Abstract
Here we report a microfluidics method that enriches cancer stem cells (CSCs) or tumor-initiating cells based on cell adhesion properties. In our on-chip enrichment system, cancer cells were driven by hydrodynamic forces to flow through basement membrane extract-coated microchannels. Highly adhesive cells were captured by the functionalized microchannels, and less adhesive cells were collected from the outlets. The separation of two heterogeneous breast cancer cell lines (SUM-149 and SUM-159) into enriched subpopulations by their adhesive capacity was also successfully achieved and the enriched cancer stem cells were confirmed with flow cytometry biomarker analyses and tumor formation assays. Our findings show that the less adhesive phenotype is associated with a higher percentage of CSCs, higher cancer cell motility, and higher resistance to chemotherapeutic drugs.
Keywords: High-throughput, microfluidics, cancer stem cell, biomaterials, cell adhesion
Accurate isolation and better understanding of cancer stem cells (CSCs) are very important in basic and clinical cancer research [1]. These are also key areas in the development of novel therapeutic strategies [2]. Current methods to isolate CSCs largely rely on CSC-specific cell surface markers or long-term culture; however, the CSC-specific cell surface markers remain unclear in many cancer types [3]. Label free CSC enrichment methods may be able to replace biomarker–based isolation and have a broad application to different cancer types. Recently, it was reported that isolated CSC populations tend to re-express all the original general markers and reverse themselves to a mixed population after days of cultivation. Cancer cells may transform phenotypes stochastically [4]. Given the challenge in both obtaining and maintaining CSCs, an efficient, rapid and convenient CSC enrichment method is thus desired for the field.
Several studies have revealed a relationship between adhesiveness and stemness in keratinocytes and human mammary epithelial cells [5]. Because CSCs are cancer cells that possess characteristics associated with normal stem cells, we used cell adhesiveness as a biophysical marker to make a tool for CSC isolation. Microfluidic systems provide unique opportunities to investigate the influence of physical (adhesiveness) and biological (phenotype) parameters on advanced-stage cancer [6]. Using microfabrication technology, we designed a unique cell purification system to enrich the less adhesive subpopulation of breast cancer and melanoma cells. The separation device, which we termed high-throughput cell adhesion chip (HCA-Chip), employs artificial microchannels in combination with hydrodynamic force and adhesive extracellular matrix materials to separate less adhesive cells from parental cells.
Our experiments demonstrate the effectiveness of the HCA-Chip for separating the following cells into enriched subpopulations of low and high adhesive capacity: (i) eight breast cancer cell types with distinct adhesive capacity and metastatic potentials; and (ii) two heterogeneous breast cancer cell lines (SUM-149 and SUM-159). The low-adhesive phenotype was associated with a higher percentage of CSCs, higher cancer cell motility, and increased resistance to chemotherapeutic drugs. We also used the mammosphere formation efficiency assay and flow cytometry to assess the tumorigenicity of the subpopulations of SUM-149 and SUM-159 cells. Our study provides an example of how a microfluidics-based approach can be used for CSC and cancer metastasis research. To the best of our knowledge, it is the first time using cell adhesiveness as a biophysical marker to effectively isolate cancer stem cell.
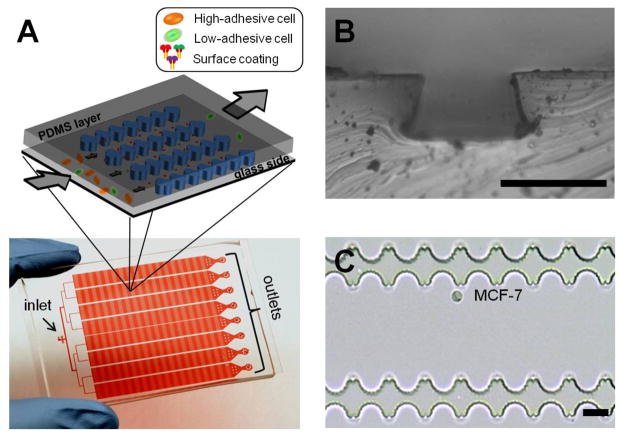
In a typical experiment, the HCA-Chip was fabricated with poly(dimethylsiloxane) (PDMS) using a photolithographic process, as summarized in Fig. S1 and described previously [7]. The HCA-Chip contained two layers: the PDMS layer and a 75×50-mm2 glass slide that was coated with a thin polystyrene layer (Fig. S2). This treatment increased protein adsorption onto the surface of the glass slide. The PDMS layer consisted of eight microchannels, and the depth of each microchannel can be set to any size from 25 to 60 μm (Fig. S3). The depth was controlled by changing the fabrication procedure. To mimic the cell-matrix adhesion and natural cellular microenvironments in vivo, the internal surface of the HCA-Chip was first covered with the polystyrene layer and then pre-coated with selected biomaterials, such as collagen type I, collagen type IV, and basement membrane extract (BME) [8].
