Abstract
Spatial reorientation by humans and other animals engages geometric representations of surface layouts as well as featural landmarks; however, the two types of information are thought to be behaviorally and neurally separable. In this paper, we examine the use of these two types of information during reorientation among children and adults with Williams syndrome (WS), a genetic disorder accompanied by abnormalities in brain regions that support use of both geometry and landmarks. Previous studies of reorientation in adolescents and adults with WS have shown deficits in the ability to use geometry for reorientation, but intact ability to use features, suggesting that the two systems can be differentially impaired by genetic disorder. Using a slightly modified layout, we found that many WS participants could use geometry, and most could use features along with geometry. However, the developmental trajectories for the two systems were quite different from one other, and different from those found in typical development. Purely geometric responding was not correlated with age in WS, and search processes appeared similar to those in typically developing (TD) children. In contrast, use of features in combination with geometry was correlated with age in WS, and search processes were distinctly different from TD children. The results support the view that use of geometry and features stem from different underlying mechanisms, that the developmental trajectories and operation of each are altered in WS, and that combination of information from the two systems is atypical. Given brain abnormalities in regions supporting the two kinds of information, our findings suggest that the co-operation of the two systems is functionally altered in this genetic syndrome.
Abundant evidence has shown that when humans and other species become spatially disoriented, they can re-establish their orientation using a geometric representation of the surrounding space (Cheng, 1986; Cheng & Newcombe, 2005; Gallistel, 1990; Gallistel & Matzel, 2013; Tommasi, Chiandetti, Pecchia, Sovrano, & Vallortigara, 2012; Wang & Spelke, 2002). It is also known that landmarks are of great importance in both oriented navigation and in re-establishing orientation (Aguirre & D’Esposito, 1999; Epstein, 2008; Newcombe & Huttenlocher, 2000). Although representations of geometry and landmarks usually work seamlessly together at the behavioral level, evidence now suggests that the two systems are separable, both in their contributions to spatial navigation and as they are instantiated in the brain (Bullens et al., 2010; Burgess, 2008; Doeller & Burgess, 2008; Doeller et al., 2008; Janzen & van Turennout, 2004; Sutton, Joanisse, & Newcombe, 2010). The learning and remembering of objects relative to the boundaries of an environment (which define its geometric shape) has been specifically linked to right posterior hippocampal activation, while learning and remembering of landmark-related locations is linked to right dorsal striatal activation (Doeller, King, & Burgess, 2008). In development, geometric sensitivity emerges early, and in some species appears to be independent of experience (Chiandetti & Vallortigara, 2008; 2010; Chiandetti, Spelke, & Vallortigara, 2014, but see Twyman, Newcombe, & Gould, 2012 for contrasting results with mice). By contrast, featural landmark use is highly susceptible to training and practice in children (Twyman, Friedman, & Spetch, 2007) and animals (Kelly & Spetch, 2004) and is supported by different learning mechanisms in adults (Doeller & Burgess, 2008). How information from the two systems is combined for reorientation over development still remains unknown, although fMRI evidence strongly implicates the role of the hippocampus in mature adults (Sutton et al., 2010). Behavioral evidence suggests that geometric representations are primary, used by children at all ages and across many contexts. Combination of geometry with a featural landmark is somewhat more variable (depending on age and size of the reorientation chamber), appearing anywhere from age 2 through 5, but stably present by age 6 (Hermer-Vasquez, Moffet, & Munkholm, 2001; Hupbach & Nadel, 2005; Learmonth, Newcombe, & Huttenlocher, 2001; Learmonth, Nadel, & Newcombe, 2002; Learmonth, Newcombe, Sheridan, & Jones, 2008).
In this paper, we probe the nature of the two systems by examining the use of geometry and features among people with Williams syndrome (WS), a genetic disorder that is characterized by a deletion of 26 genes on chromosome 7q11.23 (Hillier et al., 2003; Morris, 2006; Osborne, 2006). People with WS have a unique cognitive profile of mild to moderate mental retardation along with severe spatial impairments (Mervis et al., 2000). Across a broad range of spatial functions, they show a profile that is overall quite similar to that of TD 4–6 year-olds (Landau & Hoffman, 2012). However, perhaps the most striking aspect of the WS spatial profile is their performance on reorientation tasks. Unlike the ubiquitous pattern of geometric reorientation throughout typical human development and in all animal species studied (e.g., Cheng & Newcombe, 2005; Gallistel & Matzel, 2013; Hermer & Spelke, 1996; Tommasi et al., 2012), people with WS show severe limits in this capacity. Using the now-classic reorientation task developed by Cheng (1986) for testing rats, and adapted by Hermer and Spelke (1994; 1996) for testing children, Lakusta, Dessalegn, and Landau (2010) found that WS adolescents and adults could not reliably use geometric layout information to reorient in a rectangular room with four black walls. However, almost all were able to use a specific featural landmark (a single colored wall, hereafter referred to as a “feature”) to do so. Only 3 individuals showed use of both geometry and a feature across the two room environments.
These findings suggest selective impairment to representation of geometric layout in people with WS, but not to representation of features, a pattern which is consistent with theories and evidence suggesting that the two navigational systems are separable in terms of both behavioral mechanism (Julian, Keinath, Muzzio, & Epstein, 2014; Lee & Spelke, 2010; Lee, Shusterman, & Spelke, 2006) and underlying neural instantiation (Doeller & Burgess, 2008; Doeller et al., 2008). The WS pattern differs qualitatively from that of TD individuals, for whom geometry is used from a very early point in development, and features are integrated with geometry in both small and large spaces from about age 5 onward (Hermer-Vasquez, Moffet, & Munkholm, 2001; Learmonth et al., 2002). Consistent with the deficit in spatial navigation, WS individuals show structural and functional abnormalities in the parietal lobe and the intraparietal sulcus (Eckert et al., 2005; Kippenhan, Olsen, & Mervis, 2005; Meyer-Lindenberg et al., 2004) and also in the hippocampus (Meyer-Lindenburg et al., 2005, 2006; Reiss et al., 2000).
Hippocampal damage from lesions has been linked to deficits in using geometry for reorientation among rats, pigeons and chicks (McGregor, Hayward, Pearce, & Good, 2004; Vargas, Petruso, & Bingham, 2004; Tommasi et al. (2003). Moreover, in an fMRI study of human adults using virtual reality, the hippocampal region was found to show greater activation when participants were required to reorient according to both room geometry and a featural cue, which suggests that this region is involved in the effective combination of these sources of information (Sutton et al., 2010). People with WS show gross preservation of hippocampal volume compared to age and gender-matched controls, but consistently unusual morphology. In particular, they show local volume reduction at the posterior apex (the tail-end), and expansion at the anterior base (extending from the midsection to the dorsal hippocampal head) (Meyer-Lindenberg et al., 2005). The converse of this pattern is observed in humans proficient in spatial navigation (i.e., London taxi drivers), who show increased posterior and decreased anterior hippocampal volume (Maguire et al., 2000). A similar result has also been found for college students, for whom size of the right posterior hippocampus predicts relative position estimation of landmarks in a real-world environment (Schinazi et al., 2013).
The evidence to date for neural and behavioral separation of the two systems in WS depends on the assumption that geometric representation of layout is severely impaired in these individuals, but that feature use remains intact. Several aspects of the original data raise questions about these assumptions, however, and suggest new questions about how the two systems function in this genetic disorder. First, although Lakusta et al. (2010) found that most individuals with WS failed to use geometry when reorienting in an all-black room (i.e., with no featural information), a few individuals may have used geometry. This suggests that sensitivity to geometry may not be entirely absent in WS. Second, the use of features by the majority of WS individuals hints that they may have had access to some geometric layout information, even though this was not often shown in the all-black room. This is because successful use of the feature likely requires some kind of spatial representation of the layout. Participants used the colored wall as a true landmark and not as a beacon; that is, they did not search at the colored wall itself, but rather, used the wall to infer the target location at a specific corner. It is unclear whether spatial information was used in combination with the landmark, because it is also possible to use sense information (e.g., “when facing the red wall, search to my left”). There are hints that this spatial representation could have been geometric, at least for some people, as errors tended to accumulate in the rotationally equivalent corner to the target corner in the feature condition, which is the signature of geometric reorientation.
