Abstract
Most invasive breast cancers arise from ductal carcinoma in-situ (DCIS), a non-obligate precursor of invasive breast cancer. Given that the natural history of individual DCIS lesions is unpredictable, many women with DCIS receive extensive treatments, which may include surgery, radiation and endocrine therapy, even though many of these lesions may have limited potential to progress to invasion and metastasize. In contrast to valid concerns about over-treatment, the fact that invasive breast cancers outnumber DCIS lesions by more than three-to-one, suggests that many cancer precursors (particularly DCIS, but LCIS also) progress to invasion prior to detection. Thus, DCIS poses a dual problem of over diagnosis among some women and failure of early detection among others. These concerns are heightened by the multi-fold increase in rates of DCIS in conjunction with widespread use of mammographic screening and access to outpatient radiologically-guided biopsies. Accordingly, methods are needed to both specifically detect and identify DCIS lesions with potential to progress to invasive cancer and to discover techniques to triage and conservatively manage indolent cases of DCIS.
Keywords: Ductal carcinoma in-situ, Lobular carcinoma in-situ, epidemiology, pathogenesis, biomarkers
Introduction
Controversies about the clinical significance of breast cancer precursors generally, and ductal carcinoma in-situ (DCIS) specifically, lie at the heart of a larger debate about breast cancer screening, prevention, treatment and use of the term “carcinoma” in reference to precursors [6, 19-20, 41, 45]. The term indolent lesions of epithelial origin (“IDLE”) has been proposed as an alternative to DCIS in an effort to highlight the non-lethality of these lesions when successfully treated [19]. Although detection of DCIS has increased multifold with increased screening, rates of invasive cancer have declined only minimally, and the ratio of invasive to in-situ breast carcinomas in the U.S. exceeds three-to-one. Thus, increased radiological detection has likely led to “overdiagnosis” (detection of disease that lacks clinical significance), but has had a smaller impact on prevention of invasive cancer through detection and eradication of precursors [63]. Intensive screening may lead to earlier detection of DCIS, but if a lesion is not destined to progress to invasion, this will not substantially alter outcomes (i.e., lead time bias). However, given that the fate of individual lesions is unpredictable, many women opt for aggressive management, even at the risk of overtreatment. Accordingly, developing methods of detecting DCIS lesions with the greatest potential to progress to lethal invasive carcinoma, better estimating the risk of progression for specific DCIS lesions, and discovering methods of preventing or treating DCIS with fewer adverse effects are needed. Molecular epidemiological studies of DCIS can contribute to reaching these goals; however, conducting this research is challenging and will require trans-disciplinary approaches.
Reflecting this perspective, we summarize current knowledge about the molecular pathological and epidemiological characteristics of DCIS and present ideas and considerations for research in the following inter-related areas: 1) descriptive epidemiology; 2) etiology, 3) detection and diagnosis and 4) pathogenesis, molecular characterization and clinical behavior. This review will focus on conceptual issues in DCIS research. Readers are referred to an exhaustive review and publicly available white paper for detailed epidemiological and clinical data [62-63].
Overview of diagnosis of DCIS
DCIS arises within terminal duct lobular units (TDLUs), which are hormonally responsive, physiologically active structural units that produce milk. DCIS is an abnormal epithelial proliferation confined to pre-existing basement membrane-bound spaces, generally consisting of large cells with variable degrees of nuclear pleomorphism [49, 57] (Figure 1). Pure DCIS is curable if treated, whereas some invasive breast cancers metastasize and kill patients despite therapy. DCIS is routinely distinguished clinically from the related, numerically less frequent lesion, lobular carcinoma in-situ (LCIS) by light microscopy, although loss of e-cadherin immunohistochemical expression in LCIS is also characteristic [49, 57].
Figure 1.
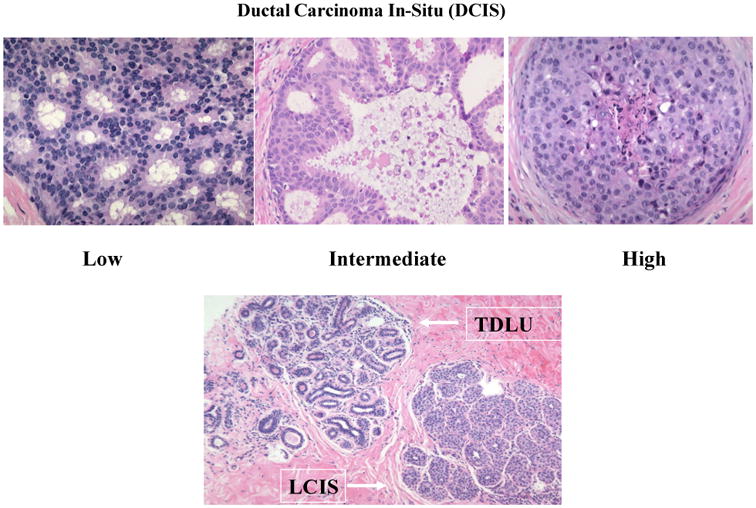
Top panel: Ductal carcinoma in-situ (DCIS) low-, intermediate and high-grade. Lower panel: Terminal duct lobular unit (TDLU) containing numerous small acini within specialized stroma (upper left); lobular carcinoma in-situ (LCIS) with filling of acini by a monotonous proliferation of small cells (lower right).
DCIS is definitively diagnosed by microscopic examination, which most often occurs on a biopsy performed to investigate suspicious mammographic calcifications. In contrast to LCIS, which is typically composed of small uniform cells, DCIS grows in multiple architectural patterns, even within the same breast, and can be characterized as low-, intermediate- or high-grade based on severity of nuclear abnormalities. High-grade DCIS with comedo-type necrosis (named because of its resemblance to comedones) is composed of mitotically active cells with extremely abnormal appearing nuclei growing in solid arrangements with central necrosis and dystrophic calcification. This type of DCIS produces the most striking abnormalities on mammograms.
