Abstract
Nonmelanoma skin cancer (NMSC) is a major health concern worldwide. With increasing numbers in high-risk groups such as organ transplant recipients and patients taking photosensitizing medications, the incidence of NMSC continues to rise. Mouse models of NMSC allow us to better understand the molecular signaling cascades involved in skin tumor development in order to identify novel therapeutic strategies. Here we review the models designed to determine the role of the polyamines in NMSC development and maintenance. Elevated polyamines are absolutely required for tumor growth, and dysregulation of their biosynthetic and catabolic enzymes has been observed in NMSC. Studies using mice with genetic alterations in epidermal polyamines suggest that they play key roles in tumor promotion and epithelial cell survival pathways, and recent clinical trials indicate that pharmacological inhibitors of polyamine metabolism show promise in individuals at high risk for NMSC.
Keywords: nonmelanoma skin cancer, mouse models, chemical carcinogenesis, UVB, polyamines
Introduction
Nonmelanoma skin cancer (NMSC) is a major health concern worldwide and accounts for 40% of all diagnosed cancers in the United States.1,2 Recent studies estimate a more than 300% increase in NMSC in the United States since 1994, with an annual cost of $650 million.3 There are two major forms of NMSC, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), which account for 80% and 16% of all diagnosed skin cancers, respectively.1 BCCs are slow-growing and rarely metastasize, whereas SCCs are more invasive and metastasize at a higher frequency.1,4 As with most cancers, NMSC prevalence increases with age. It has been estimated that 80% of NMSC cases occur in individuals aged 60 or older.4,5 However, another estimate has shown NMSC to be on the rise in young adults.5 Overall, a 3%–8% increase in NMSC incidence has been reported worldwide since 1960.4 These alarming statistics emphasize the importance of studying the mechanisms underlying this disease by implementing physiologically relevant animal models in order to identify novel preventative and therapeutic strategies.
Risks of NMSC
Exposure to ultraviolet radiation (UVR) is the primary risk factor for NMSC, as well as melanoma.1,6 The risk of SCC is increased in fair-skinned individuals, those with increased cumulative sun exposure, those living in geographic locations closer to the equator, and older populations (reviewed by Kim and Armstrong).7 The 3-year risk of SCC in patients previously diagnosed with SCC is more than 10 times the rate in the general population,8 and patients previously diagnosed with actinic keratosis (AK), intraepidermal malignancies that exist on a continuum with SCC, are also at increased risk for SCC.9
In addition to the increasing overall rate of NMSC, there are also specific groups who are at particularly high risk for developing SCC. Xeroderma pigmentosum is an autosomal recessive genetic disease resulting from mutations in the DNA damage repair machinery.10 These patients have hypersensitivity to UV light and significantly increased (2,000-fold higher) risk of SCC, BCC, and melanoma.10–12 Organ transplant recipients taking immunosuppressive drugs to prevent rejection are at a higher general cancer risk, with SCC being the most common neoplasm.13 The incidence of SCC is 60- to 100-fold greater in organ transplant recipients than the general population, and frequently patients are diagnosed with multiple SCCs.14,15 Finally, a recent large case–control study found an increased risk for developing SCC and BCC associated with photosensitizing medications, particularly antimicrobials used for skin conditions and thiazide diuretics.16 These high-risk populations not only provide valuable information about the pathways involved in NMSC development but also highlight the critical need to develop strategies for prevention and therapy.
Mouse Models of NMSC
Multistage chemical carcinogenesis model
The mouse multistage skin chemical carcinogenesis model is one of the most extensively studied in vivo models of epithelial tumorigenesis.17 This highly reproducible system is seen as a prototypical model for the initiation, promotion, and progression phases of epithelial tumorigenesis and provides the opportunity to study the effects of chemopreventive and chemotherapeutic agents, as well as genetic mutations and dietary manipulations, at various stages of tumor development.18 Chemical carcinogenesis in mouse skin has been confirmed as a valid tool to study human epithelial cancers, as humans are naturally exposed to low doses of carcinogens and tumor-promoting agents.19 Furthermore, the model is thought to be relevant to human NMSC development because progression of benign lesions to SCCs occurs in a stepwise manner, and it induces intracellular signaling alterations similar to those produced by UVR exposure. Activating mutations in codon 61 of Ha-ras are found in mouse chemical carcinogenesis,19,20 while Ha-ras mutations at codon 12 have been reported in human SCC in skin as well as head and neck cancers.21–25 Ras is an essential component of several receptor-mediated signal transduction pathways crucial to normal cell growth and differentiation, and constitutively active Ras point mutations have been implicated in at least 20% of all human cancers (reviewed by Downward, Malumbres and Barbacid).26,27 Alterations in other genetic pathways that also occur in human cancer have been linked to the various stages of tumor development in mouse skin. These include cyclin D1 overexpression, loss of heterozygosity or mutation in p53, homozygous deletions of Rb and p16INK4A, and downregulation of E-cadherin.28,29 These molecular similarities make the mouse skin chemical carcinogenesis model a valuable and relevant tool for the study of human disease.
Early predecessors of the multistage chemical carcinogenesis model date back to the 1910s when Yamagiwa and Ichikawa painted rabbit skin with coal tar to induce tumors (reviewed by Marks and Furstenberger).30 In the 1920s, Deel-man discovered that wounding after application of carcinogenic tar caused the development of skin tumors in mice.31 Friedwald and Rous were the first to define the terms “initiation” and “promotion” when describing skin carcinogenesis. Using rabbits, they demonstrated that tumor cells were initiated by a single treatment of the carcinogen 3-methylcholanthrene. The initiated cells could be promoted into tumors after subsequent treatment of the skin with agents that induce proliferation but were not able to cause neoplastic transformation when applied alone.32 In the 1940s, the initiation and promotion approach was established in mice. This occurred after the discovery that croton oil was a potent promoting agent. Tumor initiation was achieved by treating mice with a subcarcinogenic dose of carcinogen, most commonly 7,12-dimethylbenz[a]anthracene (DMBA). Promotion occurred via the chronic application of croton oil.33,34 The use of this model revealed that initiation was irreversible and that the sequence of initiation and promotion was not interchangeable.35,36 The discovery that phorbol diesters, particularly 12-O-tetradecanoylphorbol-13-acetate (TPA), are active ingredients in croton oil ultimately led to the development of the multistage chemical carcinogenesis model used today in which an initiating carcinogen is first applied to the mouse skin followed by repeated applications of TPA.17
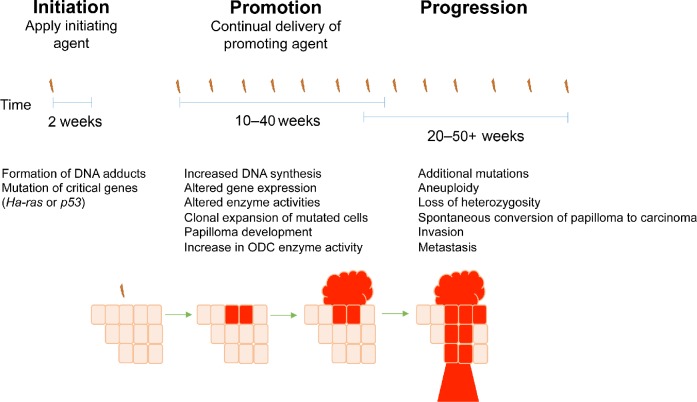
The multistage chemical carcinogenesis model is depicted in Figure 1. The polycyclic aromatic hydrocarbon DMBA is typically applied topically as the initiating agent during the resting phase of the mouse hair cycle (telogen). This application results in mutations to regulatory genes in epidermal keratinocytes by DMBA interacting with DNA and forming N6-dAdo DNA adducts.19 One hallmark of this model is A to T transversions that frequently occur in the Ha-ras proto-oncogene at codon 61 after DMBA application, which results in a constitutively active Ras protein.20 In the mouse model, Ha-ras mutations can be observed in the epidermis 1 week after the application of DMBA.37 The importance of the Ha-ras mutation and the subsequent activation of the Ras pathway in the development of skin tumors was highlighted in two studies. Balmain et al demonstrated that Ha-ras expression was increased in mouse papillomas when compared with the normal epidermal tissue.38 Spalding et al showed that mice overexpressing v-Ha-ras in the skin developed tumors after treatment with promoting agents in the absence of initiation, suggesting that Ras-activating mutations are early and critical events during skin tumorigenesis.39
Figure 1.
Multistep skin tumorigenesis. The evolution of benign papillomas and squamous cell carcinomas in response to treatment of normal skin with initiating and promoting agents is shown. Important genetic and biochemical changes associated with tumor initiation, promotion, and progression are listed.
During tumor promotion, initiated cells that have acquired a growth advantage clonally expand to form papillomas. Promotion occurs after repeated applications of a tumor-promoting agent, usually TPA. The application of tumor-promoting agents primarily induces biochemical rather than genetic alterations and often leads to skin hyperplasia and increased epidermal thickness.40 Although the exact mechanism whereby TPA induces skin tumors is not known, the hydrophobicity of the acyl chain in all phorbol esters is critical for their tumor-promoting ability, and phorbol esters have been shown to increase mRNA and protein synthesis.19 TPA is an analog of diacylglycerol and binds to protein kinase C (PKC), leading to activation of PKC downstream targets,41 and further work by Verma and colleagues has established the importance of PKC in the development of cutaneous SCC.42–44 While the contribution of chronic inflammation to the development of NMSC is not well understood, it is also known that tumor promoters induce secretion of pro-inflammatory molecules by keratinocytes. This in turn results in the recruitment of inflammatory cells into the dermis, which produce cytokines and chemokines that suppress adaptive immunity and promote tumor growth (reviewed by Rundhaug and Fischer).45
Progression is defined as the conversion of benign papillomas to carcinomas. During progression, additional genetic mutations occur and chromosomal abnormalities develop, such as aneuploidy. The resulting carcinomas may be both invasive and metastatic. The progression of papillomas to SCCs is strain dependent and occurs stochastically; there is an increased probability of additional genetic alterations as the cell population expands, and mutagen treatment or loss of p53 leads to enhanced progression.46–50 The subsequent conversion of SCCs to spindle carcinomas is a rare event.
