Abstract
Background
The National Birth Defects Prevention Study (NBDPS) is a large population-based multi-center case-control study of major birth defects in the United States.
Methods
Data collection took place from 1998 through 2013 on pregnancies ending between October 1997 and December 2011. Cases could be live born, stillborn or induced terminations, and were identified from birth defects surveillance programs in Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas and Utah. Controls were live born infants without major birth defects identified from the same geographical regions and time periods as cases via either vital records or birth hospitals. Computer-assisted telephone interviews were completed with women between 6 weeks and 24 months after the estimated date of delivery. After completion of interviews, families received buccal cell collection kits for the mother, father and infant (if living).
Results
There were 47,832 eligible cases and 18,272 eligible controls. Among these, 32,187 (67%) and 11,814 (65%) respectively, provided interview information about their pregnancies. Buccal cell collection kits with a cytobrush for at least one family member were returned by 19,065 case and 6,211 control families (65% and 59% of those who were sent a kit). More than 500 projects have been proposed by the collaborators and over 200 manuscripts published using data from the NBDPS through December 2014.
Conclusion
The NBDPS has made substantial contributions to the field of birth defects epidemiology through its rigorous design, including case classification, detailed questionnaire and specimen collection, large study population, and collaborative activities across Centers.
Major structural birth defects are common, costly and critical. About three percent of all live births in the United States are affected by birth defects (Centers for Disease and Prevention, 2008); they account for one in five infant deaths (Xu and others, 2014) and contribute substantially to childhood morbidity and long-term disability. Most birth defects are due to unknown causes or a combination of causes (Nelson and Holmes, 1989), and because the etiologies of specific phenotypes may vary, it is important to study them in homogeneous groups. However, because individual types of birth defects are relatively rare, it has been difficult in the past to conduct a study large enough to provide the necessary statistical power to assess risk factors for individual defects.
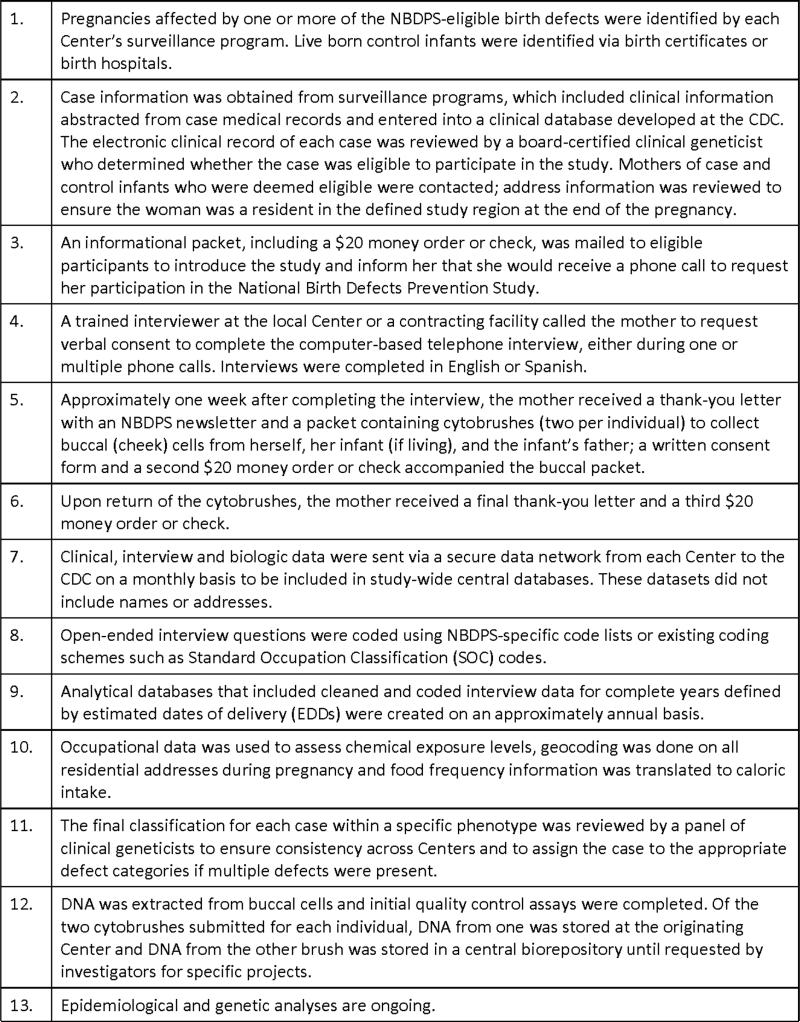
In 1996, Congress appropriated funds to the U.S. Centers for Disease Control and Prevention (CDC) to establish the Centers for Birth Defects Research and Prevention. This funding was formalized by the Birth Defects Prevention Act of 1998. A Georgia Center was established at CDC and cooperative agreements awarded with competitive renewals to Centers in nine states during one or more cycles of the cooperative agreement. From 1997 to 2013, the primary collaborative activity of these Centers was a multi-center case-control study, the National Birth Defects Prevention Study (NBDPS). In this manuscript, we summarize the methods (Figure 1) used during the data collection phase of the NBDPS.
Figure 1.
Basic study process for the National Birth Defects Prevention Study (NBDPS)
NBDPS Centers and population
Participating Centers had access to data from a birth defects surveillance program that used active case-finding for all major structural birth defects eligible for inclusion in the NBDPS (Table 1). Since each Center was expected to contribute about 300 cases per year, the minimum population base was 35,000 births per year. For most years of the study, the study sites covered a birth population between 35,000-80,000 births per year. Participating Centers were Arkansas (AR, statewide), California (CA, selected counties), Georgia (GA, selected counties), Iowa (IA, statewide), Massachusetts (MA, selected counties except for 16 months when it was statewide), North Carolina (NC, selected counties), New Jersey (NJ, statewide, some common defects were sampled), New York (NY, selected counties), Texas (TX, selected counties) and Utah (UT, statewide) (Figure A online). Abstractors at each of the birth defects surveillance programs went out to birth and children's hospitals to ascertain eligible birth defects. This information was then entered in the NBDPS clinical database for review.
Table 1.