These observations suggest two points. First, the previous study may have underestimated the degree to which geometric representations may be constructed and used by people with WS. Second, if geometry can be more systematically observed, then it would be possible to more closely examine the degree to which both systems—geometry and features—are used together, seamlessly, as seems to be the case for TD individuals after the age of 5. Even if features are combined with geometry in WS performance, we may observe a behavioral signature for this combination that differs from TD children, and this signature could further support the idea that the two systems are neurally and behaviorally separate.
To pursue these issues, we first aimed to test geometric response among a new set of WS participants belonging to a broad age range. Lakusta et al. (2010) had tested WS individuals who were between the ages of 9 and 27 (mean age 17 years), with most participants being adolescents or adults. It seems possible that dampened use of geometry among these individuals might have been a developmental product of reliance on unique features and landmarks for many years (perhaps even being encouraged to do so by parents). This could follow if geometric sensitivity is fragile, and/or if the system for features functions robustly from early in development. Therefore, in the current study, we tested a wider age range of individuals, looking specifically for any evidence of geometric sensitivity and its potential change over age. We also introduced minor changes to the reorientation room layout to enhance the salience of geometric structure (see Methods).
Second, we aimed to replicate Lakusta et al.’s (2010) finding of robust feature use, and in particular, to examine more closely how, if at all, feature use is combined with geometry in people with WS. Recall that most of the individuals tested in Lakusta et al. (2010) successfully used a featural cue (a single blue wall) to reorient themselves, despite their failure to use geometry. This pattern is consistent with the evidence supporting different neural bases for navigation by geometry vs. featural landmarks. But it also raises the question of how the feature was used, especially since the WS geometric representation of layout in some individuals appeared to be absent.
We have already noted that WS participants did not use the feature as a beacon; rather, they used the uniquely colored wall to establish their orientation in some way and then proceeded to recover the target. For TD children and adults, the process of combining features with geometry appears to operate seamlessly and in parallel at the behavioral level. When adults re-establish orientation, they likely establish their current heading direction by comparing it to a stored global representation of the spatial layout (Lew et al., 2014). Like adults, young children appear to combine geometry with features efficiently, presumably comparing their current heading to a stored geometric representation. Huttenlocher and colleagues (Huttenlocher & Vasilyeva, 2003; Lourenco & Huttenlocher, 2006) analyzed the search patterns of children following disorientation and found that they are not likely to carry out an extensive survey of the room’s layout. That is, they do not turn their bodies or heads to view multiple walls and corners in order to recover their original perspective. Instead, the majority of the time children take a direct “beeline” to a particular corner. This pattern of search suggests that children draw upon a global representation of the space to infer their relation to a geometrically appropriate corner.
It is possible that people with WS also combine features with some spatial information—perhaps geometric structure—about layout rather automatically. However, people with WS show hippocampal abnormalities (Meyer-Lindenburg et al., 2005, 2006; Reiss et al., 2000) and research suggest that this structure is involved in the combination of features and geometry during reorientation (Sutton et al., 2010). Thus, it seems possible that the two kinds of information may be less automatically combined in this population, especially if the two systems of information are functionally more separated than is typically the case. If this were so, then we might expect search patterns to show an atypical profile (e.g., failure to reorient in a direct “beeline” fashion). Following Huttenlocher and colleagues’ analysis of search patterns, we aim to examine more closely what happens when people with WS use a feature during reorientation.
In sum, we aim to probe the WS developmental profile for the use of geometry, features, and their potential combination during reorientation. We compare patterns of search among these individuals to those of TD children who are 3–4 years of age. The results will elucidate the mechanisms of the development of the two systems of information and how they are altered as a consequence of variation in genetic and neural substrates.
Method
Participants
Sixteen TD 3.5- to 4.5-year-olds participated (8 girls, M = 3.97 years; Range = 3.5–4.42 years). At the end of the study, children chose a small toy to take home. Sixteen individuals with WS also participated (8 girls, M = 16.01 years; Range = 5.67–32.67 years). Three of these people had participated in the Lakusta et al. (2010) study, approximately 7 years prior. WS participants received monetary compensation for their time.
To assess the overall cognitive profile of the WS participants, we administered the Kauffman Brief Intelligence test (K-BIT II; Kaufman & Kaufman, 2004), as well as the Pattern Construction and Digit Span (Forward) subtests of the Differential Abilities Scale (DAS II; Elliott, 1990). The Kaufman Brief Intelligence test yields an overall IQ score and is commonly used in studies of cognitive function in WS. The Pattern Construction subtest of the DAS requires participants to copy a model by assembling sets of blocks. The Digit Span (Forward) subtest requires participants to repeat ordered sequences of numbers that have been dictated to them by an experimenter. WS participants had an average IQ of 75.14 (SD = 14.04; Range = 49–102), as indicated by their performance on the K-BIT II. Average performance on the Pattern Construction subtest was at the 8th percentile for chronological age (Range = 0.01%–62%), and average performance on the Digit Span (Forward) subtest was at the 11th percentile for chronological age (Range = 0.01%–66%). Collectively, these scores reflect the typical IQs, spatial and digit span profiles that have been observed for individuals with WS (Mervis et al., 2000). All WS participants have the characteristic genetic deletion on the long arm of chromosome 7, as determined by the FISH (fluorescent in situ hybridization) test.
Design
Participants were tested in both a small chamber (1.2 × 1.8 m), as in Hermer and Spelke (1996), and a larger one (2.4 × 3.7 m), as in Learmonth et al. (2002). The walls of each chamber stood 2.03 m high. Previous research suggests that a larger space may serve to draw out sensitivities that appear absent in a smaller space. For example, although 18–24 month-olds do not use the featural information provided by the colored wall of a 1.2 × 1.8 m room (Hermer & Spelke 1994; 1996), they do so when required to reorient in a larger room (Learmonth et al., 2002). Studies with animal species have also demonstrated that the primary use of geometry or featural landmarks depends on the size of the experimental space (Chiandetti, Regolin, Sovrano, & Vallortigara, 2007; Sovrano & Valortigara, 2006; Sovrano, Bisazza, & Vallortigara, 2005; 2007; Vallortigara, Feruglio, & Sovrano 2005). Thus, it seemed possible that varying the size of the space might enhance the chances of uncovering fragile geometric sensitivity in our WS participants. Also in an effort to make geometric wall length information more salient, we introduced a string of equally-spaced tiny lights around the upper edge of the entire array, rendering the interior of the chamber more brightly lit.1
There were four reorientation conditions, crossing the two rooms sizes with two testing conditions. In the all-black (geometry) condition, the four walls of each room were black. In the red-wall (feature) condition, one of the walls was entirely covered with a piece of red felt, while the others remained black. Order of room size was counterbalanced over participants, with test condition counterbalanced within room size. Four trials were gathered in each condition, for a total of 16 trials per participant.
Four identical opaque white plastic cylindrical containers (12.7 cm high, 8.9 cm diameter) served as hiding locations for the target (stickers). These containers were situated flush with the corners of the room. In Lakusta et al. (2010), the hiding spaces were fabric floor-length panels, which had concealed the 90-degree intersections of the room’s walls. Both of these types of hiding structures have been used in previous studies (e.g., Lee & Spelke, 2008; 2010; Lourenco & Huttenlocher, 2006; Shusterman, Lee & Spelke, 2011), and there have been no reports that such differences in array characteristics result in different behavior. Nonetheless, it seemed possible that exposing the intersecting angles of the walls might enhance the chances of encoding geometric structure by WS individuals. Hiding location was counterbalanced across participants, but remained consistent for individual participants across test trials in each chamber and condition, in order to prevent interference from learning different target locations across trials (Hermer & Spelke, 1996).2
Procedure
Following the original reorientation procedure (Hermer & Spelke, 1994; 1996), participants were brought into a rectangular room where they observed the stickers being hidden in a particular container located at one of the four corners. Participants were led to the center of the room, and were blindfolded by a mask. The experimenter then guided participants as they turned in a circle in opposite directions. The experimenter counted aloud to 8, in a rhythm that did not coincide with the rotations of the turns. The participants turned in clockwise and counterclockwise directions on different trials. All participants started each trial facing the same wall (predetermined by the experimenter and counterbalanced across participants), but on each trial ended their rotation facing a different wall. Before removing the mask, participants were asked to point to the direction of the door to the chamber where they first entered. If they pointed in the correct direction, it was assumed that they had not been disoriented, and underwent a second turning session, after which they were questioned again.3 Participants then removed their masks and were asked to pick the container that they thought held the sticker. If they were incorrect in their choice, they were encouraged to choose another location. If they were incorrect on their second choice, the experimenter led them to the correct container. Thus, each trial ended with success and the reinforcement of a sticker.
