Abstract
The authors report the case of a 25-month-old boy who underwent endoscopic third ventriculostomy (ETV) for hydrocephalus resulting from aqueductal stenosis. The patient’s recovery was monitored longitudinally and prospectively using MR diffusion tensor imaging (DTI) and formal neuropsychological testing. Despite minimal change in ventricle size, improvement in the DTI characteristics and neurodevelopmental trajectory was observed following ETV. These data support the use of DTI as a biomarker to assess therapeutic response in children undergoing surgical treatment for hydrocephalus. In the patient featured in this report, DTI appeared to provide more information regarding postoperative neurodevelopmental outcome than ventricle size alone.
Keywords: diffusion tensor imaging, hydrocephalus, pediatric, endoscopic third ventriculostomy, neurodevelopment
Introduction
Hydrocephalus results from a pathological imbalance between CSF production and CSF absorption. Through increased intracranial pressure and related pathophysiological processes, hydrocephalus may affect the health and/or development of key white matter tracts, potentially with long-term neurological consequences. Surgical treatment is indicated to limit injury to neural structures and prevent neurological sequelae. Approaches for the treatment of hydrocephalus include CSF diversion with a conventional shunt or ETV. While ETV offers several potential advantages over shunts in select patients, including possibly lower long-term failure rates and the avoidance of shunt hardware,15–17 shunting is generally associated with a smaller posttreatment ventricle size. It remains to be determined whether this difference in ventricle size in shunting versus ETV has functional or developmental implications.
Magnetic resonance DTI provides a noninvasive quantifiable assessment of white matter microstructure. 11,22,26–28 By measuring the anisotropic diffusion properties of water molecules, DTI provides information about white matter tract integrity under normal or pathological conditions.11,22,26–28 Recent studies have shown significant changes in FA and MD in the corpus callosum and internal capsule of pediatric patients with hydrocephalus,3,30 with a trend toward normalization of these parameters occurring after shunting.2,3 The relationship between FA and MD, and neurodevelopmental outcome in pediatric hydrocephalus is under active investigation by us and others.
Herein, we report a longitudinal case study of a 25-month-old boy with aqueductal stenosis and hydrocephalus who underwent ETV. We compare preoperative and postoperative ventricle sizes, DTI parameters, and neuropsychological data to assess structural and functional response to therapy. To our knowledge, this report represents the first prospective, longitudinal investigation of ETV using DTI, as well as the first longitudinal study with parallel DTI and neuropsychological evaluations in hydrocephalus.
Case Report
Data were prospectively acquired after informed consent was obtained according to Cincinnati Children’s Human Research Protection Office guidelines.
History and Examination
A 25-month-old boy was evaluated for progressive macrocephaly noted by his pediatrician. His parents reported a history of morning irritability and an aversion to lying flat. He had walked independently and spoke several words at 1 year of age. His medical history was unremarkable. He had been born at term via vaginal birth and had no relatives with macrocephaly or hydrocephalus. Physical examination showed a well-appearing child with frontal bossing and an OFC measuring 53.5 cm (> 99% percentile for age). No focal neurological deficits were appreciated, but papilledema was observed on examination by an ophthalmologist.
Initial CT scanning demonstrated marked enlargement of the lateral and third ventricles with a moderately sized fourth ventricle (Fig. 1). A giant cisterna magna was also observed. Conventional MRI confirmed the above findings and demonstrated proximal aqueductal narrowing with triventricular enlargement. No cine phase-contrast CSF flow study was performed on initial MRI.
Figure 1.