Number of case and control subjects eligible to be included and interviewed in the National Birth Defects Prevention Study, 1997-2011a
| Birth Defects | Eligible(n) | Interviewed (n) | Birth Defects | Eligible (n) | Interviewed (n) |
|---|---|---|---|---|---|
| Total cases | 47832 | 32187 | |||
| Controls | 18272 | 11814 | |||
| Amnion rupture sequence | 590 | 383 | Craniosynostosis | 2611 | 1794 |
| Anencephaly, craniorachischisis | 1164 | 687 | Diaphragmatic hernia | 1328 | 907 |
| Spina bifida | 1854 | 1334 | Sacral agenesis | 175 | 123 |
| Encephalocele, encephalomyelocele | 431 | 264 | Omphalocele | 747 | 503 |
| Holoprosencephaly | 321 | 181 | Gastroschisis | 2305 | 1504 |
| Hydrocephalus | 995 | 573 | Laterality defects, heterotaxia | 683 | 433 |
| Dandy-Walker malformation | 417 | 268 | Ventricular septal defect (VSD)e | 2693 | 1798 |
| Anophthalmia, microphthalmia | 411 | 281 | Atrial septal defect (ASD)f | 7280 | 4629 |
| Cataractsb | 668 | 434 | Truncus arteriosus | 309 | 211 |
| Glaucoma, other anterior chamber eye defectsb,c | 323 | 190 | D-transposition great arteries/Double outlet right ventricle | 1731 | 1249 |
| Anotia, microtia | 1056 | 709 | Tetralogy of Fallot | 2041 | 1445 |
| Choanal atresia | 262 | 182 | Single ventricle | 413 | 276 |
| Cleft lip with and without cleft palate | 4537 | 3263 | Atrioventricular septal defectg | 1254 | 826 |
| Cleft palate | 2363 | 1651 | Aortic stenosis | 938 | 650 |
| Esophageal atresia with and without tracheoesophageal fistula | 1031 | 769 | Coarctation of the aorta | 2464 | 1730 |
| Biliary atresia | 315 | 216 | Hypoplastic left heart syndrome | 1034 | 724 |
| Intestinal atresia or stenosisd | 3342 | 2269 | Pulmonary stenosis (valvar) | 3023 | 2019 |
| Hypospadias (second- or third-degree) | 4061 | 2630 | Pulmonary atresia (not tetralogy of Fallot variant) | 679 | 453 |
| Renal agenesis or hypoplasia (bilateral) | 366 | 203 | Tricuspid atresia | 428 | 297 |
| Bladder exstrophy | 105 | 77 | Ebstein malformation | 330 | 208 |
| Cloacal exstrophy, persistent cloaca | 142 | 101 | Anomalous pulmonary venous return | 993 | 647 |
| Limb deficiency (intercalary) | 103 | 68 | |||
| Limb deficiency (longitudinal) | 895 | 587 | |||
| Limb deficiency (transverse) | 1603 | 1077 | |||
An infant with more than one eligible birth defects is counted in each eligible category
Included in the study as of January 1, 2000
Includes absence of the lens, spherical lens, lens coloboma, aniridia, Peters anomaly, Axenfeld anomaly, and Rieger anomaly
Includes stenosis or atresia of the small intestine, large intestine, or rectum, and atresia of the anus
Includes muscular VSD, perimembraneous/membraneous VSD, and VSD not otherwise specified
Includes secundum ASD, other specified ASD, and ASD not otherwise specified; does not include primum ASD
Includes primum ASD
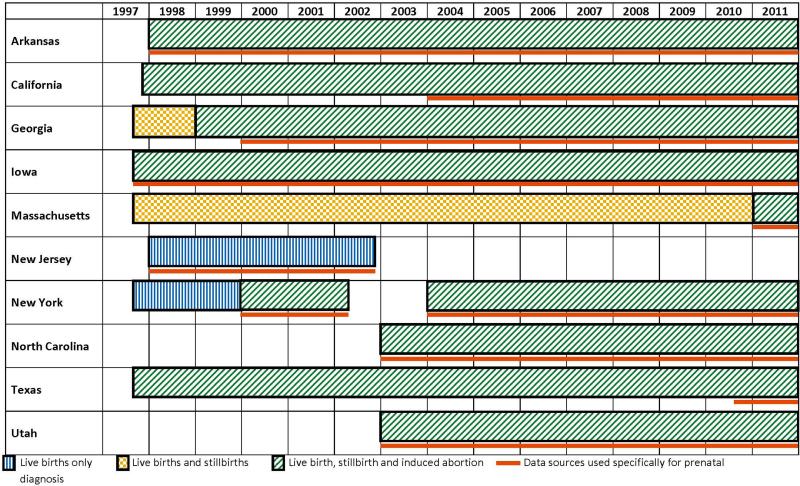
There were changes in NBDPS Centers over time (Figure 2), NJ stopped contributing data in 2003, and NC and UT started contributing data in 2003. Case ascertainment procedures also changed over time for some Centers with respect to the inclusion of cases among live births, stillbirths and induced abortions, and the use of data sources specifically to ascertain prenatal diagnoses, such as specialized ultrasound clinics (Figure 2).
Figure 2.
Inclusion of cases among live births, stillbirths and induced abortions, and data sources used specifically to ascertain prenatal diagnoses, by estimated year of delivery and Center.
Study eligibility started with pregnancies ending on or after October 1, 1997 and concluded with pregnancies with estimated dates of delivery (EDD) on or before December 31, 2011. During this study period, there were approximately six million births in the NBDPS catchment areas. Each Center's surveillance program ascertained all case infants with NBDPS-eligible birth defects that occurred in the study region. All Centers included defects diagnosed at least until the child turned one year old; some Centers included defects diagnosed up to two years old. Control infants were randomly selected from among live born infants from the same study region as the cases; the monthly number of controls selected was proportionate to the number of births in the same month in the previous year. Control infants were selected from vital records (AR [2000-2011], GA [2001-2011], IA, MA, NC, NJ, UT) or from birth hospital records (AR [1997-2000], CA, GA [1998-2001], NY, TX). Each Center interviewed mothers of approximately 100 control infants each year. A woman was not eligible to participate in the NBDPS if she already participated with a previous pregnancy, could not complete the interview in English or Spanish, was incarcerated, or did not have legal custody of the child at the time of the interview. Due to state-specific requirements for obtaining informed consent from those who were less than 18 years old at the end of their pregnancy, 3 of 10 Centers did not include eligible women less than 18 years old at the time of interview.
Study coordination
The NBDPS was managed by staff of the Birth Defects Branch at CDC's National Center on Birth Defects and Developmental Disabilities. The study-wide principal investigator, project officer, study coordinator, biologics coordinator, Georgia principal investigator, and data managers were part of the CDC management team. A Coordinating Council, established in 2004, consisted of principal investigators from participating Centers; each Center had one vote. The Coordinating Council's charge was to oversee infrastructure, address managerial issues, and establish research priorities. The Coordinating Council communicated through monthly conference calls and an annual in-person meeting.