UVR model
UVR exposure has been shown to induce oncogenic mutations in epidermal keratinocytes as well as metabolic changes in immune cells within the tumor micro-environment (reviewed by Kim and He).51 The UV spectrum is divided into UVC (200–280 nm), UVB (280–320 nm) and UVA (320–400 nm). Of these, UVB wavelengths are the most energetic and account for the majority of the biologically damaging effects of sun exposure.52 Studies in mice have shown that UVA and UVB are complete carcinogens, acting as both tumor-initiating and tumor-promoting agents.53,54 However, UVA is a weak complete carcinogen, functioning as a more potent tumor promoter than initiating agent. UVR is absorbed by macromolecules, inducing direct DNA damage by initiating the formation of cyclobutane dimers, 6–4 photoproducts, cytosine photohydrates, DNA cross-links, and DNA double-strand breaks.1 If such adducts are not repaired, then classical CC to TT and C to T transitions can ensue during normal DNA replication. Such UVR mutation signatures have been detected at a high frequency in the p53 gene for both human and mouse UV-induced skin cancers.55,56 Mutation at the dipyrimidine hot spot areas of human p53 (codons 177, 196, 278, 294, or 342) are found in 80% of AKs and greater than 90% of SCCs.57 In UVB-induced SCC in mice, p53 mutations are most often found in codon 270 (C → T). This codon corresponds with human codon 273, but there is no dipyrimidine sequence at this site in the human gene.58
Methods described for the mouse UVR model of skin carcinogenesis are more variable than the well-characterized multistage chemical carcinogenesis model. Numerous doses of UVR (usually UVB), time points, and mouse strains have been used to study the progression of NMSC, with no standard procedure being defined.59,60 It can be argued that the UV-induced mouse skin cancer model provides a more physiologically relevant method to study the underlying mechanisms of skin tumorigenesis because the carcinogens used are analogous to those that cause human disease. Moreover, while mutations in both p53 and ptch tumor suppressors have been implicated in UV-induced tumorigenesis in human and mouse,51 mutations in p53 are observed only in a minority (~30%) of tumors from DMBA/TPA-treated mice.61,62 However, UVR carcinogenesis studies are more effective in hairless mice, which complicates the ability to evaluate genetically manipulated mouse models produced in common strain backgrounds. The general principles of tumor initiation, promotion, and progression, as described above, can be applied to both chemical carcinogenesis models and those that use UVR exposure.
Polyamine Metabolism
A large number of signal transduction pathways are affected during NMSC development, and several have been the subjects of recent reviews.63–65 This review concentrates on the role of the polyamines in NMSC development and maintenance.
Polyamines, small ubiquitous polycations, are essential for normal cell growth and development,66–68 yet elevated levels of the polyamines and polyamine biosynthetic enzymes are associated with epithelial carcinogenesis and neoplastic growth.69–74 Under normal physiological conditions, intracellular polyamine levels are tightly regulated by complex metabolic, catabolic, and poorly understood transport mechanisms (Fig. 2).69,73,74 Due to the cationic nature of the polyamines, they interact with anionic molecules such as proteins, DNA, and mRNA and can modify diverse processes such as chromatin and DNA structure, DNA damage, histone acetylation, transcription, mRNA processing, stability and translation, cell cycle progression, kinase activity, and ion channel function.70,74–79 Changes in polyamine content can also induce selective rather than global effects on DNA–protein interactions80 and the translation,81 levels,82 or stability83 of specific transcripts.
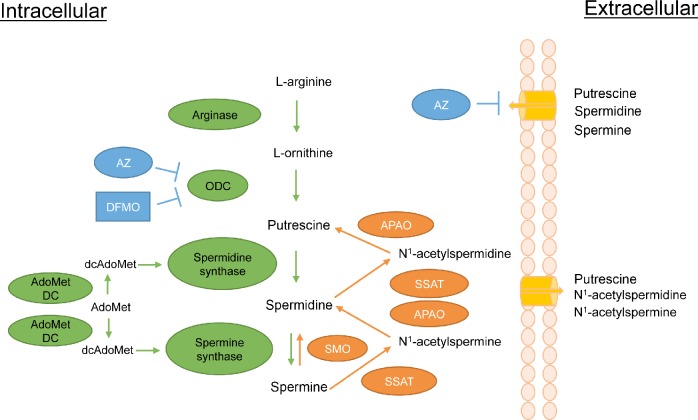
Figure 2.
The polyamine pathway in mammalian cells. Details of the pathway are described in the text. Metabolic enzymes are in green while enzymes of polyamine catabolism are in orange. Ornithine decarboxylase (ODC) inhibitors α-difluoromethylornithine (DFMO) and antizyme (AZ) are in blue. In addition to inhibiting ODC, AZ causes the ODC protein to be degraded and has the additional effect of inhibiting polyamine transport by an unknown mechanism. Other abbreviations are as follows: S-adenosylmethionine decarboxylase (AdoMetDC); S-adenosylmethionine (AdoMet); decarboxylated S-adenosylmethionine (dcAdoMet); Spermidine/spermine N1-acetyltransferase (SSAT); N1-acetylpolyamine oxidase (APAO); spermine oxidase (SMO).
In mammals, polyamines are synthesized from the amino acids l-methionine and l-arginine. l-arginine is metabolized into l-ornithine by arginase, and ornithine is subsequently decarboxylated by the rate-limiting enzyme ornithine decarboxylase (ODC) to produce the diamine putrescine. Putrescine is converted to the higher polyamines spermidine and spermine by spermidine synthase (SpdS) and spermine synthase (SpmS), respectively. The rate-limiting enzyme for the synthesis of spermidine and spermine is S-adenosylmethionine decarboxylase (AdoMetDC), a pyruvoyl-containing decarboxylase.84 AdoMetDC decarboxylates S-adenosylmethionine (AdoMet) to produce decarboxylated AdoMet (dcAdoMet). The dcAdoMet then donates its propyl amines to form spermidine and spermine via the activities of SpdS and SpmS.85,86
Both ODC and AdoMetDC activities are essential for cell growth and proliferation in a vast number of experimental models, and knockout mouse alleles for either gene are lethal at extremely early stages of embryonic development.67,87 ODC is strongly induced by proliferative stimuli and is often upregulated in cancer by various transcriptional, post-transcriptional, translational, and stability mechanisms.88–90 AdoMetDC is also a highly inducible enzyme that exhibits increased activity in association with growth-promoting stimuli,91 and it has been considered as a therapeutic target in cancer.92–94
The ODC enzyme is active as a homodimer and has a short half-life ranging from 10 to 30 minutes.90 The degradation of ODC is unique among short-lived proteins in that it is ubiquitin independent.95 Instead, the monomeric form of ODC associates noncovalently with a protein known as antizyme (AZ), which directs the ODC protein to the 26S proteasome for degradation.95 The AZ family consists of at least three differentially distributed proteins, all of which can regulate ODC.90 AZ1, the best-characterized AZ family member, is synthesized in a polyamine-dependent manner. Increases in cellular polyamine levels stimulate a +1 frameshifting event in the translation of the AZ1 mRNA, thereby increasing the expression of functional AZ1 protein.96 AZ1 not only enhances ODC degradation but also inhibits ODC activity and suppresses polyamine uptake.90,97,98 Thus, AZ1 acts as a multifunctional negative regulator of intracellular polyamine content.
Polyamine Catabolism
In addition to the highly complex biosynthetic pathway described above, the polyamines are regulated by an equally complex catabolic pathway. The relative susceptibility of spermine and spermidine to degradation/excretion is controlled by the activity of Spermidine/spermine N1-acetyltransferase (SSAT). SSAT catalyzes the formation of N1-acetylspermine or N1-acetylspermidine by transferring the acetyl group from acetyl-coenzyme A to the N1 position of spermine or spermidine.99 These acetylated polyamines can be either exported or can serve as substrates for the flavin-dependent N1-acetylpolyamine oxidase (APAO). APAO converts the acetylated polyamines to spermidine or putrescine, depending on the substrate, as well as 3-aceto-aminopropanal and hydrogen peroxide.100–102 Although APAO may also be inducible in some cases,73,102 SSAT, which is induced by a wide range of stimuli including toxins, heat shock, hormones, and polyamines (reviewed by Casero and Pegg),85 represents the rate-limiting step in this retroconversion. Acetylation of polyamines also leads to their efflux from the cell.73 SSAT induction therefore leads to the reduction of polyamine levels via their conversion to putrescine and the excretion of putrescine, N1-acetylspermidine, and N1-acetylspermine. The enzyme spermine oxidase (SMO) is able to oxidize nonacetylated spermine directly to spermidine, creating the byproducts aldehyde-3-aminopropanal and hydrogen peroxide.103 SMO is induced by several antitumorigenic polyamine analogs in cell lines derived from lung, breast, colon, and prostate tumors, suggesting that SMO is a possible target in malignancies.104,105
Polyamine Transport
As previously mentioned, intracellular polyamine content is regulated by a poorly characterized transport mechanism. Although this system is not well understood, it is evident that the polyamine transport system (PTS) plays a critical role in maintaining a specific range of cellular polyamines. The PTS is upregulated in response to polyamine depletion,106,107 and AZ has been shown to repress both ODC enzyme activity and the PTS.106 However, it has been hypothesized that this repression of the PTS is not through AZ, but via changes in the abundance of an unidentified polyamine permease.107 It is clear that additional research is needed in order to better identify the link between ODC, AZ, and the PTS. Moreover, the identification of the elusive polyamine transporter and its components is crucial for designing drugs that can target the polyamine pathway in diseases where it is dysregulated.