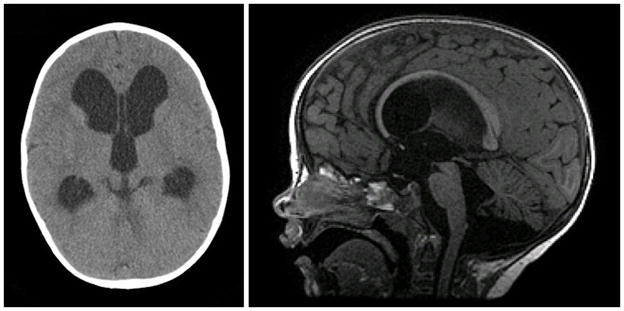
Conventional neuroimaging in a 25-month-old boy with hydrocephalus resulting from aqueductal stenosis. Left: Preoperative axial CT scan showing enlargement of the lateral and third ventricles with a moderately sized fourth ventricle. Right: Preoperative sagittal T1-weighted MR image demonstrating proximal aqueductal narrowing. A giant cisterna magna was also observed.
Operation
The patient was placed supine on the operating room table, and his head was secured in 3-point fixation. The hair in the right frontal area was clipped, and the scalp was prepared in the standard fashion. A 3-cm linear incision was made 2.5 cm lateral to the midline at the level of the coronal suture. A bur hole was made using a high-speed air drill, and the dura mater was coagulated and opened sharply. The right lateral ventricle was cannulated, and a rigid 30° Minop endoscope (Aesculap) was placed into the right lateral ventricle and through the foramen of Munro to inspect the third ventricular anatomy. Through a working channel, dissecting forceps were used to thin and open the floor of the third ventricle. The ventriculostomy was subsequently dilated with a neuroballoon catheter (Integra Neurosciences). Excellent to-and-fro flow was noted. No intraventricular hemorrhage was observed, and no immediate operative complications were identified.
Postoperative Course
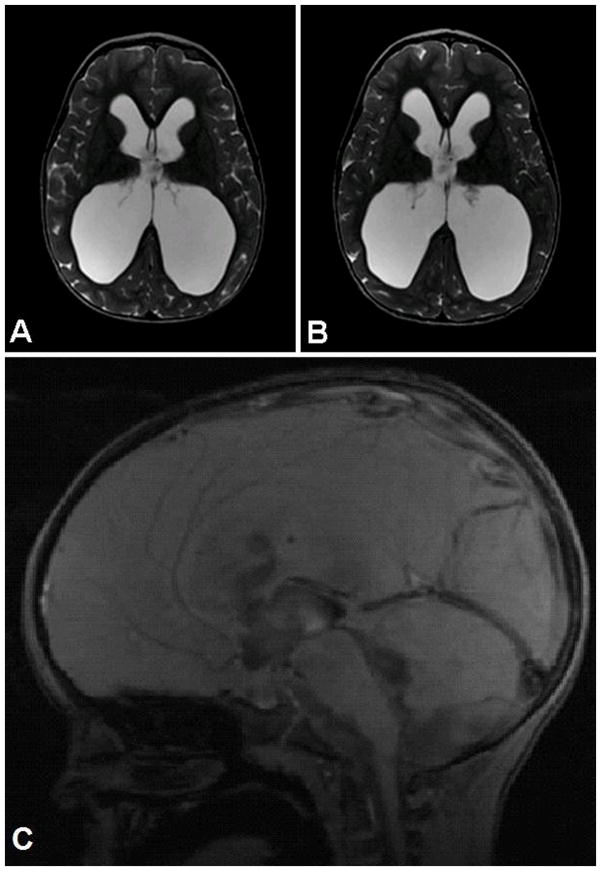
The patient was observed overnight in the pediatric intensive care unit and discharged home on postoperative Day 2. He made an excellent recovery and his symptoms resolved. He was followed serially in an outpatient setting and required no additional neurosurgical procedures. At the last follow-up 14 months postoperatively, his family reported no irritability or fussiness attributable to headaches, and they described continued language and motor progress. On physical examination, his OFC was 56 cm (> 99% percentile for age), and his neurological examination remained normal. An ophthalmologist verified resolution of his papilledema postoperatively. Neuroimaging with conventional MRI and DTI was performed 14 months postoperatively and demonstrated no change in his frontooccipital horn ratio (0.59 both preoperatively and postoperatively; Fig. 2A and B).21 Volumetric analysis of the ventricular system revealed a slight increase in ventricular volume as assessed using the ventricle/brain ratio following ETV (preoperative 0.30, postoperative 0.36). Assessment of the third ventricular shape using the third ventricular morphology index showed improvement postoperatively, which was consistent with successful ETV (preoperative 0.47, postoperative 0.41).9 Of note, a cine phase-contrast CSF flow study on the 14-month postoperative scan demonstrated excellent flow through the ventriculostomy (Fig. 2C).