Suspended cells were then applied to the HCA-Chip via a Tygon tube that was connected to the chip inlet. The flow of cells was controlled by the precision syringe pump for a total flow rate of 0.1–2 mL/h. High-adhesive cells were trapped in the device, and low-adhesive cells were collected from the outlets. After collecting the low-adhesive cells from the outlets, we can use cell dissociation buffer to wash the HCA-Chip, the high-adhesive cells in the device can be recovered (Video S1). Cells applied to the HCA-Chip were imaged by fluorescence microscopy. The separated subpopulations were then subjected to cell counting, immunostaining, flow cytometry, the cell invasion assay, or the mammosphere formation assay. As shown in Fig. S4, similar sizes of captured cells are observed in different areas of the chip. Less than 0.1% of the large-sized cells were trapped in the beginning of the chip.
To demonstrate the separation capacity of the HCA-Chip, we tested two different cancer cell types: a non-metastatic breast cancer cell line, MCF-7 (more adhesive), and a metastatic prostate cancer cell line, VAcP (less adhesive). The cells were spiked into the buffer at varying concentrations, after which they flowed through the HCA-Chip. The high-adhesive cells were captured in the chip, and the low-adhesive cells were collected from the outlets. The capture yield was calculated by counting the number of cells that were loaded into the HCA-Chip and the number of cells that were collected from the outlets. MCF-7 cells (1×104–5×106 cells/mL) were then spiked into the buffer solution and captured at a flow rate of 1 mL/h. There was little difference in capture yield when we increased the number of cells loaded (Fig. S5). Different depths of the microchannel were also applied to find the optimal dimension, and four depths were tested at a flow rate of 1 mL/h. Because increasing the depth of the microchannel reduced the chance of cells contacting with the functionalized surface and sacrificed the capture yield, an optimized channel depth of 25 μm was used in subsequent experiments (Fig. S6).
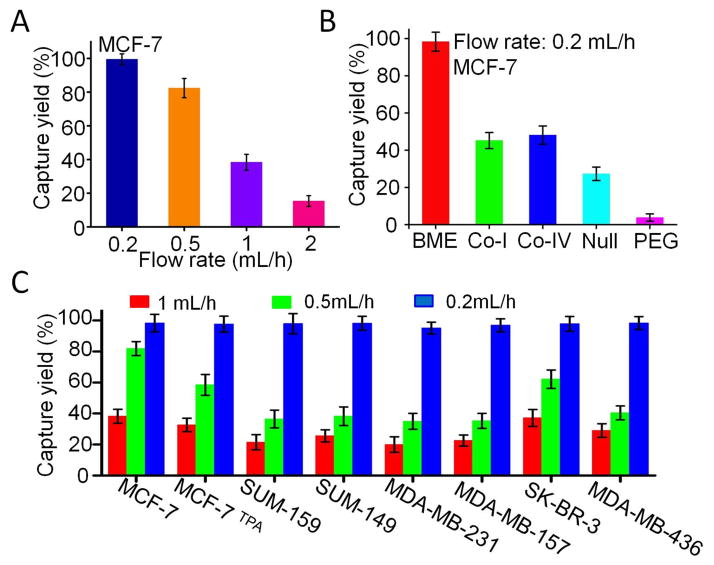
Different flow rates were also tested (Fig. 2A), and the optimal flow rate was determined to range from 0.2 to 0.5 mL/h, thus producing a capture yield of over 81.8%. Chips coated with or without different biomaterials were then compared to examine how BME might affect the capture yield. The BME-coated chip exhibited the highest capture yield (Fig. 2B and S7). To demonstrate the advantage of the HCA-Chip, the BME-coated chip and a commonly used adhesion assay were compared. For both cell lines (MCF-7 and VAcP), the capture efficiency of the HCA-Chip was much higher than that of the adhesion assay. Although almost no VAcP cells were captured in the dish when the adhesion assay was used, about 21.2% of the VAcP cells were trapped in the HCA-Chip (Fig. S6). In comparison to VAcP cells, MCF-7 cells had a higher capture yield in both the HCA-Chip and a commonly used adhesion assay. Our results confirmed the effectiveness of the HCA-Chip in isolating cells based on cell adhesion properties.