The Polyamine Pathway in NMSC
Understanding the role of the polyamine pathway in skin carcinogenesis is of great importance to the design of agents for both chemoprevention and chemotherapy of NMSC as well as epithelial tumors in general, and much progress has been made in this field. Using the mouse skin carcinogenesis model, studies by Boutwell and colleagues were the first to link the activity of ODC to cancer of any kind. These early studies showed that TPA induces a massive transient increase in epidermal ODC activity along with a more prolonged moderate increase in AdoMetDC activity.91,108,109 ODC activity and polyamine content are constitutively elevated in DMBA/TPA-induced tumors.108,110,111 Studies utilizing either the multistage chemical carcinogenesis model or UV-irradiation have demonstrated that the upregulation of ODC is necessary for the onset of skin tumors in mice.91,112–114 Mice treated with DMBA/TPA or UVB, including Xpa knockout mice, a model for Xeroderma pigmentosum, are protected from tumor promotion (but not tumor initiation) by treatment with the highly specific enzyme-activated irreversible ODC inhibitor α-difluoromethylornithine (DFMO).112–115 Moreover, human BCC and SCC of the skin both exhibit high levels of ODC and polyamines.116–118
In addition to their essential role in cellular proliferation, the polyamines have also been linked to suppression of both innate and adaptive immunity in many systems, which could provide a mechanism by which tumors evade the immune response. Recent studies have shown that both spermidine and spermine reduced neutrophil infiltration in TPA-treated skin and suppressed IL-1β and tumor necrosis factor alpha (TNF-α) production in macrophages.119 Moreover, simultaneous inhibition of ODC and polyamine transport suppressed skin tumor growth in immunocompetent mice but not in athymic nude mice lacking T-cells, suggesting that polyamine-targeted therapies may reverse tumor immunosuppression.120 These data combined with the results above indicate that the polyamine pathway is a valid target in NMSC development, and some of the pivotal genetic mouse model studies designed to support this conclusion are highlighted in the text below and summarized in Table 1.
Table 1.
Summary of key results linking polyamine metabolism and NMSC development.
| REGULATORY PROTEIN | SUMMARY OF RESULTS | REFERENCES |
|---|---|---|
| Ornithine decarboxylase | A. Transiently induced by tumor-promoting agents | 91, 108, 109 |
| B. Constitutively upregulated in skin tumors | 108, 110–112, 116–118 | |
| C. Overexpression is sufficient for NMSC promotion | 129, 130 | |
| D. Overexpression in combination with H-Ras is sufficient for spontaneous NMSC development in the absence of initiation or promotion | 134, 135 | |
| Antizyme | A. Overexpression suppresses DMBA/TPA- and MEK-induced tumors | 137, 147 |
| B. Suppresses tumor growth in ptch+/− mice exposed to UVB | 148 | |
| Spermidine/spermine N1-acetyltransferase | A. May alter keratinocyte differentiation | 156, 157 |
| B. Overexpression causes enhanced sensitivity to DMBA/TPA- induced tumors | 77, 155 | |
| C. Increase in ODC is essential to the overexpression phenotype | 77 | |
| D. May play a role in tumor progression | 77, 155 | |
| S-adenosylmethionine decarboxylase | A. Transiently induced by tumor-promoting agents | 91, 108, 109 |
| B. Overexpression reduces tumor incidence and tumor multiplicity in response to DMBA/TPA | 158 | |
| Spermine synthase | Widespread overexpression causes no change in NMSC susceptibility | 161 |
Mice with Genetically Altered Polyamines as Models to Probe NMSC Biology and Pathogenesis
In considering an in vivo model to test the biologic effects of genetically manipulating polyamine metabolism, mouse models in which transgenes are driven by keratin promoters offer the key advantage of tissue targeting based on the known expression pattern of keratin genes.121,122 The keratin 5 (K5), keratin 14 (K14), and keratin 6 (K6) promoters direct expression of transgenes to basal keratinocytes of the hair follicle outer root sheath (ORS) and the interfollicular epidermis.123,124 These regions include the critical follicular stem cell niche that supports hair follicle development and wound healing as well as the interfollicular stem cell niche that plays an important homeostatic role, and both niches are implicated in skin tumor development.49,125–127 The K5 promoter and its structural partner K14 are constitutively co-expressed in the ORS and interfollicular epidermis, while K6 expression is constitutive within the ORS and induced in the interfollicular epidermis by proliferative agents such as TPA.128 Thus, these models allow one to modify important signal transduction pathways in a small population of cells and determine what effect these genetic alterations have on tumor development and maintenance of established tumors. Many of the models discussed in this section use these promoters to direct expression of transgenes designed to modify levels of epidermal polyamines.
Ornithine decarboxylase
Transgenic mice overexpressing ODC in hair follicle keratinocytes using keratin promoters (K6-ODC mice and K5-ODC mice) were shown by O’Brien and colleagues to be much more sensitive than littermate controls to DMBA-induced carcinogenesis and did not require treatment with a tumor promoter to develop tumors, suggesting that ODC overexpression is a sufficient promoting stimulus in this model.129,130 Interestingly, it was further shown that papillomas from DMBA-treated K6-ODC mice exhibit an increased frequency of activating mutations in K-ras in addition to those typically observed in Ha-ras.131 Very recent results using 5-bromo-2′-deoxyuridine pulse-labeling suggest that high levels of ODC activity are sufficient to recruit hair follicle bulge stem cells, which is similar to the response seen after treatment with TPA.132 While Odc null mice are not viable,67 other studies have shown that heterozygous deletion of the Odc gene reduces susceptibility to DMBA/TPA carcinogenesis.133 Double-transgenic mice targeting ODC overexpression to the hair follicles in conjunction with an activated Ras protein (K6-ODC/Ras mice) develop spontaneous skin carcinomas without the need for either initiator or promoter treatment,134,135 demonstrating that Ras activation and high ODC activity are sufficient for tumor development in mouse skin. Using mice expressing a constitutively active mutant of MEK in the epidermis (K14-MEK mice), our previous studies confirmed that activation of the Ras effector pathway Raf/MEK/ERK induces ODC in the skin, leading to development of spontaneous tumors in an ODC-dependent manner.136,137
Results from the transgenic models described above have established ODC as an important factor in tumor promotion. However, the role of ODC in the later stages of tumorigenesis is less well defined. Polyamine depletion using DFMO has been shown to cause regression of both papillomas and SCCs in several transgenic models,42,135,136,138 as well as a reduction in carcinoma vascularization.135 These studies point to putrescine levels as an important regulator of tumor growth, but the molecular mechanisms are not known. DFMO-regressed tumors in K6-ODC/Ras mice showed no change in ras expression or proliferation index but did exhibit increased apoptosis, as did tumors from DFMO-treated K14-MEK mice.135,136 These results suggest that polyamines may play a key role in epithelial cell survival pathways. Another study has shown that in primary keratinocytes overexpression of a constitutively active Ras12V mutant, which activates all Ras effector pathways, did not cause invasion in tracheal xenotransplants. On the other hand, keratinocytes from K6-ODC mice acquired an invasive phenotype upon expression of a Ras12V/35S partial-loss-of-function mutant, which selectively activates Raf/MEK/ERK signaling.139 This study points to a threshold of ODC activity needed for invasion and suggested that genetic or epigenetic changes during tumor progression may be responsible for constitutive elevation of ODC activity in tumors, which is necessary to maintain a malignant phenotype. In agreement with this, Gilmour and colleagues have shown that elevated polyamines caused by ODC overexpression led to altered chromatin and elevated histone acetyltransferase activity in both skin and tumors from K6-ODC and ODC/Ras mice.79,140–143
Antizyme 1
As discussed above, AZ1 is an important endogenous regulator of ODC and polyamine homeostasis (Fig. 2). A critically important feature of AZ1 is its ability to suppress polyamine uptake, since the upregulation of polyamine transport represents one of the major limitations to the effectiveness of DFMO as a chemotherapeutic agent.69,70 In addition, studies in animal models144 and humans145,146 demonstrated a loss of AZ1 expression or activity in tumors relative to normal tissue.