Figure 2.
Stable ventricular size following successful ETV. Preoperative (A) and 14 months post-ETV (B) T2-weighted MR images used to calculate the frontooccipital horn ratio, which was 0.59 for both the preoperative and postoperative scans. Postoperative sagittal cine phase-contrast CSF flow study (C) demonstrating flow through the ventriculostomy.
Diffusion Tensor Imaging
Preoperative and postoperative DTI data were acquired with a 1.5-T scanner (Signa, GE Healthcare). A diffusion-weighted spin echo sequence with single-shot echo planar imaging was used with the following specifications: field of view = 240 × 240 mm, matrix = 96 × 96, in-plane resolution = 2.5 × 2.5 mm, slice thickness = 2.5 mm, number of slices = 76, TR = 9400 msec, TE = 93.2 msec, and ASSET factor = 2. Diffusion weighting was applied with b = 1000 seconds/mm2 along 15 independent, noncollinear orientations. One additional image with no diffusion weighting (b =0) was also acquired. Two averages were used to increase the signal-to-noise ratio. Image reconstruction, image processing, and ROI-based DTI parameter calculations were performed using the DtiStudio 2.4 software.18 Diffusion tensor imaging metrics were calculated using the standard formula given by Basser and Pierpaoli.4 Delineation of the ROIs was based on the approach described by Hermoye et al.12 and used in our previous work.2,29,30 The ROIs studied were the gCC, sCC, ALIC bilaterally, and PLIC bilaterally (Fig. 3A). The observed ROI data were compared with a previously described population of age-matched controls undergoing MRI for a headache or other nonspecific neurological symptom but without documented disease after at least 4 months of followup. 30 These control data were used to develop 99% prediction intervals for the studied ROIs at the preoperative (25 months) and follow-up (39 months) time points. The ROI data for the current patient were then assessed to determine whether they fell above, below, or within the calculated prediction interval (Table 1).
Figure 3.
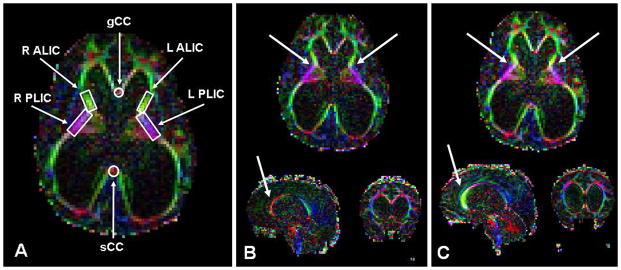
Recovery of DTI characteristics in the gCC and PLIC after ETV. A: Delineation of ROIs in key white matter tracts. B: Preoperative FA color map of the patient at 25.6 months. C: Postoperative FA color map at 39.1 months. White arrows denote areas of the gCC and PLIC, with the most improvement in DTI characteristics following ETV. L ALIC = left ALIC; L PLIC = left PLIC; R ALIC = right ALIC; R PLIC = right PLIC.
Table 1.
