palonosetron, ramosetron, and granisetron at their known optimal doses, not at equipotent doses, and (2) this was not a double-blinded study. We did not administer equipotent doses of the compounds as they were unknown at the time of commencement of the study. Investigations on a larger scale are needed to assess the equipotency of palonosetron, ramosetron, and granisetron.
Conclusions
In conclusion, our study demonstrated that palonosetron, ramosetron, and granisetron are equally effective as antiemetics against postoperative nausea and vomiting in patients undergoing laparoscopic gynecologic surgery.
Acknowledgements
None of the listed authors have any conflicts of interest or financial disclosures. We thank CKDpharm for their generous support.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LWS and CYJ contributed to conception and design of the study and wrote the manuscript. LWS and LKB contributed to conception and design of the study and revised the manuscript. LWS, LKB, and LSY contributed to acquisition, analysis, and interpretation of data. LWS revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Won-Suk Lee, Email: lws@gilhospital.com.
Kwang-Beom Lee, Email: lws@gachon.ac.kr.
Soyi Lim, Email: doug.ws.lee@gmail.com.
Young Gin Chang, Phone: +82-32-460-3272, Email: gachonlws@gmail.com.
References
- 1.Kranke P, Eberhart LH. Postoperative nausea and vomiting: rational algorithms for prevention and treatment based on current evidence. Anasthesiol Intensivmed Notfallmed Schmerzther. 2009;44:2869. doi: 10.1055/s-0029-1243019. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson H, Korttila K. Recovery profile after desflurane with or without ondansetron compared with propofol in patients undergoing outpatient gynecological laparoscopy. Anesth Analg. 1996;82:5336. doi: 10.1097/00000539-199603000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Ryu J, So Y-M, Hwang J, Do S-H. Ramosetron versus ondansetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc. 2010;24:8120. doi: 10.1007/s00464-009-0670-5. [DOI] [PubMed] [Google Scholar]
- 4.Sohi HS, Heipel J, Inman KJ, Chinnick B, Cunningham DG, Holliday RL, et al. Preoperative transdermal scopolamine does not reduce the level of nausea and frequency of vomiting after laparoscopic cholecystectomy. Can J Surg. 1994;37:3074. [PubMed] [Google Scholar]
- 5.Tramurg J. Efficacy, dose–response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials. Anesthesiology. 1997;87:1277. doi: 10.1097/00000542-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Habib AS, Gan TJ. Evidence-based management of postoperative nausea and vomiting: a review. Can J Anaesth. 2004;51:3264. doi: 10.1007/BF03018236. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie R, Kovac A. Comparison of ondansetron versus placebo to prevent postoperative nausea and vomiting in women undergoing ambulatory gynecologic surgery. Anesthesiology. 1993;78:2193. doi: 10.1097/00000542-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Park JW, Jun JW, Lim YH, Lee SS, Yoo BH, Kim K-M, et al. The comparative study to evaluate the effect of palonosetron monotherapy versus palonosetron with dexamethasone combination therapy for prevention of postoperative nausea and vomiting. Korean J Anesthesiol. 2012;63:3342. doi: 10.4097/kjae.2012.63.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak HJ, Park SK, Lee KC, Lee DC, Kim JY. High positive end-expiratory pressure preserves cerebral oxygen saturation during laparoscopic cholecystectomy under propofol anesthesia. Surg Endosc. 2012;27(2):415–20. doi: 10.1007/s00464-012-2447-5. [DOI] [PubMed] [Google Scholar]
- 10.Kazemi-Kjellberg F, Henzi I. Treatment of established postoperative nausea and vomiting: a quantitative systematic review. BMC Anesthesiol. 2001;1:2. doi: 10.1186/1471-2253-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Oliveira GS, Jr C-ALJS, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to Prevent Postoperative Nausea and Vomiting: An Updated Meta-Analysis of Randomized Controlled Trials. Anesth Analg. 2012;116(1):58–74. doi: 10.1213/ANE.0b013e31826f0a0a. [DOI] [PubMed] [Google Scholar]
- 12.Graczyk SG, McKenzie R, Kallar S, Hickok CB, Melson T, Morrill B, et al. Intravenous dolasetron for the prevention of postoperative nausea and vomiting after outpatient laparoscopic gynecologic surgery. Anesth Analg. 1997;84:3257. doi: 10.1213/00000539-199702000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Nesek-Adam V, Grizelj-Stojcić E. Comparison of dexamethasone, metoclopramide, and their combination in the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc. 2007;21:6077. doi: 10.1007/s00464-006-9122-7. [DOI] [PubMed] [Google Scholar]
- 14.Leksowski K, Peryga P, Szyca R. Ondansetron, metoclopramid, dexamethason, and their combinations compared for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a prospective randomized study. Surg Endosc. 2006;20:8786. doi: 10.1007/s00464-005-0622-7. [DOI] [PubMed] [Google Scholar]
- 15.Diebel LN, Dulchavsky SA, Wilson RF. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma. 1992;33:4592. doi: 10.1097/00005373-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Feng F, Zhang P, He Y, Li Y, Zhou M, Chen G, et al. Clinical comparison of the selective serotonin3 antagonists ramosetron and granisetron in treating acute chemotherapy-induced emesis, nausea and anorexia. Chin Med Sci J. 2002;17:1682. [PubMed] [Google Scholar]
- 17.Feng FY, Zhang P, He YJ, Li YH, Zhou MZ, Cheng G, et al. Comparison of the selective serotonin3 antagonists ramosetron and granisetron in treating acute chemotherapy-induced emesis, nausea, and anorexia: a single-blind, randomized, crossover study. Curr Ther Res. 2000;61:9010. doi: 10.1016/S0011-393X(00)90017-1. [DOI] [Google Scholar]
- 18.Pal A, Saha D, Swaika S, Chatterjee S, Dawar N. Ondansetron, ramosetron, or palonosetron: Which is a better choice of antiemetic to prevent postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy? Anesth Essays Res. 2011;5:182. doi: 10.4103/0259-1162.94761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song D, White PF. Remifentanil as an adjuvant during desflurane anesthesia facilitates early recovery after ambulatory surgery. J Clin Anesth. 1999;11:3649. doi: 10.1016/S0952-8180(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 20.Aspinall RL, Goodman NW. Denial of effective treatment and poor quality of clinical information in placebo controlled trials of ondansetron for postoperative nausea and vomiting: a review of published trials. BMJ. 1995;311:8445. doi: 10.1136/bmj.311.7009.844. [DOI] [PMC free article] [PubMed] [Google Scholar]