To determine whether suppression of ODC activity and polyamine uptake blocks the promotion of initiated target cells, we have used K5 and K6 promoter elements to drive expression of AZ1. These transgenic lines target inhibition of ODC activity to a specific subpopulation of epidermal keratinocytes, rather than the more general systemic effect seen with an inhibitor such as DFMO. K5-AZ1 and K6-AZ1 mice exhibit a substantial delay in tumor onset and a significant reduction in tumor multiplicity in response to both DMBA/TPA-induced chemical carcinogenesis and when crossed with K14-MEK mice, indicating AZ1 can act as a tumor suppressor in skin carcinogenesis.137,147 AZ1 was also found to suppress tumor growth in a UV carcinogenesis model that utilized mice heterozygous for ptch.148 Unlike DFMO, AZ1 did not activate apoptosis in tumors from K14-MEK mice, but slowed tumor proliferation by decreasing the number of cells in both S-phase and mitosis, suggesting a prolonged G2 transit time.137
Spermidine/spermine-N1-acetyltransferase
It was originally hypothesized that genetic manipulations to enhance SSAT activity would decrease tumor susceptibility by depleting the higher polyamines spermidine and spermine. The importance of SSAT in maintaining polyamine content was shown by studies using transgenic mice with constitutive and ubiquitous overexpression of SSAT under its own promoter (SSAT 165 mice).149,150 These mice show a marked alteration in polyamine content with large increases in putrescine and declines in spermidine and spermine in multiple tissues, as well as permanent hair loss by age 3 weeks and development of large dermal cysts. Interestingly, an identical phenotype was described in K6-ODC mice,151 which accompanies an increased susceptibility to skin tumorigenesis, while SSAT 165 mice were resistant to DMBA/TPA.149 However, SSAT 165 mice also suffered from severe metabolic defects.152–154
In contrast to original predictions, mice with epidermal overexpression of SSAT (K6-SSAT mice) developed significantly more and larger tumors compared to controls in response to DMBA/TPA, and these tumors were much more likely to convert to SCCs.155 This phenotype was linked to both increased putrescine levels and oxidative damage resulting from SSAT-stimulated polyamine catabolism.77 ODC activity increased in response to spermine and spermidine depletion, and DFMO treatment as well as crosses with K6-AZ1 mice demonstrated that this increase in ODC was essential to the K6-SSAT phenotype.77 Limited clinical studies have also linked SSAT induction to human skin disease. A family affected with keratosis follicularis spinulosa decalvans, a rare X-linked syndrome causing follicular hyperkeratosis, demonstrated duplication of the X-chromosome in the region containing SSAT as well as a three-fold increase in SSAT activity, accompanied by increased putrescine and decreased spermidine pools.156 This phenotype is consistent with alterations in keratinocyte differentiation, which were also observed in organotypic keratinocyte cultures from SSAT-overexpressing mice.157
AdoMetDC and SpmS
Of the polyamine biosynthetic enzymes, ODC has received the vast majority of attention in cancer studies since the initial report 40 years ago that TPA induces both ODC and AdoMetDC activity in mouse skin. To address this knowledge gap, we recently utilized a tetracycline-inducible system to achieve regulated AdoMetDC activity in mouse skin (TetO-AdoMetDC transgene (TAMD) mice).158 This was the first transgenic model to be produced that allowed manipulation of cellular AdoMetDC content in a tissue-specific and regulated manner. Given that AdoMetDC activity is rate limiting for the biosynthesis of spermidine and spermine, it was predicted that these animals would have increased tumor susceptibility if these higher polyamines drive carcinogenesis. Interestingly, upon DMBA/TPA chemical carcinogenesis, TAMD mice exhibited significantly reduced tumor incidence (percent of mice with tumors) and tumor multiplicity (number of tumors per mouse) than controls. Furthermore, latent initiated cells persist in the skin of these mice despite the lack of macroscopic tumor formation. This novel finding was demonstrated by showing that upon silencing of AdoMetDC expression, the tumor multiplicity of TAMD mice rapidly increased to levels that are nearly equivalent to those in control animals.158
Taken together with the results from ODC, AZ1, and SSAT models discussed above, studies using TAMD mice strongly support the concept that high levels of intracellular putrescine are critical for tumor promotion of initiated keratinocytes. This is in contrast to the effects seen in normal keratinocytes, where high ODC has been shown to elicit apoptosis,159 perhaps through generation of reactive oxygen species caused by induction of APAO/SMO and activation of ataxia telangiectasia mutated (ATM)-DNA damage signaling.160 These studies suggest that initiated keratinocytes respond differently to elevated putrescine. Further support for putrescine as a tumor-promoting agent was provided by findings in mice with widespread overexpression of SpmS driven by a composite cytomegalovirus-immediate early gene enhancer/chicken β-actin promoter (CAG-SpmS mice). These studies showed that elevated SpmS activity, and the resulting increase in the spermine:spermidine ratio, do not increase susceptibility to either skin chemical carcinogenesis or to spontaneous intestinal carcinogenesis in Min mice.161 These results in two of the most widely used tumorigenesis models challenge the view that spermine, which is the most highly charged polyamine, is the key driver of tumor development upon elevated polyamine biosynthesis during neoplastic growth.
Prospects for Polyamine-based Therapy in NMSC
DFMO is already approved by the US Food and Drug Administration for clinical use to prevent unwanted facial hair162 and is undergoing intensive development as a chemopreventive agent,15,163–167 where it has shown promising efficacy in phase IIb and III clinical trials for BCC and SCC in patients with a previous history of skin cancer.168,169 The topical administration of DFMO to patients with existing AKs also significantly reduced the number of lesions.170,171 The strong chemopreventive effects of DFMO, along with minimal toxicity, reinforce the idea that polyamines are more essential for the growth and survival of tumor cells than their normal counterparts. These data support the use of DFMO as a chemopreventive agent for NMSC and underscore the relevance of the polyamine pathway in skin tumor development. Evidence from animal models using DFMO in combination with polyamine transport inhibitors172,173 or cyclooxygenase (COX) inhibitors174,175 demonstrated increased efficacy in both chemoprevention and therapy of NMSC. In related clinical trials, the combination of DFMO and the COX inhibitor sulindac was both safe and remarkably effective in preventing recurrence of colorectal adenomas in resected adenoma patients followed-up for 3 years,176 suggesting this would also be a promising combination in individuals at high risk for NMSC such as organ transplant recipients or Xeroderma pigmentosum (XP) patients. Patients with XP in particular are essentially “initiated”; therefore, polyamine-targeted “antipromotion” therapy may be ideal in this population.
Conclusions and Outlook for Future Studies
Studies using the multiple mouse models designed to alter intracellular polyamines in keratinocytes have suggested that the polyamine pathway is a valid target in human NMSC. However, several hurdles remain that have so far resulted in limited therapeutic success. A greater understanding of polyamine transport is essential to design of polyamine-directed therapies and it has been a key challenge in the successful use of DFMO as a chemotherapeutic agent. As mentioned above, the combination of DFMO and a polyamine transport inhibitor was significantly more effective than DFMO alone in a mouse skin SCC model172,173 as well as a xenograft study with human breast cancer cells.177 In addition, continued studies using TAMD mice will inform the use of AdoMetDC inhibitors either alone or in combination with DFMO in a number of cancers, and similar models with spatial and temporal control of ODC and AZ will broaden the scope of questions that can be addressed regarding metabolic regulation of the polyamines in a variety of diseases. Future development of similar mouse models manipulating epidermal SMO levels would aid in evaluating the preclinical use in NMSC of polyamine analogs that have shown antitumor properties in other epithelial cancer types. Since polyamine catabolism through either SMO or SSAT/APAO may potentially contribute to oxidative DNA damage as well as putrescine accumulation, these enzymes provide additional promising targets for both prevention and therapy of NMSC. Future studies will undoubtedly employ more advanced genetic engineering technologies that allow precise control of either gene overexpression or gene deletion. Utilization of these models will enable definitive analysis of stage-specific effects of the various proteins discussed in this review, thereby increasing our ability to determine critical factors in tumor initiation, promotion, progression, and maintenance.
Abbreviations
- AdoMet
S-adenosylmethionine
- AdoMetDC
S-adenosylmethionine decarboxylase
- AK
actinic keratosis
- APAO
N1-acetylpolyamine oxidase
- AZ
ornithine decarboxylase antizyme
- BCC
basal cell carcinoma
- BrdU
5-bromo-2′-deoxyuridine
- DAG
diacylglycerol
- dcAdoMet
decarboxylated S-adenosylmethionine
- DFMO
α-difluoromethylornithine
- DMBA
7,12-dimethylbenz[a]anthracene
- Dox
doxycycline
- K5
keratin 5
- K6
keratin 6
- K14
keratin 14
- ODC
ornithine decarboxylase
- ORS
outer root sheath
- PKC
protein kinase C
- PTS
polyamine transport system
- ROS
reactive oxygen species
- SCC
squamous cell carcinoma
- SMO
spermine oxidase
- SpdS
spermidine synthase
- SpmS
spermine synthase
- SSAT
spermidine/spermine-N1-acetyltransferase
- TAMD
TetO-AdoMetDC transgene
- Tet
tetracycline
- TPA
12-O-tetradecanoylphorbol-13-acetate
- UVR
ultraviolet radiation
- XP
xeroderma pigmentosum
Footnotes
ACADEMIC EDITOR: Marc D. Basson, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 416 words, excluding any confidential comments to the academic editor.