Observed FA and MD values in a boy at 25 and 39 months of age, compared with 99% prediction intervals calculated from age-matched controls
| gCC FA | gCC MD | sCC FA | sCC MD | ALIC FA | ALIC MD | PLIC FA | PLIC MD | |
|---|---|---|---|---|---|---|---|---|
| Pre-op | ||||||||
| Value | 0.48 | 1.33 | 0.45 | 1.11 | 0.61 | 0.89 | 0.66 | 0.81 |
| 99% prediction interval | (0.618, 0.779) | (0.681, 1.248) | (0.677, 0.869) | (0.473, 1.421) | (0.461, 0.604) | (0.622, 0.953) | (0.576, 0.644) | (0.589, 0.968) |
| Relation to interval | Below | Above | Below | Within | Above | Within | Above | Within |
| Post-op | ||||||||
| Value | 0.69 | 0.95 | 0.43 | 0.97 | 0.63 | 0.76 | 0.65 | 0.83 |
| 99% prediction interval | (0.580, 0.836) | (0.823, 1.087) | (0.684, 0.851) | (0.634, 1.423) | (0.489, 0.622) | (0.587, 0.984) | (0.544, 0.722) | (0.658, 0.844) |
| Relation to interval | Within | Within | Below | Within | Above | Within | Within | Within |
Preoperative (Fig. 3B) and 14-month post-ETV (Fig. 3C) DTI images are shown. Assessment of the preoperative DTI data demonstrated FA values lower than age-matched 99% prediction intervals in gCC and sCC, whereas ALIC and PLIC FA values were elevated (Table 1). In addition, MD was elevated in the gCC. On followup DTI 14 months after successful ETV, FA values for gCC and PLIC returned to the 99% prediction interval. Mean diffusivity in the gCC also returned to control levels. Fractional anisotropy in the ALIC remained above the 99% prediction interval following treatment in the boy.
Neuropsychological Testing
A baseline, preoperative developmental assessment was retrospectively performed using the Adaptive Behavior Assessment System, Second Edition (ABAS-II) and Child Behavior Checklist (CBCL). The ABAS-II is a parent-reported measure of a child’s independence in conceptual, practical, and social skills, whereas the CBCL is a parent-reported measure of a child’s general emotional, behavioral, and social functioning. 1,10 These measures were repeated postoperatively at 3 and 14 months post-ETV. The patient also nderwent developmental testing using the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) at 3 months postsurgery and the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) at 14 months postsurgery.5,25 Postoperative assessments also included a measure of visual motor skills, the Beery-Buktenica Developmental Test of Visual-Motor Integration.6
Retrospective parent-reported measures suggested relative strengths in conceptual skills (particularly communication skills) and relative weaknesses in self-care and leisure skills preoperatively (Table 2). Postsurgery, parent responses on the ABAS-II suggested a trend toward improved independence skills overall, most notably related to social skills. Similarly, parent responses on the CBCL suggested a trend toward a reduction in internalizing symptoms of emotional distress. For each measure, the changes reached significance at the 3-month time point only but remained evident at 14 months postsurgery. Formal cognitive testing postsurgery revealed a pattern of relative verbal strengths (in the high average range or above) in the context of otherwise average abilities. Postsurgical testing also showed a relative weakness in visualmotor integration (in the low average to borderline range).
Table 2.
Neuropsychological measures before and at 3 and 14 months after ETV
| Pre-op | 3 months post-op | 14 months post-op | |
|---|---|---|---|
| ABAS-II (SS) | |||
| General Conceptual | 89 | 101 | 99 |
| Conceptual | 109 | 113 | 115 |
| Social | 90 | 108 | 99 |
| Practical | 86 | 96 | 84 |
| Motor | 95 | 80 | 95 |
| CBCL (T) | |||
| Internalizing | 52 | 37 | 43 |
| Externalizing | 52 | 37 | 43 |
| Total problems | 57 | 48 | 48 |
| Bayley-3 (SS) | |||
| Cognitive | 110 | ||
| Language | 121 | ||
| Motor | 97 | ||
| WPPSI-III (SS) | |||
| Full IQ | 104 | ||
| Verbal | 110 | ||
| Performance | 97 | ||
| VMI (SS) | |||
| VMI | 88 | 78 | |
Discussion
We present the results of a longitudinal examination of DTI characteristics and neuropsychological assessment in a young patient undergoing ETV for hydrocephalus due to aqueductal stenosis. The patient’s preoperative DTI demonstrated changes in FA and MD consistent with those previously observed in hydrocephalus.