Several types of evidence establish DCIS as an invasive breast cancer precursor: in the absence of other putative precursors (e.g. LCIS), DCIS is usually identified immediately adjacent to or engulfed by invasive breast cancer in thoroughly examined cases and the in-situ and invasive components share common morphological and molecular characteristics; invasive breast cancers that develop years after DCIS removal tend to occur near the site of the previously excised DCIS, and case series demonstrate that a substantial percentage of women with untreated DCIS develop invasive breast cancer, generally in the same quadrant as the original biopsy (reviewed in [18]). However, DCIS is not an obligate cancer precursor and does not invariably progress to invasion within a woman's lifetime. In autopsy studies, occult DCIS is detected in a median of 8.9% of women [67], supporting the view that occult DCIS lesions that do not manifest clinically during women's lifetimes are common [66].
Topics for future research
Descriptive epidemiology of DCIS and other putative breast cancer precursors
Reported DCIS incidence rates in the U.S. increased sevenfold from 1980 to 2004, paralleling the dramatic increase in mammographic screening, with notable recent increases in detection of non-comedo types (Figure 2) [62-63]. In 2012, approximately 63,300 women were diagnosed with in-situ carcinoma as compared with 226,870 women diagnosed with invasive breast cancer [3]. Like rates of invasive cancer, DCIS rates are lower among Black women than White women. Rates of “benign breast disease”, including various forms of proliferative changes (non-atypical ductal hyperplasia, sclerosing adenosis) and atypical ductal hyperplasia (ADH) and atypical lobular hyperplasia (ALH), which are not recorded in U.S. registries, also seem to have risen as a consequence of increasing performance of biopsies [12, 17, 25, 29, 36, 50].
Figure 2.
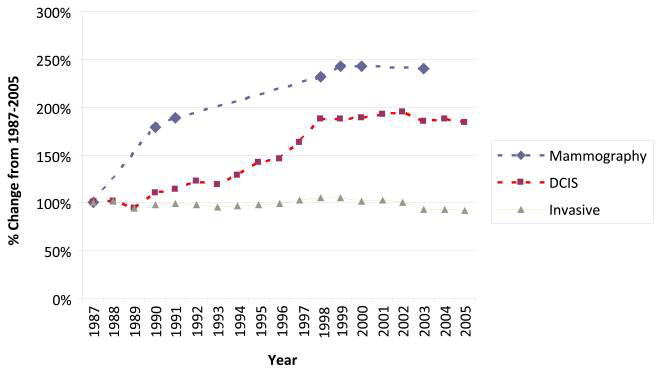
Rates of mammography and DCIS in the U.S. rose dramatically from the late 1980s through the 1990s and then plateaued, whereas rates of invasive breast cancer showed comparatively small changes over this period.
Reported “incidence rates” of DCIS or LCIS are probably better viewed as “rates of diagnosis” because some (although not all) of these lesions are likely present for years prior to detection. For example, 3% of women with a new diagnosis of DCIS harbor a contralateral neoplastic lesion (usually DCIS), which is identifiable by magnetic resonance imaging (MRI), but not by mammography [35]. Thus, newly diagnosed cases represent a mixture of incident and prevalent disease. Although invasive breast carcinomas also represent a mixture of incident and prevalent cases (some present for years prior to diagnosis), radiological detection of invasive carcinomas, which tend to form masses, may be easier than identification of DCIS, which is generally identified through the recognition of abnormal patterns of microcalcifications, which may be masked by dense tissue. Furthermore, many ducts containing DCIS do not show calcifications and remain radiologically occult, and sometimes none of the ducts in a given case shows microscopic calcifications.
The rising rates of DCIS diagnosis (and other benign breast diseases) reflects (primarily) an increase in the number of women screened and widespread adoption of digital mammography, which detects more DCIS than film-based methods in most studies [7, 14-15, 30, 46, 55, 60]. Temporal changes in screening methods and the prevalence of exposures related to breast cancer risk may affect the reported incidence of DCIS and other lesions.
Monitoring temporal trends in DCIS rates may provide useful public health information. Increased detection of clinically significant forms of DCIS should presage decreasing rates of invasive cancer, all else being equal. Moreover, assessment of DCIS rates, stratified by age, race, grade, molecular markers and mammographic density may provide useful etiological insights, complementing data for invasive breast cancer [2, 37, 69] (see “Pathogenesis, molecular characterization and clinical behavior of DCIS” below). Accounting for screening frequency is important in assessing rates of DCIS.
ER negative cancers generally, and basal breast cancers specifically, are more common among Black women than White women [4, 42]. However, data related to rates of DCIS and invasive carcinoma stratified by race and ER status have not been fully explored and could shed light on contributions of etiological factors and early detection to the excessive invasive ER negative breast cancer rates among Blacks. Specifically, it is unknown whether Blacks have proportionately higher rates than Whites of specific molecular subtypes of DCIS, such as ER negative, Human Epidermal Growth Factor 2 (HER2) amplified or basal.
Monitoring rates of DCIS through population-based registries may be important for health services research and etiological investigations; however, substantial resources may be required, particularly if pathology panel reviews and assessment of molecular markers is warranted. Advances in digital pathology and the development of a virtual registry might make this work affordable.
Etiology of DCIS
A meta-analysis has shown that a positive family history of breast cancer, elevated mammographic density and several lifestyle factors that have been linked to increased risk of invasive breast cancer share similar associations with risk of DCIS [62-63]. Recently, the Million Women Study, which focused on analysis of postmenopausal women, reported similar results, apart from the finding that obesity was associated only with increased risk of invasive breast cancer [47]. Overall, these data suggest that most breast cancer risk factors act at the pre-invasive stage of carcinogenesis.