Several committees, comprised of individuals representing each Center, assisted with the coordination of NBDPS activities. The Clinicians Committee was composed of clinical geneticists who conducted case review and classification (described below). The Questionnaire and Methods Committee was responsible for ongoing quality control and improvements to the questionnaire and other aspects of the study protocol. The Interviewers and Study Coordinators Committee provided an opportunity for interviewers from different Centers to share their experiences and to be guided by peers and CDC staff on how to handle unique interviewing situations. The Biologics Committee, Epidemiologists/Analysts Committee, and the Data Sharing Committee have on-going activities even after the completion of data collection. The Biologics Committee continues to oversee the storage and retrieval of biological specimens. The latter two committees are described in detail below.
Clinical NBDPS data: Case classification
For major structural birth defects included in the NBDPS (Table 1), all clinical information was reviewed by a clinical geneticist to confirm eligibility prior to requesting an interview. Because the goal of the study was to identify unknown causes of birth defects, cases with defects of known etiology, such as single gene conditions or chromosomal abnormalities were excluded.
The methodology for case classification has been described previously (Rasmussen and others, 2003). Cases were classified as isolated, multiple, or complex. A case was considered isolated if there was only one major defect diagnosed, regardless of whether minor defects were also diagnosed. If two or more major defects were diagnosed and the defects were developmentally related to one another, then the pattern of defects represented a sequence and the case was classified as isolated. When two or more major defects were diagnosed in the same organ system, the case was usually classified as isolated, with the exception being defects of the gastrointestinal tract. If two or more major defects occurred in different organ systems and the defects did not represent a sequence or a complex case, the case was classified as multiple. A complex case was defined as a pattern of major defects that are embryologically related and likely represent an early problem in morphogenesis, often akin to a developmental field defect. The causes of defects that are part of a complex case are generally not known, whereas patterns of defects that occur in sequences more often have a suspected cause (Table 2).
Table 2.
Complex cases in the National Birth Defects Prevention Study and the birth defect category within which they are analyzed
| Sequence | Birth Defect category |
|---|---|
| Amniotic band (AB) limb defects +/− AB craniofacial malformations | Amnion rupture sequence |
| Limb-body wall complex | Amnion rupture sequence |
| Urorectal septum malformation sequence | Intestinal atresia or stenosis |
| Imperforate anus-sacral anomalies | Intestinal atresia or stenosis |
| Omphalocele-Exstrophy-Imperforate anus-Spina bifida (OEIS complex) | Cloacal exstrophy, persistent cloaca |
| Pentalogy of Cantrell | Diaphragmatic hernia, Heart defects, and Omphalocele |
| Heterotaxy | Laterality defects, heterotaxia |
| Sacral agenesis with caudal regression | Sacral agenesis |
Some birth defect phenotypes were classified by the same clinical geneticist for all cases for the entire study period. For other defect types, classification was completed by more than one person, but the clinical geneticists developed guidelines that detailed the (1) inclusion and exclusion criteria for each defect eligible for the NBDPS; (2) rationale for including certain diagnostic codes for defects and related defects; (3) instructions and rationale for designating the final case classification (isolated, multiple, complex); and (4) instructions and recommendations to analysts on how the defect type could be analyzed in epidemiologic studies. For cases of congenital heart defects, four clinicians with expertise in pediatric cardiology and clinical genetics developed a classification of just the heart defects (Botto and others, 2007), and this classification was in addition to the classification of the overall constellation of defects that was completed by the clinical geneticists. Eligibility criteria for most defects were consistent over the study period, but for certain defects the criteria for inclusion in analyses changed (Table 3).
Table 3.
Birth defects with eligibility changes for inclusion in the National Birth Defects Prevention Study
| Birth Defect | Date of Eligibility Change(s) (EDD) | Criteria Prior to Change | Criteria After Change |
|---|---|---|---|
| Aneurysm of atrial septum with shunt | 1/1/2006 | Cases were included in the study and coded as atrial septal defect secundum type. | Cases of isolated aneurysm of the atrial septum with atrial shunt were excluded from the study. |
| Atrial septal defect, secundum type (ASD2) or not otherwise specified (ASD, NOS) | 1/1/2006 | All isolated ASD2 and ASD, NOS were included in the study. | Cases of isolated ASD2 or ASD, NOS were excluded from the study if (1) the diameter of the defect was ≤4 mm; (2) no size was provided for the defect, but it was described as “small,” “mild,” or “trivial;” or (3) no size or description of the defect was provided, and the only echocardiograms identifying the defect were performed when the infant was <6 weeks of age. |
| Cataract | 1/1/2006 | Cases with a family history consistent with Mendelian inheritance were included in the study, but they were classified as “syndromic” so as not to be included in risk factor analyses. | Cases of isolated cataract that had a first-degree relative with congenital cataract were excluded from the study. |
| Craniosynostosis | 1/1/2003 and 11/1/2005 | Presumptive isolated cases without confirmation by head computerized tomography (CT) or x-ray report were excluded from the study. | Presumptive cases without confirmation by head CT or x-ray report, but with a corrective surgical procedure for craniosynostosis were included in the study after 1/1/2003. Presumptive cases without confirmation by head CT or x-ray report, or surgery, but with histopathological diagnosis based solely on autopsy were included in the study after 11/1/2005. |
| Duodenal atresia | 1/1/2006 | Isolated cases associated with duodenal web were excluded from the study. | All cases of isolated duodenal atresia, type I, including those described as duodenal web, membrane, diaphragm, or windsock, were included in the study. |
| Pulmonary stenosis (valvar) | 1/1/2005 | All cases were included in the study. | Cases of isolated pulmonary valve stenosis were excluded from the study if one of the criteria was met: (1) peak gradient on echocardiogram or cardiac catheterization of < 15 mmHg; or (2) no peak gradient information, but the defect was described as “trivial,” “whiff,” or “mild.” However, cases that met one of the exclusion criteria, but had an abnormal pulmonary valve (described as thickened, dysplastic, doming in systole, etc.) were still included in the study. |
| Ventricular septal defect (VSD) muscular type or VSD not otherwise specified (VSD, NOS) | 10/1/1998 and 1/1/1999 | Muscular VSD and VSD, NOS without another NBDPS-eligible defect (isolated cases) were included in the study | Isolated cases of muscular VSD and VSD, NOS were excluded from the study if the EDD was after 10/1/1998 for cases from California, Georgia, Iowa, Massachusetts, New York and Texas, or after 1/1/1999 for cases from Arkansas and New Jersey. |
| Ventricular septal defect, other subtypes (e.g., canal type VSD, perimembranous VSD, malalignment VSD) | 1/1/2006 | Isolated VSD, other subtypes, without another NBDPS-eligible defect (isolated cases) were included in the study | VSD, other subtypes, without another NBDPS-eligible defect (isolated cases) were excluded from the study. |
Causative genetic factors for some defects studied in NBDPS have been identified during the course of the NBDPS, necessitating changes in eligibility criteria. For instance, initially, the cause of CHARGE syndrome was not certain, and it was classified as an association (a group of anomalies that occur with a statistical clustering, but not representing a recognizable syndrome, sequence, or developmental field defect). Therefore, cases of potential CHARGE association having at least one NBDPS-eligible defect (e.g., choanal atresia, heart defect) were included. Later, CHD7 mutations were identified as causative for some cases of CHARGE syndrome (Vissers and others, 2004). Therefore, cases with the phenotype of CHARGE syndrome with EDDs prior to January 1, 2006 remained in the study, but cases with EDDs after January 1, 2006 that had a CHD7 mutation were excluded. Cases with CHARGE syndrome phenotype that had no CHD7 mutation identified or did not have mutation testing performed were included throughout the study.