FUNDING: Work described in this article from the authors’ laboratories was supported by grants from the National Cancer Institute (CA082768 and CA142051 to LMSand CA018138 to DJF). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: SLN. Contributed to the writing of the manuscript: SLN, DJF, LMS. Agree with manuscript results and conclusions: SLN, DJF, LMS. Jointly developed the structure and arguments for the paper: SLN, LMS. Made critical revisions and approved final version: DJF. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4(1):23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Xiang F, Lucas R, Hales S, Neale R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: empirical relationships. JAMA Dermatol. 2014;150(10):1063–1071. doi: 10.1001/jamadermatol.2014.762. [DOI] [PubMed] [Google Scholar]
- 4.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375(9715):673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 5.Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294(6):681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong BK, Kricker A. How much melanoma is caused by sun exposure? Melanoma Res. 1993;3(6):395–401. doi: 10.1097/00008390-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Kim RH, Armstrong AW. Nonmelanoma skin cancer. Dermatol Clin. 2012;30(1):125–139. ix. doi: 10.1016/j.det.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136(12):1524–1530. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 9.Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1 pt 2):4–7. doi: 10.1067/mjd.2000.103342. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg EC, Henning KA. The conundrum of xeroderma pigmentosum—a rare disease with frequent complexities. Mutat Res. 1993;289(1):47–53. doi: 10.1016/0027-5107(93)90129-4. [DOI] [PubMed] [Google Scholar]
- 11.Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52(6):1119–1136. doi: 10.1111/j.1751-1097.1990.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg EC, Aguilera A, Gellert M, et al. DNA repair: from molecular mechanism to human disease. DNA Repair. 2006;5(8):986–996. doi: 10.1016/j.dnarep.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 14.Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47(1):1–17. doi: 10.1067/mjd.2002.125579. [DOI] [PubMed] [Google Scholar]
- 15.Athar M, Walsh SB, Kopelovich L, Elmets CA. Pathogenesis of nonmelanoma skin cancers in organ transplant recipients. Arch Biochem Biophys. 2011;508(2):159–163. doi: 10.1016/j.abb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson SN, Zens MS, Perry AE, Spencer SK, Duell EJ, Karagas MR. Photo-sensitizing agents and the risk of non-melanoma skin cancer: a population-based case-control study. J Invest Dermatol. 2013;133(8):1950–1955. doi: 10.1038/jid.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4(9):1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balmain A, Yuspa SH. Milestones in skin carcinogenesis: the biology of multistage carcinogenesis. J Invest Dermatol. 2014;134(e1):E2–E7. doi: 10.1038/skinbio.2014.2. [DOI] [PubMed] [Google Scholar]
- 19.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54(1):63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 20.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 21.Pierceall WE, Goldberg LH, Tainsky MA, Mukhopadhyay T, Ananthaswamy HN. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4(3):196–202. doi: 10.1002/mc.2940040306. [DOI] [PubMed] [Google Scholar]
- 22.Kreimer-Erlacher H, Seidl H, Back B, Kerl H, Wolf P. High mutation frequency at Ha-ras exons 1–4 in squamous cell carcinomas from PUVA-treated psoriasis patients. Photochem Photobiol. 2001;74(2):323–330. doi: 10.1562/0031-8655(2001)074<0323:hmfahr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Boukamp P. UV-induced skin cancer: similarities—variations. J Dtsch Dermatol Ges. 2005;3(7):493–503. doi: 10.1111/j.1610-0387.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang NJ, Sanborn Z, Arnett KL, et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci U S A. 2011;108(43):17761–17766. doi: 10.1073/pnas.1114669108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 27.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Pandolfi PP. Mouse models for multistep tumorigenesis. Trends Cell Biol. 2001;11(11):S2–S9. doi: 10.1016/s0962-8924(01)02127-4. [DOI] [PubMed] [Google Scholar]
- 29.Balmain A, Harris CC. Carcinogenesis in mouse and human cells: parallels and paradoxes. Carcinogenesis. 2000;21(3):371–377. doi: 10.1093/carcin/21.3.371. [DOI] [PubMed] [Google Scholar]
- 30.Marks F, Furstenberger G. Experimental evidence that skin carcinogenesis is a multistep phenomenon. Br J Dermatol. 1986;115(suppl 31):1–8. doi: 10.1111/j.1365-2133.1986.tb02100.x. [DOI] [PubMed] [Google Scholar]
- 31.Deelman HT. Some histological remarks on skin carcinoma. Ned Tijdschr Geneeskd. 1959;103:2453–2457. [PubMed] [Google Scholar]
- 32.Friedewald WF, Rous P. The initiating and promoting elements in tumor production: an analysis of the effects of tar, benzpyrene, and methylcholanthrene on rabbit skin. J Exp Med. 1944;80(2):101–126. doi: 10.1084/jem.80.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mottram J. A sensitising factor in experimental histogenesis. J Pathol Bacteriol. 1944;56:391–402. [Google Scholar]
- 34.Berenblum I, Shubik P. The role of croton oil applications, associated with a single painting of a carcinogen, in tumour induction of the mouse’s skin. Br J Cancer. 1947;1(4):379–382. doi: 10.1038/bjc.1947.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berenblum I, Shubik P. The persistence of latent tumour cells induced in the mouse’s skin by a single application of 9:10-dimethyl-1:2-benzanthracene. Br J Cancer. 1949;3(3):384–386. doi: 10.1038/bjc.1949.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berenblum I, Haran N. The significance of the sequence of initiating and promoting actions in the process of skin carcinogenesis in the mouse. Br J Cancer. 1955;9(2):268–271. doi: 10.1038/bjc.1955.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson MA, Futscher BW, Kinsella T, Wymer J, Bowden GT. Detection of mutant Ha-ras genes in chemically initiated mouse skin epidermis before the development of benign tumors. Proc Natl Acad Sci U S A. 1992;89(14):6398–6402. doi: 10.1073/pnas.89.14.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balmain A, Ramsden M, Bowden GT, Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- 39.Spalding JW, Momma J, Elwell MR, Tennant RW. Chemically induced skin carcinogenesis in a transgenic mouse line (TG.AC) carrying a v-Ha-ras gene. Carcinogenesis. 1993;14(7):1335–1341. doi: 10.1093/carcin/14.7.1335. [DOI] [PubMed] [Google Scholar]
- 40.Kruszewski FH, Naito M, Naito Y, DiGiovanni J. Histologic alterations produced by chrysarobin (1,8-dihydroxy-3-methyl-9-anthrone) in SENCAR mouse skin: relationship to skin tumor promoting activity. J Invest Dermatol. 1989;92(1):64–71. doi: 10.1111/1523-1747.ep13071228. [DOI] [PubMed] [Google Scholar]
- 41.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7(4):281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 42.Wheeler DL, Ness KJ, Oberley TD, Verma AK. Inhibition of development of metastatic squamous cell carcinoma in protein kinase C transgenic mice by α-difluoromethylornithine accompanied by marked hair follicle degeneration and hair loss. Cancer Res. 2003;63:3037–3042. [PubMed] [Google Scholar]
- 43.Sand JM, Aziz MH, Dreckschmidt NE, et al. PKCε overexpression, irrespective of genetic background, sensitizes skin to UVR-induced development of squamous-cell carcinomas. J Invest Dermatol. 2009;130(1):270–277. doi: 10.1038/jid.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler DL, Martin KE, Ness KJ, et al. Protein kinase C ε is an endogenous photosensitizer that enhances ultraviolet radiation-induced cutaneous damage and development of squamous cell Carcinomas. Cancer Res. 2004;64(21):7756–7765. doi: 10.1158/0008-5472.CAN-04-1881. [DOI] [PubMed] [Google Scholar]
- 45.Rundhaug JE, Fischer SM. Molecular mechanisms of mouse skin tumor promotion. Cancers (Basel) 2010;2(2):436–482. doi: 10.3390/cancers2020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bremner R, Kemp CJ, Balmain A. Induction of different genetic changes by different classes of chemical carcinogens during progression of mouse skin tumors. Mol Carcinog. 1994;11(2):90–97. doi: 10.1002/mc.2940110206. [DOI] [PubMed] [Google Scholar]
- 47.Hennings H, Glick AB, Lowry DT, Krsmanovic LS, Sly LM, Yuspa SH. FVB/N mice: an inbred strain sensitive to the chemical induction of squamous cell carcinomas in the skin. Carcinogenesis. 1993;14(11):2353–2358. doi: 10.1093/carcin/14.11.2353. [DOI] [PubMed] [Google Scholar]
- 48.Woodworth CD, Michael E, Smith L, et al. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 2004;25(9):1771–1778. doi: 10.1093/carcin/bgh170. [DOI] [PubMed] [Google Scholar]
- 49.Gerdes MJ, Yuspa SH. The contribution of epidermal stem cells to skin cancer. Stem Cell Rev. 2005;1(3):225–231. doi: 10.1385/SCR:1:3:225. [DOI] [PubMed] [Google Scholar]