One year after successful ETV, follow-up DTI demonstrated a return of the FA and MD values for gCC and PLIC to the 99% prediction interval, which was consistent with the resolution of white matter tract compression. It is not clear why FA in the ALIC remained elevated; this finding may suggest limitations in the resilience of this white matter tract, a requirement for a longer interval for recovery, or irrecoverable injury prior to ETV. It is also possible that the ETV did not provide sufficient decompression for the recovery of this white matter tract. Of note, in prior studies of surgically treated hydrocephalus (ETV or shunting), the recovery of FA values for the ALIC was inconsistent.2,3
Long-term intellectual disability is common in hydrocephalic patients.13 Despite this risk, neuropsychological assessment in the patient in the present case was consistent with a normal developmental trajectory and appropriate cognitive functioning following ETV, even though his ventricular volume was minimally changed postoperatively. Thus, ventricle size may be less predictive of developmental outcome than DTI characteristics and white matter tract integrity. Of note, the patient’s relative verbal strengths suggest a potential emerging pattern of stronger verbal than nonverbal reasoning abilities, as is often seen in children with treated hydrocephalus.7,8 We also observed a relative weakness in visual-motor integration; this has been previously reported as a common area of weakness in children with treated hydrocephalus at ages similar to our patient.24 While there are challenges to conducting neuropsychological testing in very young children, as well as developmental attributes that limit the predictive power of neuropsychological tasks at the earliest age ranges (for example, wider normal variation), we are confident that our data captured this patient’s abilities for several reasons. First, the selected measures are most commonly used for his age at each time point, with proven reliability and validity. Second, although different measures were used at 3 and 14 months postsurgery, those selected have an acceptable level of correlation: Bayley-III Cognitive and WPPSI-III FSIQ r(57) = 0.79; Bayley-III Language and WPPSI-III VIQ r(57) = 0.82.5 Finally, the use of certain tasks to test the current patient’s emerging strengths and weaknesses has precedence in the literature on young children and correlates well with commonly used measures in studies demonstrating similar findings in older children with hydrocephalus.
How the observed DTI changes in the gCC and PLIC—and their resolution after ETV—might affect long-term neurodevelopment remains speculative, and definitive conclusions regarding this association will require additional study. However, it is worth noting that anatomical studies have shown that the gCC contains crossing fibers from the prefrontal cortex, a region that has long been implicated in cognition, executive function, verbal fluency, and memory.14,19 Accordingly, pathology within the gCC has been associated with low IQ as well as developmental and psychiatric illnesses, including autism and depression.14,19 Alternatively, the PLIC contains descending corticospinal fibers, and abnormalities in DTI parameters in this region would be expected to have implications for motor function. While no motor deficits were noted preoperatively in the patient featured in this report, formal psychomotor testing was not performed until after ETV. Thus, our ability to comment on the resolution of FA values in the PLIC is limited.
Successful ETV may be associated with long-term persistent enlargement of the ventricular system when compared with normal controls or hydrocephalic patients treated with shunts. Endoscopic third ventriculostomy often results in stability or, in some cases, an increase in ventricular size.20,23 This report shows for the first time improvements in white matter tract DTI parameters and strong intellectual development following ETV, despite minimal change in ventricular size—although, notably, the third ventricular morphology index improved. We believe this report provides additional support for the role of DTI as a candidate biomarker of subtle neurobehavioral and cognitive deficits not evident on clinical examination. While this patient’s preoperative symptoms were limited to morning irritability and an aversion to lying flat (physical signs included macrocephaly and papilledema), neuropsychological testing and DTI identified evidence of subtle preoperative deficits and white matter tract damage, respectively. In patients with a relatively mild clinical presentation such as that described in our case, DTI may have utility in complementing clinical examination and close monitoring in assessing therapeutic response and postoperative outcomes. Further work with a larger sample size will be required to define the relationship between ventricular size and neurodevelopmental outcome and, in particular, the efficacy of ETV versus shunting in the recovery of white matter tracts and the optimization of neurodevelopmental potential. Importantly, this report supports the key role of DTI in assessing white matter recovery and anticipating neurodevelopmental outcomes in clinical studies evaluating surgical approaches for the treatment of hydrocephalus.