Another area of technological development that impacted NBDPS case classification was the comparative genomic hybridization (CGH) microarray, used to identify submicroscopic gains or losses of chromosomal material. Because these microduplications or microdeletions are potentially causative for birth defects, eligibility criteria based on a consensus statement (Miller and others, 2010) were developed for cases of duplication or deletion identified by CGH microarray with EDDs on or after January 1, 2009. If a phenotypically normal parent had the identical microduplication or microdeletion, then the infant's defect was presumed to represent a benign copy number variant, and the case infant was included in the study. If neither parent had the microduplication or microdeletion, then the chromosomal anomaly was presumed to be de novo and potentially pathogenic, so the case was excluded. However, if parental studies were not performed, then the case was excluded from the study if at least one of the following criteria was met for the microduplication or microdeletion: (1) it was associated with a previously characterized phenotype; (2) it contained at least one gene known or strongly suspected to be dosage sensitive; or (3) it was >400 kb in size. If none of these 3 criteria was met for the microduplication or microdeletion, the case was included.
Interview recruitment
Contact information for women eligible for study participation could be obtained from three sources: the Center's birth defects surveillance program, hospital records, or state birth certificates. Individual Centers sent out the introductory packet 6 weeks or more after the EDD; interviews had to be initiated by 24 months after the EDD. The introductory packet contained a letter introducing the study, which was signed by the local principal investigator, a calendar to assist women in accurately reporting exposures relative to their timing during her pregnancy, a “Frequently Asked Questions” document, and a “Rights of Human Subjects” document and a twenty dollar incentive. Materials were developed in collaboration with all Centers to ensure that the same information was delivered in the same way, although modifications for locally relevant information were allowed when required by the local Institutional Review Board (IRB). All protocols, contact materials and the interview content were approved by the CDC IRB, the Office of Management and Budget (OMB), and the local IRB(s) for each Center. The contents of the introductory packet and other study materials and products underwent several improvements over the study period in an effort to update and unify the appearance of all NBDPS materials; consistent graphical elements and color schemes were developed to increase study recognition and to facilitate higher participation (online-only Figure B).
Interview Participation
Overall, of the women who were invited to participate, 67% of cases and 65% of controls decided to take part. Interviews were conducted at each of the sites, or through a contractor, and participation has varied over time and by Center. Over the course of the study, use of telephone landlines decreased while use of cell phones increased; cell phone numbers were not always available through traditional directories. Centers adapted to challenges in recruitment in a variety of ways, including the use of cell phones instead of landlines to initiate calls (in order to match the participant's cellular provider to avoid call charges) and e-mail to contact study participants. In an effort to understand the magnitude of potential selection bias due to nonresponse, Cogswell and colleagues (Cogswell and others, 2009) compared demographic characteristics of controls who participated (n=4,495) to all eligible controls (n=6,681), and to all live born infants in the base population from which the controls were selected (n=2,468,697). Compared with base populations, control participants did not differ substantively in distributions of maternal or paternal age, number of previous live births, maternal smoking, or diabetes, but they did differ in other maternal characteristics (i.e., race/ethnicity, education, entry into prenatal care), and infant characteristics (i.e., birth weight, gestational age, and plurality). Compared with eligible controls who did not participate, control participants differed in distributions of maternal, but not infant, characteristics. Overall the authors concluded that control participants were generally representative of their base populations.
Interview Revisions
The computer-assisted telephone interview (CATI) was administered to women with pregnancies ending on or after October 1, 1997. The original CATI (Yoon and others, 2001) included questions on a range of topics (Table 4). Substantial changes to the CATI were implemented beginning with births on or after January 1, 2006.
Table 4.
Computer-assisted telephone interview (CATI) sections in the order they were asked for the periods 1997-2005 and 2006-2011
| CATI 1997-2005 | CATI 2006-2011 |
|---|---|
| Index pregnancy | Index pregnancy |
| Previous pregnancies | |
| Addresses during pregnancy | |
| Contraception | |
| Prenatal care | |
| Fertility treatments - mother | |
| Fertility treatments - father | |
| Morning sickness | |
| Diarrhea | |
| Dieting | |
| Weight gain/loss during pregnancy | |
| Maternal health | Maternal health |
| Pregnancy history | Medication |
| Previous pregnancies | |
| Weight gain/loss | |
| Contraception | |
| Fertility treatments - father | |
| Fertility treatments - mother | |
| Morning sickness | |
| Morning sickness weight loss | |
| Prenatal care | |
| Medication | |
| Vitamins | Vitamins |
| Food frequency | Food frequency |
| Tobacco | Stress |
| Alcohol | Tobacco |
| Substance abuse | Alcohol |
| Home environment | Substance abuse |
| Addresses during pregnancy | Reduced questions about father |
| Hot tub/sauna use | |
| Mother's occupation | Water use (reduced) |
| Father's occupation | Mother's occupation |
| Family demographics - mother | Father's occupation |
| Family demographics - father | Physical activity |
| (added for interviews after 10/1/2010) | |
| Home water environment | Family demographics - mother |
| Drinking water at home | Family demographics - father |
| Drinking water at work/school | Family information - mother and father |
| Home water use | Closing/debriefing |
| Swimming pool use | |
| Closing/debriefing | |
Note: A PDF of the most recent version of each of these two CATIs is available upon request from the corresponding author.