Clarifying etiological associations for DCIS may enable better estimation of risk and provide women with useful guidance about screening and prevention [11].
Developing large, adequately powered analyses of risk factors for DCIS may be challenging, particularly in prospective studies in which temporal changes in the prevalence of exposures and detection methods may influence associations. Pooling data across studies may overcome this limitation, if datasets are comparable.
Detection of DCIS
Most DCIS is currently detected by mammography, manifesting generally as clustered calcifications, and less commonly as a non-palpable mass within the breast (Figure 3) [7, 15, 30, 46, 55, 60]. The predominance of invasive cancer within screening populations suggests that a significant number of DCIS (and LCIS, which is typically radiologically occult) lesions are not identified by imaging. Although MRI may detect some mammographically occult lesions, this modality is not cost-effective or practical for general screening.
Figure 3.
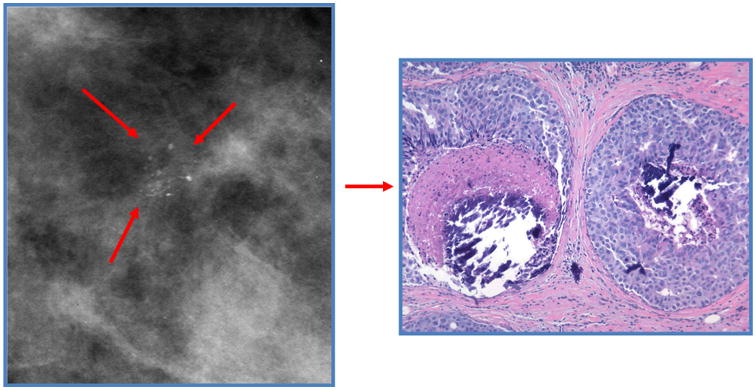
Left: Mammogram showing clustered microcalcifications typical of DCIS.
Right: histological appearance of DCIS with comedonecrosis and microcalcifications.
Most DCIS lesions arise within regions of high mammographic density [58], and pathologists have recognized for many years that DCIS lesions are sometimes surrounded by stromal reactions that could increase density in breast tissues immediately surrounding the lesion. This observation may have two disparate implications: 1) elevated regional density may mask sentinel calcifications related to DCIS and reduce detection and 2) characterizing the changes surrounding DCIS may lead to the recognition of a radiological “footprint” that is larger than the lesion itself, and therefore more readily identified. Preliminarily, we have found support for the hypothesis that peri-lesional mammographic density is increased around some DCIS lesions relative to that of the entire breast, suggesting that analysis of regional density may represent a fruitful avenue for improving detection (Figure 4) [23, 40]. Irrespective of future confirmation of this specific finding, the proposal that identifying contextual features may help identify DCIS is suggested by the histopathologic observation that many such lesions show stromal changes surrounding involved ducts. This approach could also help avert questionable biopsies if patterns of regional mammographic density are specific for neoplastic lesions.
Figure 4.
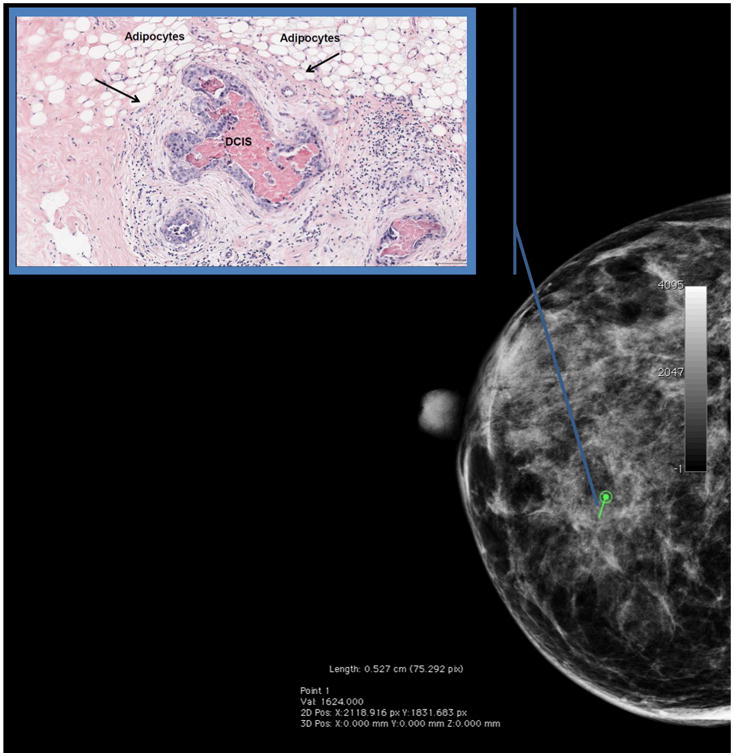
Peri-lesional mammographic density surrounding a DCIS lesion is increased relative to that of the entire breast (here, peri-lesional fibroglandular volume=85% and overall fibroglandular volume for the entire breast=68%). To compute peri-lesional mammographic density, the radiologist recorded the biopsy location and radius of the biopsy target (noted in green) on a craniocaudal view of the pre-biopsy digital mammogram. Percent dense fibroglandular volume was estimated using single x-ray absorptiometry at a volume twice the size of but excluding the biopsy target, centered at the biopsy site. We suggest that large differences between peri-lesional and total volumetric mammographic density may be associated with DCIS.
Inset: A hematoxylin and eosin stained tissue section of the biopsy target from the same patient shows that a central area of DCIS with necrosis is surrounded by a rim of reactive stroma. In a study of women who were clinically referred for an image-guided breast biopsy at the University of Vermont Breast Cancer Surveillance Consortium site, we are currently investigating whether peri-lesional mammographic density is a useful predictor of underlying pathology.