Fig. 2.
Characterization of the HCA-Chip cell capture yield with cancer cells in buffer solution. (A) Dependence of MCF-7 capture efficiency on flow rate. (B) Capture of MCF-7 cells in HCA-Chips that were coated with different biomaterials. (C) Comparison of the capture yield of different cell lines in the BME-coated HCA-Chip at different flow rates. Error bars represent the standard deviation of three replicates.
To further investigate the relationship among biomaterial coating, capture efficiency, and metastatic capacity, eight different breast cancer cell lines were tested in the HCA-Chip. MCF-7 and SK-BR-3 cells are less aggressive than SUM-159, SUM-149, MDA-MB-231, MDA-MB-157, and MDA-MB-436 cells [9]. MCF-7 cells were treated with 12-O-Tetradecanoylphorbol-13-acetate (TPA), a potent tumor promoter, to trigger epithelial-mesenchymal transition (EMT)-like cell scattering and increase the metastatic capacity (Fig. S8) [10]. In the BME-coated chip, MCF-7 and SK-BR-3 cells had higher capture yields when the flow rate was set to 0.5 mL/h, and small numbers of cells were collected from the outlets. About 81.8% of MCF-7 cells were trapped in the chip. However, the capture yield of MCF-7 was reduced after TPA treatment; only 57.1% of cells were trapped in the chip (Fig. 2C). On the contrary, all metastatic breast cancer cell lines exhibited lower capture yields at 0.5mL/h. Even the flow rate was reduced to 0.2 mL/h, and we can collect more metastatic cells from the outlets (Fig. S9). These data indicated that metastatic cells are less adhesive.
These eight breast cancer cell lines were then tested in collagen type I- and IV-coated chips (Fig. S10). Surprisingly, there were no significant differences among these cell lines when the flow rate was set to 0.5 mL/h. The capture yield of SUM-159 cells in the collagen-coated chip was higher than that in the BME-coated chip (Fig. S11). In polyethylene glycol-coated or uncoated chips, the capture yield was very low. Thus, we coated the chip’s surface with BME in subsequent experiments. The fluid flow force is the only force to compete with the adhesion between the cell membrane and the coated biomaterial. The adhesion strength is then correlated to the flow rate. In order to isolate the least adhesive cells, the flow rate was set to 0.2mL/h in the subsequent separation experiments.
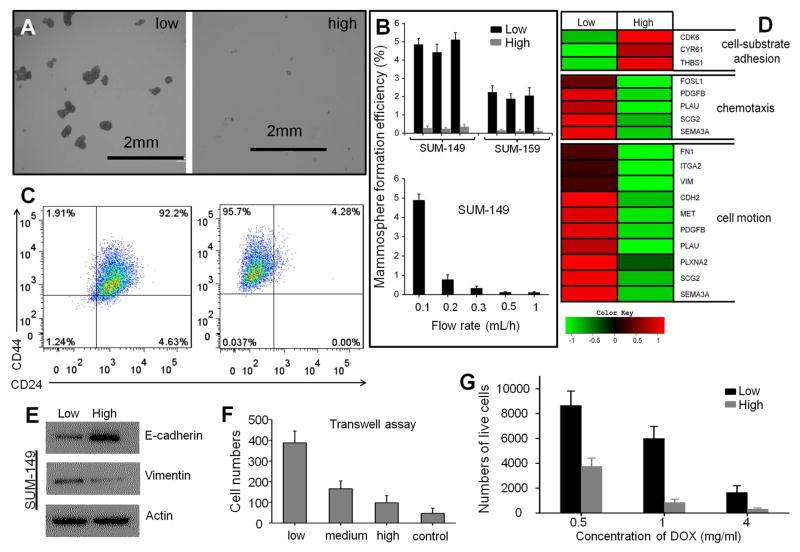
Next, we explored the mechanical subtype of SUM-149 cells in detail. The SUM-149 cell line is known for its intrinsic heterogeneity and is a well-established in vitro model of inflammatory breast cancer [11]. After low-adhesive SUM-149 cells were separated based on cell-substrate adhesion, we assessed the mammosphere formation efficiency under serum-free conditions in suspension culture to examine tumorigenicity. Less than 1% of tumor cells are able to survive under these culture conditions, and the surviving cells are capable of self-renewal, differentiation, and tumor formation upon transplantation. Results from the mammosphere formation assay demonstrated that low-adhesive cells exhibited higher growth efficiency and generated larger mammospheres, thus providing functional evidence of TIC enrichment (Fig. 3A–B) [11].