- 50.Kemp CJ, Donehower LA, Bradley A, Balmain A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell. 1993;74(5):813–822. doi: 10.1016/0092-8674(93)90461-x. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y, He YY. Ultraviolet radiation-induced non-melanoma skin cancer: regulation of DNA damage repair and inflammation. Genes Dis. 2014;1(2):188–198. doi: 10.1016/j.gendis.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ley RD. Photoreactivation in humans. Proc Natl Acad Sci U S A. 1993;90(10):4337. doi: 10.1073/pnas.90.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strickland PT. Photocarcinogenesis by near-ultraviolet (UVA) radiation in Sen-car mice. J Invest Dermatol. 1986;87(2):272–275. doi: 10.1111/1523-1747.ep12696669. [DOI] [PubMed] [Google Scholar]
- 54.Willis I, Menter JM, Whyte HJ. The rapid induction of cancers in the hairless mouse utilizing the principle of photoaugmentation. J Invest Dermatol. 1981;76(5):404–408. doi: 10.1111/1523-1747.ep12520945. [DOI] [PubMed] [Google Scholar]
- 55.Kanjilal S, Pierceall WE, Cummings KK, Kripke ML, Ananthaswamy HN. High frequency of p53 mutations in ultraviolet radiation-induced murine skin tumors: evidence for strand bias and tumor heterogeneity. Cancer Res. 1993;53(13):2961–2964. [PubMed] [Google Scholar]
- 56.Ziegler A, Leffell DJ, Kunala S, et al. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993;90(9):4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brash DE, Rudolph JA, Simon JA, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You YH, Szabo PE, Pfeifer GP. Cyclobutane pyrimidine dimers form preferentially at the major p53 mutational hotspot in UVB-induced mouse skin tumors. Carcinogenesis. 2000;21(11):2113–2117. doi: 10.1093/carcin/21.11.2113. [DOI] [PubMed] [Google Scholar]
- 59.Macias E, Rao D, Digiovanni J. Role of stat3 in skin carcinogenesis: insights gained from relevant mouse models. J Skin Cancer. 2013;2013:684050. doi: 10.1155/2013/684050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruning O, Rodenburg W, van Oostrom CT, et al. A range finding protocol to support design for transcriptomics experimentation: examples of in-vitro and in-vivo murine UV exposure. PLoS One. 2014;9(5):e97089. doi: 10.1371/journal.pone.0097089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruggeri B, DiRado M, Zhang SY, Bauer B, Goodrow T, Klein-Szanto AJ. Benzo[a]pyrene-induced murine skin tumors exhibit frequent and characteristic G to T mutations in the p53 gene. Proc Natl Acad Sci U S A. 1993;90(3):1013–1017. doi: 10.1073/pnas.90.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruggeri B, Caamano J, Goodrow T, et al. Alterations of the p53 tumor suppressor gene during mouse skin tumor progression. Cancer Res. 1991;51(24):6615–6621. [PubMed] [Google Scholar]
- 63.Ullrich SE. Sunlight and skin cancer: lessons from the immune system. Mol Carcinog. 2007;46(8):629–633. doi: 10.1002/mc.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rho O, Kim DJ, Kiguchi K, Digiovanni J. Growth factor signaling pathways as targets for prevention of epithelial carcinogenesis. Mol Carcinog. 2011;50(4):264–279. doi: 10.1002/mc.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu LS, Colegio OR. Molecularly targeted therapies for nonmelanoma skin cancers. Int J Dermatol. 2013;52(6):654–665. doi: 10.1111/ijd.12017. [DOI] [PubMed] [Google Scholar]
- 66.Pegg AE. Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 67.Pendeville H, Carpino N, Marine JC, et al. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21(19):6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nowotarski SL, Woster PM, Casero RA., Jr Polyamines and cancer: implications for chemotherapy and chemoprevention. Expert Rev Mol Med. 2013;15:e3. doi: 10.1017/erm.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casero RA Jr Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6(5):373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 70.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4(10):781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 71.Pegg AE, Feith DJ. Polyamines and neoplastic growth. Biochem Soc Trans. 2007;35(pt 2):295–299. doi: 10.1042/BST0350295. [DOI] [PubMed] [Google Scholar]
- 72.Seiler N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr Drug Targets. 2003;4(7):537–564. doi: 10.2174/1389450033490885. [DOI] [PubMed] [Google Scholar]
- 73.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376(pt 1):1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61(9):880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42(1):39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 76.Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci. 2001;58(2):244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Feith DJ, Welsh P, et al. Studies of the mechanism by which increased spermidine/spermine N1-acetyltransferase activity increases susceptibility to skin carcinogenesis. Carcinogenesis. 2007;28(11):2404–2411. doi: 10.1093/carcin/bgm162. [DOI] [PubMed] [Google Scholar]
- 78.Childs AC, Mehta DJ, Gerner EW. Polyamine-dependent gene expression. Cell Mol Life Sci. 2003;60(7):1394–1406. doi: 10.1007/s00018-003-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei G, Hobbs CA, Defeo K, Hayes CS, Gilmour SK. Polyamine-mediated regulation of protein acetylation in murine skin and tumors. Mol Carcinog. 2007;46(8):611–617. doi: 10.1002/mc.20350. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Xiao L, Thiagalingam A, Nelkin BD, Casero RA., Jr The identification of a cis-element and a trans-acting factor involved in the response to polyamines and polyamine analogues in the regulation of the human spermidine/spermine N1-acetyltransferase gene transcription. J Biol Chem. 1998;273(51):34623–34630. doi: 10.1074/jbc.273.51.34623. [DOI] [PubMed] [Google Scholar]
- 81.Nishimura K, Okudaira H, Ochiai E, et al. Identification of proteins whose synthesis is preferentially enhanced by polyamines at the level of translation in mammalian cells. Int J Biochem Cell Biol. 2009;41(11):2251–2261. doi: 10.1016/j.biocel.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 82.Ancheta AA, Hawel L, III, Byus CV. Acute increases in intracellular putrescine lead to the increase in steady-state levels of c-fos, c-jun, RING3 and Id-1 mRNAs. In: Wang JY, Casero RA Jr, editors. Polyamine Cell Signaling: Physiology, Pharmacology and Cancer Research. Totowa, New Jersey: Humana Press; 2006. pp. 25–40. [Google Scholar]
- 83.Wang JY. Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids. 2007;33(2):241–252. doi: 10.1007/s00726-007-0518-z. [DOI] [PubMed] [Google Scholar]
- 84.Stanley BA, Pegg AE, Holm I. Site of pyruvate formation and processing of mammalian S-adenosylmethionine decarboxylase proenzyme. J Biol Chem. 1989;264:21073–21079. [PubMed] [Google Scholar]
- 85.Casero RA, Pegg AE. Spermidine/spermine N1-acetyltransferase: the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 86.Ikeguchi Y, Bewley MC, Pegg AE. Aminopropyltransferases: function, structure and genetics. J Biochem. 2006;139(1):1–9. doi: 10.1093/jb/mvj019. [DOI] [PubMed] [Google Scholar]
- 87.Nishimura K, Nakatsu F, Kashiwagi K, Ohno H, Saito T, Igarashi K. Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells. 2002;7(1):41–47. doi: 10.1046/j.1356-9597.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- 88.Nowotarski SL, Shantz LM. Cytoplasmic accumulation of the RNA-binding protein HuR stabilizes the ornithine decarboxylase transcript in a murine non-melanoma skin cancer model. J Biol Chem. 2010;285(41):31885–31894. doi: 10.1074/jbc.M110.148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shantz LM. Transcriptional and translational control of ornithine decarboxylase during Ras transformation. Biochem J. 2004;377(pt 1):257–264. doi: 10.1042/BJ20030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281(21):14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 91.O’Brien T, Simsiman R, Boutwell R. Induction of the polymine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975;35:1662–1670. [PubMed] [Google Scholar]
- 92.Janne J, Alhonen-Hongisto L, Nikula P, Elo H. S-adenosylmethionine decarboxylase as target of chemotherapy. Adv Enzyme Regul. 1985;24:125–139. doi: 10.1016/0065-2571(85)90073-1. [DOI] [PubMed] [Google Scholar]
- 93.Pegg AE. S-Adenosylmethionine decarboxylase. Essays Biochem. 2009;46:25–45. doi: 10.1042/bse0460003. [DOI] [PubMed] [Google Scholar]
- 94.Pegg AE, McCann PP. S-adenosylmethionine decarboxylase as an enzyme target for therapy. Pharmacol Ther. 1992;56(3):359–377. doi: 10.1016/0163-7258(92)90025-u. [DOI] [PubMed] [Google Scholar]
- 95.Murakami Y, Matsufuji S, Kameji T, et al. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature. 1992;360:597–599. doi: 10.1038/360597a0. [DOI] [PubMed] [Google Scholar]
- 96.Matsufuji S, Matsufuji T, Miyazaki Y, et al. Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell. 1995;80(1):51–60. doi: 10.1016/0092-8674(95)90450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2(3):188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 98.Mangold U. The antizyme family: polyamines and beyond. IUBMB Life. 2005;57(10):671–676. doi: 10.1080/15216540500307031. [DOI] [PubMed] [Google Scholar]
- 99.Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J. 2009;421(3):323–338. doi: 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie X, Gillies RJ, Gerner EW. Characterization of a diamine exporter in Chinese hamster ovary cells and identification of specific polyamine substrates. J Biol Chem. 1997;272:20484–20489. doi: 10.1074/jbc.272.33.20484. [DOI] [PubMed] [Google Scholar]