Results
Preliminary analyses showed that there were no differences in search accuracy between the two room sizes for either the WS or TD groups.4 We therefore collapsed the data and analyzed performance for the all-black room, followed by the room with one red wall. We next separately analyze the search behaviors demonstrated within each condition.
All-black room (geometry)
In the all-black room, participants who demonstrate complete reliance on geometry should evenly split their searches between the correct corner and the one that is its rotational equivalent. The proportions of search to each of the four room corners are shown in Figure 1.A and 1.B for the WS and TD groups, respectively.
Figure 1.
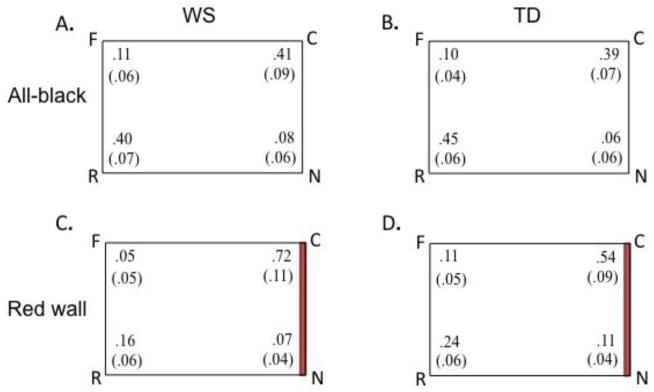
Average proportions of search (and SE’s) at each corner (correct (C), rotational equivalent (R), near (N), and far (F)) shown by the WS and TD participants in the all-black condition (A, B, respectively) and the red-wall condition (C, D, respectively).
The WS participants and TD children reoriented themselves in accord with the geometry of the enclosure. Of the 16 WS participants, 14 searched most often at the geometrically appropriate corners (C, R), 1 participant showed the opposite pattern (i.e., searched more often at the N and F corners), and 1 searched at the two corners types (geometric and non-geometric) an equal number of times (i.e., two searches to each corner type). These data significantly differ from chance (50%): χ2 (2, n = 16) of 21.13 (p < 0.001).5 Using age as a continuous variable in a multiple regression analysis, we found that age of the WS participants was not a reliable predictor of geometric search in the rooms with all-black walls, F(1,14) = 1.13, p = .31.
Of 16 TD participants, 14 searched more often at the geometrically appropriate corners and 2 searched at the two corner types the same number of times. None searched more often at the geometrically inappropriate corners. These data significantly differ from chance (50%): χ2 (2, n = 16) of 21.5 (p < 0.001). Thus, participants in both groups were sensitive to the geometry of the enclosure and constrained their searches to the correct corner and its rotational equivalent. Direct comparison of the TD and WS groups revealed that the distribution of searches over the 4 room corners did not differ between the two groups: χ2 (2, n = 32) = 1.33 (p = .51).
The results for the TD group are not surprising, as they replicate the findings of many other studies of 3–4 year-old TD children. By contrast, the results for the WS group starkly contrast with the findings of Lakusta et al. (2010), who observed only 5 participants with geometric sensitivity. An analysis of variance for the number of geometric searches made in the all-black room with study (current vs. Lakusta et al., 2010) and group (WS vs. TD) as between-subjects variables revealed a significant main effect of study, F(1, 61) = 19.71, p < .001 (Geisser-Greenhouse corrected for sphericity) and a non-significant main effect of group, F(1, 61) = 1.64, p = .21. Given these differences, we compared our findings to those of Lakusta et al. (2010), looking specifically at the proportions of searches made by individual participants to the geometric corners (C, R) in the all-black room. Figure 2 illustrates the distribution of participant’s geometric searches across the two studies in the small testing chamber, which was used in both studies.6
Figure 2.
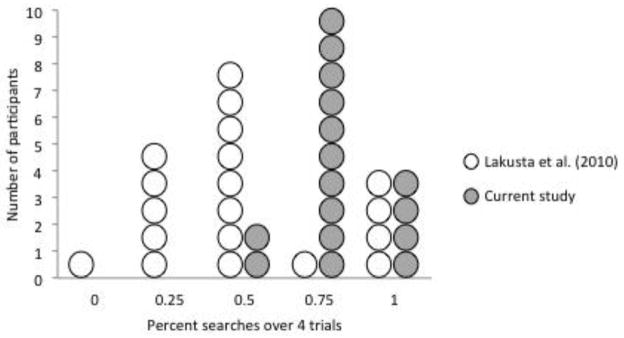
Number of subjects who search geometrically (at corners C or R) on .25, .50, .75, or all 4 trials in a 1.2 × 1.8 m room with four black walls.
Overall, the WS participants in the current study demonstrate a greater sensitivity to geometry, searching more often at the C and R corners in comparison to those tested by Lakusta et al. (2010), Wilcoxon signed-rank test, z = −2.78, p = .005, two tailed. It is notable, however, that exactly the same number of individuals (4) in each study demonstrated perfect use of the geometry of the room with all-black walls. We cannot know for sure whether the improvement observed in the present study is due to individual variation, or to the changes we made to the testing chambers, but, for several reasons, we believe the introduced layout and lighting alterations7, which rendered geometric wall-length information a more visually salient factor, made a difference for the performance of the current WS participants.8
First, consider the difference between the number of participants who searched geometrically on 3 out of the 4 (0.75) trials, where the largest improvement occurred in the present study. In Lakusta et al. (2010), only 1 person demonstrated this pattern, while in the present study, 10 people did so. This suggests that the manipulations to the physical layout of the chamber (hiding spaces divorced from the wall boundary) served to bolster geometric performance amongst members of the WS population. Second, this benefit did not extend to TD 4-year-olds, who were tested in both studies. As in previous research, children in both studies searched the geometrically appropriate corners more often than the geometrically inappropriate corners. The distribution of TD children who searched geometrically, non-geometrically, or the two corner types the same number of times did not significantly differ between the two studies, Wilcoxon signed-rank test, z = −.22, p = .89, two-tailed. Thus, it appears that the changes to the room layout enhanced performance among only the WS participants.
Red-wall room (feature)
In the red-wall room, the same geometric information is available as in the room with four black walls. A participant who relies solely geometric information in this room should search in exactly the same manner as they had in the black rooms: 50% of the time at the C corner and 50% of the time at the R corner. By contrast, participants who rely on the feature (the red wall) alone should choose the corners adjacent to the ends of the red wall or opposite from the red wall, depending on the location of the hidden target. Alternatively, participants who are sensitive to geometry and combine this information with the feature should restrict their searches to the correct corner on 100% of the trials (e.g., the corner that is left or right of the colored wall).
The proportions of search to each corner in the red-wall room are shown in Figure 1.C and 1.D for the WS and TD groups, respectively. WS participants often used the red wall as a cue for reorientation, constraining their searches to the correct corner on 72% of the trials. To consider the performance of individual participants, we analyzed the distribution of participants who searched the correct corner (C) more often than the other corners (N, F and R) over all 8 trials. Of the 16 WS participants, 10 searched most often (5 out of 8 searches or more) at the correct corner, 4 searched most often at the incorrect corners, and 1 participant searched at the target corner and the other three corners an equal number of times. These data significantly differ from chance: χ2 (2, n = 16) of 8.4 (p = 0.02) and are consistent with those of Lakusta et al. (2010), who also found that WS participants consistently used the colored wall to constrain their searches to the correct corner (no differences in the number of searches participants made to the correct corner (C) were found between the two studies, Wilcoxon signed-rank test, z = −1.34, p = .18, two tailed). The notable difference is that many of the new participants could use both geometry in the all-black room as well as the feature in the room with one red wall (11 showed this pattern), whereas many of Lakusta et al.’s participants only did so in the red-wall room.