Based on research suggesting that stressful life events may be associated with the occurrence of birth defects (Carmichael and others, 2007; de Weerth and Buitelaar, 2005; Hansen and others, 2000), a new section was added to the CATI that asked mothers about stressful life events immediately before and during pregnancy, as well as the availability of social support. The caffeine questions were changed to account for early pregnancy changes in intake and to estimate portion size. Established associations of maternal diabetes and obesity with increased risk for birth defects (Correa and others, 2008; Waller and others, 2007) prompted the addition of questions regarding fat consumption, dieting behavior, changes in weight, more detailed questions regarding diabetes management, and, in 2010, the addition of physical activity questions. In an effort to limit the interview to one hour, when new questions were added in 2006, other questions were removed. Specifically, questions about prenatal diagnoses, paternal drug use, and maternal assessment of occupational exposure to specific chemicals were excluded. A section on water use and consumption was significantly shortened, and combined with questions on use of a hot tub, Jacuzzi, or sauna.
Buccal cell collection
To better understand the role that genetic and epigenetic factors play in the etiologies of birth defects, cytobrushes were used to collect buccal (cheek) cell samples from NBDPS family triads (case- and control-infants and their parents). Following the completion of the interview, women were mailed a buccal cell collection kit that included collection instructions, documents for informed consent, six cytobrushes (two each for the mother, the child (if living), and the father), and a postage-paid return mailer. A monetary incentive ($20 money order or check) was added to each kit beginning in 1999 or 2000, depending on the Center. In 2002, the kits were redesigned to provide better clarity, organization, and convenience (Online Figure B). Buccal cell collection kits were returned by 19,065 case families and by 6,211 control families (65% and 59% of those who received the kits, respectively).
Participation in buccal cell collection
In 2002, two Centers with low buccal cell collection participation began sending families an additional $20 incentive following the return of completed buccal cell collection kits, which resulted in increased participation. The success of the additional incentive led to its implementation by all Centers by 2008. Addition of the third incentive was particularly beneficial at Centers with the lowest participation, especially among some racial-ethnic groups, and participation across Centers increased by an average of 13 and 11 percentage points in case and control families, respectively.
To assess women's attitudes toward participation in buccal cell collection, six focus group discussions were held in September 2007 with women residing in metropolitan Atlanta, Georgia who had completed the NBDPS interview and either did or did not complete buccal cell collection (Jenkins and others, 2009; Jenkins and others, 2011). Four of the focus groups were comprised solely of non-Hispanic black women because they were the racial-ethnic group with the lowest participation. Barriers to participation reported by focus group participants included concerns about how they were identified, government involvement, potential misuse of the DNA specimens, not disclosing individual genetic results, sterility of cytobrushes sent through the mail, and paternal skepticism. Motivations for participation included having a child affected by a birth defect, researcher credibility, non-invasive collection methods, monetary incentives, professional-style study materials, and positive experiences with study interviewers. To address some of the barriers, NBDPS study materials were updated with information about how infants were identified, and a website was developed that included a video of buccal cell collection and information on the use, storage, and disposal of DNA specimens. To alleviate concerns over cytobrush sterility, “Sterile if package is unopened” was added to the cytobrush packaging.
Glidewell and colleagues (Glidewell and others, 2013) examined factors associated with participation in buccal cell collection in the NBDPS. Buccal responders were more likely to be non-Hispanic white, older case mothers with higher education, higher household incomes, pregnancies that were intended, to have had a shorter interval between their EDD and interview date, to have received a redesigned buccal cell collection kit and an additional $20 incentive, to have consumed folic acid and not to have smoked cigarettes during the periconceptional period.
Buccal cell specimen quality
In response to concerns about DNA degradation during transit, cytobrushes packaged in closed plastic containers were replaced with cytobrushes packaged in paper-backed peel pouches in mid-2003. The impact of cytobrush packaging on the yield and quality of self-collected buccal cell-derived DNA was assessed (Gallagher and others, 2011; Krakowiak and others, 2003). Higher median human-specific DNA yields (1300ng vs. 60ng) and more successful short tandem repeat (STR) amplification rates (99.5% vs 59.5%) were observed when DNA was extracted from cytobrushes transported in the new pouches that allowed air-drying when compared to DNA extracted from cytobrushes transported in closed plastic containers.
To further improve DNA specimen quantity and quality, some families who previously submitted closed container cytobrushes were asked in 2006-2008 to collect new specimens using cytobrushes packaged in paper-backed peel pouches. Eligible families had pregnancies affected by spina bifida, gastroschisis, or longitudinal limb defects; and an equal number of control families were also invited. Return rates (families who returned kits /eligible families) varied by Center and case-control status (20%–83% for cases; 13%–63% for controls).
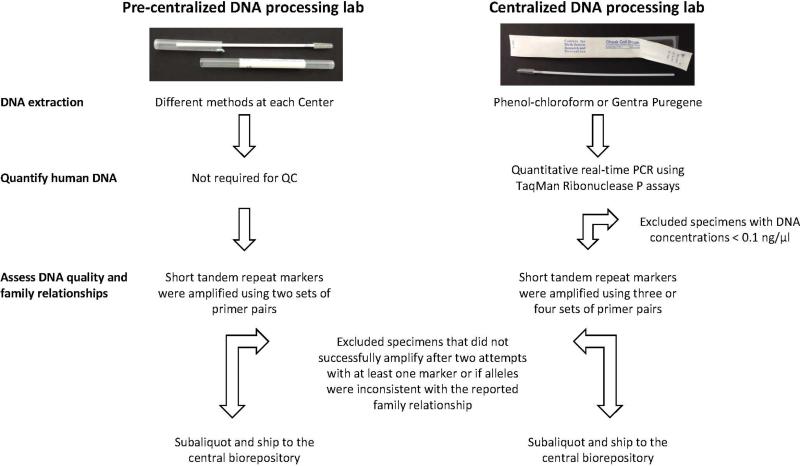
Centralized DNA processing laboratory
When DNA collection via cytobrushes was initially integrated into the NBDPS, DNA was extracted at each Center using different methods, and short tandem repeat (STR) markers were amplified using two sets of primer pairs (Figure 3). Quality control (QC) criteria included successful polymerase chain reaction (PCR) amplification after at least two attempts with at least one STR marker and alleles consistent with the reported family relationship. If those standards were met, DNA extracted from one cytobrush was divided into 10 or 20 aliquots of equal size and the aliquots were shipped to the central biorepository. DNA from the second cytobrush was retained at the local Center. In 2003, a centralized laboratory was established at the CDC to improve consistency and standardize extraction and QC methods (Figure 3). One cytobrush from each NBDPS participant was retained at the local Center, but the second cytobrush was sent to the centralized laboratory at CDC for DNA extraction using either a phenol-chloroform method (Garcia-Closas and others, 2001) or Gentra Puregene® (QIAGEN®); human genomic DNA (gDNA) yields were assessed by quantitative real-time PCR using the human RNaseP gene, and three or four STR markers were used to assess DNA quality. DNA specimens that met QC criteria (human gDNA concentration ≥0.1 ng/μl, successful PCR amplification after at least two attempts with at least one STR marker, and alleles consistent with the reported family relationship) were divided into two aliquots of equal size and shipped to the central biorepository. Both before and after establishment of the centralized lab, if a specimen did not meet QC criteria, the family was contacted and asked to provide additional specimens; however, due to low return rates and lack of resolution of the initial inconsistencies with recollected specimens, as of 2005, families whose alleles were not consistent with the reported family relationship (i.e. non-paternity) were not asked to provide additional specimens.