Advances in three-dimensional breast imaging may enable alignment and serial analysis of specific breast regions over time. Specifically, retrospective analyses of negative screening mammograms may facilitate the discovery of radiological signatures that were present at the site where an invasive cancer developed before it was detected. The diagnostic utility of these radiological findings could be prospectively validated by performing research biopsies of regions showing these changes among women with otherwise negative imaging. If successful, this strategy could also provide access to tissues from a class of precursor lesions of great interest: those missed by conventional radiological criteria that are likely to progress to invasion. Studying DCIS associated with invasive cancer may provide clues as to how to improve detection of such lesions. These analyses might also help reduce the need to biopsy equivocal lesions by defining serial radiological patterns that predict low risk of invasive carcinoma.
Diagnosis of DCIS
Pathologists can identify the vast majority of DCIS lesions reliably, with some variability in the distinction of low-grade DCIS from ADH, which is morphologically similar, tiny (1-2 mm in size) and reflects a borderline change along a morphological continuum [48, 53]. Other routine pathological assessments of DCIS include nuclear grading, size estimation, assessment of nearest approach to specimen (surgical) margin and excluding microinvasion.
Web-based studies assessing reproducibility of the diagnoses of breast cancer precursors that include community pathologists may advance research aimed at improving classifications. A prior web-based cervical cytology project provides may provide a model [54] and a project related to this topic is ongoing (Breast Pathology study, “BPath”).
Pathogenesis, molecular characterization and clinical behavior of DCIS
Over 100 years ago, surgeons described the development of DCIS, based on micro- and macroscopic observations, and its clinical behavior through case reports (reviewed in [49]). Subsequently, morphological observations linked the development of breast cancer precursors to changes in TDLUs [68] and clinically meaningful categories of benign breast disease were proposed [17]. Modern models of breast carcinogenesis generally represent adaptations of the stem cell hypothesis of carcinogenesis, which suggests that cancers arise from alterations in a minor population of pluripotential cells, or the stochastic hypothesis of carcinogenesis, which supposes that cancers arise from a wide range of cell types. Viable models of breast carcinogenesis must account for the development of multiple tumor types [8, 24] (Figure 5). Some theories propose that DCIS and invasive cancer develop in parallel; others suggest that invasive cancers arise from subclones of DCIS which have gained a growth advantage, acquired the ability to invade through the basement membrane, interact synergistically with the microenvironment and form a mass from which cells gain access to lymphatics and metastasize [1].
Figure 5. The molecular pathology of breast cancer progression.
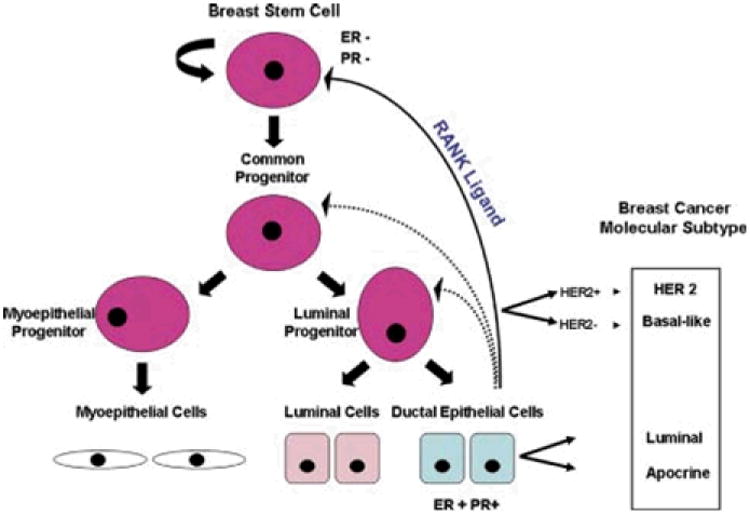
Left: Model of normal breast development. Right: Origin of different molecular subtypes of breast cancer. Stem cells give rise to common progenitor cells that in turn develop into luminal and myoepithelial cells, the two fundamental cell types within the terminal duct lobular units. Progesterone may influence secretion of RANK Ligand from progesterone receptor positive cells, promoting expansion of self-renewing stem cell pools. Transformation of normal cells may give rise to luminal A or B, basal or human epidermal growth factor 2 breast cancers. From: Bombonati A and Sigroi DC, “The molecular pathology of breast cancer progression”, J Pathol 2001; 223:307-17 (38).
Molecular studies of DCIS have used cell cultures, animal models and human tissues. In Mammary Architecture and Microenvironment Engineering studies, visual examination of cultures shows that invasion is associated with formation of structures in which proliferating DCIS cells incorporate fibroblasts that may collaborate in proteolytic digestion of stroma [51]. Similarly, other research points to interactions between fibroblasts and DCIS epithelial cells through COX-2 pathways, suggesting it may be possible to prevent or treat DCIS with anti-inflammatory agents [27]. Other data suggest that TH1 versus TH2 cellular immune responses may be related to the biology of certain forms of DCIS [32] or that testing for circulating auto-antibodies to novel tumor antigens may have value in detection [33, 39].
Limited data suggest that the process of “invasion” and metastasis may proceed biologically before its recognition by routine microscopy. Based on molecular analyses, putative tumor cells have been found in bone marrow of animals with ADH and in a subset of women with DCIS [28, 52]. Using molecular assays, metastasis have been found in sentinel lymph nodes of 4% of women diagnosed with DCIS, although in some of these cases, further sectioning of the breast revealed occult invasion [44]. These observations raise questions about whether the metastatic niche is primed prior to or coincidentally with the onset of invasion and support the view that both DCIS biology and systemic factors may be determinants of whether a DCIS lesion will give rise to an invasive metastatic carcinoma. Research that focuses exclusively on the DCIS lesion rather than DCIS in a systemic context may not identify these potential progression factors.