Unlike the results for geometry, we found a relationship between age of the WS participants and performance in the feature condition. Age was a significant predictor of search at the correct corner in the rooms with one red wall, F(1,14) = 16.63, p = .02. That is, the greater the age of a WS participant, the more likely she would be to demonstrate use of the colored wall when reorienting.
TD children also consistently used the red wall to help them reorient, searching at the correct corner on 54% of the trials. Across 8 trials, 9 of the 16 participants searched most often at the correct corner, 5 showed the opposite pattern, and 1 participant searched at the target corner and the other three an equal number of times. These data significantly differ from chance: χ2 (2, n = 16) of 6.4 (p = 0.04). Direct comparison of the WS and TD groups revealed no differences in the number of searches participants made to the correct corner (C): χ2 (2, n = 32) of .16 (p = .92), Wilcoxon signed-rank test, z = −.47, p = .64, two-tailed.
A potential benefit to performance could have been the use of the red wall as a beacon, for participants who had the target hidden along the side of the room where the wall was uniquely colored red (location of the hidden target was counterbalanced across participants). In these circumstances, it may have been easier for participants to orient themselves to face the unique and salient color red, and then use their body sense of left and right to choose between the two corners on either side of the red wall. However, this benefit was not observable for either TD or WS participants; there were no differences in the number of searches to C in the feature condition between participants for whom the target was hidden at the red wall vs. those for whom the target was hidden opposite the red wall (WS: Wilcoxon signed-rank test, z = −.68, p = .50, two tailed; TD: Wilcoxon signed-rank test, z = −.08, p = .94, two tailed). No significant differences were found when the same analyses were conducted separately for each room size (all p’s > .05). This indicates that target proximity to the red wall did not differentially benefit performance.
We also compared feature use by WS participants in the present study to those tested by Lakusta et al. (2010). In the room with one colored wall, the number of searches individual participants made to the target corner (C) did not significantly differ between the two studies, Wilcoxon signed-rank test, z = −1.35, p = .18, two tailed. This indicates that participants in the current study did not achieve a higher level of success across the board. Thus, their geometric performance cannot be attributable to global factors of increased motivation, etc., which would have contributed to a higher percentage of search to the target in the red-wall condition as well as in the all-black condition. Rather, the benefit pertains only to the use of geometry in the room with all-black walls. This suggests that the modifications in layout affected the ability of WS participants to extract geometric information from the room structure, while their use of a single uniquely colored wall as a feature was consistent across studies.
Search patterns using geometry alone and with the feature
Given that our WS participants showed sensitivity to both geometry and a feature, we asked whether the combination of these properties engages processes similar to those of TD children. Conversely, it is possible that the neural abnormalities in this syndrome disrupt the integration of information from the two systems, resulting in a reorientation mechanism in adulthood where one form of information is predominantly used over the other. To investigate this possibility, we analyzed the search behaviors of WS and TD participants following disorientation in both room conditions. We focus on the “beeline” pattern described by Huttenlocher and colleagues. This type of search behavior is indicative of a “space-centered” representation that is independent of the original heading of the viewer (Huttenlocher & Vasilyeva, 2003). Regardless of what particular portion of the room children face after being disoriented, they are able to turn their bodies to adjust their heading direction and proceed to the target corner without considering more than a minimal number of walls. Here, we ask whether WS participants demonstrate the same type of immediate search style that is characteristic of TD children and adults. If qualitative differences in search patterns emerge between the TD and WS groups, this would suggest that different underlying mechanisms are at work.
Using videotaped footage of the task that was recorded by an overhead camera (that did not provide an orientation cue), we examined the patterns of search demonstrated by participants once the blindfold was removed and they were asked to go and recover the hidden sticker. We coded the number of walls that a participant surveyed while searching. Following Huttenlocher and Vasilyeva (2003) and Lourenco and Huttenlocher (2006), we assessed surveying behaviors as evidenced by looks or movement about the room – a subject turning her head, torso, or whole body to consider another wall of the space. Next we calculated the minimum number of walls that a subject would need to consider, given the particular wall they were facing when the blindfold was removed (which was randomized for all participants across trials). We next calculated a “wall difference score” by subtracting the minimum number of walls from the actual number of walls considered. A difference score of 0, for example, reflects the search of a subject who would need to pass over at least two walls in order to arrive to the correct corner, and faced exactly two walls while they searched (2 – 2 = 0). This illustrates the most efficient search pattern possible. However, if a participant considered 3 walls when she only needed to consider 2, this would result in a difference score of 1 (3 – 2), where 1 “extra” wall was unnecessarily considered in order to locate the hidden sticker. A negative difference score would indicate a case where a participant was required to consider 2 walls to arrive at the target corner, but only considered 1 before selecting where to search (1 – 2 = −1) (this was infrequent, as is indicated by the difference scores illustrated in Figure 3 below, which are all either 0 or greater). To capture each participant’s general style of search within a particular room condition, we calculated an average wall difference score for each participant by averaging the difference scores for the 8 trials completed in each room condition.
Figure 3.
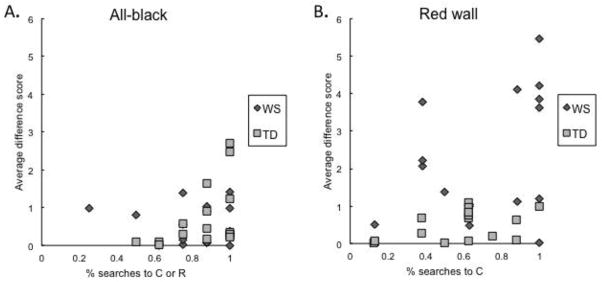
Relationship between number of walls considered and percentage of A) geometric searches (to corners C or R) in the rooms with all-black walls, and B) searches using the feature (to corner C) in the rooms with one red wall.
A 2 (group) × 2 (condition) ANOVA of these data revealed a significant main effect of condition, F(1,30) = 11.99, p = .002, where the mean wall difference score for the feature condition (M = 1.5) was higher than the all-black (M = .70). There was also a significant condition by group interaction, F(1,30) = 22.30, p < .001, reflecting higher mean wall difference scores for the WS group than TD in the red-wall condition.9 To further explore these differences, separate analyses are conducted for each of the room conditions below.
When reorienting in the rooms with all-black walls, both individuals with WS and TD children considered a similar number of walls. In Figure 3.A, the distribution of subjects amongst both groups largely overlaps. A between-subjects ANOVA revealed no significant difference between the TD and WS groups, F(1,30) = .38, p = .54. As evidenced by their search patterns following disorientation, the TD and WS participants acted upon similar representations of environmental geometry. On average, members of both groups had wall difference scores of less than 1 (TD: M = .71, SD = .86; WS: M = .55, SD = .51). This follows the pattern observed in previous research (Huttenlocher & Vasilyeva, 2003; Lourenco & Huttenlocher, 2006), where TD children routinely take a direct and non-sequential “bee line” to approach the target corner. There is some variation in the number of walls considered (2 TD child outliers considered approximately 3 extra walls on average). Review of the video footage shows that in these two cases however, both these children spent time looking back and forth between the two geometrically appropriate corners, in an attempt to deal with the ambiguous situation where both choices were geometrically correct. (None of the WS individuals tested appeared to exhibit consideration of diagonally opposite corners in the same back-and-forth fashion.)
After being disoriented in the rooms with one red wall, participants with WS showed a qualitatively different search pattern in comparison to TD children. The WS participants often showed a strategic step-by-step progression of behaviors, sequentially considering several walls before locating the target, and sometimes passing over the target corner multiple times before deciding which container to open. These differences between WS and TD search patterns are illustrated in Figure 3.B, where the distribution of the two groups are largely dissimilar. Just as in the room with all-black walls, TD children considered fewer than one extra wall (M wall difference score = .45, SD = .41), indicating an efficient “beeline” approach to one of the geometric corners. In contrast, the WS participants considered more than two extra walls on average (M wall difference score = 2.25, SD = 1.66). A between-subjects ANOVA confirmed that the WS participants looked at significantly more walls than TD children in the rooms with one red wall, F(1,30) = 17.72, p < .001. This suggests that the WS participants relied upon a different process to reorient themselves when in the presence of the feature cue. Comparing the search patterns of TD children across the two room contexts, a within-subjects ANOVA confirmed that the average wall difference scores did not significantly differ in the all-black vs. the red-wall condition, F(1,30) = 1.19, p = .28. This indicates that TD children followed similar search behaviors in both room contexts. In contrast, the average wall difference scores of WS individuals in the red-wall condition were significantly greater than those in the all-black condition (F(1,30) = 15.19, p = .001).