Fig. 3.
Analyses of the subpopulations of separated cells. (A) Mammospheres derived from SUM-149 cells of low and high adhesion. (B) Mammosphere formation efficiency of subpopulations of low and high adhesion. All three replicates showed consistently greater efficiency in flexible cells. (C) Scatter plot of CD44/CD24 expression in SUM-149 cells before and after HCA-Chip separation. (D) Heat map showing the expression levels of genes related to cell-substrate adhesion, chemotaxis, and cell motion in cells of low and high adhesion. (E) Western blot analysis showing E-cadherin and Vinmentin production. (F) Low-adhesive cells have higher cell invasive capacity. (G) Resistance of lower and higher adhesive cells to different concentrations of doxorubicin (DOX). The subpopulations of cells were separated 3 times by different HCA-Chips.
To further explore the relationship between low-adhesive capacity and tumor-initiating features (or CSC-likeness), we analyzed the expression of several breast cancer stem cell markers, including CD44, CD24, and epithelial cell adhesion molecule (EpCAM) [12]. Flow cytometric analysis confirmed a significant enrichment of the CD44+/CD24− (or CD44high/CD24−) population in low-adhesive cells. Increasing the flow rate resulted in the reduced percentage of the CD44+/CD24− population (Fig. 3C and S12). We also tested SUM-159 cells. The data confirmed a significant enrichment of the CD44+/EpCAM+ (or CD44high/CD24−) population in low-adhesive cells (Fig. S13). Immunofluorescence staining also demonstrated similar results (Fig. S14).
In addition, we applied total RNA-Seq (whole transcriptome) sequencing technology to examine the expression of cancer metastasis protein genes in cells with different adhesive properties. Total RNA-Seq can capture a broader range of gene expression changes and enables the detection of novel transcripts in both coding and non-coding RNAs [13]. The expression profile of the enriched low-adhesive SUM-149 cell subpopulation showed decreased expression levels of genes encoding cyclin-dependent kinase 6, cysteine-rich angiogenic inducer 61, and thrombospondin 1, which are related to cell-substrate adhesion(Cancer gene expression database (CGED)). This finding provides support for the rationale of HCA-Chip separation (Fig. 3D).
Our results also indicate that five highly expressed genes are relevant to chemotaxis and ten highly expressed genes were related to the regulation of tumor cell migration (Fig. 3D). We have previously shown that breast cancer cells always undergo EMT and acquire increased migratory capabilities [14]. Using Western blot analysis, we demonstrated that after separation, the low-adhesive cells lost the epithelial marker E-cadherin and gained the mesenchymal marker vimentin (Fig. 3E). Because only low-adhesive cells with a substantial fraction of CD44+/CD24− cells consistently expressed cell motion- and chemotaxis-related genes, we compared the invasive capacity of cell lines at different adhesive capacities. Interestingly, low-adhesive cells were more invasive than high-adhesive cells, thus further confirming the association between cell adhesive capacity and invasion (Fig. 3F). We also found that low-adhesive cells had a higher survival rate after 24h of anti-cancer drug treatment at the indicated concentrations (Fig. 3G).