- 101.Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J. 2003;370(pt 1):19–28. doi: 10.1042/BJ20021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu T, Yankovskaya V, McIntire WS. Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J Biol Chem. 2003;278(23):20514–20525. doi: 10.1074/jbc.M302149200. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, Murray-Stewart T, Devereux W, et al. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304(4):605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 104.Casero RA, Jr, Wang Y, Stewart TM, et al. The role of polyamine catabolism in antitumour drug response. Biochem Soc Trans. 2003;31(2):361–365. doi: 10.1042/bst0310361. [DOI] [PubMed] [Google Scholar]
- 105.Pledgie-Tracy A, Billam M, Hacker A, et al. The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemother Pharmacol. 2010;65(6):1067–1081. doi: 10.1007/s00280-009-1112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kahana C. Regulation of cellular polyamine levels and cellular proliferation by antizyme and antizyme inhibitor. Essays Biochem. 2009;46:47–61. doi: 10.1042/bse0460004. [DOI] [PubMed] [Google Scholar]
- 107.Poulin R, Casero RA, Soulet D. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids. 2012;42:2–3. 711–723. doi: 10.1007/s00726-011-0987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Brien TG. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976;36:2644–2653. [PubMed] [Google Scholar]
- 109.Yuspa SH, Lichti U, Ben T, et al. Phorbol esters stimulate DNA synthesis and ornithine decarboxylase activity in mouse epidermal cell cultures. Nature. 1976;262(5567):402–404. doi: 10.1038/262402a0. [DOI] [PubMed] [Google Scholar]
- 110.Koza R, Megosh L, Palmieri M, O’Brien T. Constitutively elevated levels of ornithine and polyamines in mouse epidermal papillomas. Carcinogenesis. 1991;12(9):1619–1625. doi: 10.1093/carcin/12.9.1619. [DOI] [PubMed] [Google Scholar]
- 111.Imamoto A, Beltran LM, Fujiki H, Chenicek KJ, DiGiovanni J. Enhanced induction of epidermal ornithine decarboxylase activity in C57BL/6 compared to DBA/2 mice by protein kinase C-activating skin tumors promoters: relevance to genetically mediated differences in promotion susceptibility. Carcinogenesis. 1992;13:177–182. doi: 10.1093/carcin/13.2.177. [DOI] [PubMed] [Google Scholar]
- 112.Weeks CE, Herrmann AL, Nelson FR, Slaga TS. α-Difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, inhibits tumor promoter-induced polyamine accumulation and carcinogenesis in mouse skin. Proc Natl Acad Sci U S A. 1982;79:6028–6032. doi: 10.1073/pnas.79.19.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verma AK, Boutwell RK. Inhibition of carcinogenesis by inhibitors of putrescine biosynthesis. In: McCann PP, Pegg AE, Sjoerdsma A, editors. Inhibition of Polyamine Metabolism Biological Significance and Basis for New Therapies. Orlando, FL. Academic Press; 1987. pp. 249–258. [Google Scholar]
- 114.Takigawa M, Verma AK, Simsiman RC, Boutwell RK. Inhibition of mouse skin tumor promotion and of promoter-stimulated epidermal polyamine biosynthesis by alpha-difluoromethylornithine. Cancer Res. 1983;43(8):3732–3738. [PubMed] [Google Scholar]
- 115.Rebel H, van Steeg H, Beems RB, Schouten R, de Gruijl FR, Terleth C. Suppression of UV carcinogenesis by difluoromethylornithine in nucleotide excision repair-deficient Xpa knockout mice. Cancer Res. 2002;62(5):1338–1342. [PubMed] [Google Scholar]
- 116.Scalabrino G, Ferioli ME. Degree of enhancement of polyamine biosynthetic decarboxylase activities in human tumors: a useful new index of degree of malignancy. Cancer Detect Prev. 1985;8:1–2. 11–16. [PubMed] [Google Scholar]
- 117.Kagoura M, Toyoda M, Matsui C, Morohashi M. Immunohistochemical localization of ornithine decarboxylase in skin tumors. J Cutan Pathol. 2000;27(7):338–343. doi: 10.1034/j.1600-0560.2000.027007338.x. [DOI] [PubMed] [Google Scholar]
- 118.Elmets CA, Athar M. Targeting ornithine decarboxylase for the prevention of nonmelanoma skin cancer in humans. Cancer Prev Res. 2010;3(1):8–11. doi: 10.1158/1940-6207.CAPR-09-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paul S, Kang SC. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res. 2013;62(7):681–688. doi: 10.1007/s00011-013-0620-5. [DOI] [PubMed] [Google Scholar]
- 120.Hayes CS, Shicora AC, Keough MP, Snook AE, Burns MR, Gilmour SK. Polyamine-blocking therapy reverses immunosuppression in the tumor micro-environment. Cancer Immunol Res. 2014;2(3):274–285. doi: 10.1158/2326-6066.CIR-13-0120-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramirez A, Vidall M, Bravo A, Larcher F, Jorcano J. A 5′-upstream region of a bovine keratin 6 gene confers tissue-specific expression and hyperproliferation-related induction in transgenic mice. Proc Natl Acad Sci U S A. 1995;92:4783–4787. doi: 10.1073/pnas.92.11.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blessing M, Jorcano J, Franke W. Enhancer elements directing cell-type-specific expression of cytokeratin genes and changes of the epithelial cytoskeleton by transfections of hybrid cytokeratin genes. EMBO J. 1989;8(1):117–126. doi: 10.1002/j.1460-2075.1989.tb03355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramirez RA, Bravo A, Jorcano JL, Vidal M. Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58:53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- 124.Casatorres J, Navarro JM, Blessing M, Jorcano J. Analysis of the control of expression and tissue specificity of the keratin 5 gene, characteristic of basal keratinocytes. J Biol Chem. 1994;269:20489–20496.2.65 pt. [PubMed] [Google Scholar]
- 125.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multi-potency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 126.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 127.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown K, Burns P, Balmain A. Transgenic approaches to understanding the mechanisms of chemical carcinogenesis in mouse skin. Toxicol Lett. 1995;8(2/83:):123–130. doi: 10.1016/0378-4274(95)03549-4. [DOI] [PubMed] [Google Scholar]
- 129.O’Brien TG, Megosh LC, Gilliard G, Peralta Soler A. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 1997;57:2630–2637. [PubMed] [Google Scholar]
- 130.Peralta Soler A, Gilliard G, Megosh L, George K, O’Brien TG. Polyamines regulate expression of the neoplastic phenotype in mouse skin. Cancer Res. 1998;58:1654–1659. [PubMed] [Google Scholar]
- 131.Megosh L, Halpern M, Farkash E, O’Brien TG. Analysis of ras gene mutational spectra in epidermal papillomas from K6/ODC transgenic mice. Mol Carcinog. 1998;22(3):145–149. doi: 10.1002/(sici)1098-2744(199807)22:3<145::aid-mc1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 132.Hayes CS, DeFeo-Mattox K, Woster PM, Gilmour SK. Elevated ornithine decarboxylase activity promotes skin tumorigenesis by stimulating the recruitment of bulge stem cells but not via toxic polyamine catabolic metabolites. Amino Acids. 2014;46(3):543–552. doi: 10.1007/s00726-013-1559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guo Y, Cleveland JL, O’Brien TG. Haploinsufficiency for odc modifies mouse skin tumor susceptibility. Cancer Res. 2005;65(4):1146–1149. doi: 10.1158/0008-5472.CAN-04-3244. [DOI] [PubMed] [Google Scholar]
- 134.Smith MK, Trempus CS, Gilmour SK. Co-operation between follicular ornithine decarboxylase and v-Ha-ras induces spontaneous papillomas and malignant conversion in transgenic skin. Carcinogenesis. 1998;19:1409–1415. doi: 10.1093/carcin/19.8.1409. [DOI] [PubMed] [Google Scholar]
- 135.Lan L, Trempus C, Gilmour SK. Inhibition of ornithine decarboxylase (ODC) decreases tumor vascularization and reverses spontaneous tumors in ODC/Ras transgenic mice. Cancer Res. 2000;60:5696–5703. [PubMed] [Google Scholar]
- 136.Feith DJ, Bol DK, Carboni JM, et al. Induction of ornithine decarboxylase activity is a necessary step for MEK-induced skin tumorigenesis. Cancer Res. 2005;65:572–578. [PubMed] [Google Scholar]
- 137.Feith DJ, Origanti S, Shoop PL, Sass-Kuhn S, Shantz LM. Tumor suppressor activity of ODC antizyme in MEK-driven skin tumorigenesis. Carcinogenesis. 2006;27(5):1090–1098. doi: 10.1093/carcin/bgi343. [DOI] [PubMed] [Google Scholar]
- 138.Arbeit JM, Riley RR, Huey B, et al. Difluoromethylornithine chemoprevention of epidermal carcinogenesis in K14-HPV16 transgenic mice. Cancer Res. 1999;59:3610–3620. [PubMed] [Google Scholar]
- 139.Hayes CS, Defeo K, Lan L, Paul B, Sell C, Gilmour SK. Elevated levels of ornithine decarboxylase cooperate with Raf/ERK activation to convert normal keratinocytes into invasive malignant cells. Oncogene. 2006;25:1543–1553. doi: 10.1038/sj.onc.1209198. [DOI] [PubMed] [Google Scholar]
- 140.Hobbs CA, Paul BA, Gilmour SK. Deregulation of polyamine biosynthesis alters intrinsic histone acetyltransferase and deacetylase activities in murine skin and tumors. Cancer Res. 2002;62(1):67–74. [PubMed] [Google Scholar]
- 141.Hobbs CA, Gilmour SK. High levels of intracellular polyamines promote his-tone acetyltransferase activity resulting in chromatin hyperacetylation. J Cell Biochem. 2000;77(3):345–360. [PubMed] [Google Scholar]