Did WS individuals who had a tendency to survey more walls in the red-wall room also do so in the all-black room? To determine if this was the case, we calculated the correlation between the number of extra walls considered in the red-wall and all-black rooms. The correlation for the two rooms was not significant (r = .38, p = .20, partial correlation to control for IQ (scores on the DAS-II and KBIT-II)). The lack of a correlation speaks against the suggestion of general search tendencies by members of the WS group that were indiscriminantly used despite of context. For TD children, the correlation between wall difference scores for the two room conditions was significant (r = .67, p = .007), suggesting that they follow similar search behaviors in both room contexts.
Another potential relationship to consider is that between chronological age and search patterns within the two room conditions. We found a significant correlation between age and the number of walls WS participants searched when reorienting in the red-wall room (r = .69, p = .009), but not in the all-black room (r = .28, p = .36). Neither correlation was significant for TD children (red-wall: r = −.17, p = .52; all-black: r = .14, p = .60). This suggests that as WS individuals grow older, they increasingly inspect more walls as they circle the room, possibly a conscious strategy to find the target. Indeed, many of the WS individuals included in this study were much older than the TD children tested, suggesting that over their lifetime, they develop explicit strategies to help them relocate hidden elements.
An alternative possibility is that such strategies become more common over age, for both typical and atypically-developing individuals. To address this, we compared the search patterns of the adult WS participants over the age of 18 years (n = 7) to those of TD adults. TD adults were recruited from the Johns Hopkins University community and earned course credit for their participation. All were over the age of 18 years (n = 7). Accuracy and search patterns of the TD adults were coded just as had been done for the TD child and WS groups. No differences were found between TD adults and WS adults in the number of geometric searches in the all-black room (TD adult M geometric search = .98, SD = .05; WS adult M geometric search = .82, SD = .26; Wilcoxon signed-rank test, z = −1.63, p = .10, two tailed), or in the number of searches to the target corner in the red-wall room (TD adult M target search = .98, SD = .05; WS adult M target search = .89, SD = .23; Wilcoxon signed-rank test, z = −.82, p = .41, two tailed).
However, there were some differences between the two participant groups in the surveying behaviors following disorientation. The TD adult mean wall difference score for the all-black condition was .69 (SD = .53) and for the feature condition was .09 (SD = .13); this compares to mean differences of .76 (SD = .61) and 3.74 (SD = 1.27) among WS adults for the two conditions. The TD and WS adult groups did not differ from one another in the number of extraneous walls considered in the all-black room, Wilcoxon signed-rank test, z = −.41, p = .69, two tailed, however this difference was significant in the red-wall room, Wilcoxon signed-rank test, z = −2.38, p = .02, two tailed. While searching for the target in the presence of the landmark, WS adults made numerous sequential turns to consider the space from a variety of perspectives. In contrast, TD adults tended to open their eyes, establish their heading orientation based on the view before them, and then turn to the target corner. These behavioral differences suggest that typical adults use a representation that combines features and global geometry, whereas WS adults use the feature cue to reorient via a sequential strategic process of realignment, perhaps based on an viewpoint-dependent representation of the target corner. This indicates that over the course of development, WS individuals have arrived at a strategy for use of featural information that is often informative and effective, yet qualitatively differs from the underlying mechanism of TD adults, which combines geometry and features in a unitary representation.
General Discussion
In this study, we used the classic reorientation paradigm to examine the use of geometry and features among people with WS. A previous study of individuals with this syndrome had suggested a clear separation of the two systems used for reorientation, with severe deficits in geometry and preservation of feature use. The results of the present study add to these findings by showing that people with WS may, under certain conditions, use both geometry and features, but that the mechanisms underlying each, as well as their combination, are different from that in typically developing individuals. Several key findings emerged. First, we found that people with WS are able to use the geometry of a rectangular chamber to reorient themselves. Second, we found that many could also use the feature cue of one colored wall to accomplish reorientation. Third, by analyzing the search behaviors following disorientation, we found evidence that the combination of information from the two systems is atypical in WS, neither like that of TD children or TD adults. As a whole, the results reinforce the conclusion that there are two separate systems underlying reorientation, but add that in this genetic disorder, each of the systems is somewhat impaired, as well as the mechanisms that underlie their combination.
In previous research, we found that only very few WS individuals were able to use the geometry of a space to reorient themselves (Lakusta et al., 2010). The present research introduced several methodological changes which served to heighten the visibility of the intersections among the wall surfaces, and hence the geometric structure of the reorientation chamber. In this simplified environment, the majority of WS individuals did demonstrate sensitivity to geometric relationships and reoriented themselves accordingly.10 Given the improved performance of WS participants in the all-black condition (compared to previous results), we speculate that people with WS might be able to enocode the layouts of only extemely simple and clear geometric configurations. If the surface junctures are rendered too complex or numerous, they may fail to encode the geometry of the layout. This finding demonstrates that the WS reorientation mechanism is not best characterized by a complete absence of geometric sensitivity, but rather that it may be fragile, requiring especially salient presentation of geometric information in order to support reorientation. Notably, the layout change of the present study did not differentially affect the reorientation performance of young TD children, as comparison of the 4-year-olds run by Lakusta et al. (2010) and those run in the present study did not reveal any significant differences. It is interesting to note that even very young children are able to reorient by the complex geometry of an octagon-shaped environment (Newcombe et al., 2010).
In terms of feature use, the present study replicated the findings of Lakusta et al. (2010). When one wall of the chamber was uniquely colored, the majority of WS participants could use this information to accomplish reorientation. These results, converging with those of Lakusta et al. (2010), demonstrate that despite other navigational impairments, people with WS are able to use a featural landmark cue to reorient themselves. Strength in the ability to reorient by one type of cue (features), compared to fragility when reorienting by another (geometry) lends support to the notion that these are two separable mechanisms of reorientation. In WS, the use of features is relatively robust, whereas the use of geometry appears to be more fragile.
Detailed analyses of search behaviors following disorientation by geometry and/or a feature adds further support to the idea that these mechanisms are separable. In the all-black room, the performance of the WS participants looked in many ways like that of TD 4-year-olds. There was no correlation between age and use of geometry in people with WS, and TD children are known to use geometry from the earliest points tested (18–24 months, Hermer & Spelke, 1996; Learmonth et al., 2001). Moreover, the search patterns among WS participants were not different from TD children; participants in both groups surveyed few walls and frequently proceeded directly to the target corner or its rotational equivalent. This behavior is the signature of a direct “beeline” style of search, originally observed by Huttenlocher and colleagues (Huttenlocher & Vasilyeva, 2003; Lourenco & Huttenlocher, 2006). Not only did people with WS arrive at the same geometrically consistent corners as TD 4-year-olds, they were also able to achieve this end result via similar search behaviors. This overlap suggests that members of both groups encode and act upon similar representations of layout geometry. In contrast, the patterns of performance for the room with the feature differed considerably between the WS individuals and TD children. For WS participants, performance in the red-wall room was correlated with age (although use of geometry was not). This developmental trajectory is clearly quite different from TD children, who combine features with geometry quite early in life, and do so regularly across different contexts by age 6.
More striking, detailed search patterns in the feature condition were quite different for WS individuals than for the other groups. TD children and typical adults showed the same “beeline” search pattern as they showed in the all-black room, suggesting that they constructed a global representation of layout that was combined with the feature, leading to direct and efficient search. In contrast, WS individuals appeared to follow a step by step, perhaps more strategic process in which they considered multiple walls before choosing a corner to search for the target. This behavioral pattern suggests the possibility that people with WS were matching particular viewpoints of the corners to their stored representation of the layout, and choosing based on the best match between the presently viewed corner and a stored representation. These viewpoint-specific representations would be characterized by fairly rich spatial information (e.g., the geometry of the intersections and the colors of the intersecting walls along with left/right sense). However, this would not require a full global representation that could be used to determine one’s current heading.11 A possible alternative explanation is that people with WS have developed strategies for navigating that invite them to search for additional information beyond geometry (which is, for them, fragile). The systematic positive correlation with age in the use of features suggests that this may be an intentional strategic behavior, rather than a more general effect of attentional resources that would be expected to remain more stable across the lifespan.