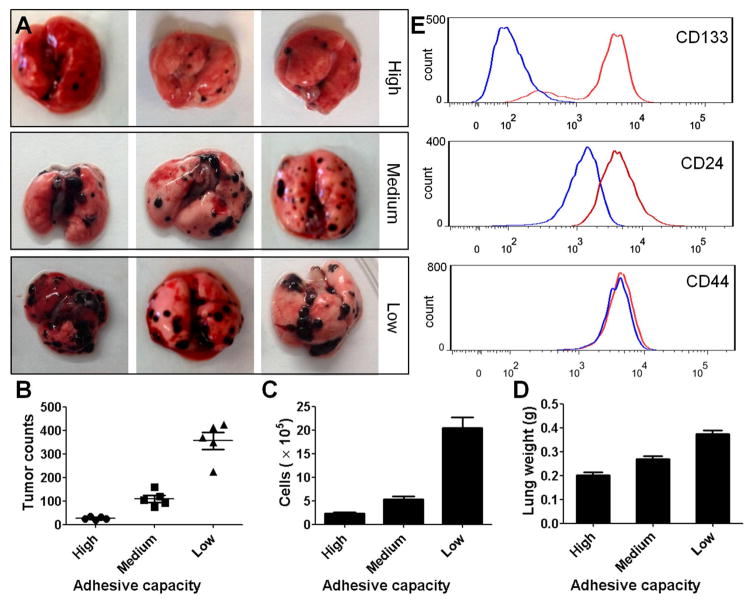
Next, we used the mammosphere formation assay to explore whether low-adhesive capacity is related to high metastatic potential in other types of tumor cells. Low-adhesive murine melanoma B16 cells showed higher growth efficiency and generated larger mammospheres, thus providing functional evidence of TIC enrichment (Fig. S15). An in vivo metastatic lung tumor model was also generated and assessed. Murine melanoma B16 cells were separated into three subpopulations by HCA-Chip, based on different adhesive capacities, and then intravenously injected into C57BL/6 mice (1×105 cells/mice). At 18 days after injection, the mice were sacrificed and analyzed for lung metastasis. C57BL/6 mice challenged with low-adhesive B16 melanoma cells showed increased tumor development, multiple tumor fusions, higher numbers of tumor foci, and increased lung weight when compared to mice that were challenged with medium- or high-adhesive B16 cells (Fig. 4A–C). These data indicate that B16 melanoma cells with lower adhesive capacity have higher metastatic potential, which is consistent with our previous observation in the breast cancer model. Moreover, results from hematoxylin and eosin staining showed that increased tumor growth was detected in the lungs of C57BL/6 mice challenged with low-adhesive B16 cells, when compared with the medium- or high-adhesive group (Fig. S16–S18). To compare the expression of CD133, CD24, and CD44, which are specific cell surface markers in CSCs, between original B16 melanoma cells and low-adhesive B16 cells, flow cytometry was used. The expression of CD133 and CD24 was increased in the HCA-Chip-isolated, low-adhesive B16 subpopulation compared to original B16 cells, whereas the expression of CD44 was comparable between groups (Fig. 4E).
Fig. 4.
Low-adhesive B16 melanoma cells demonstrate increased potential to induce tumor development. (A) Representative images of lungs are shown. (B) Numbers of tumor foci and total cells in the lung, as well as lung weights, were examined in B6 mice that were challenged with high-, medium-, or low-adhesive B16 cells. (C) Tissue pathology in the lungs of B6 mice was assessed by hematoxylin and eosin staining after challenge with low-, medium-, or high-adhesive B16 cells. Magnification, ×400. (D) Expression of CD133, CD24, and CD44 was analyzed in B16 cell subpopulations with different adhesive capacities by flow cytometry.
Overall, we used cell adhesiveness as a biophysical marker to make a tool for CSC isolation. This unique microfluidic sorting method may contribute to the advancement of CSC research and the development of CSC-targeted therapy in a variety of cancer cell types. The HCA-Chip could potentially be used to purify stem cells and CSCs based on their mechanical characteristics and thereby identify molecular signatures and genes that are essential for tumor initiation. Our device can also be used for tissue samples from cancer patients. Tissue samples often contain many stromal cells and immune cells and limit the CSC enrichment efficiency. Pre-enrichment can be used to resolve this problem, such as magnetic beads separation. Overall, HCA-Chip separation will be particularly useful in a broad range of cancers, even for the cancer types that CSC biomarkers have not yet been discovered[15].