DCIS biomarker data are largely derived from small, cross-sectional comparisons of convenience samples of DCIS and invasive carcinoma, either co-occurring within patients or identified in different patients. Analyses of mRNA chip-based profiling, RNA sequencing and miRNAs consistently identify markers that distinguish invasive tumors from DCIS; however, the molecular profile of DCIS and carcinoma overlap considerably [10, 31, 38, 61]. Thus, cross-sectional analyses of DCIS likely include lesions with invasive potential, but once treated, this is impossible to prove. Moreover, although DCIS is often a regional process, intra-lesional heterogeneity is common, and subclones that most resemble invasive carcinoma may be in the minority [26, 43]. This complexity may affect efforts to molecularly subtype DCIS.
DCIS is divisible into molecular subclasses similar to those described for invasive cancers, with some evidence that HER2 positive DCIS is relatively more common and basal DCIS comparatively less common [61]. Molecular profiling of DNA and RNA extracted from DCIS and invasive breast cancer show similar characteristic patterns, consistent with the view that some examples of the former represent direct tissue precursors of the latter [61]. Similarly, molecular data also support the status of some LCIS lesions as a direct tissue precursors of invasive cancer [59].
The way DCIS is treated critically determines risk of recurrence. For example, one study found recurrence rates with mastectomy of 2.1%, excision alone 30.1% and excision plus radiation of 13.0% [13]. Data also suggest that a margin distance of at least 2 mm provides maximal protection [16], but retrospective ascertainment of margin status is difficult because methods of margin evaluation and reporting vary, as does the use of these data to determine management (i.e. re-excision, radiation or completion mastectomy) [16]. Thus, conducting biomarker studies to define best management strategies for DCIS based on molecular characteristics is challenging.
Lari et al. comprehensively summarized the literature on the prognostic value of immunohistochemical markers in DCIS, but was unable to find strong validation for any described to date [34]. ER positive DCIS was linked to low histological grade, negative HER2 and p53 status, and positive bcl2 expression. DCIS recurrence has been linked to larger lesion size, positive margins, symptomatic presentation (as opposed to mammographic detection), elevated mammographic density, higher grade, multi-focality and treatment by excision without radiation [64-65]. A recent analysis to predict breast recurrence among women with DCIS treated by excision alone using an algorithm based on mRNA expression of selected genes found an odds ratio (OR)=2.31 for recurrent DCIS and OR=3.68 for invasive breast cancer [56]. Confirmation and extension of these findings is needed. Developing a molecular taxonomy of DCIS may be useful for advancing etiological and clinical research, but accomplishing this feat poses challenges, as discussed below.
Unlike invasive breast cancers, which form masses, DCIS is identified clinically by microscopic examination of formalin fixed paraffin embedded tissues, in which it is surrounded by non-lesional epithelium and stroma. Approaches for detailed molecular characterization of DCIS include microdissection and tissue mapping using immunohistochemical or immunoflourescent staining for proteins with topographic retention of DCIS within its stromal microenvironment [5, 9, 21]. Interpretation of mRNA levels of genes that are considered non-epithelial is complex in microdissected samples because distinguishing stromal “contamination” from epithelial mesenchymal transitions associated with invasion may be ambiguous. Immunohistochemistry is best suited for studying small numbers of targets.
Novel molecular methods that provide high throughput data using small samples of fixed cells will be important for biomarker discovery in DCIS [22]. Given that many histopathological and molecular markers are highly correlated, large sample sets may be needed to identify markers that are independently associated with risk factors or clinical outcomes.
Obstacles to studying the natural history of DCIS (and other lesions) include: alteration/removal by diagnostic biopsy and adjuvant treatment; imperfect diagnostic reproducibility; incomplete knowledge of co-factors for progression and detection biases related to heightened post-biopsy surveillance. Few women with DCIS are treated by excision only (not the standard of care), and thus using such lesions for biomarker discovery may not be representative of most DCIS. In addition, recurrences following standard treatment are uncommon, data related to site of recurrence (laterality, quadrant) are often missing and accessing tissues from pairs of initial and recurrent lesions is difficult. DCIS is rarely excised and mapped in a manner that allows detailed appreciation of the extent of disease [57]. Finally, studying pure examples of DCIS focuses attention on lesions that were discovered early (prior to invasion), ignoring those that progressed to invasion, often despite screening. The latter are the most problematic and dangerous. New inter-disciplinary studies that integrate epidemiology, radiology and pathology (see above) may help identify new forms of high-risk lesions prior to invasion. Analysis of DCIS associated with microinvasion or small associated invasive carcinomas may also provide information about biomarkers of progression.
Identifying factors associated with progression of DCIS and putative precursors may be useful, especially if they can be readily modified. Analyzing risk factors of women who develop cancers despite receiving treatment in chemoprevention trials may provide etiological insights, as would comparisons of responders and non-responders in short-term pre-surgical treatment studies of DCIS. In addition, women diagnosed with ADH or ALH who have undergone minimal surgery represent a growing pool of potential participants in prevention research. Perhaps the nexus of early detection and prevention can affect a reduction in breast cancer mortality while avoiding unnecessary treatments.
Breast carcinoma precursors: Summary
Our limited understanding of breast carcinogenesis is a barrier to cost-effective screening, prevention and management. Multi-disciplinary approaches are central in clinical management of breast diseases and offer hope for accelerating translational research. A greater emphasis on studies that incorporate imaging and collection of pre-diagnostic samples are needed to better understand the epidemiology of DCIS lesions as they develop and progress. Concerns related to detection of clinically insignificant cases of DCIS (“overdiagnosis”) are growing [19] and magnified by many patients' perceptions that DCIS is a form of cancer that implies substantial risk of lethality and our limited ability to match management strategies to clinical risk in individual patients. However, current screening techniques also miss a substantial number of DCIS lesions prior to invasion. Progress will require both scientific advances and increased understanding of the significance of different putative precursors by both health care workers and patients. Recognizing that clinical outcomes reflect a dynamic, complex interplay between patient factors and the intrinsic biological characteristics of lesions favors the inter-disciplinary model in which researchers with complementary expertise exchange ideas and pool efforts to improve patient care and develop public health policies.