Figure 3.
Comparison of DNA extraction and quality control completed at each Center (pre-centralized DNA processing lab) and at the centralized DNA processing lab on samples that were sent to the repository.
External genotyping quality assessment
To ensure proficiency of laboratories genotyping NBDPS buccal-derived DNA specimens independent of source material, NBDPS genotyping laboratories annually genotyped a standard single nucleotide polymorphism (SNP) set on a subset of the Coriell Institute for Medical Research's Polymorphism Discovery Resource (PDR) DNA specimens (Collins and others, 1998). The SNP set was determined by the NBDPS Genetic Analysis Working Group and included SNPs assayed by multiple labs and at least one SNP with publicly available genotypes. Standard 96-well plates that include 86 PDR specimens, four replicates, two negative controls, and four empty wells for internal laboratory genotyping controls were plated by Coriell and sent directly to each NBDPS laboratory. Plate formats and content were changed yearly. Laboratories were blinded to specimen type and reported genotyping results centrally to the CDC for analysis. After implementation of annual external quality assessment (EQA), call rates of 97-100% were reported across NBDPS laboratories. Concordance of 99-100% was reported between NBDPS laboratories and between NBDPS laboratories and the publicly available genotypes.
To assure data quality across laboratories using arrays from higher throughput platforms, NBDPS genotyping laboratories ran assays on a set of paired cytobrush buccal- and whole blood-derived DNA samples from 6 parent-offspring trios ascertained through the University of Washington under an IRB-approved protocol, prior to implementation of each new project. Laboratories planning to use whole genome amplified (WGA) products were required to run assays using paired gDNA and WGA products. After implementation of annual EQA, call rates of 92-100% were reported across NBDPS genotyping laboratories. Concordance between paired blood-buccal samples was 100% pre- and post-WGA. Inter-laboratory concordance for variants assayed in common was 100%. Additionally, SNP genotyping was consistent with Mendelian inheritance.
Data management
NBDPS data were stored in three major database types: the clinical databases, containing the abstracted case data; the CATI databases; and the biologic sample databases. Centers transferred data to CDC monthly to ensure the central databases were current, and to reduce the chance of substantial data loss in case of database corruption. Each Center first deposited all three current databases on CDC's secure data network; then CDC programmers added any new data to the central databases and then placed updated files for each Center in their private folder on the secure data network.
Analysis of NBDPS data is on-going. Data are accessible to NBDPS collaborators through the Data Analysis Tools (DAT), a Microsoft Access database. The DAT is a centralized effort that serves to improve data quality and efficiency of analyses conducted by investigators in different Centers. The DAT enables NBDPS analysts in all Centers to create datasets that contain only those variables that will be used for an approved project. The DAT includes a data dictionary, which provides variable names, definitions, and formatting information. The DAT contains a set of calculated variables created by combining several interview questions, including maternal age at delivery, maternal body mass index, indicators for whether the mother used alcohol or cigarettes during the periconceptional period, maternal diabetes status, and use of folic acid supplements. The DAT does not contain any names, addresses or geocodes. A specific tool using the medication database from the Slone Epidemiology Center at Boston University allows analysts to create exposure variables for specific medications or all medications with a specific component, and to define the exposure period of interest for that medication. Data from the food frequency section, which pertained to the year before pregnancy, was used in combination with data from the United States Department of Agriculture to assign levels of nutrient and calorie intake with the assistance of North Carolina Department of Nutrition Clinical Research Center, Nutrition Epidemiology Core. Analysts communicate through monthly conference calls of the Epidemiologists/Analysts Committee, a listserv and a SharePoint site. And, as a quality control measure, each analysis is replicated by an independent analyst prior to submission for publication.
The Data Sharing Committee for NBDPS has two major responsibilities: the ongoing task of deciding how data will be equitably shared for analysis and providing abstract and manuscript review prior to submission to scientific meetings or journals. This committee consists of principal investigators or co-investigators from each Center. Obtaining approval to conduct an NBDPS analysis is a two-step process. First, a two-page letter of intent is submitted containing scientific background/justification, a brief summary of the analytical plan and any conflicts with existing NBDPS analytic projects. Upon approval by the Committee, the lead investigator then submits a more detailed proposal. The proposal must contain a detailed analytical plan, including power calculations. The Committee requires annual updates from the lead investigator on the progress of each project. As of December 2014, there were more than 520 proposed projects. Senior investigators are requested to serve as NBDPS data sharing editor for six-month periods of time, where they coordinate the anonymous review of all NBDPS manuscripts. Review of abstracts to be submitted to scientific meetings is coordinated at the CDC.
Investigators from outside NBDPS who are interested in analyzing NBDPS data must collaborate with one of the NBDPS principal investigators, or they can write a letter to the Data Sharing Committee describing their research interest. The committee will let the researcher know if their interest is already covered under an existing proposed project or will forward it to NBDPS researchers interested in that topic in order to establish a formal collaboration. Collaboration with external investigators in this manner is encouraged.
Expanded occupational and environmental data
Through an agreement with the CDC's National Institute for Occupational Safety and Health (NIOSH), industrial hygienist contractors at Battelle and occupational epidemiologists at the NIOSH have used the responses to the maternal occupation sections of the NBDPS CATI to create an occupational exposure database. The database includes the estimated frequency, intensity, and duration of job exposures for over 10,000 respondents. The occupational team estimated quantified exposure to occupational chemicals including pesticides, chlorinated and aromatic solvents, polycyclic aromatic hydrocarbons, and heavy metals, resulting in several publications ((Kielb and others, 2014; Lupo and others, 2012; Rocheleau and others, 2011)).
Combining residential locations with other geographic data may inform environmental exposures such as air pollution. Therefore the NBDPS questionnaire included a complete residential history, from three months before conception through the date of delivery. Women were asked to report, to the best of their ability, the exact street address of each residence. To ensure the application of a uniform data cleaning and geocoding method, CDC requested that all NBDPS residential address data be centrally geocoded by the Agency for Toxic Substances and Disease Registry's Geographic Research, Analysis and Services Program. To protect participant privacy, CDC returned geocoded address data to the respective Centers, but this data can be requested for specific projects (Stingone and others, 2014).