Conclusions
We present evidence to support the use of DTI as a biomarker to assess treatment response in young children undergoing surgical treatment for hydrocephalus. In this case report, DTI appeared to provide more accurate information regarding postoperative neurodevelopment than basic ventricular size.
Acknowledgments
The authors thank Michael Wallendorf, Ph.D., for his assistance with statistical analysis.
Support: NIH/NINDS
Abbreviations
- ABAS-II
Adaptive Behavior Assessment System, Second Edition
- ALIC
anterior limb of the internal capsule
- Bayley-III
Bayley Scales of Infant and Toddler Development, Third Edition
- CBCL
Child Behavior Checklist
- DTI
diffusion tensor imaging
- ETV
endoscopic third ventriculostomy
- FA
fractional anisotropy
- gCC
genu of the corpus callosum
- MD
mean diffusivity
- OFC
occipitofrontal circumference
- PLIC
posterior limb of the internal capsule
- ROI
region of interest
- sCC
splenium of the corpus callosum
- WPPSIIII
Wechsler Preschool and Primary Scale of Intelligence, Third Edition
Footnotes
Author contributions to the study and manuscript preparation include the following. Conception and design: Yuan, Mangano, Limbrick. Acquisition of data: Yuan, Mangano, Phillips, McKinstry, Limbrick. Analysis and interpretation of data: Buckley, Yuan, Mangano, Powell, McKinstry, Rajagopal, Jones, Holland, Limbrick. Drafting the article: Buckley, Yuan, Mangano, Powell, Limbrick. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Buckley. Statistical analysis: Buckley.
Disclosure
This work was supported by Grant No. 1R01 NS066932 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (W.Y. and F.T.M.).
References
- 1.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth and Families; 2000. [Google Scholar]
- 2.Air EL, Yuan W, Holland SK, Jones BV, Bierbrauer K, Altaye M, et al. Longitudinal comparison of pre- and postoperative diffusion tensor imaging parameters in young children with hydrocephalus. Clinical article. J Neurosurg Pediatr. 2010;5:385–391. doi: 10.3171/2009.11.PEDS09343. [DOI] [PubMed] [Google Scholar]
- 3.Assaf Y, Ben-Sira L, Constantini S, Chang LC, Beni-Adani L. Diffusion tensor imaging in hydrocephalus: initial experience. AJNR Am J Neuroradiol. 2006;27:1717–1724. [PMC free article] [PubMed] [Google Scholar]
- 4.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 5.Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: Psychological Corporation; 2005. [Google Scholar]
- 6.Beery K, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration Manual. 5. Minneapolis, MN: NCS Pearson; 2004. [Google Scholar]
- 7.Donders J, Rourke BP, Canady AI. Neuropsychological functioning of hydrocephalic children. J Clin Exp Neuropsychol. 1991;13:607–613. doi: 10.1080/01688639108401075. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JM, Francis DJ, Thompson NM, Davidson KC, Miner ME. Verbal and nonverbal skill discrepancies in hydrocephalic children. J Clin Exp Neuropsychol. 1992;14:593–609. doi: 10.1080/01688639208402847. [DOI] [PubMed] [Google Scholar]
- 9.Foroughi M, Wong A, Steinbok P, Singhal A, Sargent MA, Cochrane DD. Third ventricular shape: a predictor of endoscopic third ventriculostomy success in pediatric patients. Clinical article. J Neurosurg Pediatr. 2011;7:389–396. doi: 10.3171/2011.1.PEDS10461. [DOI] [PubMed] [Google Scholar]