- 142.Hobbs CA, Paul BA, Gilmour SK. Elevated levels of polyamines alter chromatin in murine skin and tumors without global changes in nucleosome acetylation. Exp Cell Res. 2003;290(2):427–436. doi: 10.1016/s0014-4827(03)00352-5. [DOI] [PubMed] [Google Scholar]
- 143.Hobbs CA, Wei G, DeFeo K, Paul B, Hayes CS, Gilmour SK. Tip60 protein isoforms and altered function in skin and tumors that overexpress ornithine decarboxylase. Cancer Res. 2006;66(16):8116–8122. doi: 10.1158/0008-5472.CAN-06-0359. [DOI] [PubMed] [Google Scholar]
- 144.Tsuji T, Todd R, Meyer C, et al. Reduction of ornithine decarboxylase antizyme (ODC-Az) level in the 7,12-dimethylbenz(a)anthracene-induced hamster buccal pouch carcinogenesis model. Oncogene. 1998;16(26):3379–3385. doi: 10.1038/sj.onc.1201887. [DOI] [PubMed] [Google Scholar]
- 145.Jung MH, Kim SC, Jeon GA, et al. Identification of differentially expressed genes in normal and tumor human gastric tissue. Genomics. 2000;69(3):281–286. doi: 10.1006/geno.2000.6338. [DOI] [PubMed] [Google Scholar]
- 146.Koike C, Chao DT, Zetter BR. Sensitivity to polyamine-induced growth arrest correlates with antizyme induction in prostate carcinoma cells. Cancer Res. 1999;59(24):6109–6112. [PubMed] [Google Scholar]
- 147.Feith DJ, Shantz LM, Shoop PL, Keefer KA, Prakashagowda C, Pegg AE. Mouse skin chemical carcinogenesis is inhibited by antizyme in promotion-sensitive and promotion-resistant genetic backgrounds. Mol Carcinog. 2007;46(6):453–465. doi: 10.1002/mc.20294. [DOI] [PubMed] [Google Scholar]
- 148.Tang X, Kim AL, Feith DJ, et al. Ornithine decarboxylase is a target for chemo-prevention of basal and squamous cell carcinomas in Ptch+/− mice. J Clin Invest. 2004;113:867–875. doi: 10.1172/JCI20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pietila M, Parkkinen JJ, Alhonen L, Janne J. Relation of skin polyamines to the hairless phenotype in transgenic mice overexpressing spermidine/spermine N-acetyltransferase. J Invest Dermatol. 2001;116(5):801–805. doi: 10.1046/j.1523-1747.2001.01330.x. [DOI] [PubMed] [Google Scholar]
- 150.Suppola S, Pietilä M, Parkkinen JJ, et al. Overexpression of spermidine/spermine N1-acetyltransferase under the control of mouse metallothionein I promoter in transgenic mice: evidence for a striking post-transcriptional regulation of trans-gene expression by a polyamine analogue. Biochem J. 1999;338(pt 2):311–316. [PMC free article] [PubMed] [Google Scholar]
- 151.Peralta Soler A, Gilliard G, Megosh LC, O’Brien LG. Modulations of murine hair follicle function by alterations in ornithine decarboxylase activity. J Invest Dermatol. 1996;106:1108–1113. doi: 10.1111/1523-1747.ep12340155. [DOI] [PubMed] [Google Scholar]
- 152.Pirinen E, Kuulasmaa T, Pietilä M, et al. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol Cell Biol. 2007;27(13):4953–4967. doi: 10.1128/MCB.02034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Merentie M, Uimari A, Pietilä M, et al. Oxidative stress and inflammation in the pathogenesis of activated polyamine catabolism-induced acute pancreatitis. Amino Acids. 2007;33(2):323–330. doi: 10.1007/s00726-007-0522-3. [DOI] [PubMed] [Google Scholar]
- 154.Cerrada-Gimenez M, Pietilä M, Loimas S, et al. Continuous oxidative stress due to activation of polyamine catabolism accelerates aging and protects against hepatotoxic insults. Transgenic Res. 2011;20(2):387–396. doi: 10.1007/s11248-010-9422-5. [DOI] [PubMed] [Google Scholar]
- 155.Coleman CS, Pegg AE, Megosh LC, Guo Y, Sawicki JA, O’Brien TG. Targeted expression of spermidine/spermine N1-acetyltransferase increases susceptibility to chemically-induced skin carcinogenesis. Carcinogenesis. 2002;23:359–364. doi: 10.1093/carcin/23.2.359. [DOI] [PubMed] [Google Scholar]
- 156.Gimelli G, Giglio S, Zuffardi O, et al. Gene dosage of the spermidine/spermine N(1)-acetyltransferase (SSAT) gene with putrescine accumulation in a patient with a Xp21.1p22.12 duplication and keratosis follicularis spinulosa decalvans (KFSD) Hum Genet. 2002;111(3):235–241. doi: 10.1007/s00439-002-0791-6. [DOI] [PubMed] [Google Scholar]
- 157.Pietilä M, Pirinen E, Keskitalo S, et al. Disturbed keratinocyte differentiation in transgenic mice and organotypic keratinocyte cultures as a result of spermidine/spermine N-acetyltransferase overexpression. J Invest Dermatol. 2005;124(3):596–601. doi: 10.1111/j.0022-202X.2005.23636.x. [DOI] [PubMed] [Google Scholar]
- 158.Shi C, Cooper TK, McCloskey DE, Glick AB, Shantz LM, Feith DJ. S-adenosylmethionine decarboxylase overexpression inhibits mouse skin tumor promotion. Carcinogenesis. 2012;33(7):1310–1318. doi: 10.1093/carcin/bgs184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gilmour SK, Birchler M, Smith MK, Rayca K, Mostochuk J. Effect of elevated levels of ornithine decarboxylase on cell cycle progression in skin. Cell Growth Differ. 1999;10:739–748. [PubMed] [Google Scholar]
- 160.Wei G, DeFeo K, Hayes CS, Woster PM, Mandik-Nayak L, Gilmour SK. Elevated ornithine decarboxylase levels activate ataxia telangiectasia mutated-DNA damage signaling in normal keratinocytes. Cancer Res. 2008;68(7):2214–2222. doi: 10.1158/0008-5472.CAN-07-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Welsh PA, Sass-Kuhn S, Prakashagowda C, McCloskey D, Feith D. Spermine synthase overexpression in vivo does not increase susceptibility to DMBA/TPA skin carcinogenesis or Min-Apc intestinal tumorigenesis. Cancer Biol Ther. 2012;13(6):358–368. doi: 10.4161/cbt.19241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wolf JE, Jr, Shander D, Huber F, et al. Eflornithine HCl Study Group Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dermatol. 2007;46(1):94–98. doi: 10.1111/j.1365-4632.2006.03079.x. [DOI] [PubMed] [Google Scholar]
- 163.Pegg AE, Shantz LM, Coleman CS. Ornithine decarboxylase as a target for chemoprevention. J Cell Biochem Suppl. 1995;22:132–138. doi: 10.1002/jcb.240590817. [DOI] [PubMed] [Google Scholar]
- 164.Meyskens FL Jr Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5(5):945–951. [PubMed] [Google Scholar]
- 165.Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signalling in skin development and cancer. Exp Dermatol. 2006;15(9):667–677. doi: 10.1111/j.1600-0625.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 166.D’Ambrosio SM, Gibson-D’Ambrosio R, Milo GE, Casto B, Kelloff GJ, Steele VE. Differential response of normal, premalignant and malignant human oral epithelial cells to growth inhibition by chemopreventive agents. Anticancer Res. 2000;20(4):2273–2280. [PubMed] [Google Scholar]
- 167.Steele VE, Moon RC, Lubet RA, et al. Preclinical efficacy evaluation of potential chemopreventive agents in animal carcinogenesis models: methods and results from the NCI Chemoprevention Drug Development Program. J Cell Biochem Suppl. 1994;20:32–54. doi: 10.1002/jcb.240560905. [DOI] [PubMed] [Google Scholar]
- 168.Bailey HH, Kim K, Verma AK, et al. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of {alpha}-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res. 2010;3(1):35–47. doi: 10.1158/1940-6207.CAPR-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kreul SM, Havighurst T, Kim K, et al. A phase III skin cancer chemoprevention study of DFMO: long-term follow-up of skin cancer events and toxicity. Cancer Prev Res. 2012;5(12):1368–1374. doi: 10.1158/1940-6207.CAPR-12-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Foote JA, Ranger-Moore JR, Einspahr JG, et al. Chemoprevention of human actinic keratoses by topical DL-alpha-tocopherol. Cancer Prev Res. 2009;2(4):394–400. doi: 10.1158/1940-6207.CAPR-08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Alberts DS, Dorr RT, Einspahr JG, et al. Chemoprevention of human actinic keratoses by topical 2-(difluoromethyl)-dl-ornithine. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1281–1286. [PubMed] [Google Scholar]
- 172.Chen Y, Weeks RS, Burns MR, Boorman DW, Klein-Szanto A, O’Brien TG. Combination therapy with 2-difluoromethylornithine and a polyamine transport inhibitor against murine squamous cell carcinoma. Int J Cancer. 2006;118(9):2344–2349. doi: 10.1002/ijc.21621. [DOI] [PubMed] [Google Scholar]
- 173.Burns MR, Graminski GF, Weeks RS, Chen Y, O’Brien TG. Lipophilic lysine-spermine conjugates are potent polyamine transport inhibitors for use in combination with a polyamine biosynthesis inhibitor. J Med Chem. 2009;52(7):1983–1993. doi: 10.1021/jm801580w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fischer SM, Conti CJ, Viner J, Aldaz CM, Lubet RA. Celecoxib and difluo-romethylornithine in combination have strong therapeutic activity against UV-induced skin tumors in mice. Carcinogenesis. 2003;24(5):945–952. doi: 10.1093/carcin/bgg046. [DOI] [PubMed] [Google Scholar]
- 175.Arumugam A, Weng Z, Talwelkar SS, et al. Inhibiting cyclooxygenase and ornithine decarboxylase by diclofenac and alpha-difluoromethylornithine blocks cutaneous SCCs by targeting Akt-ERK axis. PLoS One. 2013;8(11):e80076. doi: 10.1371/journal.pone.0080076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gerner EW, Meyskens FL., Jr Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009;15(3):758–761. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weeks RS, Vanderwerf SM, Carlson CL, et al. Novel lysine-spermine conjugate inhibits polyamine transport and inhibits cell growth when given with DFMO. Exp Cell Res. 2000;261(1):293–302. doi: 10.1006/excr.2000.5033. [DOI] [PubMed] [Google Scholar]