Putting together the findings from the two room conditions suggests the following picture of reorientation in people with WS compared to TD individuals. First, geometric sensitivity is present in both groups, confirming and extending the many findings that demonstrate the ubiquity of geometric response among humans and other species. However, people with WS appear to require clearly perceptible information about the lengths of walls and their intersections. Given recent findings that demonstrate the importance of distance and direction from the target—rather than lengths and angles composing the layout (Lee, Sovrano, & Spelke, 2012; Lee et al., 2013)—we speculate that the WS deficit might be in reduced sensitivity to metric variables more generally. This would be consistent with other findings about the WS profile, which includes reduced sensitivity to estimated numerosity (Libertus, Feigneson, Halberda, & Landau, 2014). This possibility could be readily tested by examining whether the ratio of wall lengths required for WS geometric reorientation needs to be larger than it is for young TD children, who can reorient by wall length ratios as small as 8:9 (Lee, Winkler-Rhoades, & Spelke, 2012). In any case, we propose that there is reduced sensitivity to geometry among many individuals with WS, but not complete absence.
People with WS can also use a feature to reorient themselves, and they may in some circumstances combine this with a geometric representation of the space—for example, making geometric errors in the red-wall room, as some did in the study by Lakusta et al. (2010). However, due to the differences observed in the behaviors following disorientation, we speculate that WS individuals are more likely to use features separately from geometry, perhaps relying on viewpoint-dependent representations of the corners and matching those to their current viewpoint in order to identify the hiding location.
Fragile geometry and atypical use of a feature could reflect damaged portions of the neural networks involved in reorientation. As discussed in the introduction, there is evidence that people with WS have abnormalities in hippocampal structure and function, known to be important for geometric representation. They also have abnormalities in parietal areas, which form part of the larger neural network supporting navigation (Aquirre & d’Esposito, 1999; Burgess, 2008; Epstein & Kanwisher, 1998; Squire, Stark, & Clark, 2004). Such abnormalities could plausibly lead to neural weaknesses that underlie behavioral deficits in navigation. The fragile use of geometry and atypical use of features could also be a developmental product of initial biases, strengthened by learned adjustments to these biases. For example, if people with WS start out with fragile geometric sensitivity together with robust sensitivity to features, this may result in reliance on features (which could yield success in navigation and reorientation) and a reduced dependence on geometry (which may be less reliable in their case and lead to less success). It is even possible that parents of WS children might encourage the use of landmark features (which are salient and can be verbally labeled) over geometric layout properties (which are more abstract), and their verbal input may differ from parents of typically developing children (cf. Ferrara et al., 2015). Previous research has shown that route learning is improved for people with WS when they are given verbal information that calls attention to landmarks (Farran, Blades, Boucher, & Tranter, 2010). Although specific experience is not necessary to achieve geometric reorientation, landmark use is far more susceptible to training and explicit instruction. This general account is consistent with a variant of adaptive combination theory, which proposes that geometric and featural properties can both be used for reorientation, and the extent to which each is used depends on cue weighting and combination, where weights are determined by factors such as perceptual salience, reliability, and prior sucessful experiences (Newcombe & Huttenlocher, 2006; Newcombe & Ratliff, 2007). If one type of information is weighted more heavily, it will overshadow the other in performance. Support for this view includes studies showing that young children use a colored wall to reorient in a larger (but not smaller) room (Learmonth et al., 2002), and that adults who are tested in a larger room (where featural cues are more distant from the navigator, and thereby possibly more salient) show dominance of features over geometric cues (Ratliff & Newcombe, 2008). If people with WS regularly experience success with features/landmarks and are regularly encouraged to attend to them in way-finding, they may come to rely on this form of information over geometry.
A full understanding of how the WS genetic deficit comes to affect reorientation awaits further studies that unravel the complex chain between genes and behavior. The data we have provided serve as a step in this direction, suggesting that the genes deleted in WS play a vital role in establishing not only sensitivity to geometry and features, but also to the mechanisms that underlie our seamless ability to combine these two kinds of information when reorienting in space.
Highlights.
Individuals with Williams syndrome reoriented in space using geometry and features.
Use of features was correlated with age; use of geometry was not.
Behavioral use of the feature differed from TD children; use of geometry did not.
Combination of features and geometry is atypical in this disorder.
These data suggest that neural mechanisms for features vs. geometry are separable.
Acknowledgments
This research was supported in part by NIH grant R01 NS 050876 as well as an Integrative Graduate Education and Research Traineeship through NSF grant DGE 0549379.
We are grateful for the time volunteered by our participants and their families, as well as the Williams Syndrome Association for making this study possible. We thank Sang Ah Lee for fruitful discussion of the data.
Footnotes
We thank Nora Newcombe for suggesting this as a factor that could make young children more comfortable and attentive.
Reorientations trials were interspersed with standardized testing, other experimental measures, and short breaks (lasting approximately 15 minutes), such that 4 reorientation trials in one condition were never directly followed by another 4 trials in another condition. For this reason, we do not believe that the performance of participants across 16 trials with the same hiding location is a product of reference memory, due to the substantial amount of time between conditions that this memory would have to be maintained.
This was done for 4 of the WS participants (on an average of 1.75 trials) and 3 of the TD participants (on an average of 1.25 trials). These numbers (4 and 3) correspond to what may be expected by chance, which indicates that the disorientations procedure was effective for all participants.
There was no increase in the number of geometric searches (at C, R corners) in the small all-black room compared to the large one (for the TD group, M large = .83; M small = .89; Wilcoxon signed-rank test, z = −.65, p = .52, two-tailed; for WS, M large = .84; M small = .77; z = −1.40, p = .16). Nor were there any differences in the number of correct searches (C corner) across the two rooms with the single red wall (for TD, M large = .55; M small = .56; Wilcoxon signed-rank test, z = −.42, p = .67, two-tailed; for WS, M large = .68; M small = .77; z = −.06, p = .95, two-tailed). We analyzed the data following Learmonth et al. (2002), by calculating differences scores of the number of correct responses (to corner C) minus the number of responses to the opposite corner (R). Planned contrasts (repeated-measures ANOVA) for the TD group revealed no effect of room size (large or small), F(1, 6) = 3.61, p = .11, and no interaction between room size and condition (landmark or no landmark), F(1, 6) = 2.60, p = .63. Planned contrasts for the WS group also revealed no effect of room size, F(1, 6) = .52, p = .50, and no interaction between room size and condition, F(1, 6) = .58, p = .47. Thus for both groups, the use of the landmark did not differ across the large and small spaces. For both the TD and WS groups there were no effects of age, gender, or order of the room conditions (all p’s > .05).
In much of the reorientation literature, performance is analyzed using parametric statistics (such as t-tests) that evaluate the proportions of search across the four corners (e.g., Hermer & Spelke, 1996; Learmonth et al., 2001; Lee & Spelke, 2008; Hermer-Vasquez et al., 2001). In the current study we use nonparametric statistics to avoid violating the assumptions of parametric tests. Note however that results remain the same when the data presented here were analyzed using parametric statistics.
Note however that the results do not change when collapsing across both room sizes for the present study.
It is possible that reorientation was accomplished by noting differences in the number of lights along the long and short walls. However, this is likely not the explanation for geometric performance for several reasons. First, the walls of the chamber were stood 2.03 m high (well above eye level for all participants). Second, participants would have demonstrated explicit counting behavior (e.g., pointing to and tracking the lights as they counted aloud) in order to employ this strategy successfully (none did), and third, research has shown that number knowledge is impaired in this population (Libertus et al, 2014).
Three WS individuals participated in both the present study and that conducted by Lakusta et al. (2010) (approximately 3 years apart). All showed a slight improvement in geometric performance (an increase of 1–2 searches at the target corner or its rotational equivalent) in the all-black condition of the current study, although this improvement did not reach the level of significance. We believe this boost in performance to be illustrative of more than re-test effects, as the 13 non-overlapping participants also showed increased geometric performance in comparison to the remaining 16 tested by Lakusta et al. (2010). It is possible that the difference in performance could be attributable to the largely independent groups of subjects tested across the two studies, as is noted in Footnote 9; however, these participant groups did not differ on other relevant measures (see Footnote 9).