- 10.Harrison PL, Oakland T. Adaptive Behavior Assessment System. 2. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 11.Hasan KM, Eluvathingal TJ, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: a diffusion tensor tractography study of the association pathways. J Magn Reson Imaging. 2008;27:700–709. doi: 10.1002/jmri.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe-Hirsch E, Laroussinie F, Brunet L, Sainte-Rose C, Renier D, Cinalli G, et al. Late outcome of the surgical treatment of hydrocephalus. Childs Nerv Syst. 1998;14:97–99. doi: 10.1007/s003810050186. [DOI] [PubMed] [Google Scholar]
- 14.Kontis D, Catani M, Cuddy M, Walshe M, Nosarti C, Jones D, et al. Diffusion tensor MRI of the corpus callosum and cognitive function in adults born preterm. Neuroreport. 2009;20:424–428. doi: 10.1097/WNR.0b013e328325a8f9. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S. Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery. 2010;67:588–593. doi: 10.1227/01.NEU.0000373199.79462.21. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. Clinical article. J Neurosurg Pediatr. 2010;6:310–315. doi: 10.3171/2010.8.PEDS103. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni AV, Riva-Cambrin J, Browd SR. Use of the ETV Success Score to explain the variation in reported endoscopic third ventriculostomy success rates among published case series of childhood hydrocephalus. Clinical article. J Neurosurg Pediatr. 2011;7:143–146. doi: 10.3171/2010.11.PEDS10296. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Narberhaus A, Segarra D, Caldú X, Giménez M, Pueyo R, Botet F, et al. Corpus callosum and prefrontal functions in adolescents with history of very preterm birth. Neuropsychologia. 2008;46:111–116. doi: 10.1016/j.neuropsychologia.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Nowosławska E, Polis L, Kaniewska D, Mikołajczyk W, Krawczyk J, Szymański W, et al. Influence of neuroendoscopic third ventriculostomy on the size of ventricles in chronic hydrocephalus. J Child Neurol. 2004;19:579–587. doi: 10.1177/088307380401900803. [DOI] [PubMed] [Google Scholar]
- 21.O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29:245–249. doi: 10.1159/000028730. [DOI] [PubMed] [Google Scholar]
- 22.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 23.St George E, Natarajan K, Sgouros S. Changes in ventricular volume in hydrocephalic children following successful endoscopic third ventriculostomy. Childs Nerv Syst. 2004;20:834–838. doi: 10.1007/s00381-004-0939-x. [DOI] [PubMed] [Google Scholar]
- 24.Thompson NM, Fletcher JM, Chapieski L, Landry SH, Miner ME, Bixby J. Cognitive and motor abilities in preschool hydrocephalics. J Clin Exp Neuropsychol. 1991;13:245–258. doi: 10.1080/01688639108401041. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- 26.Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 27.Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan W, Deren KE, McAllister JP, II, Holland SK, Lindquist DM, Cancelliere A, et al. Diffusion tensor imaging correlates with cytopathology in a rat model of neonatal hydrocephalus. Cerebrospinal Fluid Res. 2010;7:19. doi: 10.1186/1743-8454-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan W, Holland SK, Schmithorst VJ, Walz NC, Cecil KM, Jones BV, et al. Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR Am J Neuroradiol. 2007;28:1919–1925. doi: 10.3174/ajnr.A0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan W, Mangano FT, Air EL, Holland SK, Jones BV, Altaye M, et al. Anisotropic diffusion properties in infants with hydrocephalus: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1792–1798. doi: 10.3174/ajnr.A1663. [DOI] [PMC free article] [PubMed] [Google Scholar]