Acknowledgments
Supported in part with funding from the National Cancer Institute, Intramural Research Program and funding from the Breast Cancer Stamp Act.
Footnotes
Authors have do not have relevant conflict of interest statements to report.
References
- 1.Allred DC, Wu Y, Mao S, Nagtegaal ID, Lee S, Perou CM, Mohsin SK, O'Connell P, Tsimelzon A, Medina D. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14:370–378. doi: 10.1158/1078-0432.CCR-07-1127. [DOI] [PubMed] [Google Scholar]
- 2.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- 3.I. American Cancer Society. Breast Cancer Facts & Figures 2011-2012 [Google Scholar]
- 4.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103:1397–1402. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck AH, Sangoi AR, Leung S, Marinelli RJ, Nielsen TO, van de Vijver MJ, West RB, van de Rijn M, Koller D. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci Transl Med. 2011;3:108ra113. doi: 10.1126/scitranslmed.3002564. [DOI] [PubMed] [Google Scholar]
- 6.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 7.Bluekens AM, Holland R, Karssemeijer N, Broeders MJ, den Heeten GJ. Comparison of digital screening mammography and screen-film mammography in the early detection of clinically relevant cancers: a multicenter study. Radiology. 2012;265:707–714. doi: 10.1148/radiol.12111461. [DOI] [PubMed] [Google Scholar]
- 8.Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol. 2011;223:307–317. doi: 10.1002/path.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 10.Castro NP, Osorio CA, Torres C, Bastos EP, Mourao-Neto M, Soares FA, Brentani HP, Carraro DM. Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma. Breast Cancer Res. 2008;10:R87. doi: 10.1186/bcr2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4:567–571. [PubMed] [Google Scholar]
- 12.Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. Magnitude and laterality of breast cancer risk according to histologic type of atypical hyperplasia: results from the Nurses' Health Study. Cancer. 2007;109:180–187. doi: 10.1002/cncr.22408. [DOI] [PubMed] [Google Scholar]
- 13.Cutuli C, Cohen-Solal-Le Nir C, De Lafontan B, Mignotte H, Fichet V, Fay R, Servent V, Giard S, Charra-Brunaud C, Auvray H, Penault-Llorca F, Charpentier JC. Ductal carcinoma in situ of the breast results of conservative and radical treatments in 716 patients. Eur J Cancer. 2001;37:2365–2372. doi: 10.1016/s0959-8049(01)00303-3. [DOI] [PubMed] [Google Scholar]
- 14.de Gelder R, Fracheboud J, Heijnsdijk EA, den Heeten G, Verbeek AL, Broeders MJ, Draisma G, de Koning HJ. Digital mammography screening: weighing reduced mortality against increased overdiagnosis. Prev Med. 2011;53:134–140. doi: 10.1016/j.ypmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Domingo L, Romero A, Belvis F, Sanchez M, Ferrer J, Salas D, Ibanez J, Vega A, Ferrer F, Laso MS, Macia F, Castells X, Sala M. Differences in radiological patterns, tumour characteristics and diagnostic precision between digital mammography and screen-film mammography in four breast cancer screening programmes in Spain. Eur Radiol. 2011;21:2020–2028. doi: 10.1007/s00330-011-2143-1. [DOI] [PubMed] [Google Scholar]
- 16.Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–1620. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 17.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 18.Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;97:135–144. doi: 10.1007/s10549-005-9101-z. [DOI] [PubMed] [Google Scholar]
- 19.Esserman LJ, Thompson IM, Reid B. Overdiagnosis and Overtreatment in Cancer: An Opportunity for Improvement. JAMA. 2013 doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 20.Fallowfield L, Francis A, Catt S, Mackenzie M, Jenkins V. Time for a low-risk DCIS trial: harnessing public and patient involvement. Lancet Oncol. 2012;13:1183–1185. doi: 10.1016/S1470-2045(12)70503-X. [DOI] [PubMed] [Google Scholar]
- 21.Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, Ostman A, Landegren U. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 22.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 23.Gierach GL, Johnson J, Geller B, Vacek P, Weaver D, Chicoine R, Shepherd J, Wang J, Herschorn S, Brinton L, Sherman ME. Relationship of Peri-lesional Mammographic Density to Pathologic Diagnosis. Abstracts of the 3rd North American Congress of Epidemiology, June 21-24, 2011 Montreal, Canada. American Journal of Epidemiology. 2011;173:S67. [Google Scholar]
- 24.Grizzle WE, Srivastava S, Manne U. The biology of incipient, pre-invasive or intraepithelial neoplasia. Cancer Biomark. 2010;9:21–39. doi: 10.3233/CBM-2011-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, Tlsty T, Vachon CM, Melton LJ, 3rd, Visscher DW. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez L, Wilkerson PM, Lambros MB, Campion-Flora A, Rodrigues DN, Gauthier A, Cabral C, Pawar V, Mackay A, A'Hern R, Marchio C, Palacios J, Natrajan R, Weigelt B, Reis-Filho JS. Genomic and mutational profiling of ductal carcinomas in situ and matched adjacent invasive breast cancers reveals intra-tumour genetic heterogeneity and clonal selection. J Pathol. 2012;227:42–52. doi: 10.1002/path.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Ioffe OB, Berg WA, Silverberg SG, Kumar D. Mammographic-histopathologic correlation of large-core needle biopsies of the breast. Mod Pathol. 1998;11:721–727. [PubMed] [Google Scholar]