No difference in the number of walls considered was found for the large vs. small rooms or for the order of room conditions (all p’s > .05).
It is also possible that these findings are attributable to the fact that we tested a largely independent group of WS individuals from Lakusta et al. (2010) (only 3 participants in common between the two studies). The performance of these 3 common participants did not significantly improve from Lakusta et al. (2010) to the present study. The WS groups of the two studies do not differ from one another on other measures of visuo-spatial ability (DAS II, Pattern Construction subtest) or IQ (K-BIT II).
Some have suggested that reorientation is accomplished by an “image-matching” algorithm, whereby low-level representations of the location image are stored, and then retrieved and matched to the current location image (Cheng, 2008). We doubt that such an impoverished representation could account for participant’s behavior since they are continually moving around the array, resulting in a large degree of variation in the amount of light projected to the eye (see also, Lee & Spelke, 2011; Lee, Winkler-Rhoades, & Spelke, 2012). Although there is evidence that in some conditions animals other than insects use image-matching for orientation (e.g. Pecchia, Gagliardo, & Vallortigara, 2011; Pecchia & Vallortigara, 2012), there is also clear evidence that spontaneous reorientation by TD individuals is based instead on three-dimensional environmental geometry and not on image matching (Lee, Spelke, & Vallortigara, 2012). However, it is possible the WS individuals tested in the current study employed an image-matching strategy in the presence of the single red wall, as evidenced by their turns to consider multiple viewpoints of the room before choosing where to search.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, D’Esposito M. Topographical disorientation: A synthesis and taxonomy. Brain. 1999;122:1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- Bullens J, Nardini M, Doeller CF, Braddick O, Postma A, Burgess N. The role of landmarks and boundaries in the development of spatial memory. Developmental Science. 2010;13:170–180. doi: 10.1111/j.1467-7687.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Burgess N. Spatial cognition and the brain. Annals of the New York Academy of Sciences. 2008;1124:77–97. doi: 10.1196/annals.1440.002. [DOI] [PubMed] [Google Scholar]
- Cheng K. A purely geometric module in the rats’ spatial representation. Cognition. 1986;23:149–178. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Cheng K. Whither geometry? Troubles of the geometric module. Trends in Cognitive Sciences. 2008;12:355–361. doi: 10.1016/j.tics.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Cheng K, Newcombe NS. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychonomic Bulletin & Review. 2005;12:1–23. doi: 10.3758/bf03196346. [DOI] [PubMed] [Google Scholar]
- Chiandetti C, Spelke E, Vallortigara G. Inexperienced newborn chicks use geometry to spontaneously reorient to an artificial partner. 2014:1–7. doi: 10.1111/desc.12277. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chiandetti C, Regolin L, Sovrano VA, Vallortigara G. Spatial reorientation: the effects of space size on the encoding of landmark and geometry information. Animal Cognition. 2007;10:159–168. doi: 10.1007/s10071-006-0054-3. [DOI] [PubMed] [Google Scholar]
- Chiandetti C, Vallortigara G. Is there an innate geometric module? Effects of experience with angular geometric cues on spatial reorientation based on the shape of the environment. Animal Cognition. 2008;11:139–146. doi: 10.1007/s10071-007-0099-y. [DOI] [PubMed] [Google Scholar]
- Chiandetti C, Vallortigara G. Experience and geometry: Controlled-rearing studies with chicks. Animal Cognition. 2010;13:463–470. doi: 10.1007/s10071-009-0297-x. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Burgess N. Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proceedings of the National Academy of Sciences (USA) 2008;105:5909–5914. doi: 10.1073/pnas.0711433105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proceedings of the National Academy of Sciences (USA) 2008;105:5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Hu D, Eliez S, Bellugi U, Galaburda A, Korenberg J, Mills D, Reiss AL. Evidence for superior parietal impairment in Williams syndrome. Neurology. 2005;64:152–153. doi: 10.1212/01.WNL.0000148598.63153.8A. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential abilities scale. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Jablonski K, Feiler AM. Visual scene processing in familiar and unfamiliar environments. Journal of Neurophysiology. 2007;97:3670–3683. doi: 10.1152/jn.00003.2007. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Farran EK, Blades M, Boucher J, Tranter LJ. How do individuals with Williams syndrome learn a route in a real-world environment? Developmental Science. 2010;13(3):454–468. doi: 10.1111/j.1467-7687.2009.00894.x. [DOI] [PubMed] [Google Scholar]
- Ferrara K, Silva M, Wilson C, Landau B. Adapting descriptions of spatial relationships: Parents’ tuning to child knowledge under review. [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Gallistel CR, Matzel LD. The neuroscience of learning: Beyond the Hebbian synapse. Annual Review of Psychology. 2013;64:169–200. doi: 10.1146/annurev-psych-113011-143807. [DOI] [PubMed] [Google Scholar]
- Hermer L, Spelke E. Modularity and development: The case of spatial reorientation. Cognition. 1996;61:195–232. doi: 10.1016/s0010-0277(96)00714-7. [DOI] [PubMed] [Google Scholar]
- Hermer L, Spelke E. A geometric process for spatial reorientation in young children. Nature. 1994;370:57–59. doi: 10.1038/370057a0. [DOI] [PubMed] [Google Scholar]
- Hermer-Vasquez L, Moffet A, Munkholm P. Language, space, and the development of cognitive flexibility in the case of humans: The case of two spatial memory tasks. Cognition. 2001;79:263–299. doi: 10.1016/s0010-0277(00)00120-7. [DOI] [PubMed] [Google Scholar]
- Hillier LW, Fulton RS, Fulton LA, et al. The DNA sequence of human chromosome 7. Nature. 2003;424(6945):157–64. doi: 10.1038/nature01782. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Nadel L. Reorientation in a rhombic environment: No evidence for an encapsulated geometric module. Cognitive Development. 2005;20:279–302. [Google Scholar]
- Huttenlocher J, Vasilyeva M. How toddlers represent enclosed spaces. Cognitive Science. 2003;27:749–766. [Google Scholar]
- Janzen G, van Turennout M. Selective neural representation of objects relevant for navigation. Nature Neuroscience. 2004;7:673–677. doi: 10.1038/nn1257. [DOI] [PubMed] [Google Scholar]
- Julian JB, Keinath A, Muzzio I, Epstein RA. Place recognition and heading retrieval are dissociable in mice (and possibly men). Poster presented at the annual meeting of the Vision Sciences Society; St. Pete Beach, FL. 2014. [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- Kelly DM, Spetch ML. Reorientation in a two-dimensional environment: Do pigeons (Columba livia) encode the featural and geometric properties of a two- dimensional schematic of a room? Journal of Comparative Psychology. 2004;118:384–395. doi: 10.1037/0735-7036.118.4.384. [DOI] [PubMed] [Google Scholar]
- Kippenhan JS, Olsen RK, Mervis CB, et al. Genetic contributions to human gyrification: Sulcal morphometry in Williams syndrome. Journal of Neuroscience. 2005;25:7840–7846. doi: 10.1523/JNEUROSCI.1722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakusta L, Dessalegn B, Landau B. Impaired geometric reorientation caused by genetic defect. Proceeding of the National Academy of Sciences (USA) 2010;107:2813–2817. doi: 10.1073/pnas.0909155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, Hoffman JE. Spatial Representation: From Gene to Mind. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Learmonth AE, Newcombe NS, Huttenlocher J. Toddlers’ use of metric information and landmarks to reorient. Journal of Experimental Psychology. 2001;80(3):225–244. doi: 10.1006/jecp.2001.2635. [DOI] [PubMed] [Google Scholar]
- Learmonth AE, Newcombe NS, Sheridan N, Jones M. Why size counts: Children’s spatial reorientation in large and small enclosures. Developmental Science. 2008;11:414–426. doi: 10.1111/j.1467-7687.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- Learmonth AE, Nadel L, Newcombe NS. Children’s use of landmarks: Implications for modularity theory. Psychological Science. 2002;13:337–341. doi: 10.1111/j.0956-7976.2002.00461.x. [DOI] [PubMed] [Google Scholar]