Limitations of NBDPS
There were several limitations of the NBDPS. The first is that, on average only about two-thirds of invited women participated, which could potentially introduce selection bias if nonparticipants had substantially different risk profiles from those who participated. NBDPS control participants were older, more often non-Hispanic white and more highly educated than non-participants (Cogswell and others, 2009). The fact that the inclusion of stillbirths and induced abortions changed over the data collection period is a limitation, as is the to-be-expected difference in participation based on the pregnancy outcome. Another limitation was that exposure information was self-reported. Recall of the timing and duration of exposures occurring up to 2 years in the past was challenging for some participants, especially for items requiring detailed information. Recall of use of over-the-counter and prescription medications during early pregnancy, is of particular concern. To optimize recall, women were first asked about specific diseases or medical conditions and then asked about medication use to treat conditions. In addition, women were asked about specific medications using a list of specific medication names, without reference to diseases or conditions. However, NBDPS did not capture information on medication dose, and for those medications that were reported in response to the medication list, there was no information about the indication for use. For some exposures, validation studies were conducted. One such project compared fertility treatment data reported by assisted reproductive technology clinics to NBDPS participants’ responses to questions about fertility treatment use (Liberman and others, 2014). Using the clinic data as the gold standard, the sensitivity of maternal report for use of in vitro fertilization (IVF) was 91%. Another validation study compared responses to CATI questionnaire items regarding smoking to data on birth certificates; smoking was more often reported in the NBDPS (Srisukhumbowornchai and others, 2012). Another study conducted a Bayesian bias analysis which incorporated previous research on reliability of self-reported smoking to examine the relationship of smoking and orofacial clefts reported from the NBDPS (Honein and others, 2007; MacLehose and others, 2009). Smoking recall bias has been raised as a concern in previous studies looking at the smoking-orofacial cleft association. After correcting for potential misclassification using bias modeling, this study found the likely levels of bias did not impact the relationship between smoking and orofacial clefts in NBDPS.
Lessons learned
The NBDPS has provided an unprecedented level of information for studying birth defects and for learning best practices for future birth defects research. A major strength of the study, in addition to its sample size, was the successful collaboration with multi-disciplinary teams across 10 Centers in the United States. Consistency of study methods across the sites, particularly use of the same case inclusion criteria and interview instrument allowed for the creation of a pooled dataset for analysis that is both large and internally consistent. Multiple levels of case review including a study-wide central review ensured consistency in determining case eligibility and classification. Providing centralized data management with monthly replication safe-guarded the data. One particularly beneficial lesson learned regarding buccal cell DNA collection was letting brushes air dry, which resulted in higher DNA yields and better quality DNA. A last key step in fostering this collaboration was establishing clear data sharing guidelines. Clear rationale for undertaking a project as well as critical feedback at the start of projects resulted in a more efficient review process and higher quality manuscripts.
The NBDPS has moved the field of birth defects epidemiology forward since its launch in 1996. Over 44,000 women with pregnancies in 1997-2011 participated in the study. To date, more than 350 interdisciplinary researchers have collaborated on more than 200 peer-reviewed manuscripts. The study has provided training opportunities where many masters-level students have learned about reproductive epidemiology working on NBDPS analyses and at least six doctoral dissertations have been completed using NBDPS data. And this is only the beginning. Papers on genetic risk factors for birth defects are beginning to be published using data from the NBDPS – and there are many more genetic, environmental, and gene-environment interaction analyses being planned. NBDPS will be a rich source of epidemiological and genetic data to study risk factors for birth defects for many years to come.
Supplementary Material
Online only Figure A. Maps of the included regions in each of the ten Centers for Birth Defects Research and Prevention.
Footnote: In New Jersey there was sampling of the most common defects in the early years of the study.
Online only Figure B. The first informational and buccal collection packet (2003) and the redesigned informational and buccal collection packet with consistent graphical elements.
Acknowledgments
We thank the many families who have contributed their time and efforts to further the scientific understanding of the causes and preventives of human birth defects. We also thank the many public health programs who have contributed to the success of the NBDPS. These include: Arkansas Department of Health; California Department of Public Health Maternal Child and Adolescent Health Division; Georgia Department of Public Health and the Metropolitan Atlanta Congenital Defects Program; Iowa Department of Public Health (Iowa Registry for Congenital and Inherited Disorders); Massachusetts Department of Public Health; North Carolina Department of Health and Human Services; New Jersey Department of Health; New York State Department of Health (Congenital Malformations Registry); Texas Department of State Health Services (Birth Defects Epidemiology and Surveillance Branch); Utah Department of Health (Utah Birth Defect Network)
Financial Support
The Centers for Disease Control and Prevention provided funding for the National Birth Defects Prevention Study and for the analysis of data from this study.
Funds for part of the nutrient database work were provided by NIH DK56350 granted to the University of North Carolina Department of Nutrition Clinical Research Center, Nutrition Epidemiology Core.