- 30.Karssemeijer N, Bluekens AM, Beijerinck D, Deurenberg JJ, Beekman M, Visser R, van Engen R, Bartels-Kortland A, Broeders MJ. Breast cancer screening results 5 years after introduction of digital mammography in a population-based screening program. Radiology. 2009;253:353–358. doi: 10.1148/radiol.2532090225. [DOI] [PubMed] [Google Scholar]
- 31.Kaur H, Mao S, Li Q, Sameni M, Krawetz SA, Sloane BF, Mattingly RR. RNA-Seq of human breast ductal carcinoma in situ models reveals aldehyde dehydrogenase isoform 5A1 as a novel potential target. PLoS One. 2012;7:e50249. doi: 10.1371/journal.pone.0050249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, Sorlie T, Warnberg F, Haakensen VD, Helland A, Naume B, Perou CM, Haussler D, Troyanskaya OG, Borresen-Dale AL. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A. 2012;109:2802–2807. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacombe J, Mange A, Jarlier M, Bascoul-Mollevi C, Rouanet P, Lamy PJ, Maudelonde T, Solassol J. Identification and validation of new autoantibodies for the diagnosis of DCIS and node negative early-stage breast cancers. Int J Cancer. 2013;132:1105–1113. doi: 10.1002/ijc.27766. [DOI] [PubMed] [Google Scholar]
- 34.Lari SA, Kuerer HM. Biological Markers in DCIS and Risk of Breast Recurrence: A Systematic Review. J Cancer. 2011;2:232–261. doi: 10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, Peacock S, Smazal SF, Maki DD, Julian TB, DePeri ER, Bluemke DA, Schnall MD. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 36.Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP. Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy. AJR Am J Roentgenol. 1995;164:1111–1113. doi: 10.2214/ajr.164.5.7717215. [DOI] [PubMed] [Google Scholar]
- 37.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8:R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, Zhou YX, Varnholt H, Smith B, Gadd M, Chatfield E, Kessler J, Baer TM, Erlander MG, Sgroi DC. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mange A, Lacombe J, Bascoul-Mollevi C, Jarlier M, Lamy PJ, Rouanet P, Maudelonde T, Solassol J. Serum autoantibody signature of ductal carcinoma in situ progression to invasive breast cancer. Clin Cancer Res. 2012;18:1992–2000. doi: 10.1158/1078-0432.CCR-11-2527. [DOI] [PubMed] [Google Scholar]
- 40.Markov S, Wang J, Kerlikowske K, Cummings SR, Shepherd JA. Single x-ray asborptiometry method for the quantitative mammographic measure of fibroglandular tissue volume. Med Phys. 2009;36:5525–5536. doi: 10.1118/1.3253972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCaskill-Stevens W. National Institutes of Health State-of-the-Science Conference on the Management and Diagnosis of Ductal Carcinoma in Situ: a call to action. J Natl Cancer Inst Monogr. 2010;2010:111–112. doi: 10.1093/jncimonographs/lgq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muggerud AA, Hallett M, Johnsen H, Kleivi K, Zhou W, Tahmasebpoor S, Amini RM, Botling J, Borresen-Dale AL, Sorlie T, Warnberg F. Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol Oncol. 2010;4:357–368. doi: 10.1016/j.molonc.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osako T, Iwase T, Kimura K, Horii R, Akiyama F. Detection of occult invasion in ductal carcinoma in situ of the breast with sentinel node metastasis. Cancer Sci. 2013;104:453–457. doi: 10.1111/cas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peres J. Mammography screening: after the storm, calls for more personalized approaches. J Natl Cancer Inst. 2010;102:9–11. doi: 10.1093/jnci/djp496. [DOI] [PubMed] [Google Scholar]
- 46.Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, Conant EF, Fajardo LL, Bassett L, D'Orsi C, Jong R, Rebner M. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 47.Reeves GK, Pirie K, Green J, Bull D, Beral V. Comparison of the effects of genetic and environmental risk factors on in situ and invasive ductal breast cancer. Int J Cancer. 2012;131:930–937. doi: 10.1002/ijc.26460. [DOI] [PubMed] [Google Scholar]
- 48.Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15:209–221. doi: 10.1097/00000478-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Rosen PP. Rosen's breast pathology. Lippincott-Raven Publishers; Philadelphia, PA: 1997. pp. 177–194.pp. 227–274.pp. 507–544. [Google Scholar]
- 50.Rubin E, Visscher DW, Alexander RW, Urist MM, Maddox WA. Proliferative disease and atypia in biopsies performed for nonpalpable lesions detected mammographically. Cancer. 1988;61:2077–2082. doi: 10.1002/1097-0142(19880515)61:10<2077::aid-cncr2820611024>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 51.Sameni M, Anbalagan A, Olive MB, Moin K, Mattingly RR, Sloane BF. MAME models for 4D live-cell imaging of tumor: microenvironment interactions that impact malignant progression. J Vis Exp. 2012 doi: 10.3791/3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanger N, Effenberger KE, Riethdorf S, Van Haasteren V, Gauwerky J, Wiegratz I, Strebhardt K, Kaufmann M, Pantel K. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer. 2011;129:2522–2526. doi: 10.1002/ijc.25895. [DOI] [PubMed] [Google Scholar]