- Lee SA, Vallortigara G, Flore M, Spelke ES, Sovrano VA. Navigation by environmental geometry: The use of zebrafish as a model. Journal of Experimental Biology. 2013;216:3693–3699. doi: 10.1242/jeb.088625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Sovrano VA, Spelke E. Navigation as a source of geometric knowledge: Young children’s use of length, angle, distance, and direction in a reorientation task. Cognition. 2012;123:144–161. doi: 10.1016/j.cognition.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Spelke E. Children’s use of geometry for navigation. Developmental Science. 2008;11(5):743–749. doi: 10.1111/j.1467-7687.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- Lee SA, Spelke E. A modular geometric mechanism for reorientation in children. Cognitive Psychology. 2010;61:152–176. doi: 10.1016/j.cogpsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Spelke E. Young children reorient by computing layout geometry, not by matching images of the environment. Psychonomic Bulletin & Review. 2011;18:192–198. doi: 10.3758/s13423-010-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Shusterman S, Spelke ES. Reorientation and landmark-guided search by young children: Evidence for two systems. Psychological Science. 2006;17:577–582. doi: 10.1111/j.1467-9280.2006.01747.x. [DOI] [PubMed] [Google Scholar]
- Lee SA, Winkler-Rhoades N, Spelke ES. Spontaneous reorientation is guided by perceived surface distance, not by image matching or comparison. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0051373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Spelke E, Vallortigara G. Chicks, like children, spontaneously reorient by three-dimensional environmental geometry, not by image matching. Biology Letters. 2012;8(4):492–494. doi: 10.1098/rsbl.2012.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew AR, Usherwood B, Fragkioudaki F, Koukoumi V, Smith SP, Austen JM, McGregor A. Transfer of spatial search between environments in human adults and young children (Homo sapiens): Implications for representation of local geometry by spatial systems. Developmental Psychobiology. 2014;56:421–434. doi: 10.1002/dev.21109. [DOI] [PubMed] [Google Scholar]
- Libertus ME, Feigneson L, Halberda J, Landau B. Understanding the mapping between numerical approximation and number words: Evidence from Williams syndrome and typical development. Developmental Science. 2014;17(6):905–919. doi: 10.1111/desc.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco SF, Huttenlocher JE. Using geometry to specify location: Implications for spatial coding in children and nonhuman animals. Psychological Research. 2006;71:252–264. doi: 10.1007/s00426-006-0081-3. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, Firth CD. Navigation-related structural change in the hippocampi of taxi drivers. Proceeding of the National Academy of Sciences (USA) 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP, Armstrong SC. The Williams syndrome cognitive profile. Brain and Cognition. 2000;44(3):604–628. doi: 10.1006/brcg.2000.1232. [DOI] [PubMed] [Google Scholar]
- McGregor A, Hayward AJ, Pearce JM, Good MA. Hippocampal lesions disrupt navigation based on the shape of the environment. Behavioral Neuroscience. 2004;118:1011–1021. doi: 10.1037/0735-7044.118.5.1011. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, Berman KF. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43(5):623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Sarpal D, Koch P, Steele S, Kohn P, Marenco S, Morris CA, Das S, Kippenhan S. Functional, structural, and metabolic abnormalities of the hippocampal formation in Williams syndrome. Journal of Clinical Investigation. 2005;115(7):1888–1895. doi: 10.1172/JCI24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA. The dysmorphology, genetics, and natural history of Williams-Beuren syndrome. In: Morris CA, Lenhoff H, Wang P, editors. Williams-Beuren syndrome: Research, evaluation, and treatment. Baltimore, MD: Johns Hopkins University Press; 2006. pp. 3–17. [Google Scholar]
- Newcombe NS, Ratliff KR. Explaining the development of spatial reorientation: Modularity-plus-language versus the emergence of adaptive combination. In: Plumert J, Spencer J, editors. The emerging spatial mind. New York: Oxford University Press; 2007. [Google Scholar]
- Newcombe NS, Huttenlocher J. Making space: The development of spatial representation and reasoning. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Newcombe NS, Huttenlocher J. Development of spatial cognition. In: Kuhn D, Siegler RS, editors. Handbook of child psychology. 6. New York: John Wiley and Sons; 2006. pp. 734–776. [Google Scholar]
- Osborne LR. The molecular basis of a multisystem disorder. In: Morris CA, Lenhoff HM, Wang PP, editors. Williams-Beuren Syndrome: Research, Evaluation, and Treatment. Baltimore, MD: Johns Hopkins University Press; 2006. pp. 18–58. [Google Scholar]
- Pecchia T, Gagliardo A, Vallortigara G. Stable panoramic views facilitate snap- shot like memories for spatial reorientation in homing pigeons. PLoS ONE. 2011;6(7):e22657. doi: 10.1371/journal.pone.0022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecchia T, Vallortigara G. Spatial reorientation by geometry with freestanding objects and extended surfaces: A unifying view. Proceedings of the Royal Society of London Biological Sciences. 2012;279(1736):2228–2236. doi: 10.1098/rspb.2011.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff KR, Newcombe NS. Reorienting when cues conflict: Evidence for an adaptive-combination view. Psychological Science. 2008;19(12):1301–1307. doi: 10.1111/j.1467-9280.2008.02239.x. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, Bellugi U. Neuroanatomy of Williams syndrome: A high-resolution MRI study. Journal of Cognitive Neuroscience. 2000;12:65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- Schinazi VR, Nardi D, Newcombe NS, Shipley TF, Epstein RA. Hippocampal size predicts rapid learning of a cognitive map in humans. Hippocampus. 2013;23:515–528. doi: 10.1002/hipo.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman A, Lee SA, Spelke ES. Cognitive effects of language on human navigation. Cognition. 2011;120:186–201. doi: 10.1016/j.cognition.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovrano VA, Vallortigara G. Dissecting the geometric module: A sense-linkage for metric and landmark information in animals’ spatial reorientation. Psychological Science. 2006;17:616–621. doi: 10.1111/j.1467-9280.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- Sovrano VA, Bisazza A, Vallortigara G. Animals’ use of landmarks and metric information to reorient: Effects of the size of the experimental space. Cognition. 2005;97:121–133. doi: 10.1016/j.cognition.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Sovrano VA, Bisazza A, Vallortigara G. How fish do geometry in large and in small spaces. Animal Cognition. 2007;10:47–54. doi: 10.1007/s10071-006-0029-4. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Joanisse MF, Newcombe NS. Spinning in the scanner: Neural Correlates of virtual reorientation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36(5):1097–1107. doi: 10.1037/a0019938. [DOI] [PubMed] [Google Scholar]
- Tommasi L, Gagliardo A, Andrew RJ, Vallortigara G. Spatial processing mechanisms for encoding of geometric and landmark information in the avian hippocampus. European Journal of Neuroscience. 2003;17:1695–1702. doi: 10.1046/j.1460-9568.2003.02593.x. [DOI] [PubMed] [Google Scholar]
- Tommasi L, Chiandetti C, Pecchia T, Sovrano VA, Vallortigara G. From natural geometry to spatial cognition. Neuroscience & Biobehavioral Reviews. 2012;36:799–824. doi: 10.1016/j.neubiorev.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Twyman A, Friedman A, Spetch ML. Penetrating the geometric module: Catalyzing children’s use of landmarks. Developmental Psychology. 2007;43:1523–1530. doi: 10.1037/0012-1649.43.6.1523. [DOI] [PubMed] [Google Scholar]
- Twyman AD, Newcombe NS, Gould TG. Malleability in the development of spatial reorientation. Developmental Psychobiology. 2012;55(3):243–255. doi: 10.1002/dev.21017. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Feruglio M, Sovrano VA. Reorientation by geometric and landmark information in environments of different spatial size. Developmental Science. 2005;8:393–401. doi: 10.1111/j.1467-7687.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- Vargas JP, Petruso EJ, Bingham VP. Hippocampal formation is required for geometric navigation in pigeons. European Journal of Neuroscience. 2004;20:1937–1944. doi: 10.1111/j.1460-9568.2004.03654.x. [DOI] [PubMed] [Google Scholar]
- Wang RF, Spelke ES. Human spatial representation: Insights from animals. Trends in Cognitive Sciences. 2002;6:376–382. doi: 10.1016/s1364-6613(02)01961-7. [DOI] [PubMed] [Google Scholar]