Experimental Section
Experimental Details can be found in Supporting Information.
Supplementary Material
Fig. 1.
(A) Scheme (upper) and photograph (lower) of the high-throughput cell adhesion chip (HCA-Chip). The chip was filled with red dye. (B) Optical microscopic images of the HCA-Chip. The depth of the microchannel is approximately 25 μm. Scale bar: 30 μm. (C) Optical microscopic images of the microchannel’s structure and captured MCF-7 cell. Scale bar: 20 μm.
Acknowledgments
This study was funded by the Cancer Prevention and Research Institute of Texas (CPRIT-R1007), NIH-CA180083, Emily Herman Research Fund, and Golfers Against Cancer Foundation.
References
- 1.a) Beck B, Blanpain C. Nat Rev Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]; b) Nguyen LV, Vanner R, Dirks P, Eaves CJ. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 2.a) Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Nat Rev Drug discovery. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]; b) McCubrey JA, Steelman LS, Abrams SL, Misaghian N, Chappell WH, Basecke J, Nicoletti F, Libra M, Ligresti G, Stivala F, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Laidler P, Bonati A, Evangelisti C, Cocco L, Martelli AM. Curr Pharm Des. 2012;18:1784–1795. doi: 10.2174/138161212799859701. [DOI] [PubMed] [Google Scholar]; c) Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV, Hidalgo M, Tsao M, Ailles L, Waddell TK, Maitra A, Neel BG, Matsui W. Cell stem cell. 2010;7:279–282. doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tirino V, Desiderio V, Paino F, Papaccio G, De Rosa M. Methods Mol Biol. 2012;879:513–529. doi: 10.1007/978-1-61779-815-3_32. [DOI] [PubMed] [Google Scholar]
- 4.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 5.a) Farahani E, Patra HK, Jangamreddy JR, Rashedi I, Kawalec M, Rao Pariti RK, Batakis P, Wiechec E. Carcinogenesis. 2014;35:747–759. doi: 10.1093/carcin/bgu045. [DOI] [PubMed] [Google Scholar]; b) Zetter BR. Semin Cancer Biol. 1993;4:219–229. [PubMed] [Google Scholar]
- 6.a) Zhang Y, Zhang W, Qin L. Angew Chem Int Ed. 2014;53:2344–2348. doi: 10.1002/anie.201309885. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Reategui E, Aceto N, Lim EJ, Sullivan JP, Jensen AE, Zeinali M, Martel JM, Aranyosi AJ, Li W, Castleberry S, Bardia A, Sequist LV, Haber DA, Maheswaran S, Hammond PT, Toner M, Stott SL. Adv Mater. 2015;27:1593–1599. doi: 10.1002/adma.201404677. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mohamadi RM, Besant JD, Mepham A, Green B, Mahmoudian L, Gibbs T, Ivanov I, Malvea A, Stojcic J, Allan AL, Lowes LE, Sargent EH, Nam RK, Kelley SO. Angew Chem Int Ed. 2015;54:139–143. doi: 10.1002/anie.201409376. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhou L, Qin L. J Am Chem Soc. 2014;136:15257–15262. doi: 10.1021/ja5072114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benton G, Kleinman HK, George J, Arnaoutova I. Int J Cancer. 2011;128:1751–1757. doi: 10.1002/ijc.25781. [DOI] [PubMed] [Google Scholar]
- 9.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. Cancer cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu WS, Tsai RK, Chang CH, Wang S, Wu JR, Chang YX. Mol Cancer Res. 2006;4:747–758. doi: 10.1158/1541-7786.MCR-06-0096. [DOI] [PubMed] [Google Scholar]
- 11.Zhang WJ, Kai K, Choi DS, Iwamoto T, Nguyen YH, Wong HL, Landis MD, Ueno NT, Chang J, Qin LD. Proc Natl Acad Sci USA. 2012;109:18707–18712. doi: 10.1073/pnas.1209893109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillmore CM, Kuperwasser C. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Nat Biotechnol. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalluri R, Weinberg RA. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. Trends Mol Med. 2008;14:450–460. doi: 10.1016/j.molmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.