- 53.Schnitt SJ, Connolly JL, Tavassoli FA, Fechner RE, Kempson RL, Gelman R, Page DL. Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol. 1992;16:1133–1143. doi: 10.1097/00000478-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Sherman ME, Dasgupta A, Schiffman M, Nayar R, Solomon D. The Bethesda Interobserver Reproducibility Study (BIRST): a web-based assessment of the Bethesda 2001 System for classifying cervical cytology. Cancer. 2007;111:15–25. doi: 10.1002/cncr.22423. [DOI] [PubMed] [Google Scholar]
- 55.Skaane P, Hofvind S, Skjennald A. Randomized trial of screen-film versus full-field digital mammography with soft-copy reading in population-based screening program: follow-up and final results of Oslo II study. Radiology. 2007;244:708–717. doi: 10.1148/radiol.2443061478. [DOI] [PubMed] [Google Scholar]
- 56.Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW, Jr, Davidson NE, Ingle JN, Perez EA, Wood WC, Sparano JA, Badve S. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701–710. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tavassoli FA. Pathology of the breast. Appleton & Lange; Stamford, CT: 1999. pp. 205–324.pp. 373–400. [Google Scholar]
- 58.Ursin G, Hovanessian-Larsen L, Parisky YR, Pike MC, Wu AH. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005;7:R605–608. doi: 10.1186/bcr1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkitaraman R. Lobular neoplasia of the breast. Breast J. 2010;16:519–528. doi: 10.1111/j.1524-4741.2010.00971.x. [DOI] [PubMed] [Google Scholar]
- 60.Vigeland E, Klaasen H, Klingen TA, Hofvind S, Skaane P. Full-field digital mammography compared to screen film mammography in the prevalent round of a population-based screening programme: the Vestfold County Study. Eur Radiol. 2008;18:183–191. doi: 10.1007/s00330-007-0730-y. [DOI] [PubMed] [Google Scholar]
- 61.Vincent-Salomon A, Lucchesi C, Gruel N, Raynal V, Pierron G, Goudefroye R, Reyal F, Radvanyi F, Salmon R, Thiery JP, Sastre-Garau X, Sigal-Zafrani B, Fourquet A, Delattre O. Integrated genomic and transcriptomic analysis of ductal carcinoma in situ of the breast. Clin Cancer Res. 2008;14:1956–1965. doi: 10.1158/1078-0432.CCR-07-1465. [DOI] [PubMed] [Google Scholar]
- 62.Virnig BA, Shamliyan T, Tuttle TM, Kane RL, Wilt TJ. AHRQ publication N 09-E018. Agency for Healthcare Research and Quality.; Rockville, MD: Diagnosis and management of ductal carcinoma in situ (DCIS). Evidence report/technology assessment No. 185 (Prepared by the Minnesota evidence-based practice center under contract No. 290-02-10064-I) [Google Scholar]
- 63.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 64.Wang SY, Chu H, Shamliyan T, Jalal H, Kuntz KM, Kane RL, Virnig BA. Network meta-analysis of margin threshold for women with ductal carcinoma in situ. J Natl Cancer Inst. 2012;104:507–516. doi: 10.1093/jnci/djs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang SY, Shamliyan T, Virnig BA, Kane R. Tumor characteristics as predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Breast Cancer Res Treat. 2011;127:1–14. doi: 10.1007/s10549-011-1387-4. [DOI] [PubMed] [Google Scholar]
- 66.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 67.Welch HG, Black WC. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med. 1997;127:1023–1028. doi: 10.7326/0003-4819-127-11-199712010-00014. [DOI] [PubMed] [Google Scholar]
- 68.Wellings SR, Jensen HM. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973;50:1111–1118. doi: 10.1093/jnci/50.5.1111. [DOI] [PubMed] [Google Scholar]
- 69.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, Fasching PA, Hein R, Spurdle AB, Blows F, Driver K, Flesch-Janys D, Heinz J, Sinn P, Vrieling A, Heikkinen T, Aittomaki K, Heikkila P, Blomqvist C, Lissowska J, Peplonska B, Chanock S, Figueroa J, Brinton L, Hall P, Czene K, Humphreys K, Darabi H, Liu J, Van 't Veer LJ, van Leeuwen FE, Andrulis IL, Glendon G, Knight JA, Mulligan AM, O'Malley FP, Weerasooriya N, John EM, Beckmann MW, Hartmann A, Weihbrecht SB, Wachter DL, Jud SM, Loehberg CR, Baglietto L, English DR, Giles GG, McLean CA, Severi G, Lambrechts D, Vandorpe T, Weltens C, Paridaens R, Smeets A, Neven P, Wildiers H, Wang X, Olson JE, Cafourek V, Fredericksen Z, Kosel M, Vachon C, Cramp HE, Connley D, Cross SS, Balasubramanian SP, Reed MW, Dork T, Bremer M, Meyer A, Karstens JH, Ay A, Park-Simon TW, Hillemanns P, Arias Perez JI, Menendez Rodriguez P, Zamora P, Benitez J, Ko YD, Fischer HP, Hamann U, Pesch B, Bruning T, Justenhoven C, Brauch H, Eccles DM, Tapper WJ, Gerty SM, Sawyer EJ, Tomlinson IP, Jones A, Kerin M, Miller N, McInerney N, Anton-Culver H, Ziogas A, Shen CY, Hsiung CN, Wu PE, Yang SL, Yu JC, Chen ST, Hsu GC, Haiman CA, Henderson BE, Le Marchand L, Kolonel LN, Lindblom A, Margolin S, Jakubowska A, Lubinski J, Huzarski T, Byrski T, Gorski B, Gronwald J, Hooning MJ, Hollestelle A, van den Ouweland AM, Jager A, Kriege M, Tilanus-Linthorst MM, Collee M, Wang-Gohrke S, Pylkas K, Jukkola-Vuorinen A, Mononen K, Grip M, Hirvikoski P, Winqvist R, Mannermaa A, Kosma VM, Kauppinen J, Kataja V, Auvinen P, Soini Y, Sironen R, Bojesen SE, Orsted DD, Kaur-Knudsen D, Flyger H, Nordestgaard BG, Holland H, Chenevix-Trench G, Manoukian S, Barile M, Radice P, Hankinson SE, Hunter DJ, Tamimi R, Sangrajrang S, Brennan P, McKay J, Odefrey F, Gaborieau V, Devilee P, Huijts PE, Tollenaar RA, Seynaeve C, Dite GS, Apicella C, Hopper JL, Hammet F, Tsimiklis H, Smith LD, Southey MC, Humphreys MK, Easton D, Pharoah P, Sherman ME, Garcia-Closas M. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]