Coding of drug information in NBDPS used the Slone Drug Dictionary, under license from the Slone Epidemiology Center at Boston University, Boston, MA.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A, NBDPS Seeking causes: classifying and evaluating congenital hearts defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714–727. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Yang W, Abrams B, Lammer EJ. Maternal stressful life events and risks of birth defects. Epidemiology. 2007;18(3):356–361. doi: 10.1097/01.ede.0000259986.85239.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978-2005. MMWR Morb Mortal Wkly Rep. 2008;57(1):1–5. [PubMed] [Google Scholar]
- Cogswell ME, Bitsko RH, Anderka M, Caton AR, Feldkamp ML, Hockett Sherlock SM, Meyer RE, Ramadhani T, Robbins JM, Shaw GM, Mathews TJ, Royle M, Reefhuis J. Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. Am J Epidemiol. 2009;170(8):975–985. doi: 10.1093/aje/kwp226. [DOI] [PubMed] [Google Scholar]
- Collins FS, Brooks LD, Chakravarti A. A DNA polymorphism discovery resource for research on human genetic variation. Genome Res. 1998;8(12):1229–1231. doi: 10.1101/gr.8.12.1229. [DOI] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, Cleves MA, Riehle-Colarusso TJ, Waller DK, Reece EA. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237, e231–239. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy--a review. Neurosci Biobehav Rev. 2005;29(2):295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Gallagher ML, Sturchio C, Smith A, Koontz D, Jenkins MM, Honein MA, Rasmussen SA. Evaluation of mailed pediatric buccal cytobrushes for use in a case‐control study of birth defects. Birth Defects Res A Clin Mol Teratol. 2011;91(7):642–648. doi: 10.1002/bdra.20829. [DOI] [PubMed] [Google Scholar]
- Garcia-Closas M, Egan KM, Abruzzo J, Newcomb PA, Titus-Ernstoff L, Franklin T, Bender PK, Beck JC, Le Marchand L, Lum A, Alavanja M, Hayes RB, Rutter J, Buetow K, Brinton LA, Rothman N. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10(6):687–696. [PubMed] [Google Scholar]
- Glidewell J, Reefhuis J, Rasmussen SA, Woomert A, Hobbs C, Romitti PA, Crider KS. Factors affecting maternal participation in the genetic component of the National Birth Defects Prevention Study -- United States, 1997-2007. Genet Med. 2013;16(4):329–337. doi: 10.1038/gim.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D, Lou HC, Olsen J. Serious life events and congenital malformations: a national study with complete follow-up. Lancet. 2000;356(9233):875–880. doi: 10.1016/S0140-6736(00)02676-3. [DOI] [PubMed] [Google Scholar]
- Honein MA, Rasmussen SA, Reefhuis J, Romitti PA, Lammer EJ, Sun L, Correa A. Maternal smoking and environmental tobacco smoke exposure and the risk of orofacial clefts. Epidemiology. 2007;18(2):226–233. doi: 10.1097/01.ede.0000254430.61294.c0. [DOI] [PubMed] [Google Scholar]
- Jenkins MM, Reed-Gross E, Rasmussen SA, Barfield WD, Prue CE, Gallagher ML, Honein MA. Maternal attitudes toward DNA collection for gene-environment studies: a qualitative research study. Am J Med Genet A. 2009;149A(11):2378–2386. doi: 10.1002/ajmg.a.33043. [DOI] [PubMed] [Google Scholar]
- Jenkins MM, Reed-Gross E, Barfield WD, Prue CE, Gallagher ML, Rasmussen SA, Honein MA. Qualitative assessment of study materials and communication strategies used in studies that include DNA collection. Am J Med Genet A. 2011;155(11):2721–2731. doi: 10.1002/ajmg.a.34263. [DOI] [PubMed] [Google Scholar]
- Kielb C, Lin S, Herdt-Losavio M, Bell E, Chapman B, Rocheleau CM, Lawson C, Waters M, Stewart P, Olney RS, Romitti PA, Cao Y, Druschel C. Maternal periconceptional occupational exposure to pesticides and selected musculoskeletal birth defects. Int J Hyg Environ Health. 2014;217(2-3):248–254. doi: 10.1016/j.ijheh.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Guan Y, Greenhaw J, Freeman W, Kimbrough J, Lyle R, Zhao W, Cleves M, Hobbs C. Quality of human DNA from buccal cytobrushes depends on storage conditions; 2003. Univ Chicago Press; 1427 e 60th st, Chicago, IL 60637-2954 USA: p. 411. [Google Scholar]
- Liberman RF, Stern JE, Luke B, Reefhuis J, Anderka M. Validating assisted reproductive technology self-report. Epidemiology. 2014;25(5):773–775. doi: 10.1097/EDE.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo PJ, Langlois PH, Reefhuis J, Lawson CC, Symanski E, Desrosiers TA, Khodr ZG, Agopian AJ, Waters MA, Duwe KN, Finnell RH, Mitchell LE, Moore CA, Romitti PA, Shaw GM, Study NBDP Maternal occupational exposure to polycyclic aromatic hydrocarbons: effects on gastroschisis among offspring in the National Birth Defects Prevention Study. Environ Health Perspect. 2012;120(6):910–915. doi: 10.1289/ehp.1104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLehose RF, Olshan AF, Herring AH, Honein MA, Shaw GM, Romitti PA, National Birth Defects Prevention Study Bayesian methods for correcting misclassification: an example from birth defects epidemiology. Epidemiology. 2009;20(1):27–35. doi: 10.1097/EDE.0b013e31818ab3b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, Church DM, Crolla JA, Eichler EE, Epstein CJ, Faucett WA, Feuk L, Friedman JM, Hamosh A, Jackson L, Kaminsky EB, Kok K, Krantz ID, Kuhn RM, Lee C, Ostell JM, Rosenberg C, Scherer SW, Spinner NB, Stavropoulos DJ, Tepperberg JH, Thorland EC, Vermeesch JR, Waggoner DJ, Watson MS, Martin CL, Ledbetter DH. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86(5):749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K, Holmes LB. Malformations due to presumed spontaneous mutations in newborn infants. N Engl J Med. 1989;320(1):19–23. doi: 10.1056/NEJM198901053200104. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA, the National Birth Defects Prevention Study Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67(3):193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- Rocheleau CM, Lawson CC, Waters MA, Hein MJ, Stewart PA, Correa A, Echeverria D, Reefhuis J. Inter-rater reliability of assessed prenatal maternal occupational exposures to solvents, polycyclic aromatic hydrocarbons, and heavy metals. J Occup Environ Hyg. 2011;8(12):718–728. doi: 10.1080/15459624.2011.627293. [DOI] [PubMed] [Google Scholar]
- Srisukhumbowornchai S, Krikov S, Feldkamp ML. Self-reported maternal smoking during pregnancy by source in Utah, 2003–2007. Birth Defects Res A Clin Mol Teratol. 2012;94(12):996–1003. doi: 10.1002/bdra.23058. [DOI] [PubMed] [Google Scholar]
- Stingone JA, Luben TJ, Daniels JL, Fuentes M, Richardson DB, Aylsworth AS, Herring AH, Anderka M, Botto L, Correa A, Gilboa SM, Langlois PH, Mosley B, Shaw GM, Siffel C, Olshan AF. Maternal Exposure to Criteria Air Pollutants and Congenital Heart Defects in Offspring: Results from the National Birth Defects Prevention Study. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307289. E-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, Schoenmakers EF, Brunner HG, Veltman JA, van Kessel AG. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36(9):955–957. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, Siega-Riz AM, Gallaway MS, Correa A. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161(8):745–750. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- Xu J, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief. 2014;(168):1–8. [PubMed] [Google Scholar]
- Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael SL, Costa P, Druschel C, Hobbs CA, Romitti PA, Langlois PH, Edmonds LD. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online only Figure A. Maps of the included regions in each of the ten Centers for Birth Defects Research and Prevention.
Footnote: In New Jersey there was sampling of the most common defects in the early years of the study.
Online only Figure B. The first informational and buccal collection packet (2003) and the redesigned informational and buccal collection packet with consistent graphical elements.